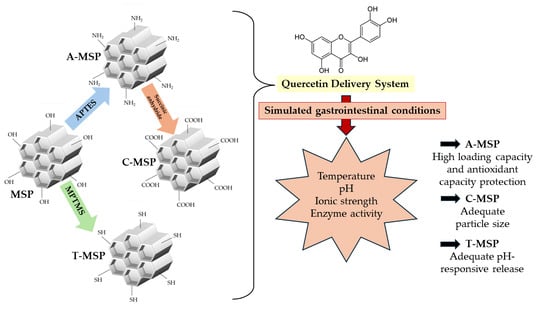
Effect of Amine, Carboxyl, or Thiol Functionalization of Mesoporous Silica Particles on Their Efficiency as a Quercetin Delivery System in Simulated Gastrointestinal Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Functionalization of Mesoporous Silica Particles (MSPs)
2.3. Physicochemical Characterization of F-MSPs
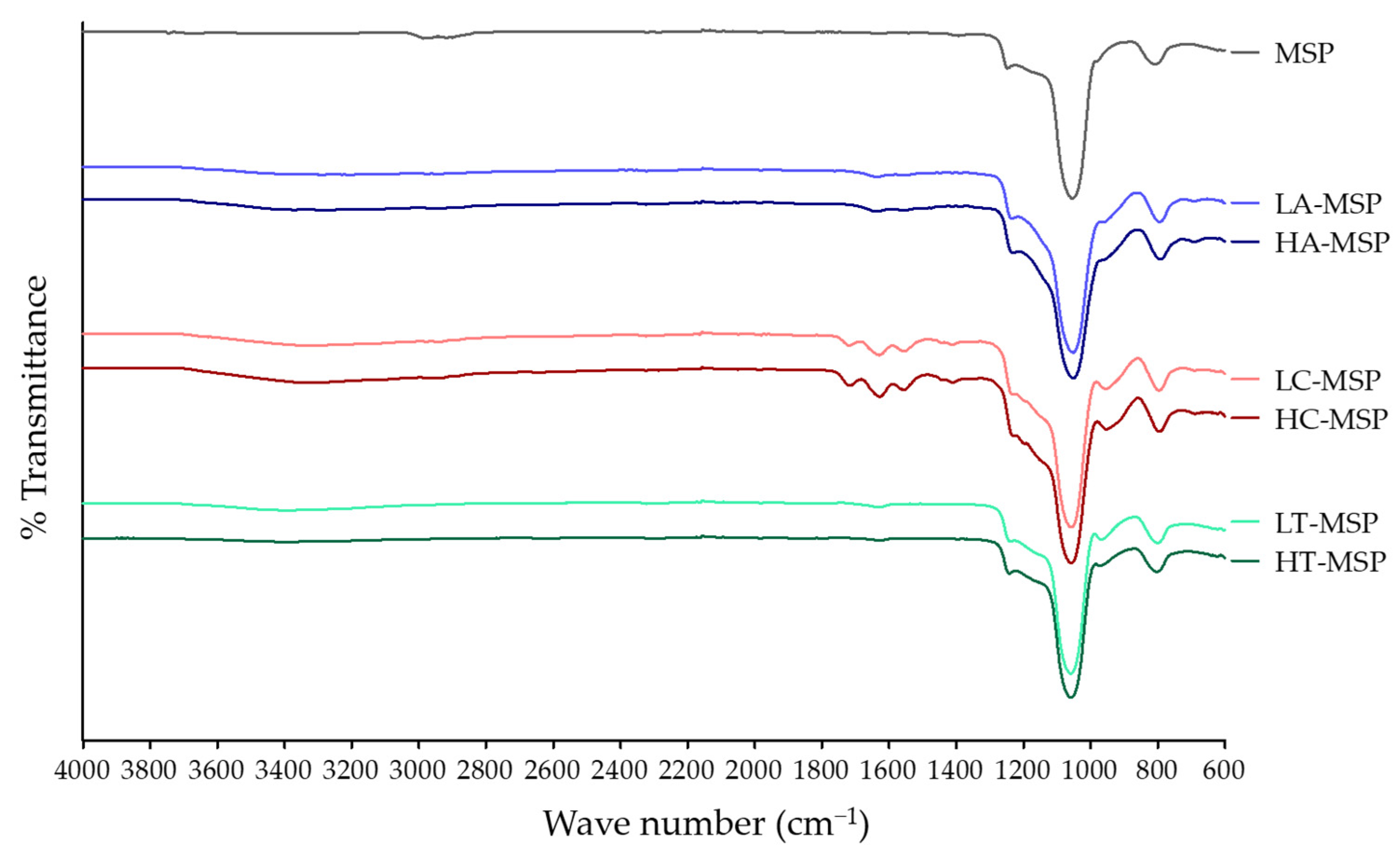
2.3.1. FTIR Spectroscopy
2.3.2. Loading Capacity
2.3.3. Particle Size, Polydispersity Index, and ζ Potential
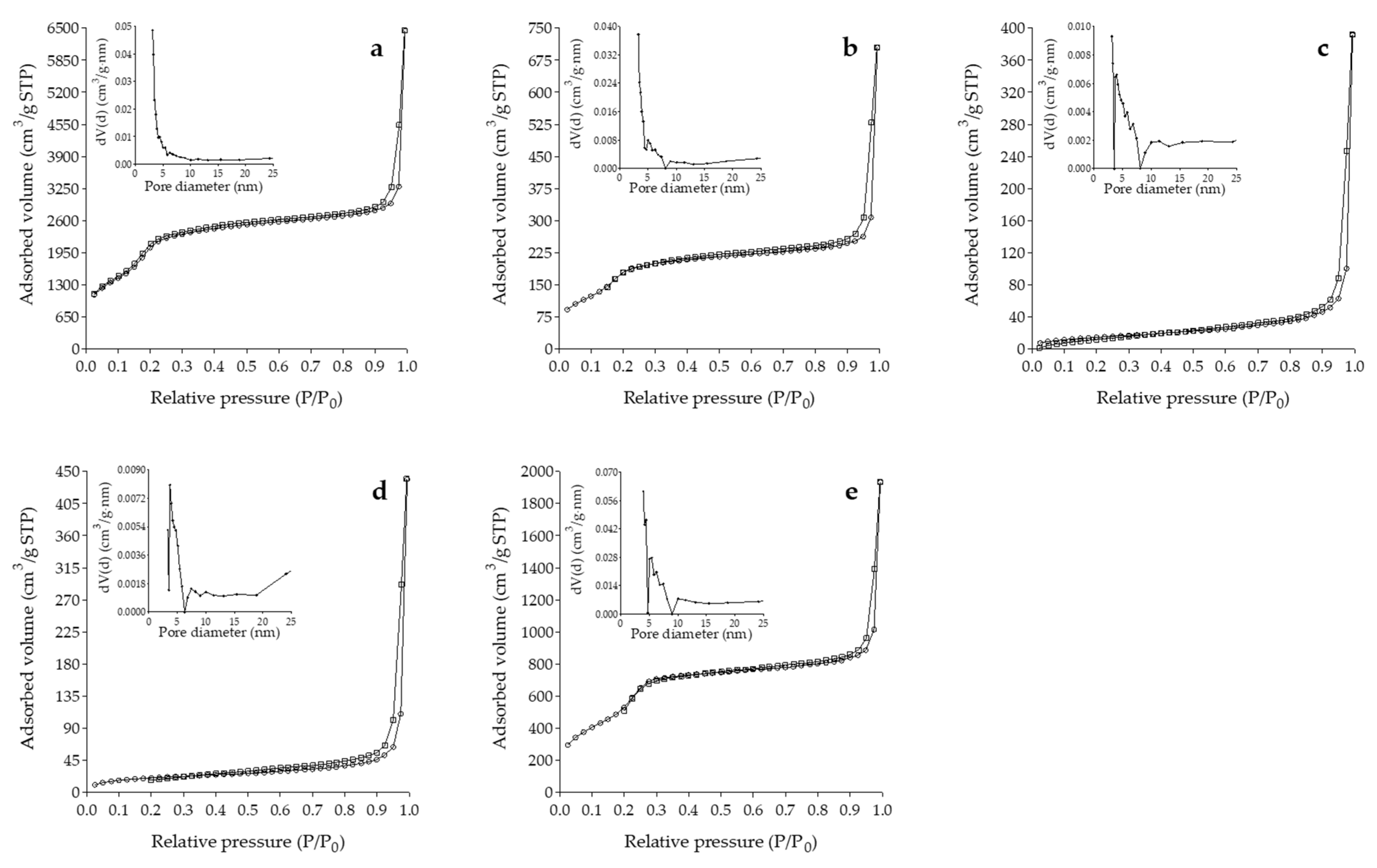
2.3.4. Gas Physisorption Analysis
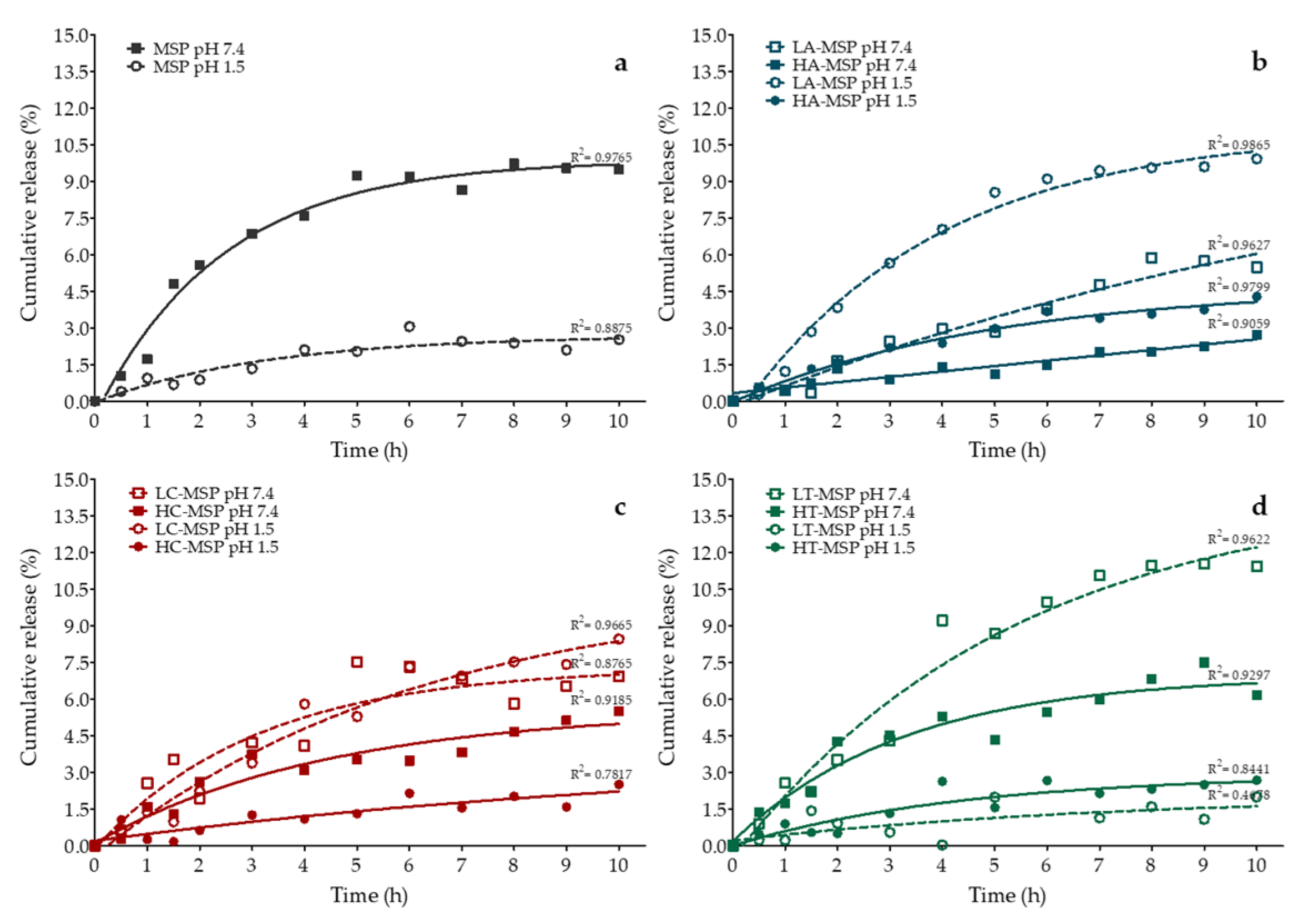
2.4. Quercetin In Vitro Release from F-MSP
2.5. Simulated Gastrointestinal Digestion of Q-Loaded F-MSP
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of F-MSP
3.1.1. FTIR Spectroscopy
3.1.2. Loading Capacity
3.1.3. Particle Size, Polydispersity Index, and ζ Potential
3.1.4. Gas Physisorption Analysis
3.2. In Vitro Release of Q from F-MSP
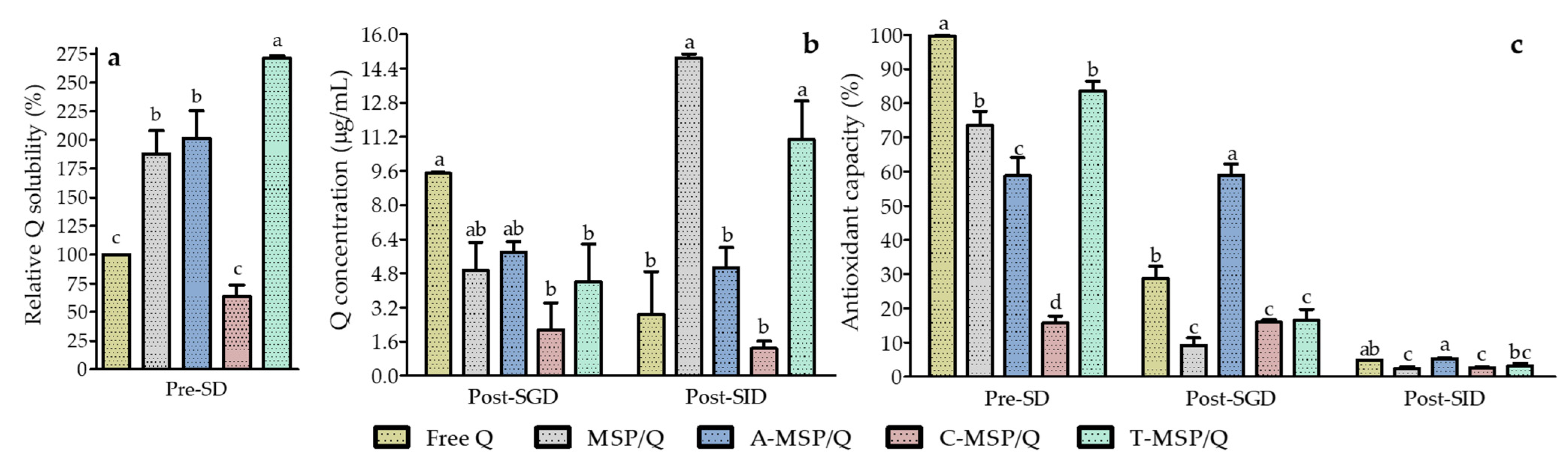
3.3. Simulated Gastrointestinal Digestion of Q-Loaded F-MSP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, C.G.; Haenen, G.R.; Oostveen, R.M.; Stalpers, E.M.; Bast, A. Protection of flavonoids against lipid peroxidation: The structure-activity relationship revisited. Free Radic. Res. 2002, 36, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Florek, J.; Caillard, R.; Kleitz, F. Evaluation of mesoporous silica nanoparticles for oral drug delivery—current status and perspective of MSNs drug carriers. Nanoscale 2017, 9, 15252–15277. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M.K.; Dorraji, M.S.S.; Dodangeh, F.; Ashjari, H.R.; Mousavi, S.N.; Rasoulifard, M.H. Design of a new light curable starch-based hydrogel drug delivery system to improve the release rate of quercetin as a poorly water-soluble drug. Eur. J. Pharm. Sci. 2022, 174, 106191. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety aspects of the use of quercetin as a dietary supplement. Mol. Nutr. Food Res. 2017, 62, 1700447. [Google Scholar] [CrossRef] [PubMed]
- Stephen, S.; Gorain, B.; Choudhury, H.; Chatterjee, B. Exploring the role of mesoporous silica nanoparticle in the development of novel drug delivery systems. Drug Deliv. Transl. Res. 2021, 12, 105–123. [Google Scholar] [CrossRef]
- Sreeharsha, N.; Philip, M.; Krishna, S.S.; Viswanad, V.; Sahu, R.K.; Shiroorkar, P.N.; Aasif, A.H.; Fattepur, S.; Asdaq, S.M.B.; Nair, A.B.; et al. Multifunctional mesoporous silica nanoparticles for oral drug delivery. Coatings 2022, 12, 358. [Google Scholar] [CrossRef]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef]
- Vallet-Regı, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous silica nanoparticles for drug delivery: Current insights. Molecules 2017, 23, 47. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Du Cao, V.; Nguyen, T.N.Q.; Hoang, D.T.; Ngo, V.C.; Nguyen, D.H. Functionalized mesoporous silica nanoparticles and biomedical applications. Mat. Sci. Eng. C 2019, 99, 631–656. [Google Scholar] [CrossRef]
- Wibowo, F.R.; Saputra, O.A.; Lestari, W.W.; Koketsu, M.; Mukti, R.R.; Martien, R. pH-triggered drug release controlled by poly(styrene sulfonate) growth hollow mesoporous silica nanoparticles. ACS Omega 2020, 5, 4261–4269. [Google Scholar] [CrossRef]
- Chen, N.; Cheng, S.; Souris, J.S.; Chen, C.; Mou, C.; Lo, L. Theranostic applications of mesoporous silica nanoparticles and their organic/inorganic hybrids. J. Mater. Chem. B 2013, 1, 3128. [Google Scholar] [CrossRef]
- Siddiqui, B.; Rehman, A.U.; Ul-Haq, I.; Al-Dossary, A.A.; ElaıSsari, A.; Ahmed, N. Exploiting recent trends for the synthesis and surface functionalization of mesoporous silica nanoparticles towards biomedical applications. Int. J. Pharm. X 2022, 4, 100116. [Google Scholar] [CrossRef] [PubMed]
- Saputra, O.A.; Lestari, W.A.; Kurniansyah, V.; Lestari, W.W.; Sugiura, T.; Mukti, R.R.; Martien, R.; Wibowo, F.R. Organically surface engineered mesoporous silica nanoparticles control the release of quercetin by pH stimuli. Sci. Rep. 2022, 12, 20661. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Kettiger, H.; Schoubben, A.M.M.; Rosenholm, J.M.; Ambrogi, V.; Hamidi, M. Mesoporous silica materials: From physico-chemical properties to enhanced dissolution of poorly water-soluble drugs. J. Control. Release 2017, 262, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Bernardos, A.; Kouřimská, L. Applications of mesoporous silica materials in food—A review. Czech J. Food Sci. 2013, 31, 99–107. [Google Scholar] [CrossRef]
- Morante-Zarcero, S.; Endrino, A.; Casado, N.; Pérez-Quintanilla, D.; Sierra, I. Evaluation of mesostructured silica materials with different structures and morphologies as carriers for quercetin and naringin encapsulation. J. Porous Mater. 2021, 29, 33–48. [Google Scholar] [CrossRef]
- Popova, M.; Trendafilova, I.; Szegedi, Á.; Mihály, J.; Németh, P.; Marinova, S.G.; Aleksandrov, H.A.; Vayssilov, G.N. Experimental and theoretical study of quercetin complexes formed on pure silica and Zn-modified mesoporous MCM-41 and SBA-16 materials. Microporous Mesoporous Mater. 2016, 228, 256–265. [Google Scholar] [CrossRef]
- Berlier, G.; Gastaldi, L.; Ugazio, E.; Miletto, I.; Iliade, P.; Sapino, S. Stabilization of quercetin flavonoid in MCM-41 mesoporous silica: Positive effect of surface functionalization. J. Colloid Interface Sci. 2013, 393, 109–118. [Google Scholar] [CrossRef]
- Shabana, N.; Taju, G.; Majeed, S.; Ahmed, A.N.; Karthika, M.S.; Ramasubramanian, V.; Hameed, A.S. Preparation and evaluation of mesoporous silica nanoparticles loaded quercetin against bacterial infections in Oreochromis niloticus. Aquac. Rep. 2021, 21, 100808. [Google Scholar] [CrossRef]
- Zaharudin, N.S.; Isa, E.D.M.; Ahmad, H.; Rahman, M.B.A.; Jumbri, K. Functionalized mesoporous silica nanoparticles templated by pyridinium ionic liquid for hydrophilic and hydrophobic drug release application. J. Saudi Chem. Soc. 2020, 24, 289–302. [Google Scholar] [CrossRef]
- Trendafilova, I.; Szegedi, Á.; Mihály, J.; Momekov, G.; Lihareva, N.; Popova, M. Preparation of efficient quercetin delivery system on Zn-modified mesoporous SBA-15 silica carrier. Mater. Sci. Eng. C 2017, 73, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Ghosh, S.; Chowdhury, S.; Pandey, B.; Sil, P.C. Targeted delivery of quercetin loaded mesoporous silica nanoparticles to the breast cancer cells. Biochim. Biophys. Acta 2016, 1860, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tao, B.; Wan, Y.; Sun, Y.; Wang, L.; Sun, J.; Li, C. Drug delivery based pharmacological enhancement and current insights of quercetin with therapeutic potential against oral diseases. Biomed. Pharmacother. 2020, 128, 110372. [Google Scholar] [CrossRef] [PubMed]
- Petrișor, G.; Motelică, L.; Ficai, D.; Truşcă, R.; Surdu, V.; Voicu, G.; Oprea, O.; Ficai, A.; Andronescu, E. New mesoporous silica materials loaded with polyphenols: Caffeic acid, ferulic acid and p-coumaric acid as dietary supplements for oral administration. Materials 2022, 15, 7982. [Google Scholar] [CrossRef]
- Garrido-Cano, I.; Candela-Noguera, V.; Herrera, G.; Cejalvo, J.M.; Lluch, A.; Marcos, M.D.; Sancenón, F.; Eroles, P.; Martínez-Máñez, R. Biocompatibility and internalization assessment of bare and functionalised mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2021, 310, 110593. [Google Scholar] [CrossRef]
- Tng, D.J.H.; Low, J.G.H. Current status of silica-based nanoparticles as therapeutics and its potential as therapies against viruses. Antiviral Res. 2023, 210, 105488. [Google Scholar] [CrossRef]
- Ros-Lis, J.V.; Bernardos, A.; Pérez, É.; Baviera, J.M.B.; Martínez-Máñez, R. Functionalized Silica Nanomaterials as a New Tool for New Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 165–196. [Google Scholar] [CrossRef]
- Schoonjans, R.; Castenmiller, J.; Chaudhry, Q.; Cubadda, F.; Daskaleros, T.; Franz, R.; Gott, D.M.; Mast, J.; Mortensen, A.; Oomen, A.G.; et al. Regulatory safety assessment of nanoparticles for the food chain in Europe. Trends Food Sci. Technol. 2023, 134, 98–111. [Google Scholar] [CrossRef]
- Fernandez-Bats, I.; Di Pierro, P.; Villalonga-Santana, R.; García-Almendárez, B.E.; Porta, R. Bioactive mesoporous silica nanocomposite films obtained from native and transglutaminase-crosslinked bitter vetch proteins. Food Hydrocoll. 2018, 82, 106–115. [Google Scholar] [CrossRef]
- Li, J.; Shen, S.; Kong, F.; Jiang, T.; Tang, C.; Chen, Y. Effects of pore size on in vitro and in vivo anticancer efficacies of mesoporous silica nanoparticles. RSC Adv. 2018, 8, 24633–24640. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Chang, C.; Liu, Z.; Chen, K.; Zhou, Y.; Zheng, G.; Huang, Z.; Xu, H.; Xu, P.; Lü, B. Polydopamine coated hollow mesoporous silica nanoparticles as pH-sensitive nanocarriers for overcoming multidrug resistance. Colloids Surf. B Biointerfaces 2019, 183, 110427. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Sun, X.; Raza, H.; Khan, M.A.; Ma, H.; Ren, X. Fabrication and characterization of quercetin loaded casein phosphopeptides-chitosan composite nanoparticles by ultrasound treatment: Factor optimization, formation mechanism, physicochemical stability and antioxidant activity. Ultrason. Sonochem. 2021, 80, 105830. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ou, S.; Tang, C. Core–shell soy protein–soy polysaccharide complex (nano)particles as carriers for improved stability and sustained release of curcumin. J. Agric. Food Chem. 2016, 64, 5053–5059. [Google Scholar] [CrossRef] [PubMed]
- Rumman, G.A.; Al-Musawi, T.J.; Sillanpää, M.; Balarak, D. Adsorption performance of an amine-functionalized MCM–41 mesoporous silica nanoparticle system for ciprofloxacin removal. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100536. [Google Scholar] [CrossRef]
- Gou, K.; Wang, Y.; Guo, X.; Wang, Y.; Bian, Y.; Zhao, H.; Guo, Y.; Pang, Y.; Xie, L.; Li, S.; et al. Carboxyl-functionalized mesoporous silica nanoparticles for the controlled delivery of poorly water-soluble non-steroidal anti-inflammatory drugs. Acta Biomater. 2021, 134, 576–592. [Google Scholar] [CrossRef]
- Velusamy, P.; Srinivasa, C.M.; Kumar, G.V.; Qurishi, Y.; Su, C.; Gopinath, S.C.B. A pH stimuli thiol modified mesoporous silica nanoparticles: Doxorubicin carrier for cancer therapy. J. Taiwan Inst. Chem. Eng. 2018, 87, 264–271. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Fan, N.; Li, J.; Zhang, H.; Sun, J. Multimodal nanoporous silica nanoparticles functionalized with aminopropyl groups for improving loading and controlled release of doxorubicin hydrochloride. Mater. Sci. Eng. C 2017, 78, 370–375. [Google Scholar] [CrossRef]
- Dogra, P.; Adolphi, N.L.; Wang, Z.; Yu, L.; Butler, K.S.; Durfee, P.N.; Croissant, J.G.; Noureddine, A.; Coker, E.N.; Bearer, E.L.; et al. Establishing the effects of mesoporous silica nanoparticle properties on in vivo disposition using imaging-based pharmacokinetics. Nat. Commun. 2018, 9, 4551. [Google Scholar] [CrossRef]
- Mazzotta, E.; De Santo, M.; Lombardo, D.; Leggio, A.; Pasqua, L. Mesoporous silicas in materials engineering: Nanodevices for bionanotechnologies. Mater. Today Bio 2022, 17, 100472. [Google Scholar] [CrossRef] [PubMed]
- Saputra, O.A.; Wibowo, F.R.; Lestari, W.W. High storage capacity of curcumin loaded onto hollow mesoporous silica nanoparticles prepared via improved hard-templating method optimized by Taguchi DoE. Eng. Sci. Technol. Int. J. 2022, 33, 101070. [Google Scholar] [CrossRef]
- Oh, N.; Park, J. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9 (Suppl. S1), 51–63. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M. Gas Adsorption Characterization of ordered organic−inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Chiang, Y.; Lian, H.; Leo, S.; Wang, S.; Yamauchi, Y.; Wu, K.C. Controlling particle size and structural properties of mesoporous silica nanoparticles using the Taguchi method. J. Phys. Chem. C. 2011, 115, 13158–13165. [Google Scholar] [CrossRef]
- Kamarudin, N.H.N.; Jalil, A.A.; Triwahyono, S.; Salleh, N.F.M.; Karim, A.H.; Mukti, R.R.; Hameed, B.; Ahmad, A. Role of 3-aminopropyltriethoxysilane in the preparation of mesoporous silica nanoparticles for ibuprofen delivery: Effect on physicochemical properties. Microporous Mesoporous Mater. 2013, 180, 235–241. [Google Scholar] [CrossRef]
- Tran, V.A.; Lee, S. A prominent anchoring effect on the kinetic control of drug release from mesoporous silica nanoparticles (MSNs). J. Colloid Interface Sci. 2018, 510, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Trendafilova, I.; Tsacheva, I.; Mitova, V.; Kyulavska, M.; Koseva, N.; Mihály, J.; Momekova, D.; Momekov, G.; Aleksandrov, H.A.; et al. Amino-modified KIT-6 mesoporous silica/polymer composites for quercetin delivery: Experimental and theoretical approaches. Microporous Mesoporous Mater. 2018, 270, 40–47. [Google Scholar] [CrossRef]
- Cheah, W.; Sim, Y.; Yeoh, F. Amine-functionalized mesoporous silica for urea adsorption. Mater. Chem. Phys. 2016, 175, 151–157. [Google Scholar] [CrossRef]
- Patiño-Herrera, R.; Louvier-Hernández, J.F.; Escamilla-Silva, E.M.; Chaumel, J.; Palestino, G.; Pérez, E. Prolonged release of metformin by SiO2 nanoparticles pellets for type II diabetes control. Eur. J. Pharma. Sci. 2019, 131, 1–8. [Google Scholar] [CrossRef]
- Szewczyk, A.; Prokopowicz, M. Mesoporous silica pellets—A promising oral drug delivery system? J. Drug Deliv. Sci. Technol. 2020, 56, 101491. [Google Scholar] [CrossRef]
- Bahrami, Z.; Badiei, A.; Atyabi, F. Surface functionalization of SBA-15 nanorods for anticancer drug delivery. Chem. Eng. Res. Des. 2014, 92, 1296–1303. [Google Scholar] [CrossRef]
- Pool, H.; Mendoza, S.; Xiao, H. Encapsulation and release of hydrophobic bioactive components in nanoemulsion-based delivery systems: Impact of physical form on quercetin bioaccessibility. Food Funct. 2013, 4, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, A.; Sato, K.; Brouwers, H.J.; Geus, J. Pore structure development of silica particles below the isoelectric point. Microporous Mesoporous Mater. 2018, 267, 257–264. [Google Scholar] [CrossRef]
- Kim, M.K.; Park, K.; Lee, C.; Park, H.; Choo, H.; Chong, Y. Enhanced stability and intracellular accumulation of quercetin by protection of the chemically or metabolically susceptible hydroxyl groups with a pivaloxymethyl (POM) promoiety. J. Med. Chem. 2010, 53, 8597–8607. [Google Scholar] [CrossRef] [PubMed]
- AbouAitah, K.; Farghali, A.A. Mesoporous silica materials in drug delivery system: Ph/glutathione- responsive release of poorly water-soluble pro-drug quercetin from two and three-dimensional pore-structure nanoparticles. J. Nanomed. Nanotechnol. 2016, 7, 360. [Google Scholar] [CrossRef]
- Hartono, S.B.; Hadisoewignyo, L.; Yang, Y.; Meka, A.K.; Antaresti; Yu, C. Amine functionalized cubic mesoporous silica nanoparticles as an oral delivery system for curcumin bioavailability enhancement. Nanotechnology 2016, 27, 505605. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Haworth, I.S.; Zuo, Z.; Chow, M.S.; Chow, A.H. Physicochemical and structural characterization of quercetin-β-cyclodextrin complexes. J. Pharm. Sci. 2005, 94, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Dey, M.; Ghosh, B.; Giri, T.K. Enhanced intestinal stability and pH sensitive release of quercetin in GIT through gellan gum hydrogels. Colloids Surf. B Biointerfaces. 2020, 196, 111341. [Google Scholar] [CrossRef]
- Zvezdanović, J.; Stanojevic, J.; Marković, D.; Cvetković, D. Irreversible UV-induced quercetin and rutin degradation in solution, studied by UV-spectrophotometry and HPLC chromatography. J. Serb. Chem. Soc. 2012, 77, 297–312. [Google Scholar] [CrossRef]
- Jurasekova, Z.; Domingo, C.; García-Ramos, J.V.; Sánchez-Cortés, S. Effect of pH on the chemical modification of quercetin and structurally related flavonoids characterized by optical (UV-visible and Raman) spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 12802–12811. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Garmendia, A.; Madrigal-Pérez, L.A.; Regalado-González, C. The multifaceted role of quercetin derived from its mitochondrial mechanism. Mol. Cell. Biochem. 2023. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, Y.; Liang, Y.; Ma, X.; Xiao, Q.; Xiao, J.; Wang, X.; Luo, Y.; Yue, T. Advance on the absorption, metabolism, and efficacy exertion of quercetin and its important derivatives. Food Frontiers. 2020, 1, 420–434. [Google Scholar] [CrossRef]
- Li, H.; Zhao, X.; Ma, Y.; Zhai, G.; Li, L.; Lou, H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control. Release. 2009, 133, 238–244. [Google Scholar] [CrossRef]
| PS (µm) | PI | ζ (mV) | |||
|---|---|---|---|---|---|
| Bare | pH 1.5 | MSP | 1.79 ± 0.22 bcCDE | 0.83 ± 0.16 abcAB | 1.57 ± 1.75 cdE |
| LA-MSP | 0.69 ± 0.04 efF | 0.56 ± 0.05 cdeBCDE | 31.37 ± 0.70 aA | ||
| HA-MSP | 0.87 ± 0.07 defF | 0.6 ± 0.04 bcdeBCDE | 32.93 ± 2.50 aA | ||
| LC-MSP | 1.57 ± 0.06 bcdE | 0.54 ± 0.12 cdeCDE | 6.69 ± 1.36 bCD | ||
| HC-MSP | 2.05 ± 0.15 bBC | 0.64 ± 0.09 abcdABCDE | 6.19 ± 0.997 bcCD | ||
| LT-MSP | 1.95 ± 0.14 bBCD | 0.62 ± 0.11 bcdeBCDE | 1.31 ± 1.41 dE | ||
| HT-MSP | 2.21 ± 0.14 bB | 0.64 ± 0.1 abcdBCDE | 1.62 ± 0.76 cdE | ||
| pH 7.4 | MSP | 1.03 ± 0.15 deDE | 0.63 ± 0.14 bcdeCD | −15.83 ± 1.10 fBC | |
| LA-MSP | 3.93 ± 0.54 aA | 0.97 ± 0.06 aA | −7.65 ± 1.86 eA | ||
| HA-MSP | 3.33 ± 0.28 aAB | 0.86 ± 0.22 abcABC | −6.71 ± 2.23 eA | ||
| LC-MSP | 0.23 ± 0.004 fE | 0.34 ± 0.003 deEF | −38 ± 1.95 gDE | ||
| HC-MSP | 0.21 ± 0.01 fE | 0.3 ± 0.01 eF | −44.1 ± 2.17 hF | ||
| LT-MSP | 1.16 ± 0.41 cdeDE | 0.83 ± 0.13 abcABCD | −14.23 ± 0.95 fBC | ||
| HT-MSP | 1.92 ± 0.37 bCD | 0.92 ± 0.12 abAB | −16.1 ± 0.57 fBC | ||
| Loaded with Q | pH 1.5 | MSP | 1.62 ± 0.07 cdefDE | 0.64 ± 0.06 cdeBCDE | 4.13 ± 2.01 cDE |
| LA-MSP | 1.86 ± 0.13 bcdCDE | 0.78 ± 0.1 bcABC | 13.93 ± 1.68 aB | ||
| HA-MSP | 2.72 ± 0.12 bA | 0.92 ± 0.05 abA | 15.33 ± 0.31 aB | ||
| LC-MSP | 1.63 ± 0.08 cdefDE | 0.48 ± 10 efDE | 8.85 ± 1.10 bC | ||
| HC-MSP | 1.70 ± 0.03 cdeDE | 0.42 ± 0.03 fgE | 8.65 ± 1.39 bC | ||
| LT-MSP | 1.79 ± 0.12 cdCDE | 0.64 ± 0.06 cdeBCDE | 1.22 ± 1.27 cE | ||
| HT-MSP | 1.73 ± 0.12 cdeCDE | 0.73 ± 0.12 cdABCD | 1.66 ± 1.13 cE | ||
| pH 7.4 | MSP | 0.86 ± 0.03 efgDE | 0.66 ± 0.05 cdeBCD | −13.9 ± 0.85 deBC | |
| LA-MSP | 2.43 ± 0.42 bcBC | 0.96 ± 0.06 abA | −17.4 ± 1.71 eC | ||
| HA-MSP | 3.96 ± 1.02 aA | 0.997 ± 0.01 aA | −11.43 ± 1.02 dAB | ||
| LC-MSP | 0.21 ± 0.004 gE | 0.24 ± 0.01 ghF | −34.6 ± 0.20 fD | ||
| HC-MSP | 0.21 ± 0.004 gE | 0.22 ± 0.01 hF | −41.83 ± 3.09 gEF | ||
| LT-MSP | 1.17 ± 0.005 defDE | 0.62 ± 0.06 cdeCD | −16.87 ± 1.80 eC | ||
| HT-MSP | 0.73 ± 0.03 fgE | 0.57 ± 0.04 defDE | −14.57 ± 0.67 deBC |
| SBET (m2/g) | VP (cm3/g) | DP (nm) | |
|---|---|---|---|
| MSPs | 871.508 | 0.659 | 3.136 |
| MSP/Q | 767.986 | 0.808 | 3.315 |
| A-MSP/Q | 56.480 | 0.606 | 3.136 |
| C-MSP/Q | 76.961 | 0.669 | 3.714 |
| T-MSP/Q | 2022.822 | 1.946 | 3.933 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matadamas-Ortiz, A.; Pérez-Robles, J.F.; Reynoso-Camacho, R.; Amaya-Llano, S.L.; Amaro-Reyes, A.; Di Pierro, P.; Regalado-González, C. Effect of Amine, Carboxyl, or Thiol Functionalization of Mesoporous Silica Particles on Their Efficiency as a Quercetin Delivery System in Simulated Gastrointestinal Conditions. Foods 2024, 13, 1208. https://doi.org/10.3390/foods13081208
Matadamas-Ortiz A, Pérez-Robles JF, Reynoso-Camacho R, Amaya-Llano SL, Amaro-Reyes A, Di Pierro P, Regalado-González C. Effect of Amine, Carboxyl, or Thiol Functionalization of Mesoporous Silica Particles on Their Efficiency as a Quercetin Delivery System in Simulated Gastrointestinal Conditions. Foods. 2024; 13(8):1208. https://doi.org/10.3390/foods13081208
Chicago/Turabian StyleMatadamas-Ortiz, Alexis, Juan F. Pérez-Robles, Rosalía Reynoso-Camacho, Silvia L. Amaya-Llano, Aldo Amaro-Reyes, Prospero Di Pierro, and Carlos Regalado-González. 2024. "Effect of Amine, Carboxyl, or Thiol Functionalization of Mesoporous Silica Particles on Their Efficiency as a Quercetin Delivery System in Simulated Gastrointestinal Conditions" Foods 13, no. 8: 1208. https://doi.org/10.3390/foods13081208