Use of Pea Proteins in High-Moisture Meat Analogs: Physicochemical Properties of Raw Formulations and Their Texturization Using Extrusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Recipes
2.2. Moisture and Protein Contents
2.3. Particle Size
2.4. Protein Solubility
2.5. Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.6. Rapid-Visco Analysis
2.7. Least Gelation Concentration
2.8. Differential Scanning Calorimetry
2.9. Size Exclusion Chromatography by High Performance Liquid Chromatography (SEC-HPLC)
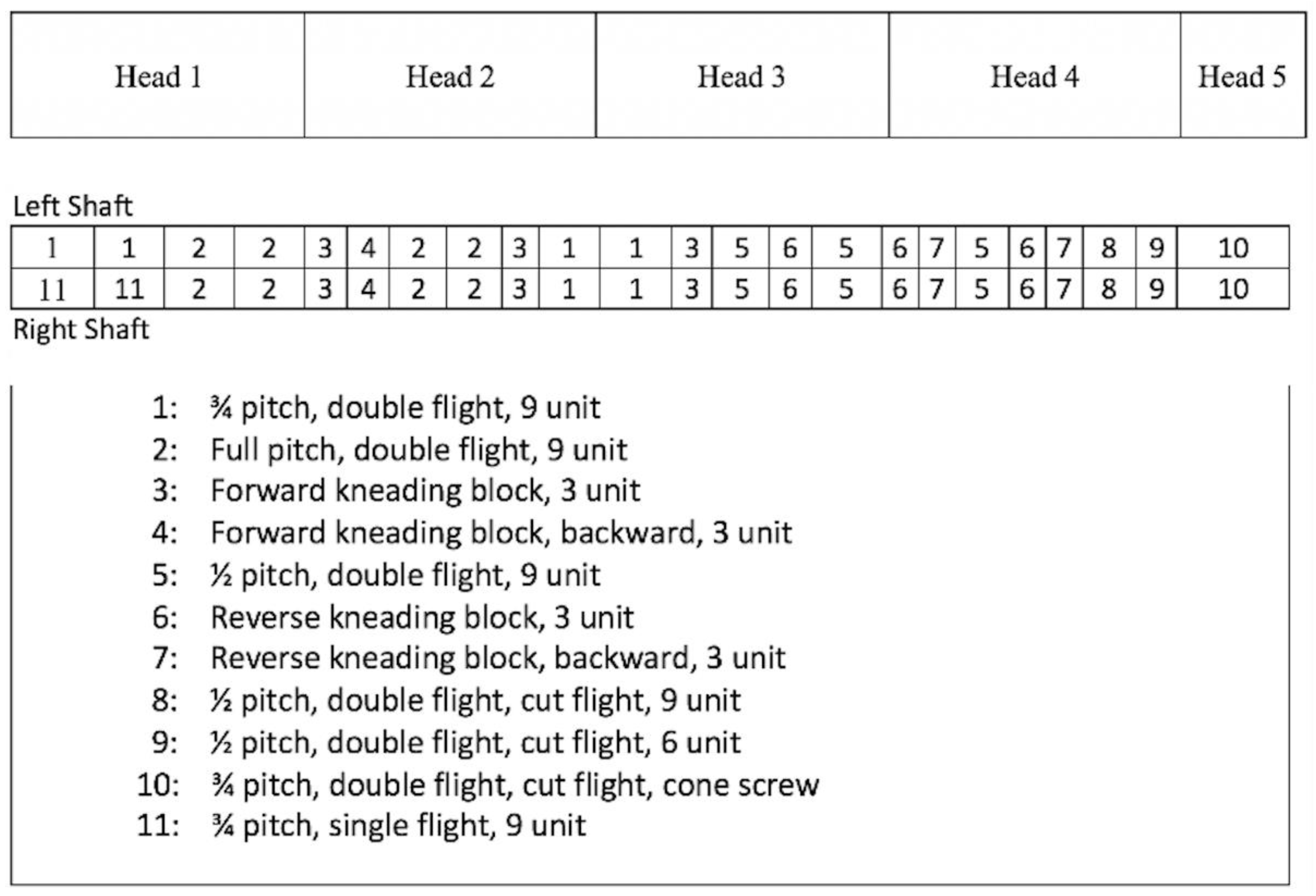
2.10. Extrusion Processing
2.11. Texture Analysis
2.12. Sensory Analysis
2.13. Statistical Analysis
3. Results and Discussion
3.1. Protein Content
3.2. Particle Size
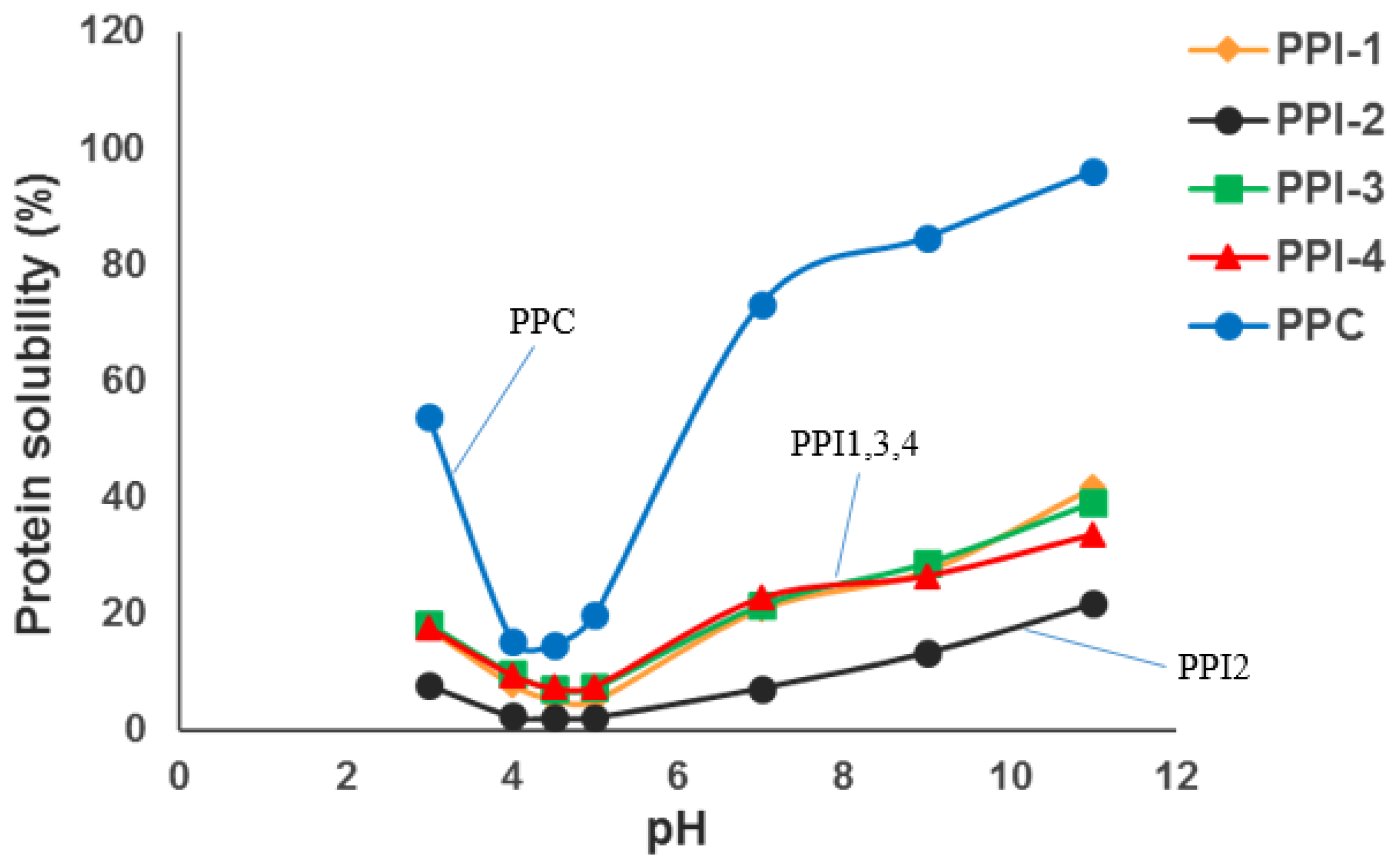
3.3. Protein Solubility
3.4. Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis
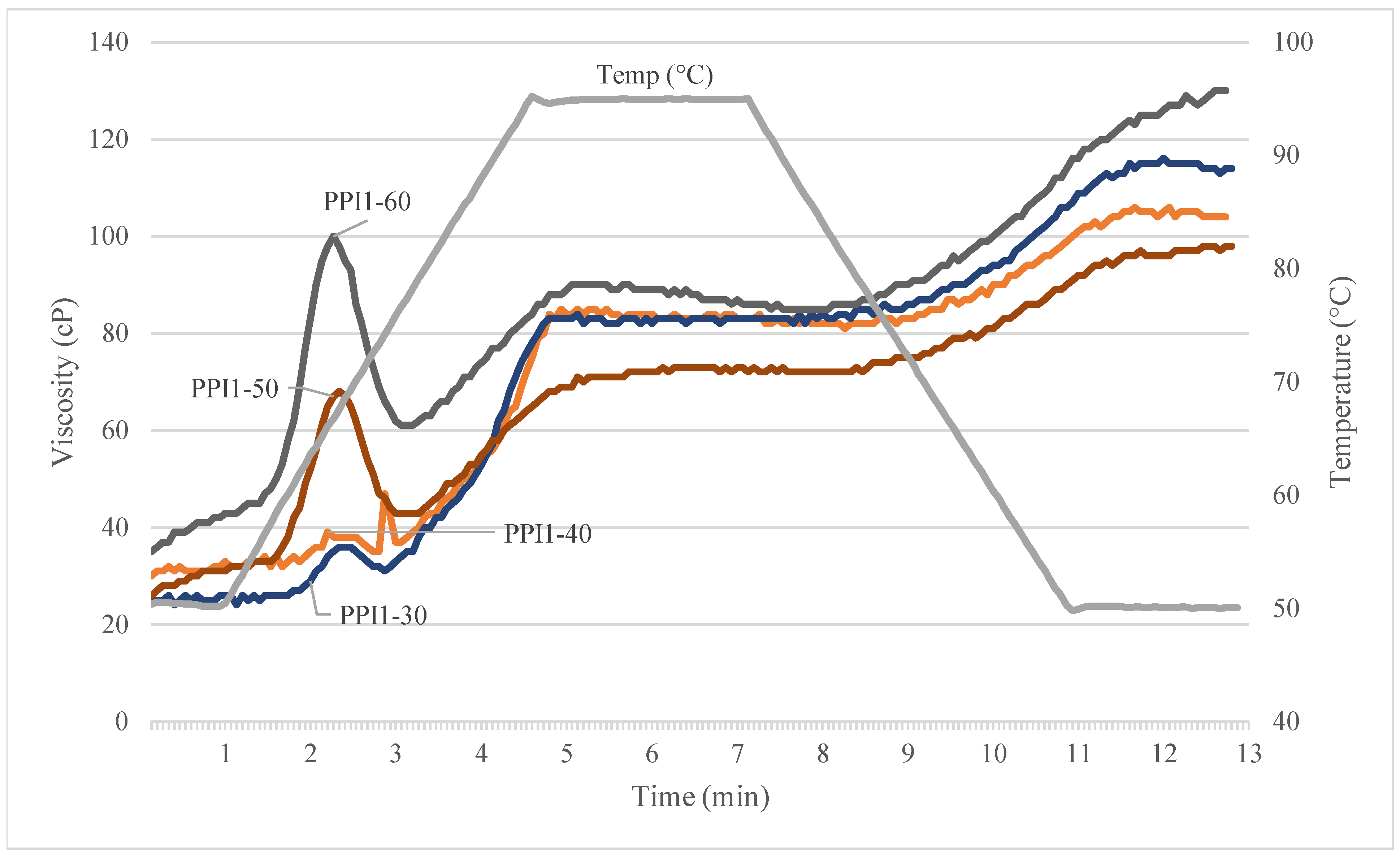
3.5. Rapid-Visco Analysis
3.6. Least Gelation Concentration
3.7. Differential Scanning Calorimetry
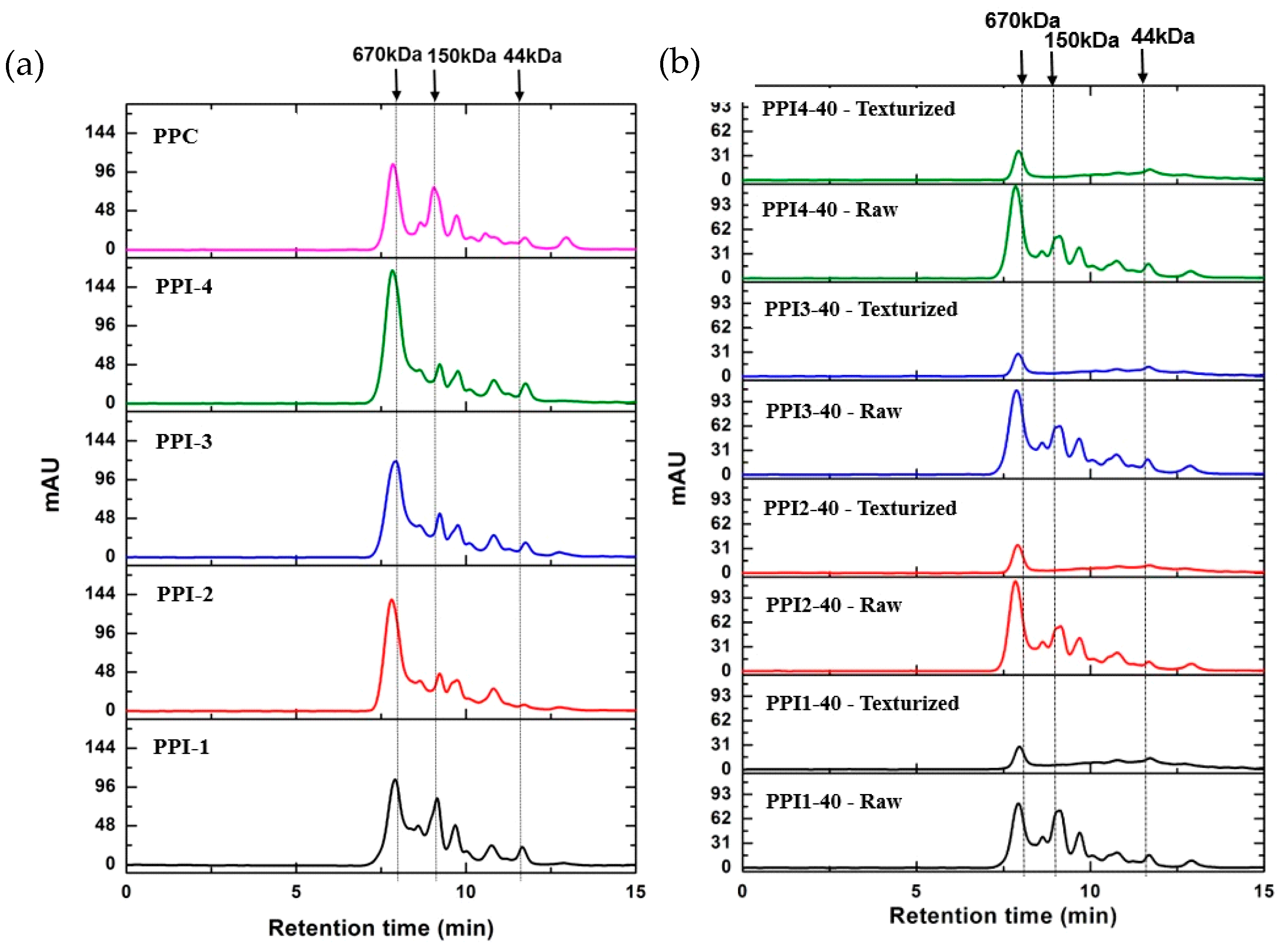
3.8. Size Exclusion Chromatography by High Performance Liquid Chromatography (SEC-HPLC)
3.9. Extrusion Parameters
3.9.1. Moisture
3.9.2. Mechanical Energy
3.10. Texture Analysis
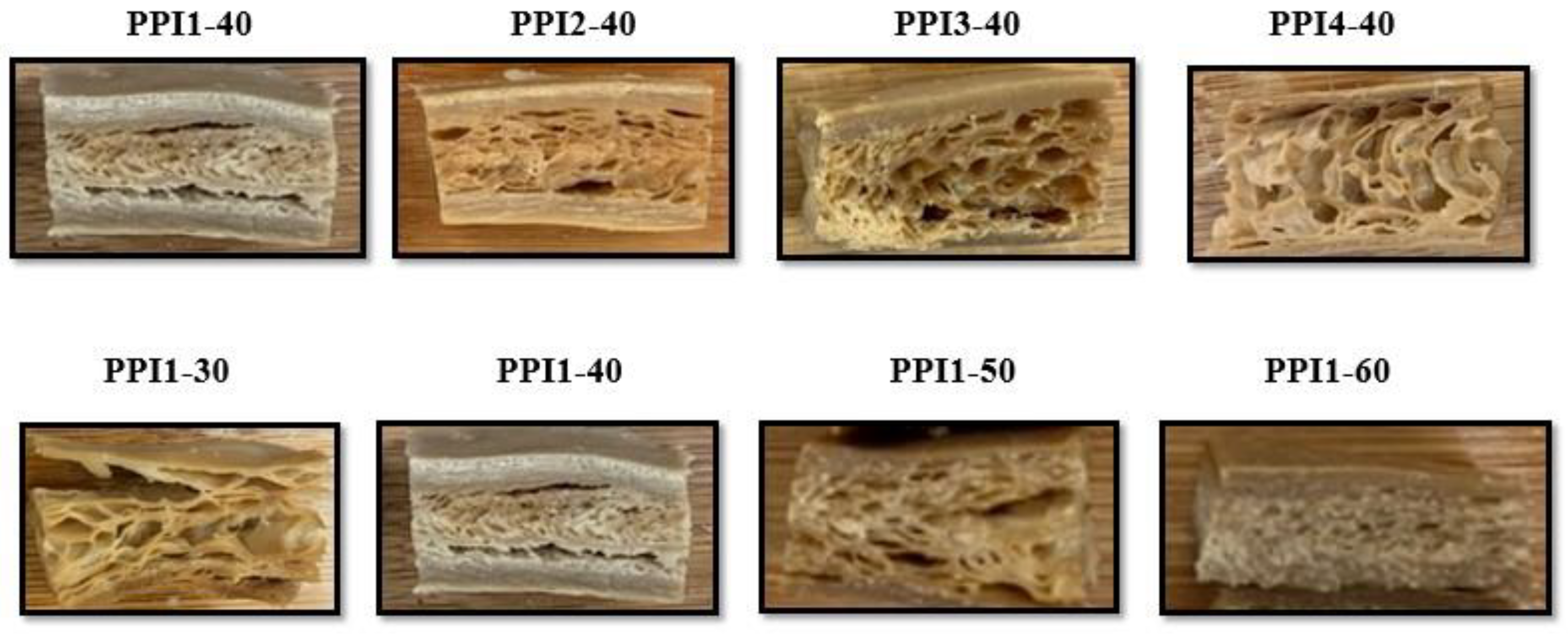
3.10.1. Cutting Test
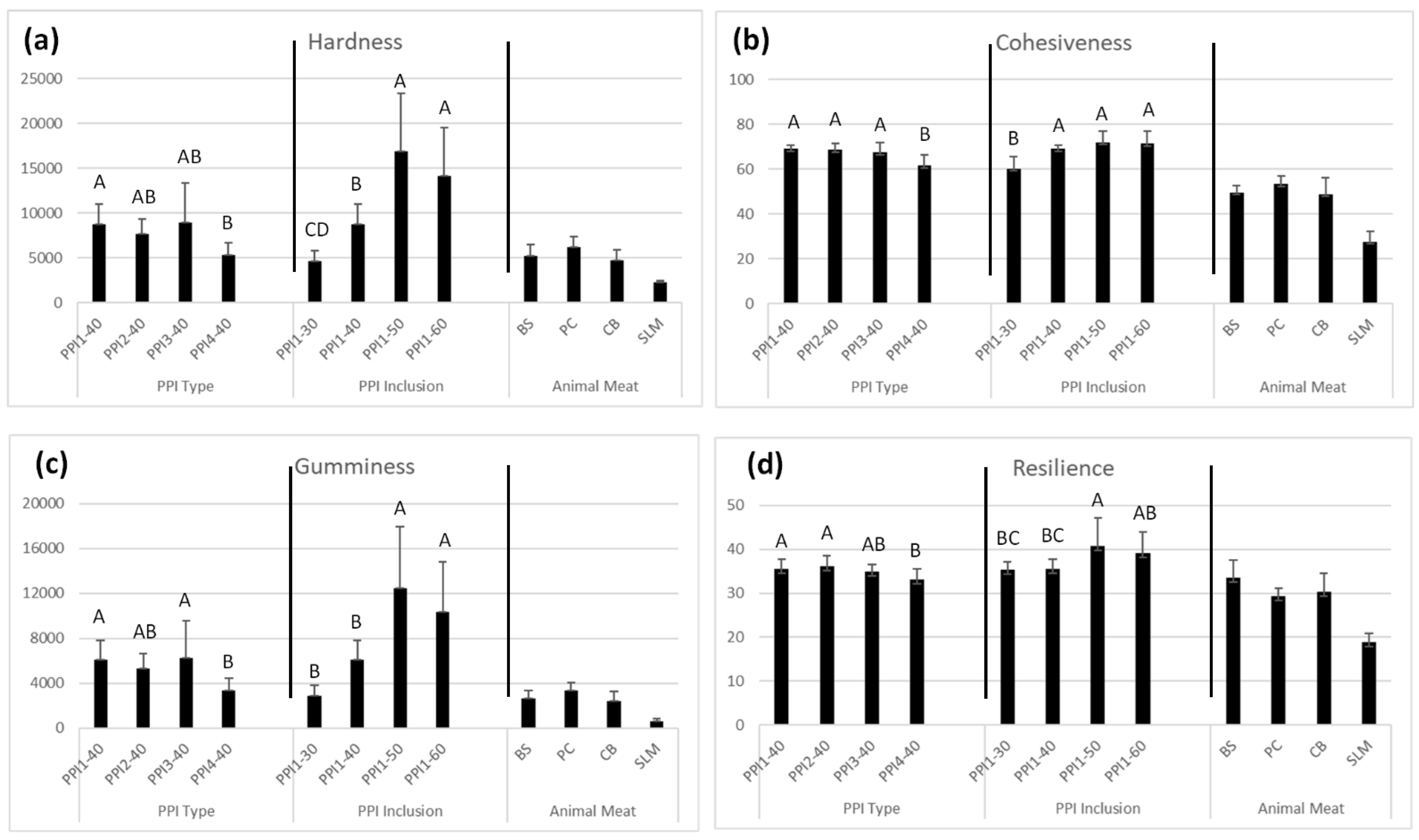
3.10.2. Texture Profile Analysis
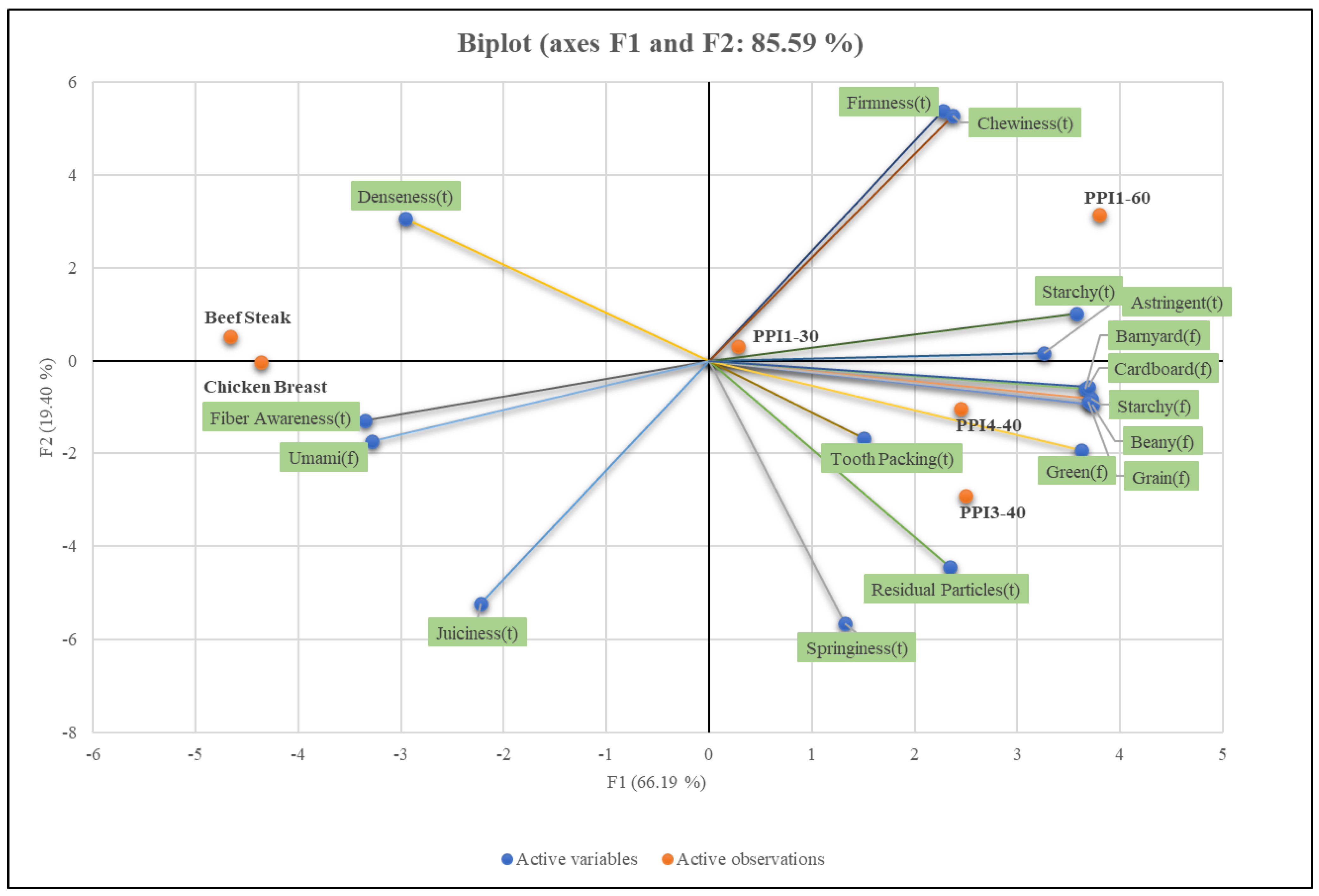
3.11. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Practical Application
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Textured Vegetable Protein Market Size, Share, Global Trends, and Forecasts to 2027. Available online: https://www.marketsandmarkets.com/Market-Reports/textured-vegetable-protein-market-264440297.html (accessed on 8 October 2023).
- Plattner, B. Extrusion Processing Technologies for Textured Vegetable Protein. In Manual of Textured Vegetable Protein and Other Soy Products; Riaz, M.M., Ed.; Texas A&M University: College Station, TX, USA, 2009. [Google Scholar]
- Riaz, M.N. 15—Texturized Vegetable Proteins. In Handbook of Food Proteins; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2011; pp. 395–418. ISBN 978-1-84569-758-7. [Google Scholar]
- Alexander, P.; Brown, C.; Dias, C.; Moran, D.; Rounsevell, M.D.A. Chapter 1—Sustainable Proteins Production. In Proteins: Sustainable Source, Processing and Applications; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–39. ISBN 978-0-12-816695-6. [Google Scholar]
- Samard, S.; Gu, B.Y.; Ryu, G.H. Effects of Extrusion Types, Screw Speed and Addition of Wheat Gluten on Physicochemical Characteristics and Cooking Stability of Meat Analogues. J. Sci. Food Agric. 2019, 99, 4922–4931. [Google Scholar] [CrossRef]
- Webb, D.; Plattner, B.J.; Donald, E.; Funk, D.; Plattner, B.S.; Alavi, S. Role of Chickpea Flour in Texturization of Extruded Pea Protein. J. Food Sci. 2020, 85, 4180–4187. [Google Scholar] [CrossRef]
- Asgar, M.A.; Fazilah, A.; Huda, N.; Bhat, R.; Karim, A.A. Nonmeat Protein Alternatives as Meat Extenders and Meat Analogs. Compr. Rev. Food Sci. Food Saf. 2010, 9, 513–529. [Google Scholar] [CrossRef]
- Vatansever, S.; Tulbek, M.C.; Riaz, M.N. Low- and High-Moisture Extrusion of Pulse Proteins as Plant-Based Meat Ingredients: A Review. CFW 2020, 65. [Google Scholar] [CrossRef]
- Osen, R.; Toelstede, S.; Wild, F.; Eisner, P.; Schweiggert-Weisz, U. High Moisture Extrusion Cooking of Pea Protein Isolates: Raw Material Characteristics, Extruder Responses, and Texture Properties. J. Food Eng. 2014, 127, 67–74. [Google Scholar] [CrossRef]
- Osen, R. Texturization of Pea Protein Isolates Using High Moisture Extrusion Cooking. Ph.D. Thesis, Technische Universität München, München, Germany, 2017. [Google Scholar]
- Samard, S.; Ryu, G.H. Physicochemical and Functional Characteristics of Plant Protein-Based Meat Analogs. J. Food Process. Preserv. 2019, 43, e14123. [Google Scholar] [CrossRef]
- Ferawati, F.; Zahari, I.; Barman, M.; Hefni, M.; Ahlström, C.; Witthöft, C.; Östbring, K. High-Moisture Meat Analogues Produced from Yellow Pea and Faba Bean Protein Isolates/Concentrate: Effect of Raw Material Composition and Extrusion Parameters on Texture Properties. Foods 2021, 10, 843. [Google Scholar] [CrossRef]
- Cereals & Grains Association; AACCI. 44-19.01 Moisture–Air-Oven Method, Drying at 135 Degrees. In AACC International Approved Methods. AACC International; Cereals & Grains Association: St. Paul, MN, USA, 2009; Volume 10. [Google Scholar]
- AACC International. Method 46-30.01. Crude Protein—Combustion Method. In Approved Methods of Analysis; Cereals & Grains Association: St. Paul, MN, USA, 1999. [Google Scholar]
- Shen, Y.; Tang, X.; Li, Y. Drying Methods Affect Physicochemical and Functional Properties of Quinoa Protein Isolate. Food Chem. 2021, 339, 127823. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- AACC. Method 76–21.02. General Pasting Method for Wheat or Rye Flour of Starch Using the Rapid Visco Analyser. In AACC Approved Methods of Analysis; Cereals & Grains Association: St. Paul, MN, USA, 1997. [Google Scholar]
- Zhu, S.M.; Lin, S.L.; Ramaswamy, H.S.; Yu, Y.; Zhang, Q.T. Enhancement of Functional Properties of Rice Bran Proteins by High Pressure Treatment and Their Correlation with Surface Hydrophobicity. Food Bioprocess Technol. 2017, 10, 317–327. [Google Scholar] [CrossRef]
- Brishti, F.; Zarei, M.; Muhammad, K.; Ismail-Fitry, M.R.; Shukri, R.; Saari, N. Evaluation of the Functional Properties of Mung Bean Protein Isolate for Development of Textured Vegetable Protein. Int. Food Res. J. 2017, 24, 1595–1605. [Google Scholar]
- Kim, T.; Riaz, M.N.; Awika, J.; Teferra, T.F. The Effect of Cooling and Rehydration Methods in High Moisture Meat Analogs with Pulse Proteins-Peas, Lentils, and Faba Beans. J. Food Sci. 2021, 86, 1322–1334. [Google Scholar] [CrossRef]
- Zahari, I.; Ferawati, F.; Helstad, A.; Ahlström, C.; Östbring, K.; Rayner, M.; Purhagen, J.K. Development of High-Moisture Meat Analogues with Hemp and Soy Protein Using Extrusion Cooking. Foods 2020, 9, 772. [Google Scholar] [CrossRef]
- USDA. Cooking Meat? Check the New Recommended Temperatures. Available online: https://www.usda.gov/media/blog/2011/05/25/cooking-meat-check-new-recommended-temperatures (accessed on 14 January 2024).
- Texture Profile Analysis. Available online: https://texturetechnologies.com/resources/texture-profile-analysis (accessed on 8 October 2023).
- Hootman, R.C. Manual on Descriptive Analysis Testing for Sensory Evaluation; ASTM: Philadelphia, PA, USA, 1992. [Google Scholar]
- Society of Sensory Professionals (SSP). Recommendations for Publications Containing Sensory Data. Available online: https://www.sensorysociety.org/pages/default.aspx (accessed on 14 January 2024).
- Shen, Y.; Li, Y. Acylation Modification and/or Guar Gum Conjugation Enhanced Functional Properties of Pea Protein Isolate. Food Hydrocoll. 2021, 117, 106686. [Google Scholar] [CrossRef]
- Tang, X.; Shen, Y.; Zhang, Y.; Schilling, M.W.; Li, Y. Parallel Comparison of Functional and Physicochemical Properties of Common Pulse Proteins. LWT 2021, 146, 111594. [Google Scholar] [CrossRef]
- Izadi, M.; Aminlari, M.; Tavana, M. Changes in the Solubility and SDS-PAGE Profile of Whey Proteins during Storage at Different Temperatures: A Kinetic Study. J. Food Agric. Sci. 2011, 1, 15–21. [Google Scholar]
- Webb, D.M. Physicochemical Properties of Pea Proteins, Texturization Using Extrusion, and Application in Plant-Based Meats. Master’s Thesis, Kansas State University, Manhattan, KS, USA, 2021. [Google Scholar]
- Riaz, M.N. Texturized Soy Protein as an Ingredient. In Proteins in Food Processing; Woodhead Publishing: Cambridge, UK, 2004; pp. 517–558. [Google Scholar]
- Onwulata, C.I.; Tunick, M.H.; Thomas-Gahring, A.E. Rapid Visco Analysis of Food Protein Pastes. J. Food Process. Preserv. 2014, 38, 2083–2089. [Google Scholar] [CrossRef]
- Yang, J.; Zamani, S.; Liang, L.; Chen, L. Extraction Methods Significantly Impact Pea Protein Composition, Structure and Gelling Properties. Food Hydrocoll. 2021, 117, 106678. [Google Scholar] [CrossRef]
- Nicolai, T.; Chassenieux, C. Heat-Induced Gelation of Plant Globulins. Curr. Opin. Food Sci. 2019, 27, 18–22. [Google Scholar] [CrossRef]
- Tulbek, M.C.; Lam, R.S.H.; Wang, Y.C.; Asavajaru, P.; Lam, A. Chapter 9—Pea: A Sustainable Vegetable Protein Crop. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 145–164. ISBN 978-0-12-802778-3. [Google Scholar]
- Liu, Y.-X.; Cao, M.-J.; Liu, G.-M. 17—Texture Analyzers for Food Quality Evaluation. In Evaluation Technologies for Food Quality; Zhong, J., Wang, X., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2019; pp. 441–463. ISBN 978-0-12-814217-2. [Google Scholar]
- Zhang, J.; Liu, L.; Jiang, Y.; Faisal, S.; Wang, Q. A New Insight into the High-Moisture Extrusion Process of Peanut Protein: From the Aspect of the Orders and Amount of Energy Input. J. Food Eng. 2020, 264, 109668. [Google Scholar] [CrossRef]
- Palanisamy, M.; Franke, K.; Berger, R.G.; Heinz, V.; Töpfl, S. High Moisture Extrusion of Lupin Protein: Influence of Extrusion Parameters on Extruder Responses and Product Properties. J. Sci. Food Agric. 2019, 99, 2175–2185. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Zhang, Y.; Meng, S.; Wang, Q. Rheological Properties of Pea Protein Isolate-Amylose/Amylopectin Mixtures and the Application in the High-Moisture Extruded Meat Substitutes. Food Hydrocoll. 2021, 117, 106732. [Google Scholar] [CrossRef]
- Yongsawatdigul, J.; Carvajal-Rondanelli, P.; Lanier, T. Surimi Gelation Chemistry. In Surimi and Surimi Seafood; CRC Press: Boca Raton, FL, USA, 2005; pp. 435–489. ISBN 978-0-8247-2649-2. [Google Scholar]
- Flory, J.; Alavi, S. Use of Hydration Properties of Proteins to Understand Their Functionality and Tailor Texture of Extruded Plant-Based Meat Analogues. J. Food Sci. 2024, 89, 245–258. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Q.; Zhang, N.; Bak, K.H.; Soladoye, O.P.; Aluko, R.E.; Fu, Y.; Zhang, Y. Insights into Formation, Detection and Removal of the Beany Flavor in Soybean Protein. Trends Food Sci. Technol. 2021, 112, 336–347. [Google Scholar] [CrossRef]



| Recipes a | Recipes b | |||||||
|---|---|---|---|---|---|---|---|---|
| Ingredients: | PPI1-40 | PPI2-40 | PPI3-40 | PPI4-40 | PPI1-30 | PPI1-40 | PPI1-50 | PPI1-60 |
| Pea Protein Isolate | 40 | 40 | 40 | 40 | 30 | 40 | 50 | 60 |
| Pea Protein Concentrate | 38 | 38 | 38 | 38 | 48 | 38 | 28 | 18 |
| Pea Flour | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 13 |
| Pea Fiber | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Salt | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Oil | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Recipes a | Recipes b | |||||||
|---|---|---|---|---|---|---|---|---|
| Nutrients: | PPI1-40 | PPI2-40 | PPI3-40 | PPI4-40 | PPI1-30 | PPI1-40 | PPI1-50 | PPI1-60 |
| Protein (%) | 54.00 | 52.88 | 53.50 | 52.76 | 51.33 | 54.00 | 56.68 | 59.36 |
| Starch (%) | 10.36 | 10.36 | 10.36 | 10.36 | 10.56 | 10.36 | 10.16 | 9.96 |
| Fiber (%) | 8.62 | 8.62 | 8.62 | 8.62 | 10.12 | 8.62 | 7.12 | 5.62 |
| Fat (%) | 1.65 | 1.65 | 1.65 | 1.65 | 2.05 | 1.65 | 1.25 | 0.85 |
| Extrusion Conditions: | ||||||||
| In-Barrel Moisture (%) | 41.99 | 42.52 | 41.01 | 41.12 | 40.57 | 41.99 | 44.64 | 45.31 |
| Specific Mechanical Energy (kJ/kg) | 812.57 | 750.86 | 699.43 | 709.71 | 678.86 | 812.57 | 514.29 | 493.71 |
| Die Temperature (°C) | 163 | 161 | 163 | 158 | 161 | 163 | 155 | 151 |
| Die Pressure (psig) | 850 | 825 | 650 | 575 | 575 | 850 | 400 | 425 |
| Cumulative (%) | |||||
|---|---|---|---|---|---|
| Sieves (μm) | PPI1 | PPI2 | PPI3 | PPI4 | PPC |
| 25 | 1.5 | 3.9 | 4.2 | 2.3 | 0.3 |
| 32 | 5.2 | 11.5 | 4.9 | 4.8 | 1.4 |
| 53 | 27.8 | 81.5 | 93.4 | 23.3 | 71.6 |
| 63 | 33.8 | 92.1 | 95.1 | 33.4 | 92.3 |
| 75 | 41.4 | 96.4 | 96.2 | 43.2 | 99.9 |
| 90 | 50.7 | 98.6 | 97.1 | 54.6 | 100.0 |
| 106 | 57.9 | 99.5 | 97.7 | 63.0 | 100.0 |
| 125 | 66.0 | 100.0 | 98.2 | 71.4 | 100.0 |
| 250 | 96.2 | 100.0 | 99.9 | 97.3 | 100.0 |
| Peak Viscosity (cP) | Final Viscosity (cP) | Pasting Time (s) | Pasting Temp (°C) | |
|---|---|---|---|---|
| PPI1 | 525 ± 59 A | 68 ± 3 D | 24 ± 11 E | 50 ± 0 C |
| PPI2 | 115 ± 14 C | 90 ± 6 C | 293 ± 5 C | 95 ± 0 A |
| PPI3 | 63 ± 2 C | 83 ± 3 CD | 349 ± 0 A | 95 ± 0 A |
| PPI4 | 293 ± 19 B | 145 ± 9 B | 232 ± 0 D | 85 ± 0 B |
| PPC | 133 ± 5 C | 182 ± 5 A | 312 ± 0 B | 95 ± 0 A |
| Recipe | ||||
| PPI1-40 | 39 ± 7 BC | 106 ± 5 A | 153 ± 6 B | 69 ± 1 B |
| PPI2-40 | 118 ± 9 A | 117 ± 8 A | 304 ± 4 A | 95 ± 0 A |
| PPI3-40 | 23 ± 1 C | 81 ± 10 B | 165 ± 16 B | 71 ± 3 B |
| PPI4-40 | 52 ± 6 B | 119 ± 10 A | 161 ± 2 B | 70 ± 1 B |
| LGC | |||||
|---|---|---|---|---|---|
| 12% | 14% | 16% | 18% | 20% | |
| PPI1 | - | - | + | + | ++ |
| PPI2 | - | / | + | ++ | ++ |
| PPI3 | - | / | + | ++ | ++ |
| PPI4 | + | + | ++ | ++ | ++ |
| PPC | + | + | ++ | ++ | ++ |
| Onset Temperature (°C) | Peak Denaturation Temperature (°C) | End Temperature (°C) | 1 Enthalpy (J/g) | |
|---|---|---|---|---|
| PPI1 | 161.50 A | 190.82 A | 206.75 A | 18.88 |
| PPI2 | 159.59 AB | 183.76 B | 199.32 B | 19.75 |
| PPI3 | 153.93 C | 181.38 BC | 196.04 BC | 15.30 |
| PPI4 | 154.42 BC | 180.20 C | 192.48 BC | 13.75 |
| PPC | 156.85 ABC | 175.79 D | 190.81 C | 20.58 |
| Recipe | Firmness Ratio | Toughness Ratio |
|---|---|---|
| PPI1-40 | 1.11 | 1.16 |
| PPI2-40 | 1.18 | 1.10 |
| PPI3-40 | 1.17 | 1.14 |
| PPI4-40 | 1.30 | 1.29 |
| PPI1-30 | 1.53 | 1.69 |
| PPI1-40 | 1.11 | 1.16 |
| PPI1-50 | 1.29 | 1.20 |
| PPI1-60 | 0.94 | 1.13 |
| Attribute | Definition | Reference |
|---|---|---|
| Springiness (Texture) | Degree to which sample returns to its original height when compressed once partially with molar teeth and slowly released. | Oscar Mayer Uncured Bun-Length Wieners |
| Denseness (Texture) | The degree of compactness of the cross section. | Oscar Mayer Uncured Bun-Length Wieners |
| Juiciness (Texture) | The amount of liquid expressed from the sample at the maximum intensity from 5 chews. | Hormel Cure 81 Ham |
| Residual Particles (Texture) | Particles remaining in mouth after mastication and swallowing. Maybe fibers, flakes and/or granules. | Hormel Cure 81 Ham |
| Firmness (Texture) | The force required to bite completely through the meat pieces with the molar teeth. | Hormel Cure 81 Ham |
| Chewiness (Texture) | Difficulty with which the sample can be broken down with the molars for swallowing. | Hormel Cure 81 Ham |
| Fiber Awareness (Texture) | The perception of filaments or strands of muscle tissue in product during mastication. | Hillshire Farms Lit’l Beef Smokies |
| Tooth Packing (Texture) | The amount of sample packed in a between the molar teeth after swallowing. | Wheaties |
| Astringent (Texture) | A drying puckering or tingling sensation on the surface and/or edge of the tongue and mouth. | Alum Solution |
| Starchy (Texture) | Degree to which the sample mixes with saliva to form a starchy, pasty slurry that coats mouth surfaces after swallowing. | American Beauty Elbo-Roni |
| Beany (Flavor) | A slightly brown, musty, slightly nutty, and starchy flavor associated with cooked beans. | Bush’s Best Pinto Beans |
| Starchy (Flavor) | The dry aromatics associated with starch and starch-based grain products such as wheat, rice, oats, and other grains. | Bush’s Best Pinto Beans |
| Grain (Flavor) | A general term used to describe the aromatic which includes musty, dusty, slightly brown, slightly sweet and is associated with harvested grains and dry grain stems. | Cereal Mixture (dry) |
| Green (Flavor) | A green aromatic associated with fresh green peapods. May include beany, increased pungent, musty/earthy, bitter and astringent. | Great Value Frozen Baby Lima Beans |
| Umami (Flavor) | A general term for aromatics associated with juices from cooked seafood, meat and/or vegetables. | Button Mushroom Broth |
| Barnyard (Flavor) | Combination of pungent, slightly sour, hay-like aromatics associated with farm animals and the inside of a barn. | White Pepper in water |
| Cardboard (Flavor) | The flat aromatics that may be associated with cardboard or paper packaging. | Cardboard soaked in water |
| Recipe | 1 Springiness | Denseness | Juiciness | Residual Particles | Firmness | Chewiness | Fiber Awareness | Tooth Packing | Astringent | Starchy |
|---|---|---|---|---|---|---|---|---|---|---|
| PPI3-40 | 5.19 | 6.94 B | 5.94 | 4.25 | 8.94 CD | 8.25 b | 7.25 | 2.06 | 2.81 | 2.19 AB |
| PPI4-40 | 4.31 | 7.94 AB | 5.50 | 3.81 | 9.69 bc | 9.00 ab | 7.19 | 2.19 | 2.81 | 2.69 a |
| PPI1-30 | 4.56 | 8.81 ab | 5.00 | 3.63 | 10.56 b | 9.31 ab | 6.88 | 2.19 | 2.13 | 1.00 BC |
| PPI1-60 | 3.19 | 8.31 AB | 3.88 | 3.56 | 13.00 a | 11.13 a | 6.75 | 2.00 | 3.00 | 3.38 a |
| Beef Steak | 3.75 | 11.00 a | 6.19 | 3.56 | 9.44 bcD | 8.63 b | 8.31 | 1.63 | 2.25 | 0.25 C |
| Chicken Breast | 3.31 | 8.94 ab | 5.63 | 3.19 | 8.25 D | 7.75 b | 7.88 | 2.19 | 1.69 | 0.25 C |
| Recipe | Beany | Starchy | Grain | Green | 1 Umami | Barnyard | Cardboard |
|---|---|---|---|---|---|---|---|
| PPI3-40 | 5.06 a | 3.13 a | 3.44 a | 2.81 a | 2.13 | 2.06 | 2.56 a |
| PPI4-40 | 4.88 a | 3.00 a | 3.69 a | 2.63 a | 2.31 | 2.13 | 3.06 a |
| PPI1-30 | 3.69 a | 1.69 AB | 2.19 ab | 1.56 ab | 2.00 | 1.50 | 1.75 ab |
| PPI1-60 | 4.88 a | 3.19 a | 3.50 a | 2.31 a | 1.81 | 2.19 | 2.81 a |
| Beef Steak | 0.25 b | 0.00 B | 1.00 b | 0.25 b | 2.63 | 0.69 | 0.50 b |
| Chicken Breast | 0.25 b | 0.50 B | 0.81 b | 0.50 b | 2.88 | 1.13 | 0.25 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plattner, B.J.; Hong, S.; Li, Y.; Talavera, M.J.; Dogan, H.; Plattner, B.S.; Alavi, S. Use of Pea Proteins in High-Moisture Meat Analogs: Physicochemical Properties of Raw Formulations and Their Texturization Using Extrusion. Foods 2024, 13, 1195. https://doi.org/10.3390/foods13081195
Plattner BJ, Hong S, Li Y, Talavera MJ, Dogan H, Plattner BS, Alavi S. Use of Pea Proteins in High-Moisture Meat Analogs: Physicochemical Properties of Raw Formulations and Their Texturization Using Extrusion. Foods. 2024; 13(8):1195. https://doi.org/10.3390/foods13081195
Chicago/Turabian StylePlattner, Blake J., Shan Hong, Yonghui Li, Martin J. Talavera, Hulya Dogan, Brian S. Plattner, and Sajid Alavi. 2024. "Use of Pea Proteins in High-Moisture Meat Analogs: Physicochemical Properties of Raw Formulations and Their Texturization Using Extrusion" Foods 13, no. 8: 1195. https://doi.org/10.3390/foods13081195
APA StylePlattner, B. J., Hong, S., Li, Y., Talavera, M. J., Dogan, H., Plattner, B. S., & Alavi, S. (2024). Use of Pea Proteins in High-Moisture Meat Analogs: Physicochemical Properties of Raw Formulations and Their Texturization Using Extrusion. Foods, 13(8), 1195. https://doi.org/10.3390/foods13081195








