Akkermansia muciniphila: A Potential Target for the Prevention of Diabetes
Abstract
1. Introduction
2. Mechanism of Action of A. muciniphila in Diabetes Mellitus
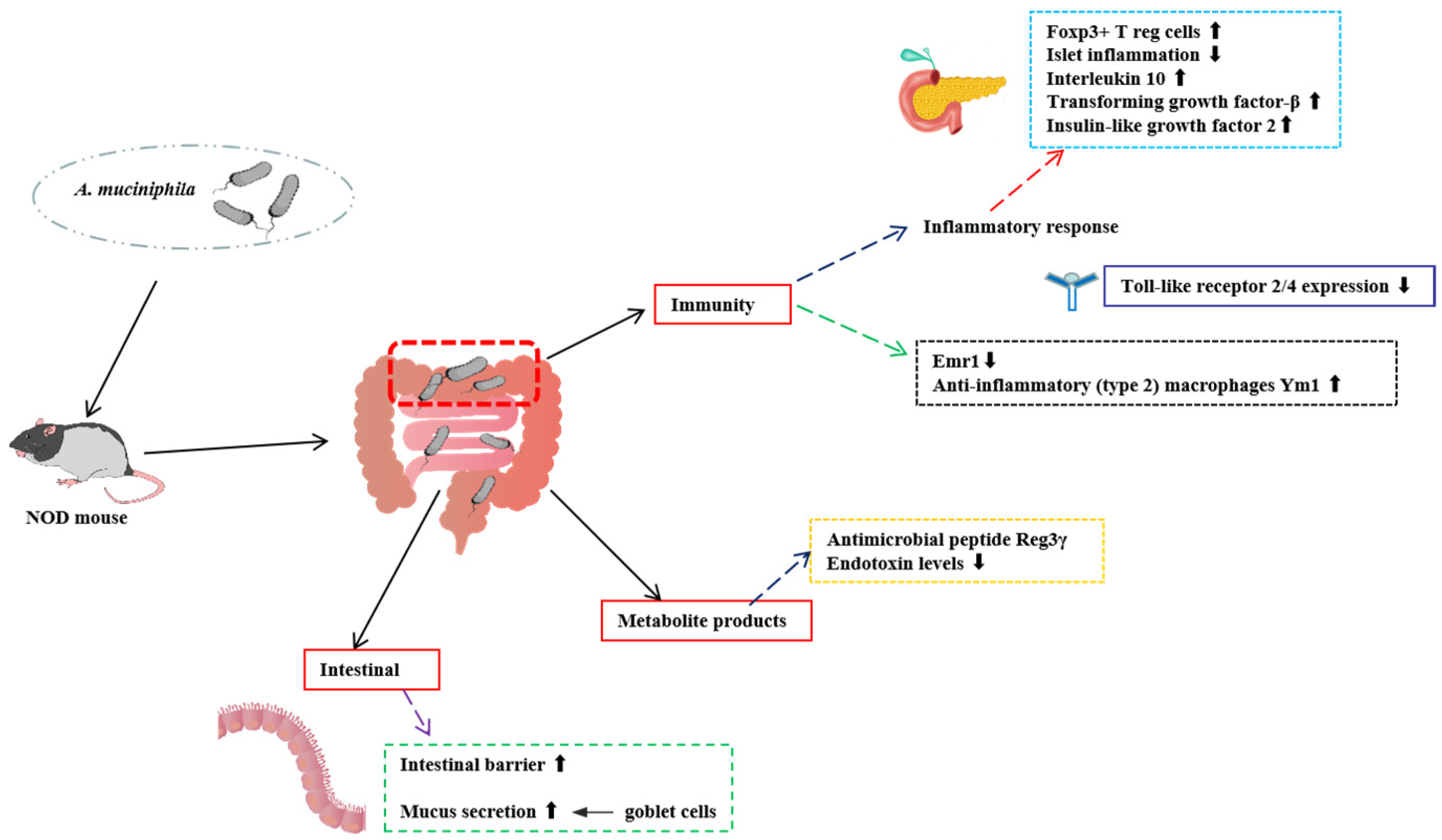
2.1. Role of A. muciniphila in T1D
2.2. Role of A. muciniphila in T2D
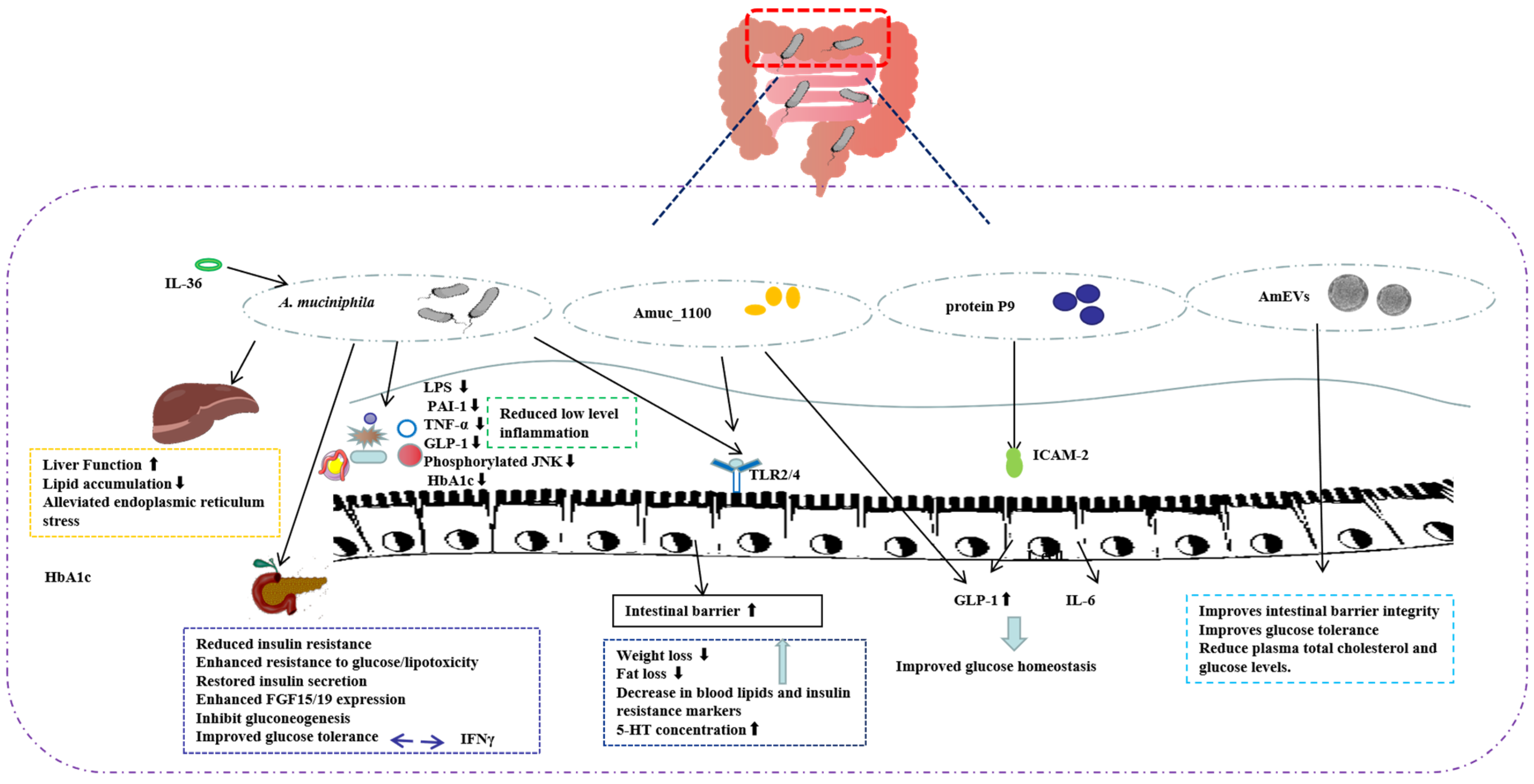
2.2.1. A. muciniphila and T2D
2.2.2. A. muciniphila Outer Membrane Protein and T2D
2.2.3. A. muciniphila-Secreted Protein and T2D
2.2.4. A. muciniphila-Derived Extracellular Vesicles (AmEVs) and T2D
2.3. Role of A. muciniphila in GDM
3. Modulation of Diabetes Development by Drug-Induced Changes in the Abundance of A. muciniphila
| Drugs | Models | Periods | Effect of Treatment on Microbes | Beneficial Changes | References |
|---|---|---|---|---|---|
| Metformin | HFD mice | 10 weeks | A. muciniphila ↑ Clostridium cocleatum ↑ | Improved serum glucose levels | [58] |
| Metformin | Normal chow diet or HFD mice | 6 weeks | A. muciniphila ↑ | Reduced serum LPS, enhanced glucose tolerance, attenuated adipose tissue inflammation | [59] |
| Metformin | 28 patients with T2D | Not mentioned | A. muciniphila ↑ Butyrivibrio ↑ Bifidobacterium bifidum ↑ Megasphaera ↑ | Significant differences were found in the comparison of β-diversity of microbial groups | [60] |
| GLP-1 AR (i.e., liraglutide) | 37 patients with T2D (18 treated with metformin and 19 treated with GLP-1 mimetics) | 6 weeks | A. muciniphila ↑ | Patients receiving a GLP-1 agonist had higher Akkermansia abundances than those on metformin | [68] |
| Andrographolide | db/db mice | 8 weeks | A. muciniphila ↑ Bacteroidetes/Firmicutes ↑ | Improved glucose tolerance and insulin resistance, and reduced redox disorders and inflammation | [73] |
| Rhubarb extract | HFHS mice | 8 weeks | A. muciniphila ↑ | Increased Reg3γ expression in the colon, prevented insulin resistance and liver steatosis | [74] |
| Huang-Lian-Jie-Du-Decoction | HFD- and streptozotocin-induced type 2 diabetic rats | 4 weeks | A. muciniphila ↑ Parabacteroides ↑ Blautia ↑ Aerococcus ↓ Staphylococcus ↓ Corynebacterium ↓ | Improved impaired glucose tolerance | [75] |
| JinQi Jiangtang Tablet | Streptozotocin-induced type 2 diabetic rats | 5 weeks | A. muciniphila ↑ Desulfovibrio ↓ | Down-regulated fasting glucose and HbA1c levels, reduced TNF-α and IL-6 levels, increased insulin sensitivity, and inhibited inflammation | [76] |
| WBF-011 | 76 patients with T2D | Average of 78 days | Not mentioned | Improved glycated hemoglobin and blood glucose levels | [79] |
4. Modulation of Diabetes Development by Diet-Induced Changes in the Abundance of A. muciniphila
| Source | Models | Treatment | Effect of Treatment on Microbes | Beneficial Changes | References |
|---|---|---|---|---|---|
| CE | HFHS mice | 200 mg/kg for 8 weeks | A. muciniphila ↑ (2% to over 30% in feces) | Improved insulin tolerance, lower homeostasis model assessment of insulin resistance, and decreased glucose-induced hyperinsulinemia | [92] |
| CE | DIO mice | CE (200 mg/kg, Chow + CE, HFHS + CE) or vehicle (Chow, HFHS) for 8 weeks | A. muciniphila ↑ | Reverse HFHS diet-induced insulin resistance and hepatic steatosis | [88] |
| GP | C57BL/6J mice | HFD containing 1% Concord grape polyphenols | A. muciniphila ↑ Firmicutes to Bacteroidetes ↓ | Lowered intestinal expression of inflammatory markers (TNFα, IL-6, inducible nitric oxide synthase); attenuated glucose intolerance | [89] |
| GPs(proanthocyanidi) | C57BL/6J mice | 10 days with GPE (delivering 360 mg total PACs/kg), PAC standard (360 mg/kg) | A. muciniphila ↑ | Attenuated glucose intolerance | [90] |
| Oligofructose | DIO mice | 0.3 g/d with a standard diet for 8 weeks | A. muciniphila ↑ (100-fold decrease in feces) | Reversed metabolic endotoxemia, improved mucosal barrier function | [24] |
| Oligofructose | ob/ob mice | 0.3 g/d with a standard diet for 8 weeks | A. muciniphila ↑ (80-fold decrease in feces) | Reduced plasma LPS, improved gut barrier function, improved glucose tolerance | [96] |
| Mannan and metformin | HFD- and streptozotocin-induced type 2 diabetic rats | 5 weeks | A. muciniphila ↑ Bifidobacterium pseudolongum ↑ | Improved insulin resistance and glucose tolerance | [97] |
| DF | HFD and streptozotocin-induced type 2 diabetic rats | 10% or 2,5% modified DF into HFD for 4 weeks | A. muciniphila ↑ Firmicutes to Bacteroidetes ↓ | Increased the serum insulin content, recovery effect on islet β-cells | [98] |
| NaBut and inulin | 60 overweight and obese diabetic patients | 600 mg/d NaBut (group A), 10 g/d inulin powder (group B), both inulin and NaBut (group C) | A. muciniphila ↑ | Reduced TNF-α mRNA expression | [99] |
| Walnut green husk polysaccharide extracts | High-fructose diet-induced obese mice | 200, 400, and 800 mg per kg bw (0.4 mL, i.g.) once daily | A. muciniphila ↓ Lachnoclostridium↓ | Suppressed weight gain, liver and fat weight, TG levels, TC levels, insulin levels, and glucose levels | [101] |
| Mediterranean diet | Twenty-nine overweight/obese individuals of both genders, aged 20–60 years | Mediterranean diet for 8 weeks | A. muciniphila ↑ Intestinimonas butyriciproducens↑ | Reduced glucose and insulin responses, improved insulin sensitivity | [103] |
| Functional food-based diet | 81 patients with T2D | Dietary portfolio or portfolio treatment combined with reduced energy diet for 1 month | P. Copri ↓ A. muciniphila ↑ Faecalibacterium prausnitzii ↑ | Reduced metabolic endotoxemia mediated by LPS, increased serum antioxidant activity | [104] |
5. Limitations of A. muciniphila-Related Studies
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Salpea, P.; Karuranga, S.; Petersohn, I.; Malanda, B.; Gregg, E.W.; Unwin, N.; Wild, S.H.; Williams, R. Mortality attributable to diabetes in 20–79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2020, 162, 108086. [Google Scholar] [CrossRef] [PubMed]
- Bielka, W.; Przezak, A.; Pawlik, A. The Role of the Gut Microbiota in the Pathogenesis of Diabetes. Int. J. Mol. Sci. 2022, 23, 480. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, J.; Hao, Y.; Zhou, H.; Hu, Y.; Zhang, C.; Zheng, H.; Wang, X.; Zeng, F.; Hu, J.; et al. Akkermansia muciniphila plays critical roles in host health. Crit. Rev. Microbiol. 2023, 49, 82–100. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Davids, M.; Suarez-Diez, M.; Boeren, S.; Schaap, P.J.; Martins Dos Santos, V.A.P.; Smidt, H.; Belzer, C.; de Vos, W.M. Genome-Scale Model and Omics Analysis of Metabolic Capacities of Akkermansia muciniphila Reveal a Preferential Mucin-Degrading Lifestyle. Appl. Environ. Microbiol. 2017, 83, e01014–e01017. [Google Scholar] [CrossRef]
- Yan, J.; Sheng, L.; Li, H. Akkermansia muciniphila: Is it the Holy Grail for ameliorating metabolic diseases. Gut Microbes. 2021, 13, 1984104. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, F.; Keshavarz Azizi Raftar, S.; Lari, A.; Shahryari, A.; Abdollahiyan, S.; Moradi, H.R.; Masoumi, M.; Davari, M.; Khatami, S.; Omrani, M.D.; et al. Extracellular vesicles and pasteurized cells derived from Akkermansia muciniphila protect against high-fat induced obesity in mice. Microb. Cell Fact. 2021, 20, 219. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; Herold, K.; Eisenbarth, G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 2010, 464, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Hänninen, A.; Toivonen, R.; Pöysti, S.; Belzer, C.; Plovier, H.; Ouwerkerk, J.P.; Emani, R.; Cani, P.D.; De Vos, W.M. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut 2018, 67, 1445–1453. [Google Scholar] [CrossRef]
- Chen, D.; Thayer, T.C.; Wen, L.; Wong, F.S. Mouse Models of Autoimmune Diabetes: The Nonobese Diabetic (NOD) Mouse. Methods Mol. Biol. 2020, 2128, 87–92. [Google Scholar] [PubMed]
- Hansen, C.H.; Krych, L.; Nielsen, D.S.; Vogensen, F.K.; Hansen, L.H.; Sørensen, S.J.; Buschard, K.; Hansen, A.K. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia 2012, 55, 2285–2294. [Google Scholar] [CrossRef]
- Fassatoui, M.; Lopez-Siles, M.; Díaz-Rizzolo, D.A.; Jmel, H.; Naouali, C.; Abdessalem, G.; Chikhaoui, A.; Nadal, B.; Jamoussi, H.; Abid, A.; et al. Gut microbiota imbalances in Tunisian participants with type 1 and type 2 diabetes mellitus. Biosci. Rep. 2019, 39, BSR20182348. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Liu, X.; Liu, X.; Liu, T.; Feng, Y.; Yuan, Z.; Jia, Z.; Zhang, Y. Akkermansia muciniphila administration ameliorates streptozotocin-induced hyperglycemia and muscle atrophy by promoting IGF2 secretion from mouse intestine. IMeta 2024, 3, e237. [Google Scholar] [CrossRef]
- Huang, J.; Peng, J.; Pearson, J.A.; Efthimiou, G.; Hu, Y.; Tai, N.; Xing, Y.; Zhang, L.; Gu, J.; Jiang, J.; et al. Toll-like receptor 7 deficiency suppresses type 1 diabetes development by modulating B-cell differentiation and function. Cell Mol. Immunol. 2021, 18, 28–338. [Google Scholar] [CrossRef]
- Tai, N.; Wong, F.S.; Wen, L. The role of the innate immune system in destruction of pancreatic beta cells in NOD mice and humans with type I diabetes. J. Autoimmun. 2016, 71, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Mithieux, G. Does Akkermansia muciniphila play a role in type 1 diabetes? Gut 2018, 67, 1373–1374. [Google Scholar] [CrossRef] [PubMed]
- Harreiter, J.; Roden, M. Diabetes mellitus—Definition, Klassifikation, Diagnose, Screening und Prävention (Update 2023). Wien. Klin. Wochenschr. 2023, 135 (Suppl. S1), 7–17. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.F.; Elias-Oliveira, J.; Pereira, Í.S.; Pereira, J.A.; Barbosa, S.C.; Machado, M.S.G.; Carlos, D. Akkermansia muciniphila and Gut Immune System: A Good Friendship That Attenuates Inflammatory Bowel Disease, Obesity, and Diabetes. Front. Immunol. 2022, 13, 934695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, Q.; Cheng, L.; Buch, H.; Zhang, F. Akkermansia muciniphila is a promising probiotic. Microb. Biotechnol. 2019, 12, 1109–1125. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Ellekilde, M.; Krych, L.; Hansen, C.H.; Hufeldt, M.R.; Dahl, K.; Hansen, L.H.; Sørensen, S.J.; Vogensen, F.K.; Nielsen, D.S.; Hansen, A.K. Characterization of the gut microbiota in leptin deficient obese mice—Correlation to inflammatory and diabetic parameters. Res. Vet. Sci. 2014, 96, 241–250. [Google Scholar] [CrossRef]
- Letchumanan, G.; Abdullah, N.; Marlini, M.; Baharom, N.; Lawley, B.; Omar, M.R.; Mohideen, F.B.S.; Addnan, F.H.; Nur Fariha, M.M.; Ismail, Z.; et al. Gut Microbiota Composition in Prediabetes and Newly Diagnosed Type 2 Diabetes: A Systematic Review of Observational Studies. Front. Cell Infect. Microbiol. 2022, 12, 943427. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Q.; Liu, M.; Zhang, X.; He, F.; Wang, G. Akkermansia muciniphila can reduce the damage of gluco/lipotoxicity, oxidative stress and inflammation, and normalize intestine microbiota in streptozotocin-induced diabetic rats. Pathog. Dis. 2018, 76, fty028. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.T.; Yeh, Y.T.; Lin, C.C.; Yang, L.Y.; Chiang, C.P. Akkermansia muciniphila is Negatively Correlated with Hemoglobin A1c in Refractory Diabetes. Microorganisms 2020, 8, 1360. [Google Scholar] [CrossRef]
- Zhang, L.; Chu, J.; Hao, W.; Zhang, J.; Li, H.; Yang, C.; Yang, J.; Chen, X.; Wang, H. Gut Microbiota and Type 2 Diabetes Mellitus: Association, Mechanism, and Translational Applications. Mediators Inflamm. 2021, 2021, 5110276. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, W.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Liu, R.; Hong, J. Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J. Mol. Endocrinol. 2017, 58, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sanjiwani, M.I.D.; Aryadi, I.P.H.; Semadi, I.M.S. Review of Literature on Akkermansia muciniphila and its Possible Role in the Etiopathogenesis and Therapy of Type 2 Diabetes Mellitus. J. ASEAN Fed. Endocr. Soc. 2022, 37, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Chen, L.; Li, N.; Wei, X.; Wang, J.; Dong, W.; Wang, Y.; Shi, J.; Ding, X.; Peng, Y. Effect of Akkermansia muciniphila on pancreatic islet β-cell function in rats with prediabetes mellitus induced by a high-fat diet. Bioresour. Bioprocess. 2024, 11, 51. [Google Scholar] [CrossRef]
- Altalhi, R.; Pechlivani, N.; Ajjan, R.A. PAI-1 in Diabetes: Pathophysiology and Role as a Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 3170. [Google Scholar] [CrossRef]
- Yarmolinsky, J.; Bordin Barbieri, N.; Weinmann, T.; Ziegelmann, P.K.; Duncan, B.B.; Inês Schmidt, M. Plasminogen activator inhibitor-1 and type 2 diabetes: A systematic review and meta-analysis of observational studies. Sci. Rep. 2016, 6, 17714. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Liu, W.; Pan, X.; Liu, L.; Yang, Y.; Wang, D.; Xu, P.; Huang, M.; Chen, Z. Specific inhibition of plasminogen activator inhibitor 1 reduces blood glucose level by lowering TNF-α. Life Sci. 2020, 246, 117404. [Google Scholar] [CrossRef]
- Holst, J.J.; Gasbjerg, L.S.; Rosenkilde, M.M. The Role of Incretins on Insulin Function and Glucose Homeostasis. Endocrinology 2021, 162, bqab065. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; Naya, I.; García-Honduvilla, N.; Álvarez-Mon, M.; Buján, J.; Asúnsolo, Á.; de la Torre, B. Type 2 Diabetes Mellitus Associated with Obesity (Diabesity). The Central Role of Gut Microbiota and Its Translational Applications. Nutrients 2020, 12, 2749. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Galiero, R.; Caturano, A.; Rinaldi, L.; Criscuolo, L.; Di Martino, A.; Albanese, G.; Vetrano, E.; Catalini, C.; Sardu, C.; et al. Current Knowledge on the Pathophysiology of Lean/Normal-Weight Type 2 Diabetes. Int. J. Mol. Sc. 2022, 24, 658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ni, Y.; Qian, L.; Fang, Q.; Zheng, T.; Zhang, M.; Gao, Q.; Zhang, Y.; Ni, J.; Hou, X.; et al. Decreased Abundance of Akkermansia muciniphila Leads to the Impairment of Insulin Secretion and Glucose Homeostasis in Lean Type 2 Diabetes. Adv. Sci. 2021, 8, e2100536. [Google Scholar] [CrossRef] [PubMed]
- Greer, R.L.; Dong, X.; Moraes, A.C.; Zielke, R.A.; Fernandes, G.R.; Peremyslova, E.; Vasquez-Perez, S.; Schoenborn, A.A.; Gomes, E.P.; Pereira, A.C.; et al. Akkermansia muciniphila mediates negative effects of IFNγ on glucose metabolism. Nat. Commun. 2016, 7, 13329. [Google Scholar] [CrossRef] [PubMed]
- Giannoudaki, E.; Hernandez-Santana, Y.E.; Mulfaul, K.; Doyle, S.L.; Hams, E.; Fallon, P.G.; Mat, A.; O’Shea, D.; Kopf, M.; Hogan, A.E.; et al. Interleukin-36 cytokines alter the intestinal microbiome and can protect against obesity and metabolic dysfunction. Nat. Commun. 2019, 10, 4003. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietilä, T.E.; Kainulainen, V.; Klievink, J.; Huuskonen, L.; Aalvink, S.; Skurnik, M.; Boeren, S.; et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE 2017, 12, e0173004. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Wang, R.; Cheng, R.; Tang, Z.; Zhang, M. The outer membrane protein Amuc_1100 of Akkermansia muciniphila promotes intestinal 5-HT biosynthesis and extracellular availability through TLR2 signalling. Food Funct. 2021, 12, 3597–3610. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Jin, Y.L.; Pei, W.L.; Li, J.C.; Zhang, R.L.; Wang, J.J.; Lin, W. Amuc_1100 pretreatment alleviates acute pancreatitis in a mouse model through regulating gut microbiota and inhibiting inflammatory infiltration. Acta Pharmacol. Sin. 2024, 45, 570–580. [Google Scholar] [CrossRef]
- Gu, Z.; Pei, W.; Shen, Y.; Wang, L.; Zhu, J.; Zhang, Y.; Fan, S.; Wu, Q.; Li, L.; Zhang, Z. Akkermansia muciniphila and its outer protein Amuc_1100 regulates tryptophan metabolism in colitis. Food Funct. 2021, 12, 10184–10195. [Google Scholar] [CrossRef]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.H.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Di, W.; Zhang, Y.; Zhang, X.; Han, L.; Zhao, L.; Hao, Y.; Zhai, Z. Heterologous expression of P9 from Akkermansia muciniphila increases the GLP-1 secretion of intestinal L cells. World J. Microbiol. Biotechnol. 2024, 40, 199. [Google Scholar] [CrossRef]
- Kang, E.J.; Kim, J.H.; Kim, Y.E.; Lee, H.; Jung, K.B.; Chang, D.H.; Lee, Y.; Park, S.; Lee, E.Y.; Lee, E.J.; et al. The secreted protein Amuc_1409 from Akkermansia muciniphila improves gut health through intestinal stem cell regulation. Nat. Commun. 2024, 15, 2983. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, G.; Zhang, Q.; Liu, Z.; Jiang, X.; Xin, Y. Function of Akkermansia muciniphila in type 2 diabetes and related diseases. Front. Microbiol. 2023, 14, 1172400. [Google Scholar] [CrossRef]
- Chelakkot, C.; Choi, Y.; Kim, D.K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.S.; Jee, Y.K.; Gho, Y.S.; et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, F.; Shahriary, A.; Behrouzi, A.; Moradi, H.R.; Keshavarz Azizi Raftar, S.; Lari, A.; Hadifar, S.; Yaghoubfar, R.; Ahmadi Badi, S.; Khatami, S.; et al. Akkermansia muciniphila-Derived Extracellular Vesicles as a Mucosal Delivery Vector for Amelioration of Obesity in Mice. Front. Microbiol. 2019, 10, 2155. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Lin, C.; Feng, Y.; You, Y.; Jin, Z.; Li, M.; Zhou, Y.; Chen, K. Akkermansia muciniphila-derived small extracellular vesicles attenuate intestinal ischemia-reperfusion-induced postoperative cognitive dysfunction by suppressing microglia activation via the TLR2/4 signaling. Biochimica et biophysica acta. Mol. Cell Res. 2024, 1871, 119630. [Google Scholar] [CrossRef] [PubMed]
- Menafra, D.; Proganò, M.; Tecce, N.; Pivonello, R.; Colao, A. Diet and gut microbiome: Impact of each factor and mutual interactions on prevention and treatment of type 2, type 1, and gestational diabetes. Hum. Nutr. Metab. 2024, 38. [Google Scholar] [CrossRef]
- Cortez, R.V.; Taddei, C.R.; Sparvoli, L.G.; Ângelo, A.G.S.; Padilha, M.; Mattar, R.; Daher, S. Microbiome and its relation to gestational diabetes. Endocrine 2019, 64, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, G.; Cui, L.; Zhang, L.; Zhou, Q.; Mu, C.; Chi, R.; Zhang, N.; Ma, G. Dynamic changes in gut microbiota during pregnancy among Chinese women and influencing factors: A prospective cohort study. Front. Microbiol. 2023, 14, 1114228. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, J.; Wu, W.; Su, C.; Wu, Y.; Li, Q. Xylooligosaccharides ameliorate insulin resistance by increasing Akkermansia muciniphila and improving intestinal barrier dysfunction in gestational diabetes mellitus mice. Food Funct. 2024, 15, 3122–3129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, N. Effects of Metformin on the Gut Microbiota in Obesity and Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2020, 13, 5003–5014. [Google Scholar] [CrossRef]
- Kaneto, H.; Kimura, T.; Obata, A.; Shimoda, M.; Kaku, K. Multifaceted Mechanisms of Action of Metformin Which Have Been Unraveled One after Another in the Long History. Int. J. Mol. Sci. 2021, 22, 2596. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ko, G. Effect of metformin on metabolic improvement and gut microbiota. Appl. Environ. Microbiol. 2014, 80, 5935–5943. [Google Scholar] [CrossRef]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef]
- de la Cuesta-Zuluaga, J.; Mueller, N.T.; Corrales-Agudelo, V.; Velásquez-Mejía, E.P.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Metformin Is Associated with Higher Relative Abundance of Mucin-Degrading Akkermansia muciniphila and Several Short-Chain Fatty Acid-Producing Microbiota in the Gut. Diabetes Care 2017, 40, 54–62. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: Update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. 2023, 19, 460–476. [Google Scholar] [CrossRef] [PubMed]
- He, K.Y.; Lei, X.Y.; Wu, D.H.; Zhang, L.; Li, J.Q.; Li, Q.T.; Yin, W.T.; Zhao, Z.L.; Liu, H.; Xiang, X.Y.; et al. Akkermansia muciniphila protects the intestine from irradiation-induced injury by secretion of propionic acid. Gut Microbes 2023, 15, 2293312. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.; Li, F.; Deng, W.; Li, Z.; Wang, S.; Lv, P.; Chen, Y. Metformin Exerts Anti-inflammatory and Mucus Barrier Protective Effects by Enriching Akkermansia muciniphila in Mice with Ulcerative Colitis. Front. Pharmacol. 2021, 12, 726707. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Shen, J.; Feng, S.; Huang, C.; Wang, H.; Huo, F.; Liu, H. Akkermansia muciniphila, which is enriched in the gut microbiota by metformin, improves cognitive function in aged mice by reducing the proinflammatory cytokine interleukin-6. Microbiome 2023, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Rosell-Díaz, M.; Petit-Gay, A.; Molas-Prat, C.; Gallardo-Nuell, L.; Ramió-Torrentà, L.; Garre-Olmo, J.; Pérez-Brocal, V.; Moya, A.; Jové, M.; Pamplona, R.; et al. Metformin-induced changes in the gut microbiome and plasma metabolome are associated with cognition in men. Metab. Clin. Exp. 2024, 157, 155941. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Price, J.K.; Owrangi, S.; Gundu-Rao, N.; Satchi, R.; Paik, J.M. The Global Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis Among Patients with Type 2 Diabetes. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2024, 22, 1999–2010.e8. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Stephens, J.W.; Harris, D.A. Gut microbiota influence in type 2 diabetes mellitus (T2DM). Gut Pathog. 2021, 13, 50. [Google Scholar] [CrossRef]
- Wang, Z.; Saha, S.; Van Horn, S.; Thomas, E.; Traini, C.; Sathe, G.; Rajpal, D.K.; Brown, J.R. Gut microbiome differences between metformin- and liraglutide-treated T2DM subjects. Endocrinol. Diabetes Metab. 2017, 1, e00009. [Google Scholar] [CrossRef] [PubMed]
- Moreira, G.V.; Azevedo, F.F.; Ribeiro, L.M.; Santos, A.; Guadagnini, D.; Gama, P.; Liberti, E.A.; Saad, M.; Carvalho, C. Liraglutide modulates gut microbiota and reduces NAFLD in obese mice. J. Nutr. Biochem. 2018, 62, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Zheng, Z.; Zhang, D.; He, S.; Shen, J. Efficacy of liraglutide in treating type 2 diabetes mellitus complicated with non-alcoholic fatty liver disease. Biosci. Rep. 2018, 38, BSR20181304. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Tian, W.; Lin, L.; Xu, X. Liraglutide or insulin glargine treatments improves hepatic fat in obese patients with type 2 diabetes and nonalcoholic fatty liver disease in twenty-six weeks: A randomized placebo-controlled trial. Diabetes Res. Clin. Pract. 2020, 170, 108487. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Rongjiong, Z.; Kahaer, M.; Chunhui, J.; Wulasihan, M. Therapeutic efficacy of liraglutide versus metformin in modulating the gut microbiota for treating type 2 diabetes mellitus complicated with nonalcoholic fatty liver disease. Front. Microbiol. 2023, 14, 1088187. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Mo, J.; Ni, J.; Ke, H.; Bao, T.; Xie, J.; Xu, Y.; Xie, L.; Chen, W. Andrographolide Exerts Antihyperglycemic Effect through Strengthening Intestinal Barrier Function and Increasing Microbial Composition of Akkermansia muciniphila. Oxid. Med. Cell Longev. 2020, 2020, 6538930. [Google Scholar] [CrossRef]
- Régnier, M.; Rastelli, M.; Morissette, A.; Suriano, F.; Le Roy, T.; Pilon, G.; Delzenne, N.M.; Marette, A.; Van Hul, M.; Cani, P.D. Rhubarb Supplementation Prevents Diet-Induced Obesity and Diabetes in Association with Increased Akkermansia muciniphila in Mice. Nutrients 2020, 12, 2932. [Google Scholar] [CrossRef]
- Chen, M.; Liao, Z.; Lu, B.; Wang, M.; Lin, L.; Zhang, S.; Li, Y.; Liu, D.; Liao, Q.; Xie, Z. Huang-Lian-Jie-Du-Decoction Ameliorates Hyperglycemia and Insulin Resistant in Association with Gut Microbiota Modulation. Front. Microbiol. 2018, 9, 2380. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yao, G.; Sheng, Y.; Yang, L.; Wang, Z.; Yang, Z.; Zhuang, P.; Zhang, Y. JinQi Jiangtang Tablet Regulates Gut Microbiota and Improve Insulin Sensitivity in Type 2 Diabetes Mice. J. Diabetes Res. 2019, 2019, 1872134. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Ni, Y.; Cheung, C.K.Y.; Lam, K.S.L.; Wang, Y.; Xia, Z.; Ye, D.; Guo, J.; Tse, M.A.; et al. Gut Microbiome Fermentation Determines the Efficacy of Exercise for Diabetes Prevention. Cell Metab. 2020, 31, 77–91.e5. [Google Scholar] [CrossRef]
- Meng, X.; Xv, C.; Lv, J.; Zhang, S.; Ma, C.; Pang, X. Optimizing Akkermansia muciniphila Isolation and Cultivation: Insights into Gut Microbiota Composition and Potential Growth Promoters in a Chinese Cohort. Microorganisms 2024, 12, 881. [Google Scholar] [CrossRef] [PubMed]
- Perraudeau, F.; McMurdie, P.; Bullard, J.; Cheng, A.; Cutcliffe, C.; Deo, A.; Eid, J.; Gines, J.; Iyer, M.; Justice, N.; et al. Improvements to postprandial glucose control in subjects with type 2 diabetes: A multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation. BMJ Open Diabetes Res. Care 2020, 8, e001319. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Stoeva, M.K.; Justice, N.; Nemchek, M.; Sieber, C.M.K.; Tyagi, S.; Gines, J.; Skennerton, C.T.; Souza, M.; Kolterman, O.; et al. Increased circulating butyrate and ursodeoxycholate during probiotic intervention in humans with type 2 diabetes. BMC Microbiol. 2022, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Dimba, N.R.; Mzimela, N.; Sosibo, A.M.; Khathi, A. Effectiveness of Prebiotics and Mediterranean and Plant-Based Diet on Gut Microbiota and Glycemic Control in Patients with Prediabetes or Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 3272. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kanwal, R.; Yue, X.; Li, M.; Xie, A. Polyphenols and Intestinal Microorganisms: A Review of Their Interactions and Effects on Human Health. Food Biosci. 2024, 62, 105220. [Google Scholar] [CrossRef]
- Xie, A.; Zhao, S.; Liu, Z.; Yue, X.; Shao, J.; Li, M.; Li, Z. Polysaccharides, proteins, and their complex as microencapsulation carriers for delivery of probiotics: A review on carrier types and encapsulation techniques. Int. J. Biol. Macromol. 2023, 242 Pt 1, 124784. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Xie, A.; Li, Z.; Jiang, C.; Wu, J.; Li, M.; Yue, X. Research Progress for Probiotics Regulating Intestinal Flora to Improve Functional Dyspepsia: A Review. Foods 2024, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; An, F.; Zhang, T.; Lou, M.; Guo, J.; Liu, K.; Zhu, Y.; Wu, J.; Wu, R. Antimicrobial peptides: An alternative to traditional antibiotics. Eur. J. Med. Chem. 2024, 265, 116072. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Feng, S.; Arjan, N.; Chen, W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019, 59, 3227–3236. [Google Scholar] [CrossRef]
- Hagi, T.; Belzer, C. The interaction of Akkermansia muciniphila with host-derived substances, bacteria and diets. Appl. Microbiol. Biotechnol. 2021, 105, 4833–4841. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Nachbar, R.T.; Varin, T.V.; Vilela, V.; Dudonné, S.; Pilon, G.; Fournier, M.; Lecours, M.A.; Desjardins, Y.; Roy, D.; et al. A polyphenol-rich cranberry extract reverses insulin resistance and hepatic steatosis independently of body weight loss. Mol. Metab. 2017, 6, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Carmody, R.N.; Kalariya, H.M.; Duran, R.M.; Moskal, K.; Poulev, A.; Kuhn, P.; Tveter, K.M.; Turnbaugh, P.J.; Raskin, I.; et al. Grape proanthocyanidin-induced intestinal bloom of Akkermansia muciniphila is dependent on its baseline abundance and precedes activation of host genes related to metabolic health. J. Nutr. Biochem. 2018, 56, 142–151. [Google Scholar] [CrossRef]
- Pheiffer, C.; Riedel, S.; Dias, S.; Adam, S. Gestational Diabetes and the Gut Microbiota: Fibre and Polyphenol Supplementation as a Therapeutic Strategy. Microorganisms 2024, 12, 633. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yue, C.; Tian, R.; Yu, L.; Tian, F.; Zhao, J.; Chen, W.; Zhai, Q. Akkermansia muciniphila-directed polyphenol chlorogenic acid intervention for obesity in mice. Food Sci. Hum. Wellness 2023, 13, 90–100. [Google Scholar] [CrossRef]
- Power, K.A.; Lepp, D.; Zarepoor, L.; Monk, J.M.; Wu, W.; Tsao, R.; Liu, R. Dietary flaxseed modulates the colonic microenvironment in healthy C57Bl/6 male mice which may alter susceptibility to gut-associated diseases. J. Nutr. Biochem. 2016, 28, 61–69. [Google Scholar] [CrossRef]
- Livingston, D.B.H.; Sweet, A.; Rodrigue, A.; Kishore, L.; Loftus, J.; Ghali, F.; Mahmoodianfard, S.; Celton, C.; Hosseinian, F.; Power, K.A. Dietary Flaxseed and Flaxseed Oil Differentially Modulate Aspects of the Microbiota Gut-Brain Axis Following an Acute Lipopolysaccharide Challenge in Male C57Bl/6 Mice. Nutrients 2023, 15, 3542. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; François, P.; de Vos, W.M.; et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Li, H.; Zhang, X.; Jiang, M.; Luo, C.; Lu, Z.; Xu, Z.; Shi, J. Prebiotic Mannan-Oligosaccharides Augment the Hypoglycemic Effects of Metformin in Correlation with Modulating Gut Microbiota. J. Agric. Food Chem. 2018, 66, 5821–5831. [Google Scholar] [CrossRef]
- Li, X.X.; Zhang, X.X.; Zhang, R.; Ni, Z.J.; Elam, E.; Thakur, K.; Cespedes-Acuña, C.L.; Zhang, J.G.; Wei, Z.J. Gut modulation based anti-diabetic effects of carboxymethylated wheat bran dietary fiber in high-fat diet/streptozotocin-induced diabetic mice and their potential mechanisms. Food Chem. Toxicol. 2021, 152, 112235. [Google Scholar] [CrossRef] [PubMed]
- Roshanravan, N.; Mahdavi, R.; Alizadeh, E.; Ghavami, A.; Rahbar Saadat, Y.; Mesri Alamdari, N.; Alipour, S.; Dastouri, M.R.; Ostadrahimi, A. The effects of sodium butyrate and inulin supplementation on angiotensin signaling pathway via promotion of Akkermansia muciniphila abundance in type 2 diabetes; A randomized, double-blind, placebo-controlled trial. J. Cardiovasc. Thorac. Res. 2017, 9, 183–190. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Tan, H.; Zhong, Y.; Nie, S. Akkermansia muciniphila, an important link between dietary fiber and host health. Curr. Opin. Food Sci. 2022, 47, 100905. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Y.; Zhang, R.; Pan, J.; Qi, D.; Wang, J.; Yang, X. The protective effects of walnut green husk polysaccharide on liver injury, vascular endothelial dysfunction and disorder of gut microbiota in high fructose-induced mice. Int. J. Biol. Macromol. 2020, 162, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Hu, Q.; Han, Y.; Du, H.; Yang, W.; Pan, C.; Cao, X.; Muinde Kimatu, B.; Pei, F.; Xiao, H. Inhibitory effects of β-type glycosidic polysaccharide from Pleurotus eryngii on dextran sodium sulfate-induced colitis in mice. Food Funct. 2021, 12, 3831–3841. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.; Giacco, R.; Laiola, M.; Della Pepa, G.; Luongo, D.; Mangione, A.; Salamone, D.; Vitaglione, P.; Ercolini, D.; Rivellese, A.A. Acute and chronic improvement in postprandial glucose metabolism by a diet resembling the traditional Mediterranean dietary pattern: Can SCFAs play a role? Clin. Nutr. 2021, 40, 428–437. [Google Scholar] [CrossRef]
- Medina-Vera, I.; Sanchez-Tapia, M.; Noriega-López, L.; Granados-Portillo, O.; Guevara-Cruz, M.; Flores-López, A.; Avila-Nava, A.; Fernández, M.L.; Tovar, A.R.; Torres, N. A dietary intervention with functional foods reduces metabolic endotoxaemia and attenuates biochemical abnormalities by modifying faecal microbiota in people with type 2 diabetes. Diabetes Metab. 2019, 45, 122–131. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Seregin, S.S.; Golovchenko, N.; Schaf, B.; Chen, J.; Pudlo, N.A.; Mitchell, J.; Baxter, N.T.; Zhao, L.; Schloss, P.D.; Martens, E.C.; et al. NLRP6 Protects Il10 Mice from Colitis by Limiting Colonization of Akkermansia muciniphila. Cell Rep. 2017, 19, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Druart, C.; Plovier, H.; Van Hul, M.; Brient, A.; Phipps, K.R.; de Vos, W.M.; Cani, P.D. Toxicological safety evaluation of pasteurized Akkermansia muciniphila. J. Appl. Toxicol. 2021, 41, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Eid, J.; Cheng, A.; Lynch, B.; Bauter, M. Lack of genotoxicity and subchronic toxicity in safety assessment studies of Akkermansia muciniphila formulation. Toxicol. Rep. 2024, 13, 101790. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, F.; Keshavarz Azizi Raftar, S.; Shahryari, A.; Behrouzi, A.; Yaghoubfar, R.; Lari, A.; Moradi, H.R.; Khatami, S.; Omrani, M.D.; Vaziri, F.; et al. Comparative effects of alive and pasteurized Akkermansia muciniphila on normal diet-fed mice. Sci. Rep. 2021, 11, 17898. [Google Scholar] [CrossRef]
- Du, Y.; An, Y.; Song, Y.; Li, N.; Zheng, J.; Lu, Y. Live and pasteurized Akkermansia muciniphila ameliorates diabetic cognitive impairment by modulating gut microbiota and metabolites in db/db mice. Exp. Neurol. 2024, 378, 114823. [Google Scholar] [CrossRef]
- Hong, R.; Xie, A.; Jiang, C.; Guo, Y.; Zhang, Y.; Chen, J.; Shen, X.; Li, M.; Yue, X. A review of the biological activities of lactoferrin: Mechanisms and potential applications. Food Funct. 2024, 15, 8182–8199. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Li, M.; Liu, Y.; Zhang, X.M.; Zheng, Y. Microbial diversity and function of soybean paste in East Asia: What we know and what we don’t. Curr. Opin. Food Sci. 2021, 37, 145–152. [Google Scholar] [CrossRef]
- Zhao, S.; Yue, J.; Wang, Y.; Shao, J.; Li, Z.; Li, M. The regulation of volatile flavor compounds in fermented meat products mediated by microorganisms: A review. Food Biosci. 2024, 62, 105180. [Google Scholar] [CrossRef]
- Corb Aron, R.A.; Abid, A.; Vesa, C.M.; Nechifor, A.C.; Behl, T.; Ghitea, T.C.; Munteanu, M.A.; Fratila, O.; Andronie-Cioara, F.L.; Toma, M.M.; et al. Recognizing the Benefits of Pre-/Probiotics in Metabolic Syndrome and Type 2 Diabetes Mellitus Considering the Influence of Akkermansia muciniphila as a Key Gut Bacterium. Microorganisms 2021, 9, 618. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, K.; An, F.; Zhang, H.; Yan, D.; Li, T.; Wu, J.; Wu, R. Akkermansia muciniphila: A Potential Target for the Prevention of Diabetes. Foods 2025, 14, 23. https://doi.org/10.3390/foods14010023
He K, An F, Zhang H, Yan D, Li T, Wu J, Wu R. Akkermansia muciniphila: A Potential Target for the Prevention of Diabetes. Foods. 2025; 14(1):23. https://doi.org/10.3390/foods14010023
Chicago/Turabian StyleHe, Kairu, Feiyu An, Henan Zhang, Danli Yan, Tong Li, Junrui Wu, and Rina Wu. 2025. "Akkermansia muciniphila: A Potential Target for the Prevention of Diabetes" Foods 14, no. 1: 23. https://doi.org/10.3390/foods14010023
APA StyleHe, K., An, F., Zhang, H., Yan, D., Li, T., Wu, J., & Wu, R. (2025). Akkermansia muciniphila: A Potential Target for the Prevention of Diabetes. Foods, 14(1), 23. https://doi.org/10.3390/foods14010023







