Relationships among Structure, Physicochemical Properties and In Vitro Digestibility of Starches from Ethiopian Food Barley Varieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Starch Isolation
2.3. Amylose Content
2.4. Chain Length Distributions
2.5. X-ray Diffraction (XRD)
2.6. Fourier Transform Infrared Spectroscopy (FT-IR)
2.7. Pasting Properties
2.8. Thermal Properties
2.9. In Vitro Starch Digestibility
2.10. Statistical Analysis
3. Results and Discussion
3.1. Amylose Contents
3.2. Amylopectin Chain Length Distributions
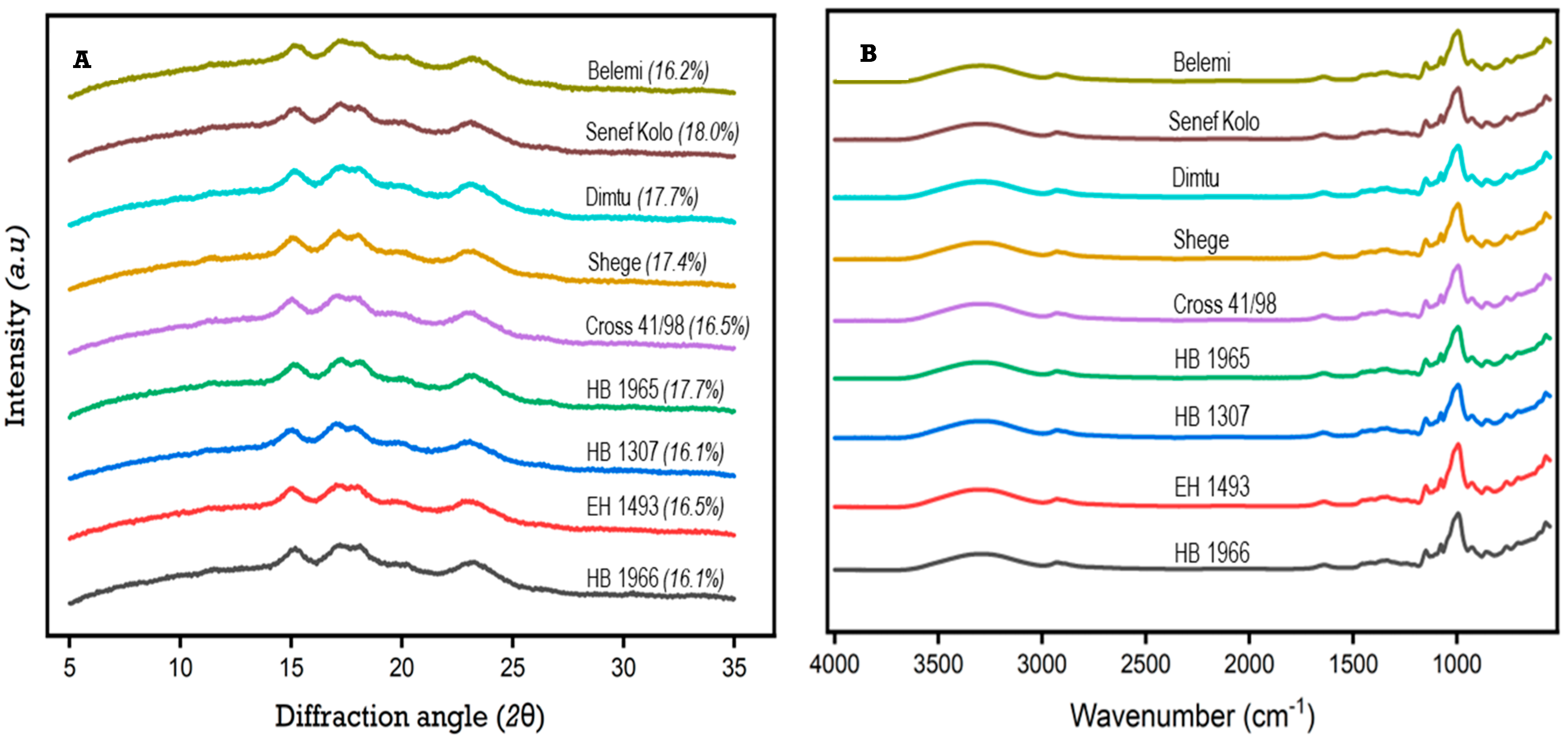
3.3. X-ray Diffraction (XRD)
3.4. Fourier Transform Infrared Spectroscopy (FT-IR)
3.5. Pasting Properties
3.6. Thermal Properties
3.7. In Vitro Digestibility
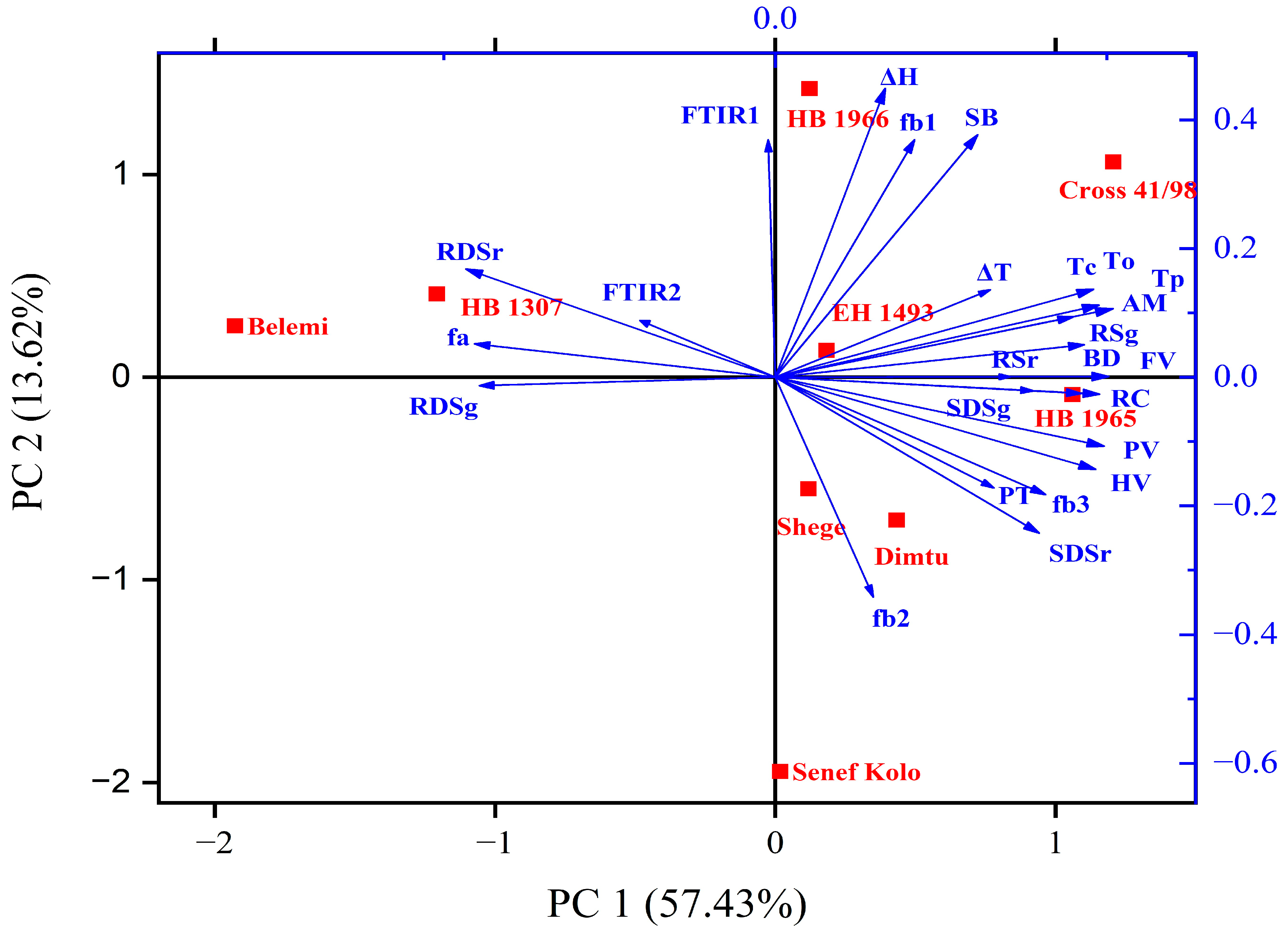
3.8. Structure, Physicochemical, and In Vitro Digestibility Relationships
3.9. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, F. Barley starch: Composition, structure, properties, and modifications. Compr. Rev. Food Sci. 2017, 16, 558–579. [Google Scholar] [CrossRef] [PubMed]
- Obadi, M.; Sun, J.; Xu, B. Highland barley: Chemical composition, bioactive compounds, health effects, and applications. Food Res. Int. 2021, 140, 110065. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Xiao, J.; Abdallah, H.M. Nutritional value of barley cereal and better opportunities for its processing as a value-added food: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 1092–1104. [Google Scholar] [CrossRef] [PubMed]
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and functionality of starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Waleed, A.A.; Mushtaq, B.S.; Mahdi, A.A.; Al-Maqtari, Q.A.; Abduqader, A.A.; Ahmed, A.; Fan, M.; Li, Y.; Qian, H.; Jinxin, L.; et al. Molecular structure, morphological, and physicochemical properties of highlands barley starch as affected by natural fermentation. Food Chem. 2021, 356, 129665. [Google Scholar]
- Yang, L.; Liu, Y.; Wang, S.; Zhang, X.; Yang, J.; Du, C. The relationship between amylopectin fine structure and the physicochemical properties of starch during potato growth. Int. J. Biol. Macromol. 2021, 182, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Pycia, K.; Gałkowska, D.; Juszczak, L.; Fortuna, T.; Witczak, T. Physicochemical, thermal and rheological properties of starches isolated from malting barley varieties. J. Food Sci. Technol. 2015, 52, 4797–4807. [Google Scholar] [CrossRef] [PubMed]
- Källman, A.; Vamadevan, V.; Bertoft, E.; Koch, K.; Seetharaman, K.; Åman, P.; Andersson, R. Thermal properties of barley starch and its relation to starch characteristics. Int. J. Biol. Macromol. 2015, 81, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shao, S.; Chen, M.; Hou, C.; Yu, X.; Xiong, F. Morphology and physicochemical properties of starch from waxy and non-waxy barley. Stärke 2020, 72, 1900206. [Google Scholar] [CrossRef]
- Liang, W.; Ding, L.; Guo, K.; Liu, Y.; Wen, X.; Kirkensgaard, J.J.K.; Khakimov, B.; Enemark-Rasmussen, K.; Hebelstrup, K.H.; Herburger, K.; et al. The relationship between starch structure and digestibility by time-course digestion of amylopectin-only and amylose-only barley starches. Food Hydrocoll. 2023, 139, 108491. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, L.; Zhang, H.; Wu, G.; Cheng, L.; Li, J. Effects of composition, starch structural orders, and kernel structure on starch in vitro digestion of highland barley. Carbohydr. Polym. 2023, 301, 120274. [Google Scholar] [CrossRef] [PubMed]
- Gujral, H.S.; Sharma, P.; Kaur, H.; Singh, J. Physiochemical, pasting, and thermal properties of starch isolated from different barley cultivars. Int. J. Food Prop. 2013, 16, 1494–1506. [Google Scholar] [CrossRef]
- Bera, S.; Sabikhi, L.; Singh, A.K. Assessment of malting characteristics of different Indian barley cultivars. J. Food Sci. Technol. 2018, 55, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xiao, X.; Zhang, W.; Zheng, J.; Luo, Q.; Ouyang, S.; Zhang, G. Compositional, morphological, structural and physicochemical properties of starches from seven naked barley cultivars grown in China. Food Res. Int. 2014, 58, 7–14. [Google Scholar] [CrossRef]
- Yangcheng, H.; Gong, L.; Zhang, Y.; Jane, J. Pysicochemical properties of Tibetan hull-less barley starch. Carbohydr. Polym. 2016, 137, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Kasapis, S.; Zhu, P.; Sui, Z.; Bao, J.; Corke, H. Physicochemical and structural characteristics of starches from Chinese hull-less barley cultivars. Int. J. Food Sci. Technol. 2016, 51, 509–518. [Google Scholar] [CrossRef]
- Li, K.; Zhang, T.; Sui, Z.; Narayanamoorthy, S.; Jin, C.; Li, S.; Corke, H. Genetic variation in starch physicochemical properties of Chinese foxtail millet (Setaria italica Beauv.). Int. J. Biol. Macromol. 2019, 133, 337–345. [Google Scholar] [CrossRef]
- Holtekjølen, A.; Uhlen, A.K.; Bråthen, E.; Sahlstrøm, S.; Knutsen, S. Contents of starch and non-starch polysaccharides in barley varieties of different origin. Food Chem. 2006, 94, 348–358. [Google Scholar] [CrossRef]
- Song, Y.; Jane, J.L. Characterization of barley starches of waxy, normal, and high amylose varieties. Carbohydr. Polym. 2000, 41, 365–377. [Google Scholar] [CrossRef]
- Contreras-Jiménez, B.; Del Real, A.; Millan-Malo, B.M.; Gaytán-Martínez, M.; Morales-Sánchez, E.; Rodríguez-García, M.E. Physicochemical changes in barley starch during malting. J. Inst. Brew. 2019, 125, 10–17. [Google Scholar] [CrossRef]
- Edney, M.; MacLeod, A.; LaBerge, D. Evolution of a quality testing program for improving malting barley in Canada. Can. J. Plant Sci. 2014, 94, 535–544. [Google Scholar] [CrossRef]
- Idehen, E.; Tang, Y.; Sang, S. Bioactive phytochemicals in barley. J. Food Drug Anal. 2017, 25, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.R.; Tangsrianugul, N.; Sriprablom, J.; Wongsagonsup, R.; Wansuksri, R.; Suphantharika, M. Effect of heat-moisture treatment on the physicochemical properties and digestibility of proso millet flour and starch. Carbohydr. Polym. 2023, 307, 120630. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Bertoft, E.; Bao, J.; Corke, H. Molecular structure of amylopectin from amaranth starch and its effect on physicochemical properties. Int. J. Biol. Macromol. 2008, 43, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Chen, Y.; Zhu, P.; Sui, Z.; Corke, H.; Bao, J. Relationships among genetic, structural, and functional properties of rice starch. J. Agric. Food Chem. 2015, 63, 6241–6248. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, J.J.; Tournois, H.; de Wit, D.; Vliegenthart, J.F. Short-range structure in (partially) crystalline potato starch determined with attenuated total reflectance Fourier-transform IR spectroscopy. Carbohydr. Polym. 1995, 279, 201–214. [Google Scholar] [CrossRef]
- Sun, L.; Xu, Z.; Song, L.; Ma, M.; Zhang, C.; Chen, X.; Xu, X.; Sui, Z.; Corke, H. Removal of starch granule associated proteins alters the physicochemical properties of annealed rice starches. Int. J. Biol. Macromol. 2021, 185, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.; Yao, T.; Ye, X.; Bao, J.; Kong, X.; Wu, Y. Physicochemical properties and starch digestibility of in-kernel heat-moisture-treated waxy, low-, and high-amylose rice starch. Stärke 2017, 69, 1600164. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.; Cummings, J. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. S2), S33–S50. [Google Scholar] [PubMed]
- Gebre, B.A.; Zhang, C.; Li, Z.; Sui, Z.; Corke, H. Impact of starch chain length distributions on physicochemical properties and digestibility of starches. Food Chem. 2023, 435, 137641. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hu, Y.; Li, S.; Yi, X.; Shao, S.; Yu, W.; Li, E. Biological factors controlling starch digestibility in human digestive system. Food Sci. Hum. Wellness 2023, 12, 351–358. [Google Scholar] [CrossRef]
- Akonor, P.; Osei Tutu, C.; Arthur, W.; Adjebeng-Danquah, J.; Affrifah, N.; Budu, A.; Saalia, F. Granular structure, physicochemical and rheological characteristics of starch from yellow cassava (Manihot esculenta) genotypes. Int. J. Food Prop. 2023, 26, 259–273. [Google Scholar] [CrossRef]
- Sun, X.; Saleh, A.S.; Sun, Z.; Ge, X.; Shen, H.; Zhang, Q.; Yu, X.; Yuan, L.; Li, W. Modification of multi-scale structure, physicochemical properties, and digestibility of rice starch via microwave and cold plasma treatments. LWT 2022, 153, 112483. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, H.; Guo, B.; Xu, K.; Dai, Q.; Wei, C.; Zhou, G.; Huo, Z. Effects of nitrogen level on structure and physicochemical properties of rice starch. Food Hydrocoll. 2017, 63, 525–532. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Zhang, W.; Li, C.; Yu, J.; Wang, S. Molecular order and functional properties of starches from three waxy wheat varieties grown in China. Food Chem. 2015, 181, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.M.C.; Copeland, L. Genotype and environmental influences on pasting properties of rice flour. Cereal Chem. 2004, 81, 486–489. [Google Scholar] [CrossRef]
- Li, E.; Lv, J.; Huo, D.; Jia, B.; Li, C. Importance of amylose chain-length distribution in determining starch gelatinization and retrogradation property of wheat flour in the presence of different salts. Carbohydr. Polym. 2023, 308, 120648. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.; Wang, L.; Wang, L.; Li, Z.; Qiu, J. Multi-scale structure, rheological and digestive properties of starch isolated from highland barley kernels subjected to different thermal treatments. Food Hydrocoll. 2022, 129, 107630. [Google Scholar] [CrossRef]
- Zhong, Y.; Qu, J.; Li, Z.; Tian, Y.; Zhu, F.; Blennow, A.; Liu, X. Rice starch multi-level structure and functional relationships. Carbohydr. Polym. 2022, 275, 118777. [Google Scholar] [CrossRef]
- Chang, Q.; Zheng, B.; Zhang, Y.; Zeng, H. A comprehensive review of the factors influencing the formation of retrograded starch. Int. J. Biol. Macromol. 2021, 186, 163–173. [Google Scholar] [CrossRef] [PubMed]
| Variety | Type | Grain Color | Row Type | Year of Release | Grain Yield at Research Field (tons/ha) |
|---|---|---|---|---|---|
| HB 1966 | Released Variety | White | 6 | 2017 | 3.5–5.4 |
| EH 1493 | Released Variety | White | 6 | 2012 | 2.5–5.6 |
| HB 1307 | Released Variety | White | 6 | 2006 | 4.8 |
| HB 1965 | Released Variety | White | 6 | 2017 | 3.0–5.0 |
| Cross 41/98 | Released Variety | White | 6 | 2012 | 2.5–5.6 |
| Shege | Released Variety | White | 6 | 1995 | 3.2–5.5 |
| Dimtu | Released Variety | Purple | Irregular | 2001 | 2.0–4.0 |
| Senef Kolo | Farmer variety | White | 6 | - | - |
| Belemi | Farmer variety | Dark Gray | 2 | - | - |
| Variety | Amylose (%) | FT-IR Ratios | Chain Length Distribution (CLD) (%) | ||||
|---|---|---|---|---|---|---|---|
| 1041/1014 | 1014/993 | fa (DP: 6–12) | fb1 (DP: 13–24) | fb2 (DP: 25–36) | fb3 (DP > 36) | ||
| HB 1966 | 27.6 ± 0.55 cd | 0.58 ± 0.02 a | 0.85 ± 0.01 e | 27.2 ± 0.27 bc | 49.3 ± 0.30 ab | 15.0 ± 0.02 b | 8.5 ± 0.43 bc |
| EH 1493 | 27.6 ± 0.51 cd | 0.55 ± 0.01 c | 0.86 ± 0.01 de | 26.7 ± 0.48 c | 49.7 ± 0.49 a | 15.0 ± 0.52 b | 8.6 ± 0.49 bc |
| HB 1307 | 24.5 ± 0.50 f | 0.57 ± 0.01 ab | 0.87 ± 0.01 bc | 29.0 ± 0.15 a | 48.5 ± 0.16 de | 15.0 ± 0.04 b | 7.5 ± 0.04 d |
| HB 1965 | 29.1 ± 0.32 b | 0.55 ± 0.01 c | 0.87 ± 0.01 cd | 26.4 ± 0.77 c | 49.4 ± 0.29 ab | 15.3 ± 0.19 b | 8.9 ± 0.54 ab |
| Cross 41/98 | 30.3 ± 0.61 a | 0.58 ± 0.01 a | 0.89 ± 0.01 ab | 26.3 ± 0.26 c | 49.3 ± 0.10 ab | 15.5 ± 0.40 ab | 8.9 ± 0.53 ab |
| Shege | 28.3 ± 0.38 bc | 0.57 ± 0.01 ab | 0.87 ± 0.01 bc | 26.5 ± 0.49 c | 48.8 ± 0.21 bc | 15.9 ± 0.21 a | 8.7 ± 0.42 bc |
| Dimtu | 28.0 ± 0.10 bc | 0.56 ± 0.02 bc | 0.86 ± 0.00 cd | 26.6 ± 0.52 c | 48.7 ± 0.62 cd | 15.1 ± 0.12 b | 9.5 ± 0.21 a |
| Senef Kolo | 26.7 ± 0.79 de | 0.56 ± 0.01 c | 0.86 ± 0.01 cd | 27.3 ± 1.03 bc | 48.0 ± 0.01 e | 15.9 ± 0.50 a | 8.8 ± 0.53 ab |
| Belemi | 26.3 ± 0.57 e | 0.56 ± 0.01 bc | 0.90 ± 0.01 a | 28.0 ± 0.05 b | 48.8 ± 0.15 bc | 15.1 ± 0.09 b | 8.0 ± 0.03 cd |
| Min | 24.0 | 0.54 | 0.85 | 25.5 | 48.0 | 14.5 | 7.5 |
| Max | 31.0 | 0.60 | 0.91 | 29.1 | 50.2 | 16.4 | 9.7 |
| Mean | 27.6 | 0.57 | 0.87 | 27.1 | 48.9 | 15.3 | 8.6 |
| SD | 1.67 | 0.013 | 0.015 | 0.96 | 0.56 | 0.43 | 0.65 |
| CV (%) | 6.1 | 2.3 | 1.7 | 3.5 | 1.2 | 2.8 | 7.6 |
| Variety | Raw Starch | Gelatinized Starch | ||||
|---|---|---|---|---|---|---|
| RDS (%) | SDS (%) | RS (%) | RDS (%) | SDS (%) | RS (%) | |
| HB 1966 | 24.7 ± 0.74 c | 60.3 ± 1.00 cd | 15.1 ± 1.04 a | 89.2 ± 0.70 d | 4.0 ± 0.64 ab | 6.9 ± 0.57 b |
| EH 1493 | 21.7 ± 1.54 d | 62.8 ± 2.29 bc | 15.6 ± 0.92 a | 89.5 ± 0.80 cd | 3.5 ± 0.46 bc | 7.0 ± 0.81 b |
| HB 1307 | 27.7 ± 0.86 b | 62.4 ± 0.70 bc | 9.9 ± 1.19 c | 92.6 ± 0.87 ab | 1.9 ± 0.57 d | 5.6 ± 0.55 cd |
| HB 1965 | 21.7 ± 1.00 d | 66.0 ± 1.01 a | 12.3 ± 0.12 b | 86.5 ± 0.85 e | 5.0 ± 0.74 a | 8.6 ± 0.30 a |
| Cross 41/98 | 19.7 ± 0.57 de | 64.5 ± 0.82 ab | 15.9 ± 0.70 a | 86.8 ± 1.19e | 5.1 ± 1.20 a | 8.1 ± 0.57 a |
| Shege | 20.1 ± 1.32 de | 64.3 ± 2.42 ab | 15.6 ± 1.10 a | 92.1 ± 1.00 ab | 2.7 ± 0.57 cd | 5.3 ± 0.52 d |
| Dimtu | 18.8 ± 1.50 e | 66.8 ± 1.30 a | 14.3 ± 0.64 a | 91.0 ± 1.02 bc | 2.5 ± 0.55 cd | 6.5 ± 0.59 bc |
| Senef Kolo | 21.7 ± 1.39 d | 64.1 ± 2.06 ab | 14.2 ± 0.81 a | 88.1 ± 1.40 de | 5.0 ± 0.85 a | 6.9 ± 0.55 b |
| Belemi | 30.5 ± 0.70 a | 58.3 ± 0.52 d | 11.2 ± 0.91 bc | 93.7 ± 1.11 a | 2.1 ± 0.59 d | 4.2 ± 0.70 e |
| Min | 17.1 | 57.7 | 9.1 | 85.4 | 1.4 | 3.4 |
| Max | 31.2 | 68.1 | 16.6 | 94.7 | 6.3 | 8.9 |
| Mean | 22.9 | 63.3 | 13.8 | 89.9 | 3.5 | 6.6 |
| SD | 3.9 | 2.9 | 2.18 | 2.62 | 1.38 | 1.41 |
| CV (%) | 17.0 | 4.6 | 15.8 | 2.9 | 39.4 | 21.4 |
| Variety | Pasting Properties | Thermal Properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PV (mPa·s) | HV (mPa·s) | BD (mPa·s) | FV (mPa·s) | SB (mPa·s) | PT (min) | To (°C) | Tp (°C) | Tc (°C) | ΔT (°C) | ΔH (J/g) | |
| HB 1966 | 943 ± 16.9 c | 772 ± 14.2 e | 171 ± 3.1 b | 997 ± 20.3 b | 225 ± 6.1 b | 6.63 ± 0.06 b | 55.5 ± 0.12 a | 59.7 ± 0.44 b | 63.4 ± 0.40 cd | 7.9 ± 0.31 ef | 9.9 ± 0.31 a |
| EH 1493 | 936 ± 20.2 c | 798 ± 17.9 d | 138 ± 3.5 c | 1008 ± 20.0 b | 210 ± 3.0 d | 6.67 ± 0.15 b | 55.3 ± 0.26 ab | 59.6 ± 0.21 b | 63.4 ± 0.46 cd | 8.1 ± 0.21 de | 8.3 ± 0.30 de |
| HB 1307 | 807 ± 5.0 e | 716 ± 2.5 f | 90 ±6.7 d | 909 ± 6.0 d | 192 ± 7.6 f | 6.87 ± 0.06 a | 53.9 ± 0.15 c | 58.0 ± 0.36 c | 62.2 ± 0.44 e | 8.3 ± 0.29 cd | 9.1 ± 0.31 bc |
| HB 1965 | 1076 ± 22.0 a | 885 ± 17.2 a | 190 ± 5.8 a | 1088 ± 23.0 a | 203 ± 6.9 e | 6.90 ± 0.10 a | 55.9 ± 0.67 a | 60.4 ± 0.71 a | 64.4 ± 0.56 ab | 8.5 ± 0.12 bc | 9.4 ± 0.36 ab |
| Cross 41/98 | 1008 ± 7.2 b | 821 ± 4.6 c | 187 ± 3.6 a | 1073 ± 3.6 a | 252 ± 8.2 a | 7.00 ± 0.20 a | 55.6 ± 0.42 a | 61.0 ± 0.15 a | 64.7 ± 0.49 a | 9.1 ± 0.10 a | 9.8 ± 0.59 a |
| Shege | 897 ± 21.5 d | 763 ± 12.1 e | 134 ± 9.5 c | 944 ± 23.0 c | 181 ± 11.1 g | 7.00 ± 0.10 a | 54.9 ± 0.25 b | 59.5 ± 0.42 b | 63.8 ± 0.20 bc | 8.9 ± 0.21 ab | 8.8 ± 0.53 bc |
| Dimtu | 989 ± 8.5 b | 845 ± 4.5 b | 144 ± 4.4 c | 1064 ± 6.8 a | 219 ± 2.6 c | 6.80 ± 0.10 b | 55.5 ± 0.12 a | 59.6 ± 0.10 b | 63.1 ± 0.17 d | 7.6 ± 0.25 f | 8.2 ± 0.36 de |
| Senef Kolo | 998 ± 18.6 b | 821 ± 12.1 c | 177 ± 6.6 b | 984 ± 19.6 b | 163 ± 7.5 h | 6.93 ± 0.06 a | 54.2 ± 0.06 c | 58.5 ± 0.10 c | 61.9 ± 0.21 e | 7.7 ± 0.20 f | 7.9 ± 0.12 e |
| Belemi | 744 ± 10.5 f | 648 ± 4.5 g | 96 ± 6.0 d | 827 ± 8.2 e | 179 ± 3.8 g | 6.43 ± 0.06 c | 52.8 ± 0.20 d | 56.2 ± 0.30 d | 60.0 ± 0.06 f | 7.2 ± 0.15 g | 8.5 ± 0.10 cd |
| Min. | 734 | 644 | 86 | 820 | 156 | 6.40 | 52.6 | 55.9 | 59.9 | 7.0 | 7.8 |
| Max. | 1098 | 901 | 197 | 1112 | 259 | 7.20 | 56.6 | 61.2 | 65.3 | 9.2 | 10.5 |
| Mean | 933 | 786 | 147.5 | 988 | 203 | 6.80 | 54.9 | 59.2 | 63.0 | 8.1 | 8.9 |
| SD | 100.7 | 69.0 | 36.0 | 82.7 | 26.8 | 0.21 | 1.01 | 1.41 | 1.43 | 0.63 | 0.76 |
| CV (%) | 10.8 | 8.8 | 24.4 | 8.4 | 13.2 | 3.1 | 1.8 | 2.4 | 2.3 | 7.8 | 8.5 |
| Molecular Structures | Crystalline Structures | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AM | fa | fb1 | fb2 | fb3 | RC | FTIR1 | FTIR2 | ||
| Molecular structures | AM | 1 | −0.929 ** | 0.548 | 0.308 | 0.746 * | 0.773 * | 0.099 | 0.095 |
| fa | −0.929 ** | 1 | −0.485 | −0.362 | −0.872 ** | −0.763 * | 0.118 | 0.142 | |
| fb1 | 0.548 | −0.485 | 1 | −0.449 | 0.149 | 0.262 | −0.079 | 0.000 | |
| fb2 | 0.308 | −0.362 | −0.449 | 1 | 0.317 | 0.333 | 0.106 | 0.064 | |
| fb3 | 0.746 * | −0.872 ** | 0.149 | 0.317 | 1 | 0.743 * | −0.174 | −0.290 | |
| Crystalline structures | RC | 0.773 * | −0.763 * | 0.262 | 0.333 | 0.743 * | 1 | 0.138 | −0.274 |
| FTIR1 | 0.099 | 0.118 | −0.079 | 0.106 | −0.174 | 0.138 | 1 | 0.070 | |
| FTIR2 | 0.095 | 0.142 | 0.000 | 0.064 | −0.290 | −0.274 | 0.070 | 1 | |
| Pasting properties | PV | 0.715 * | −0.759 * | 0.253 | 0.301 | 0.791 * | 0.824 ** | −0.148 | −0.426 |
| HV | 0.669 * | −0.726 * | 0.227 | 0.245 | 0.794 * | 0.843 ** | −0.248 | −0.468 | |
| BD | 0.763 * | −0.736 * | 0.273 | 0.375 | 0.693 * | 0.692 * | 0.063 | −0.296 | |
| FV | 0.739 * | −0.748 * | 0.402 | 0.082 | 0.776 * | 0.884 ** | −0.074 | −0.393 | |
| SB | 0.614 | −0.435 | 0.658 | −0.382 | 0.345 | 0.553 | 0.418 | −0.004 | |
| PT | 0.356 | −0.336 | −0.166 | 0.604 | 0.306 | 0.756 * | 0.166 | −0.158 | |
| Thermal properties | To | 0.744 * | −0.771 * | 0.580 | 0.008 | 0.691 * | 0.874 ** | 0.046 | −0.498 |
| Tp | 0.802 ** | −0.775 * | 0.525 | 0.173 | 0.675 * | 0.923 ** | 0.173 | −0.360 | |
| Tc | 0.760 * | −0.721 * | 0.554 | 0.172 | 0.669 * | 0.907 ** | 0.187 | −0.318 | |
| ΔT | 0.551 | −0.416 | 0.341 | 0.387 | 0.109 | 0.680 * | 0.360 | 0.076 | |
| ΔH | 0.379 | −0.097 | 0.512 | −0.205 | −0.152 | 0.308 | 0.630 | 0.099 | |
| In vitro digestibility of raw starch | RDS | −0.720 * | 0.839 ** | −0.160 | −0.459 | −0.865 ** | −0.900 ** | 0.039 | 0.389 |
| SDS | 0.501 | −0.592 | −0.037 | 0.368 | 0.722 * | 0.867 ** | −0.231 | −0.306 | |
| RS | 0.694 * | −0.794 * | 0.356 | 0.374 | 0.669 * | 0.558 | 0.228 | −0.326 | |
| In vitro digestibility of gelatinized starch | RDS | −0.675 * | 0.614 | −0.354 | −0.241 | −0.534 | −0.635 | 0.040 | 0.237 |
| SDS | 0.637 | −0.573 | 0.235 | 0.400 | 0.472 | 0.503 | 0.014 | −0.152 | |
| RS | 0.658 | −0.602 | 0.445 | 0.072 | 0.542 | 0.720 * | −0.078 | −0.311 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebre, B.A.; Xu, Z.; Ma, M.; Lakew, B.; Sui, Z.; Corke, H. Relationships among Structure, Physicochemical Properties and In Vitro Digestibility of Starches from Ethiopian Food Barley Varieties. Foods 2024, 13, 1198. https://doi.org/10.3390/foods13081198
Gebre BA, Xu Z, Ma M, Lakew B, Sui Z, Corke H. Relationships among Structure, Physicochemical Properties and In Vitro Digestibility of Starches from Ethiopian Food Barley Varieties. Foods. 2024; 13(8):1198. https://doi.org/10.3390/foods13081198
Chicago/Turabian StyleGebre, Bilatu Agza, Zekun Xu, Mengting Ma, Berhane Lakew, Zhongquan Sui, and Harold Corke. 2024. "Relationships among Structure, Physicochemical Properties and In Vitro Digestibility of Starches from Ethiopian Food Barley Varieties" Foods 13, no. 8: 1198. https://doi.org/10.3390/foods13081198
APA StyleGebre, B. A., Xu, Z., Ma, M., Lakew, B., Sui, Z., & Corke, H. (2024). Relationships among Structure, Physicochemical Properties and In Vitro Digestibility of Starches from Ethiopian Food Barley Varieties. Foods, 13(8), 1198. https://doi.org/10.3390/foods13081198