A Critical Review on Immobilized Sucrose Isomerase and Cells for Producing Isomaltulose
Abstract
:1. Introduction
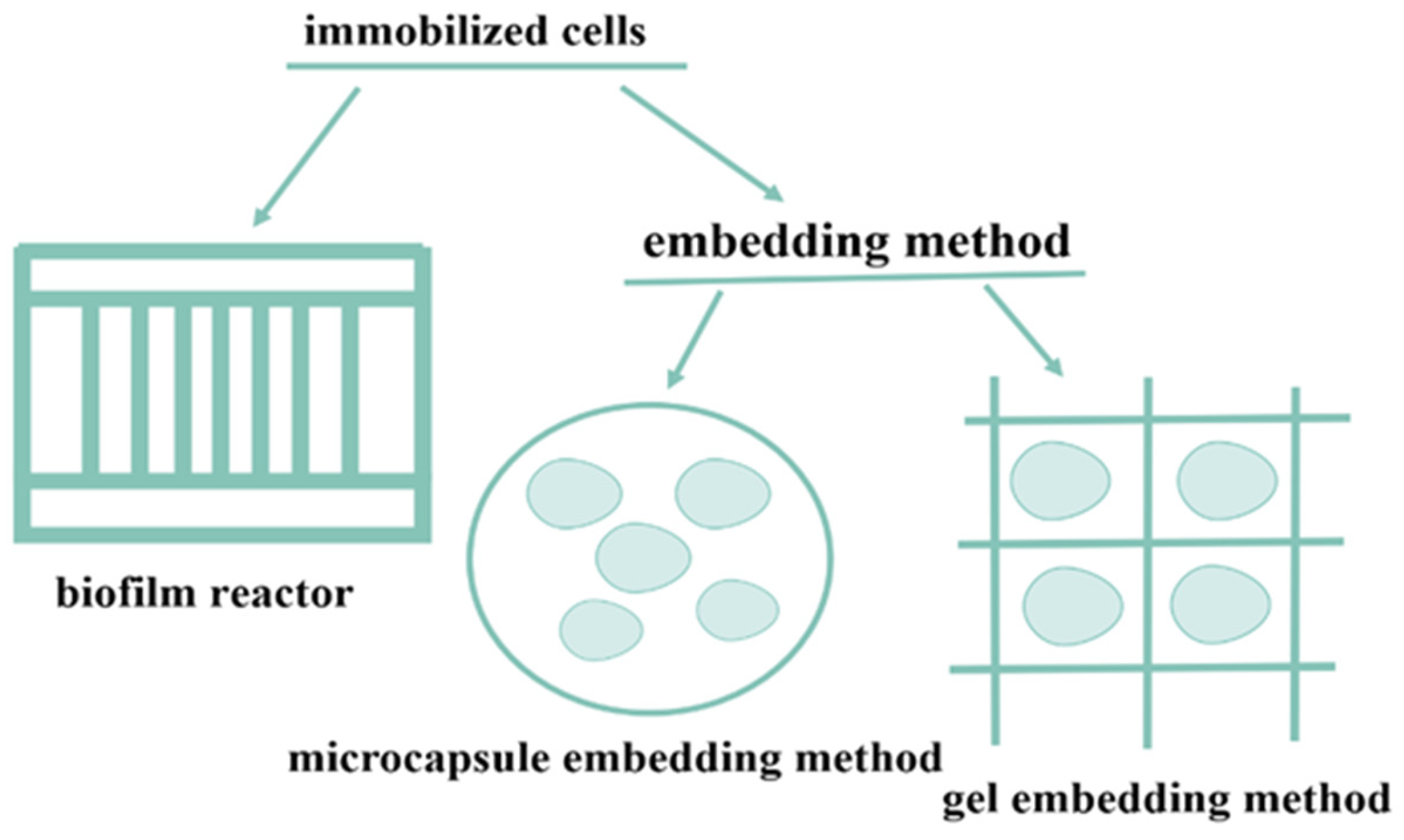
2. Immobilized Cells
2.1. Embedding Method
2.2. Membrane Reactor
3. Immobilized Enzyme
3.1. Innovations in Traditional Immobilizations of Sucrose Isomerase
3.1.1. Adsorption
3.1.2. Embedding
3.1.3. Covalent Binding
3.1.4. Cross-Linking
3.2. Novel Strategies for the Immobilization of Sucrose Isomerase
3.2.1. Surface Modification
3.2.2. Carrier-Free Immobilization
Cross-Linked Enzyme Aggregates
Inclusion Body
Surface Display
3.2.3. Nanoflowers
3.2.4. Directional Immobilization
3.3. Novel Material
4. Expanding the Application of Isomaltulose
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Yang, X.; Yang, S.; Zhu, M.; Wang, X. Technology prospecting on enzymes: Application, marketing and engineering. Comput. Struct. Biotechnol. J. 2012, 2, e201209017. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Li, H.; Ohair, J.; Bhatti, S.; Chen, F.-C.; Al Nasr, K.; Johnson, T.; Zhou, S. Biochemical Characteristics of Microbial Enzymes and Their Significance from Industrial Perspectives. Mol. Biotechnol. 2019, 61, 579–601. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial Applications of Enzymes: Recent Advances, Techniques, and Outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Madhavan, A.; Sindhu, R.; Binod, P.; Sukumaran, R.K.; Pandey, A. Strategies for design of improved biocatalysts for industrial applications. Bioresour. Technol. 2017, 245, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial enzymes: Industrial progress in 21st century. 3 Biotech 2016, 6, 174. [Google Scholar] [CrossRef]
- Mehta, P.K.; Sehgal, S. Microbial Enzymes in Food Processing. In Biocatalysis: Enzymatic Basics and Applications; Husain, Q., Ullah, M.F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 255–275. ISBN 978-3-030-25023-2. [Google Scholar]
- Choct, M. Enzymes for the feed industry: Past, present and future. World’s Poult. Sci. J. 2006, 62, 5–16. [Google Scholar] [CrossRef]
- Choi, J.-M.; Han, S.-S.; Kim, H.-S. Industrial applications of enzyme biocatalysis: Current status and future aspects. Biotechnol. Adv. 2015, 33, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Yin, J. Progress of enzyme immobilization and its potential application. Desalination Water Treat. 2009, 1, 157–171. [Google Scholar] [CrossRef]
- Kawaguti, H.Y.; Sato, H.H. Produção de isomaltulose, um substituto da sacarose, utilizando glicosiltransferase microbiana. Quim. Nova 2008, 31, 134–143. [Google Scholar] [CrossRef]
- Sawale, P.D.; Shendurse, A.M.; Mohan, M.S.; Patil, G. Isomaltulose (Palatinose)—An emerging carbohydrate. Food Biosci. 2017, 18, 46–52. [Google Scholar] [CrossRef]
- Moreira, P.I. High-sugar diets, type 2 diabetes and Alzheimer’s disease. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Van Can, J.G.P.; Ijzerman, T.H.; van Loon, L.J.C.; Brouns, F.; Blaak, E.E. Reduced glycaemic and insulinaemic responses following trehalose ingestion: Implications for postprandial substrate use. Br. J. Nutr. 2009, 102, 1395–1399. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Z.-P.; Sheng, J.; Zheng, Y.; Ji, X.-F.; Zhou, H.-X.; Liu, X.-Y.; Chi, Z.-M. High and efficient isomaltulose production using an engineered Yarrowia lipolytica strain. Bioresour. Technol. 2018, 265, 577–580. [Google Scholar] [CrossRef]
- Mu, W.; Li, W.; Wang, X.; Zhang, T.; Jiang, B. Current studies on sucrose isomerase and biological isomaltulose production using sucrose isomerase. Appl. Microbiol. Biotechnol. 2014, 98, 6569–6582. [Google Scholar] [CrossRef] [PubMed]
- Lina, B.A.R.; Jonker, D.; Kozianowski, G. Isomaltulose (Palatinose®): A review of biological and toxicological studies. Food Chem. Toxicol. 2002, 40, 1375–1381. [Google Scholar] [CrossRef]
- Periche, A.; Heredia, A.; Escriche, I.; Andrés, A.; Castelló, M. Potential use of isomaltulose to produce healthier marshmallows. LWT—Food Sci. Technol. 2015, 62, 605–612. [Google Scholar] [CrossRef]
- Castelló, M.L.; Echevarrías, A.; Rubio-Arraez, S.; Ortolá, M.D. How isomaltulose and oligofructose affect physicochemical and sensory properties of muffins? J. Texture Stud. 2021, 52, 410–419. [Google Scholar] [CrossRef]
- Maresch, C.C.; Petry, S.F.; Theis, S.; Bosy-Westphal, A.; Linn, T. Low Glycemic Index Prototype Isomaltulose—Update of Clinical Trials. Nutrients 2017, 9, 381. [Google Scholar] [CrossRef] [PubMed]
- Peltroche-Llacsahuanga, H.; Hauk, C.; Kock, R.; Lampert, F.; Lütticken, R.; Haase, G. Assessment of Acid Production by Various Human Oral Micro-organisms when Palatinose or Leucrose is Utilized. J. Dent. Res. 2001, 80, 378–384. [Google Scholar] [CrossRef]
- Periche, A.; Heredia, A.; Escriche, I.; Andrés, A.; Castelló, M. Optical, mechanical and sensory properties of based-isomaltulose gummy confections. Food Biosci. 2014, 7, 37–44. [Google Scholar] [CrossRef]
- Holub, I.; Gostner, A.; Theis, S.; Nosek, L.; Kudlich, T.; Melcher, R.; Scheppach, W. Novel findings on the metabolic effects of the low glycaemic carbohydrate isomaltulose (Palatinose™). Br. J. Nutr. 2010, 103, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Low, N.H.; Sporns, P. Analysis and Quantitation of Minor Di- and Trisaccharides in Honey, Using Capillary Gas Chromatography. J. Food Sci. 1988, 53, 558–561. [Google Scholar] [CrossRef]
- Takazoe, I. New trends on sweeteners in Japan. Int. Dent. J. 1985, 35, 58–65. [Google Scholar] [PubMed]
- Li, S.; Xu, H.; Yu, J.; Wang, Y.; Feng, X.; Ouyang, P. Enhancing isomaltulose production by recombinant Escherichia coli producing sucrose isomerase: Culture medium optimization containing agricultural wastes and cell immobilization. Bioprocess Biosyst. Eng. 2013, 36, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Kawaguti, H.Y.; Celestino, É.M.; Moraes, A.L.; Yim, D.K.; Yamamoto, L.K.; Sato, H.H. Characterization of a glucosyltransferase from Erwinia sp. D12 and the conversion of sucrose into isomaltulose by immobilized cells. Biochem. Eng. J. 2010, 48, 211–217. [Google Scholar] [CrossRef]
- Krastanov, A.; Blazheva, D.; Yanakieva, I.; Kratchanova, M. Conversion of sucrose into palatinose in a batch and continuous processes by immobilized Serratia plymuthica cells. Enzym. Microb. Technol. 2006, 39, 1306–1312. [Google Scholar] [CrossRef]
- Lee, H.C.; Kim, J.H.; Kim, S.Y.; Lee, J.K. Isomaltose Production by Modification of the Fructose-Binding Site on the Basis of the Predicted Structure of Sucrose Isomerase from “Protaminobacter rubrum”. Appl. Environ. Microbiol. 2008, 74, 5183–5194. [Google Scholar] [CrossRef]
- Li, S.; Cai, H.; Qing, Y.; Ren, B.; Xu, H.; Zhu, H.; Yao, J. Cloning and Characterization of a Sucrose Isomerase from Erwinia rhapontici NX-5 for Isomaltulose Hyperproduction. Appl. Biochem. Biotechnol. 2011, 163, 52–63. [Google Scholar] [CrossRef]
- Wu, L.; Birch, R. Characterization of Pantoea dispersa UQ68J: Producer of a highly efficient sucrose isomerase for isomaltulose biosynthesis. J. Appl. Microbiol. 2004, 97, 93–103. [Google Scholar] [CrossRef]
- Zhang, D.; Li, X.; Zhang, L.-H. Isomaltulose Synthase from Klebsiella sp. Strain LX3: Gene Cloning and Characterization and Engineering of Thermostability. Appl. Environ. Microbiol. 2002, 68, 2676–2682. [Google Scholar] [CrossRef]
- Liu, L.; Bilal, M.; Luo, H.; Zhao, Y.; Duan, X. Studies on Biological Production of Isomaltulose Using Sucrose Isomerase: Current Status and Future Perspectives. Catal. Lett. 2020, 151, 1868–1881. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, F.; Jia, D.-X.; Gu, Y.-H.; Liu, Z.-Q.; Zheng, Y.-G. Characterization of a recombinant sucrose isomerase and its application to enzymatic production of isomaltulose. Biotechnol. Lett. 2020, 43, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, C.; An, Q.; Zhang, D. Substrate induction of isomaltulose synthase in a newly isolated Klebsiella sp. LX3. J. Appl. Microbiol. 2003, 95, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Eskandarloo, H.; Abbaspourrad, A. Production of galacto-oligosaccharides from whey permeate using β-galactosidase immobilized on functionalized glass beads. Food Chem. 2018, 251, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Palai, T.; Kumar, A.; Bhattacharya, P.K. Kinetic studies and model development for the formation of galacto-oligosaccharides from lactose using synthesized thermo-responsive bioconjugate. Enzym. Microb. Technol. 2015, 70, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Vaghari, H.; Jafarizadeh-Malmiri, H.; Mohammadlou, M.; Berenjian, A.; Anarjan, N.; Jafari, N.; Nasiri, S. Application of magnetic nanoparticles in smart enzyme immobilization. Biotechnol. Lett. 2016, 38, 223–233. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Wang, K.; Duan, F.; Lu, L. Carrier-Free Immobilization of α-Galactosidase as Nano-Biocatalysts for Synthesizing Prebiotic α-Galacto-Oligosaccharides. Molecules 2021, 26, 1248. [Google Scholar] [CrossRef]
- De Andrade, B.C.; Gennari, A.; Renard, G.; Nervis, B.D.R.; Benvenutti, E.V.; Costa, T.M.H.; Nicolodi, S.; da Silveira, N.P.; Chies, J.M.; Volpato, G.; et al. Synthesis of magnetic nanoparticles functionalized with histidine and nickel to immobilize His-tagged enzymes using β-galactosidase as a model. Int. J. Biol. Macromol. 2021, 184, 159–169. [Google Scholar] [CrossRef]
- Wu, X.; Yang, C.; Ge, J. Green synthesis of enzyme/metal-organic framework composites with high stability in protein denaturing solvents. Bioresour. Bioprocess. 2017, 4, 24. [Google Scholar] [CrossRef]
- Ke, C.; Fan, Y.; Chen, Y.; Xu, L.; Yan, Y. A new lipase–inorganic hybrid nanoflower with enhanced enzyme activity. RSC Adv. 2016, 6, 19413–19416. [Google Scholar] [CrossRef]
- Wang, A.; Wang, M.; Wang, Q.; Chen, F.; Zhang, F.; Li, H.; Zeng, Z.; Xie, T. Stable and efficient immobilization technique of aldolase under consecutive microwave irradiation at low temperature. Bioresour. Technol. 2011, 102, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Koo, B.-S.; Lee, H.-C.; Yoon, Y. Improved production of isomaltulose by a newly isolated mutant of Serratia sp. cells immobilized in calcium alginate. Can. J. Microbiol. 2015, 61, 193–199. [Google Scholar] [CrossRef]
- Su, H.-H.; Xu, R.-Y.; Yang, Z.-D.; Guo, Y.-S.; Gao, J.-Y.; Mo, L.-Z.; Gao, Y.-F.; Cheng, H.; Zhang, P.-J.; Huang, J.-S. Green synthesis of isomaltulose from cane molasses by an immobilized recombinant Escherichia coli strain and its prebiotic activity. LWT 2021, 143, 111054. [Google Scholar] [CrossRef]
- Hu, M.; Liu, F.; Wang, Z.; Shao, M.; Xu, M.; Yang, T.; Zhang, R.; Zhang, X.; Rao, Z. Sustainable isomaltulose production in Corynebacterium glutamicum by engineering the thermostability of sucrose isomerase coupled with one-step simplified cell immobilization. Front. Microbiol. 2022, 13, 979079. [Google Scholar] [CrossRef] [PubMed]
- De Souza, W.F.C.; Pereira, I.; de Lucena, F.A.; Martins, L.P.; Furtado, R.F.; de Castro, R.J.S.; Sato, H.H. A new system of Erwinia sp. D12 cells immobilized in a matrix of alginate and algaroba gum (Prosopis juliflora): An efficient way to improve isomaltulose production. Process. Biochem. 2022, 114, 52–58. [Google Scholar] [CrossRef]
- Kawaguti, H.Y.; Buzzato, M.F.; Orsi, D.C.; Suzuki, G.T.; Sato, H.H. Effect of the additives polyethylenimine and glutaraldehyde on the immobilization of Erwinia sp. D12 cells in calcium alginate for isomaltulose production. Process. Biochem. 2006, 41, 2035–2040. [Google Scholar] [CrossRef]
- De Oliva-Neto, P.; Menão, P.T. Isomaltulose production from sucrose by Protaminobacter rubrum immobilized in calcium alginate. Bioresour. Technol. 2009, 100, 4252–4256. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.H.; Kawaguti, H.Y.; de Souza, W.F.C.; Sato, H.H. Immobilization of Serratia plymuthica by ionic gelation and cross-linking with transglutaminase for the conversion of sucrose into isomaltulose. Bioprocess Biosyst. Eng. 2021, 44, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- De Souza, W.F.C.; de Castro, R.J.S.; Sato, H.H. Sequential optimization strategy for the immobilization of Erwinia sp. D12 cells and the production of isomaltulose with high stability and prebiotic potential. Bioprocess Biosyst. Eng. 2022, 45, 999–1009. [Google Scholar] [CrossRef]
- Hellmers, F.; Takors, R.; Thum, O. Robust enzyme immobilizates for industrial isomalt production. Mol. Catal. 2018, 445, 293–298. [Google Scholar] [CrossRef]
- Krastanov, A.; Blazheva, D.; Stanchev, V. Sucrose conversion into palatinose with immobilized Serratia plymuthica cells in a hollow-fibre bioreactor. Process. Biochem. 2007, 42, 1655–1659. [Google Scholar] [CrossRef]
- Wu, L.; Wu, S.; Qiu, J.; Xu, C.; Li, S.; Xu, H. Green synthesis of isomaltulose from cane molasses by Bacillus subtilis WB800-pHA01-palI in a biologic membrane reactor. Food Chem. 2017, 229, 761–768. [Google Scholar] [CrossRef]
- Contesini, F.J.; Ibarguren, C.; Grosso, C.R.F.; Carvalho, P.d.O.; Sato, H.H. Immobilization of glucosyltransferase from Erwinia sp. using two different techniques. J. Biotechnol. 2012, 158, 137–143. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Z.-P.; Liu, S.; Wang, Y.-L.; Zhang, Z.-F.; Liu, X.-M.; Du, Y.-M.; Yuan, X.-L. Overexpression of secreted sucrose isomerase in Yarrowia lipolytica and its application in isomaltulose production after immobilization. Int. J. Biol. Macromol. 2018, 121, 97–103. [Google Scholar] [CrossRef]
- Wang, Q.-Q.; Yang, M.; Hao, J.-H.; Ma, Z.-C. Direct Isomaltulose Synthesis from Beet Molasses by Immobilized Sucrose Isomerase. Front. Bioeng. Biotechnol. 2021, 9, 691547. [Google Scholar] [CrossRef]
- Wu, L.; Qiu, J.; Wu, S.; Liu, X.; Liu, C.; Xu, Z.; Li, S.; Xu, H. Bioinspired Production of Antibacterial Sucrose Isomerase-Sponge for the Synthesis of Isomaltulose. Adv. Synth. Catal. 2016, 358, 4030–4040. [Google Scholar] [CrossRef]
- Chen, N.; Chang, B.; Shi, N.; Lu, F.; Liu, F. Preparation and characterization of adsorption-crosslinking immobilized sucrose isomerase. Food Ferment. Ind. 2022, 48, 9–15. [Google Scholar] [CrossRef]
- Geng, M.; Chen, S.; Wu, J.; Wu, D. Immobilization of Sucrose Isomerase by Adsorption and Crosslinking Method for the Synthesis of Isomaltulose. J. Food Sci. Biotechnol. 2019, 38, 104–110. [Google Scholar]
- Wu, L.; Liu, Y.; Chi, B.; Xu, Z.; Feng, X.; Li, S.; Xu, H. An innovative method for immobilizing sucrose isomerase on ε-poly-l-lysine modified mesoporous TiO2. Food Chem. 2015, 187, 182–188. [Google Scholar] [CrossRef]
- Chen, N.; Chang, B.; Shi, N.; Lu, F.; Liu, F. Robust and recyclable cross-linked enzyme aggregates of sucrose isomerase for isomaltulose production. Food Chem. 2023, 399, 134000. [Google Scholar] [CrossRef]
- Gao, X.; Wang, C.; Pang, J.; Li, X. Heterologous expression and characterization of active inclusion bodies of sucrose iso-merase fused with amyloid fragments. J. Dalian Dalian Polytech. Univ. 2022, 41, 167–172. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, X.; Pang, J.; Liu, Y.; Li, M.; Wang, C.; Li, X. Heterologous expression and enzymatic properties of active inclusion bodies of sucrose isomerase fused with coiled-coil domain. Chin. J. Biol. 2023, 36, 793–799. [Google Scholar] [CrossRef]
- Meng, H.; Zhao, Y.; Li, X.; Li, R. Preparation, structure and performance of sucrose isomerase PalⅠ-Cu2+ nanoflower as an immobilized enzyme. Chin. J. Biol. 2021, 34, 286–289+293. [Google Scholar] [CrossRef]
- Zhang, F.; Cai, X.; Cheng, F.; Yu, J.-M.; Wang, B.; Liu, Z.-Q.; Zheng, Y.-G. Immobilization of Sucrose Isomerase from Erwinia sp. with Graphene Oxide and Its Application in Synthesizing Isomaltulose. Appl. Biochem. Biotechnol. 2021, 194, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-Y.; Jung, J.-H.; Seo, D.-H.; Hansin, J.; Ha, S.-J.; Cha, J.; Kim, Y.-S.; Park, C.-S. Isomaltulose production via yeast surface display of sucrose isomerase from Enterobacter sp. FMB-1 on Saccharomyces cerevisiae. Bioresour. Technol. 2011, 102, 9179–9184. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, H.; Cheng, H.; Deng, Z. Isomaltulose production by yeast surface display of sucrose isomerase from Pantoea dispersa on Yarrowia lipolytica. J. Funct. Foods 2017, 32, 208–217. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Z.; Ji, X.; Sheng, J. Display of a sucrose isomerase on the cell surface of Yarrowia lipolytica for synthesis of isomaltulose from sugar cane by-products. 3 Biotech 2019, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Zheng, M.; Wu, X.; Chen, N.; Jiang, L.; Chang, B.; Lu, F.; Liu, F. Construction and Catalytic Study of Affinity Peptide Orientation and Light Crosslinking Immobilized Sucrose Isomerase. J. Agric. Food Chem. 2023, 71, 13401–13408. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-M.; Chen, J.; Shi, Y.-P. Advances on methods and easy separated support materials for enzymes immobilization. TrAC Trends Anal. Chem. 2018, 102, 332–342. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Neeraj, G.; Ravi, S.; Somdutt, R.; Ravi, S.K.; Kumar, V.V. Immobilized inulinase: A new horizon of paramount importance driving the production of sweetener and prebiotics. Crit. Rev. Biotechnol. 2017, 38, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Shakerian, F.; Zhao, J.; Li, S.-P. Recent development in the application of immobilized oxidative enzymes for bioremediation of hazardous micropollutant—A review. Chemosphere 2019, 239, 124716. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R. Cross-linked enzyme aggregates (CLEA®s): Stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Qi, W.; Jia, C.; Ren, Y.; Su, R.; He, Z. Enhancement of activity of cross-linked enzyme aggregates by a sugar-assisted precipitation strategy: Technical development and molecular mechanism. J. Biotechnol. 2011, 156, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.B.; Mondal, K.; Singh, T.P.; Gupta, M.N. Designing cross-linked lipase aggregates for optimum performance as biocatalysts. Biocatal. Biotransform. 2008, 26, 235–242. [Google Scholar] [CrossRef]
- Krauss, U.; Jäger, V.D.; Diener, M.; Pohl, M.; Jaeger, K.-E. Catalytically-active inclusion bodies—Carrier-free protein immobilizates for application in biotechnology and biomedicine. J. Biotechnol. 2017, 258, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Rinas, U.; Garcia-Fruitós, E.; Corchero, J.L.; Vázquez, E.; Seras-Franzoso, J.; Villaverde, A. Bacterial Inclusion Bodies: Discovering Their Better Half. Trends Biochem. Sci. 2017, 42, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Cherf, G.M.; Cochran, J.R. Applications of Yeast Surface Display for Protein Engineering. In Yeast Surface Display; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2015; Volume 1319. [Google Scholar] [CrossRef]
- Schüürmann, J.; Quehl, P.; Festel, G.; Jose, J. Bacterial whole-cell biocatalysts by surface display of enzymes: Toward industrial application. Appl. Microbiol. Biotechnol. 2014, 98, 8031–8046. [Google Scholar] [CrossRef]
- Patel, S.K.; Gupta, R.K.; Kim, I.-W.; Lee, J.-K. Coriolus versicolor laccase-based inorganic protein hybrid synthesis for application in biomass saccharification to enhance biological production of hydrogen and ethanol. Enzym. Microb. Technol. 2023, 170, 110301. [Google Scholar] [CrossRef]
- Koo, B.; Dolan, N.S.; Wucherer, K.; Munch, H.K.; Francis, M.B. Site-Selective Protein Immobilization on Polymeric Supports through N-Terminal Imidazolidinone Formation. Biomacromolecules 2019, 20, 3933–3939. [Google Scholar] [CrossRef]
- Duan, F.; Sun, T.; Zhang, J.; Wang, K.; Wen, Y.; Lu, L. Recent innovations in immobilization of β-galactosidases for industrial and therapeutic applications. Biotechnol. Adv. 2022, 61, 108053. [Google Scholar] [CrossRef] [PubMed]
- Shyam, S.; Ramadas, A.; Chang, S.K. Isomaltulose: Recent evidence for health benefits. J. Funct. Foods 2018, 48, 173–178. [Google Scholar] [CrossRef]
- Sekartini, R.; Wiguna, T.; Bardosono, S.; Novita, D.; Arsianti, T.; Calame, W.; Schaafsma, A. The effect of lactose–isomaltulose-containing growing-up milks on cognitive performance of Indonesian children: A cross-over study. Br. J. Nutr. 2013, 110, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-D.; Guo, Y.-S.; Huang, J.-S.; Gao, Y.-F.; Peng, F.; Xu, R.-Y.; Su, H.-H.; Zhang, P.-J. Isomaltulose Exhibits Prebiotic Activity, and Modulates Gut Microbiota, the Production of Short Chain Fatty Acids, and Secondary Bile Acids in Rats. Molecules 2021, 26, 2464. [Google Scholar] [CrossRef]
- König, D.; Theis, S.; Kozianowski, G.; Berg, A. Postprandial substrate use in overweight subjects with the metabolic syndrome after isomaltulose (Palatinose™) ingestion. Nutrition 2012, 28, 651–656. [Google Scholar] [CrossRef]
- Stevenson, E.J.; Watson, A.; Theis, S.; Holz, A.; Harper, L.D.; Russel, M. A comparison of isomaltulose versus maltodextrin ingestion during soccer-specific exercise. Eur. J. Appl. Physiol. 2017, 117, 2321–2333. [Google Scholar] [CrossRef]


| Synthetic Reaction |  Isomaltulose | SIase |  Sucrose | SIase | 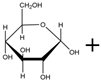 Glucose |  Fructose |
|---|---|---|---|---|---|---|
| Chemical formula | C12H22O11 | C12H22O11 | C6H12O6 | C6H12O6 | ||
| Molar mass (g/mol) | 342.30 | 342.30 | 180.16 | 180.16 | ||
| Density (g/cm3) | 1.7 | 1.77 | 1.58 | 1.694 | ||
| Melting point (°C) | 122~124 | 185–187 | 146 | 103~105 | ||
| Solubility | Easily soluble in water and has a lower solubility in water than sucrose | Soluble in water, insoluble in organic solvents | Soluble in water, slightly soluble in alcohol and acetone, insoluble in ether | Soluble in water, hot acetone, slightly soluble in cold acetone | ||
| Source | Methods | Substrate Conversion Rate | Isomaltulose Yield | Reusability | Refs. |
|---|---|---|---|---|---|
| Serratia sp. | Alginate embedding | 76% | After 35 cycles of use, the yield of isomaltulose is about 60% | [43] | |
| P. dispersa UQ68J | Alginate embedding | 94% | After 30 cycles of use, the relative enzyme activity remains around 80% | [44] | |
| P. dispersa UQ68J | Alginate embedding | 90.6% | After 26 cycles, the sucrose conversion rate remains at 83.2% | [45] | |
| Erwinia sp. D12 | Algae gel and alginate embedding | 61.94% | When converted for 72 h, the yield of isomaltulose is 47.86% | [46] | |
| Erwinia sp. D12 | Alginate glutaraldehyde | 71.42% | At 282 h, the yield of isomaltulose is higher than 55% | [47] | |
| P. rubrum | Alginate embedding, cross-linking with glutaraldehyde and polyethylene imine | 100% | 94.5% | After 24 cycles of recycling, the yield of isomaltulose remains above 80% | [48] |
| S. plymuthica | Alginate and gelatin encapsulation, transglutaminase cross-linking | 71.04% | [49] | ||
| Erwinia sp. D12 | Alginate and gelatin encapsulation, transglutaminase cross-linking | 93.66% | [50] | ||
| P. rubrum | Sipernat 320 and Eudragit NM | 80% | [51] | ||
| S. plymuthicaa ATCC 15928 | Hollow fiber membrane reactor | 100% | After continuous operation for 90 days, the activity loss is 11% | [52] | |
| E. rhapontici NX-5 | Biofilm reactor | 92.4% | After 12 cycles, the sucrose conversion rate is above 90% | [53] |
| Methods | Advantages | Disadvantages |
|---|---|---|
| Embedding | Non-toxic and harmless, simple operation | Cell leakage |
| Composite encapsulation | Improved mechanical strength, decreased cell leakage | Reduced the cell contact rate with the substrate and reduced the conversion efficiency |
| Membrane reactors | Reducing the cost of separation and purification in downstream processing, automated operation | Uneven distribution of cells on the membrane, when the pressure is high, the membrane is prone to rupture, membrane fouling |
| Source | Methods | Substrate Conversion Rate | Isomaltulose Yield | Reusability | References |
|---|---|---|---|---|---|
| Erwinia sp. D12 | Low methoxy pectin and fat microcapsules embedding | 30% | After 9 cycles, the yield of isomaltulose is less than 5% | [54] | |
| P. dispersa UQ68J | Polyvinyl alcohol alginate embedding | 96% | After 13 batches, the sucrose conversion rate remains above 90% | [55] | |
| P. dispersa UQ68J | Polyvinyl alcohol alginate embedding | 97.5% | 94% | After 11 cycles, the sucrose conversion rate remains above 94% | [56] |
| E. rhapontici NX-5 | ε-poly-L-lysine and gelatin | 83.58% | At 300 h, the yield of isomaltulose is 48% | [57] | |
| P. dispersa UQ68J | Silicon ball glutaraldehyde | After 15 cycles, the relative enzyme activity is 77.9% | [58] | ||
| S. plymuthica | Chitosan glutaraldehyde | 87.8% | After 16 cycles, the yield of isomaltulose remains at 87.52% | [59] | |
| E. rhapontici NX-5 | ε-Poly L-lysine mesoporous titanium dioxide | Over 95% | After 16 cycles, the sucrose conversion rate is about 95% | [60] | |
| P. dispersa UQ68J | Cross-linked enzyme aggregate | After 10 cycles, the relative enzyme activity is 91.7% | [61] | ||
| Klebsiella sp. LX3 | Active inclusion body | 80.66 ± 0.82% | [62] | ||
| Klebsiella sp. LX3 | Active inclusion body | 82.9 ± 0.92% | [63] | ||
| Klebsiella sp. LX3 | Cu2+ nanoflower | After 6 cycles, only 40% of the relative enzyme activity remains | [64] | ||
| Erwinia sp. Ejp617 | Graphene oxide | 95.3% | After 10 cycles, the relative enzyme activity is 80.5% | [65] | |
| Enterobacter sp. FMB-1 | Yeast surface display | 7.4% | [66] | ||
| Pantoea dispersa | Yeast surface display | 93 ± 2% | At the 16th cycle of use, its relative enzyme activity is 50% | [67] | |
| Pantoea dispersa | Yeast surface display | 92.4% | After 9 cycles of use, the conversion rate of isomaltulose remains above 85% | [68] | |
| P. dispersa UQ68J | Epoxy resin oriented photo-cross-linking | After 11 cycles, the relative enzyme activity is above 50% | [69] |
| Methods | Advantages | Disadvantages |
|---|---|---|
| Adsorption | Simple operation | Binding between the carrier and the enzyme is relatively weak |
| Embedding | Simple to operate, non-toxic, and harmless | Enzyme leakage |
| Covalent binding | Covalent bonds are stable | Decreased enzyme activity |
| Cross-linking | Covalent bonds are stable | Decreased enzyme activity |
| Surface modification | Enhancing enzyme loading capacity | Require additional carriers |
| Cross-linked enzyme aggregates | Simple to operate, does not require additional carriers | Decreased enzyme activity |
| Inclusion body | Does not require additional carriers or cross-linking agents | Poor reusability |
| Surface display | Replace the tedious process of purifying and immobilized enzymes, do not require additional carriers | Dependent on the type, structure, and application of the enzymes |
| Nanoflower | High specific surface area, simple to operate | Poor structural stability |
| Directional immobilization | High enzyme activity | Require additional carriers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, W.; Hou, F.; Wu, X.; Zheng, M.; Zheng, Y.; Lu, F.; Liu, F. A Critical Review on Immobilized Sucrose Isomerase and Cells for Producing Isomaltulose. Foods 2024, 13, 1228. https://doi.org/10.3390/foods13081228
Jing W, Hou F, Wu X, Zheng M, Zheng Y, Lu F, Liu F. A Critical Review on Immobilized Sucrose Isomerase and Cells for Producing Isomaltulose. Foods. 2024; 13(8):1228. https://doi.org/10.3390/foods13081228
Chicago/Turabian StyleJing, Wenjie, Feihong Hou, Xinming Wu, Mingqiang Zheng, Yue Zheng, Fuping Lu, and Fufeng Liu. 2024. "A Critical Review on Immobilized Sucrose Isomerase and Cells for Producing Isomaltulose" Foods 13, no. 8: 1228. https://doi.org/10.3390/foods13081228





