New Perspectives on Canned Fish Quality and Safety on the Road to Sustainability
Abstract
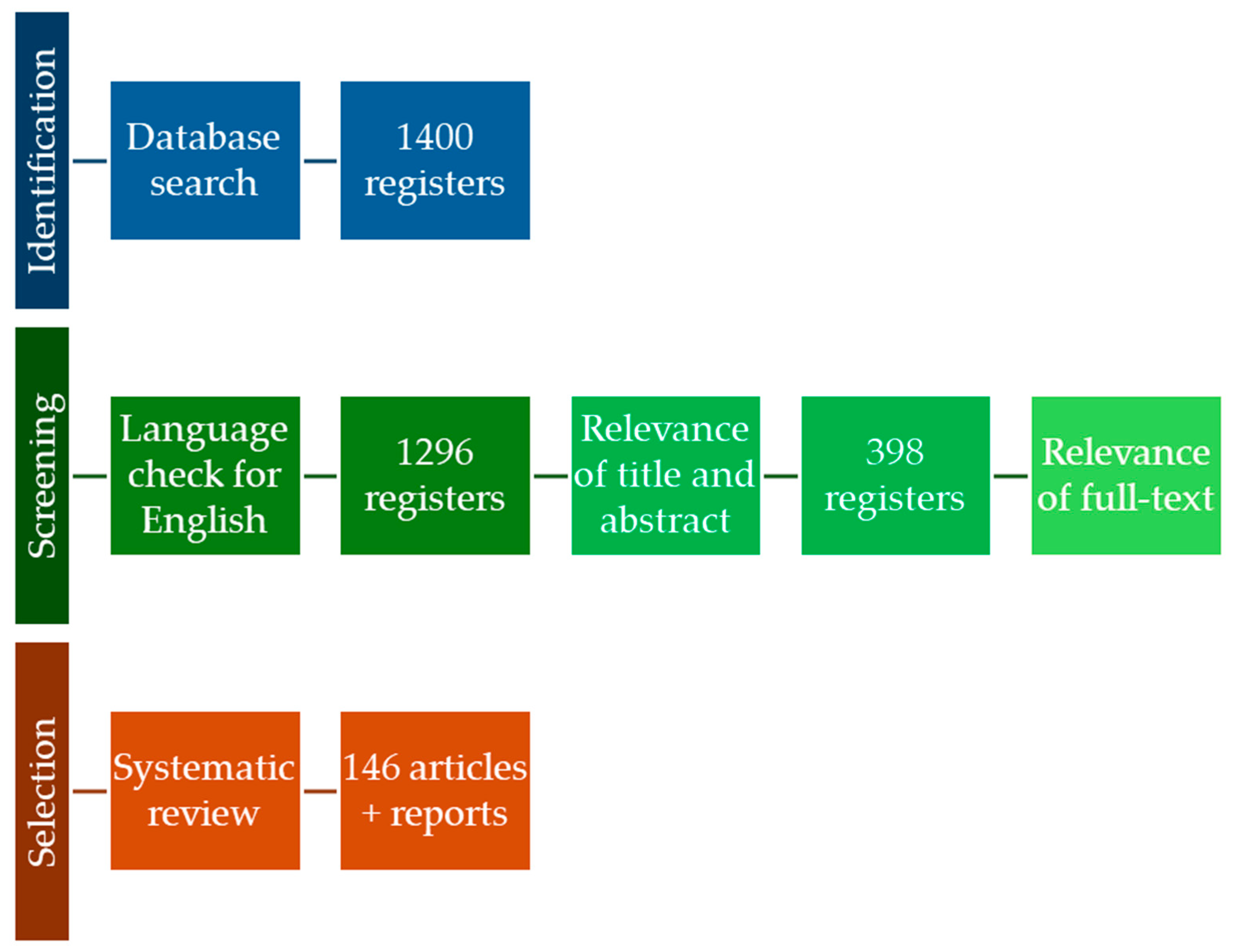
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Impacts of Seafood Canning Chain on Quality
3.2. Hazards in Canned Seafood
3.2.1. Biological Hazards
Histamine
Thermostable Allergens
Marine Biotoxins
3.2.2. Chemical Hazards
Bisphenols
Toxic Elements
| Origin | Species | n | Toxic Metals | References | ||||
|---|---|---|---|---|---|---|---|---|
| Cd | Hg | Pb | Al | Sn | ||||
| European countries | Tuna | 279 | <LOD*–110 | <0.001–0.29 | <0.007–3.05 | <LOD*–14.45 | <0.001–0.19 | [100,101,108,109,110] |
| Sardines | 100 | <0.001–113 | <0.001–0.45 | - | - | <0.001–0.16 | ||
| Mackerels | 53 | <0.001–0.12 | <0.001–0.21 | <0.001–3.05 | - | <0.001–0.39 | ||
| non-European countries | Tuna | 457 | <LOD*–0.63 | 0.01–0.79 | 0.02–2.80 | <LOD*–47.33 | 4.9–157.90 | [93,102,103,104,105,106,107,111,112,113] |
| Sardines | 201 | <LOD*–0.42 | - | <LOD*–2.50 | <LOD*–5.12 | - | ||
| Fish | 200 | 0.02–0.15 | 0.02–0.18 | 0.04–1.60 | - | - | ||
Other Contaminants
Microplastics
3.3. Innovative Canned Fish Products
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Al | Aluminum |
| BaP | Benzo[a]pyrene |
| Cd | Cadmium |
| CdB | Cyclo-di-BADGE |
| DHA | Docosahexaenoic Acid |
| DTX | Dinophysistoxin |
| EPA | Eicosapentaenoic Acid |
| Hg | Mercury |
| IgE | Immunoglobulin E |
| LDPE | Low-Density Polyethylene |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| MPLs | Maximum Permissible Limits |
| MPs | Microplastics |
| NaCl | Sodium Chloride |
| OA | Okadaic Acid |
| PA | Polyamide |
| Pb | Lead |
| PE | Polyethylene |
| PET | Polyethylene Terephthalate |
| PFHxS | Perfluorohexane Sulfonate |
| PFNA | Perfluorononanoic Acid |
| PFOA | Perfluorooctanoic Acid |
| PFOS | Perfluorooctane Sulfonate |
| PMAME | Polymethacrylic Acid Methyl Ester |
| PP | Polypropylene |
| PS | Polystyrene |
| PTX | Pectenotoxin |
| PVC | Polyvinyl Chloride |
| PVS | Poly(vinyl stearate) |
| Sn | Tin |
| TDI | Tolerable Daily Intake |
| Acronyms | |
| BADGE | Bisphenol A Diglycidyl Ether |
| BPA | Bisphenol A |
| BPAF | Bisphenol AF |
| BPB | Bisphenol B |
| BPC | Bisphenol C |
| BPE | Bisphenol E |
| BPF | Bisphenol F |
| BPG | Bisphenol G |
| BPM | Bisphenol M |
| BPP | Bisphenol P |
| BPS | Bisphenol S |
| EVOH | Ethylene-Vinyl Alcohol |
| POPs | Persistent Organic Pollutants |
| Initialisms | |
| CD | Chlorinated Derivative |
| DST | Diarrhetic Shellfish Toxin |
| EC | European Commission |
| EFSA | European Food and Safety Authority |
| EU | European Union |
| FAO | Food and Agriculture Organization |
| HACCP | Hazard Analysis and Critical Control Points |
| HD | Hydroxyl Derivative |
| IEAA | Essential Amino Acid Index |
| LF | Liquid Fraction |
| n.s. | Not Specified |
| N/A | Not Applicable |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| PAN | Polyacrylonitrile |
| PCBs | Polychlorinated Biphenyls |
| PCDD/F-TEQ | Polychlorinated Dibenzo-para-Dioxin/Furan Toxic Equivalence |
| PFASs | Perfluorinated Alkyl Substances |
| PFHxS | Perfluorohexane Sulfonate |
| PFNA | Perfluorononanoic Acid |
| PFOA | Perfluorooctanoic Acid |
| PFOS | Perfluorooctane Sulfonate |
| POF | Polyolefin |
| PSP | Paralytic Shellfish Poisoning |
| SF | Solid Fraction |
| SML | Specific Migration Limit |
| WHO | World Health Organization |
References
- Pitarch, J.L.; Vilas, C.; De Prada, C.; Palacín, C.G.; Alonso, A.A. Optimal Operation of Thermal Processing of Canned Tuna under Product Variability. J. Food Eng. 2021, 304, 110594. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Dave, D.; Budge, S.; Brooks, M.S. Fish Spoilage Mechanisms and Preservation Techniques: Review. Am. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef]
- Zelasney, J.; Ford, A.; Westlund, L.; Ward, A.; Riego Peñarubia, O. Securing Sustainable Small-Scale Fisheries: Showcasing Applied Practices in Value Chains, Post-Harvest Operations and Trade; FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2020; ISBN 978-92-5-132350-2. [Google Scholar]
- Aubourg, S.P. Enhancement of Lipid Stability and Acceptability of Canned Seafood by Addition of Natural Antioxidant Compounds to the Packing Medium—A Review. Antioxidants 2023, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Canned Seafood Market Size, Share, Trends & Forecast. 2031. Available online: https://www.skyquestt.com/report/canned-seafood-market (accessed on 3 October 2024).
- Eissa, F.; Younes, A. Fish Contamination: Analysis of the EU RASFF Notifications over the Last 23 Years. Food Control 2024, 161, 110404. [Google Scholar] [CrossRef]
- DeBeeR, J.; Bell, J.W.; Nolte, F.; Arcieri, J.; Correa, G. Histamine Limits by Country: A Survey and Review. J. Food Prot. 2021, 84, 1610–1628. [Google Scholar] [CrossRef]
- EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP); Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; et al. Re-evaluation of the Risks to Public Health Related to the Presence of Bisphenol A (BPA) in Foodstuffs. EFS2 2023, 21, e06857. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) (Draft) on the Use of Bisphenol A (BPA) and Other Bisphenols and Their Derivatives with Harmonised Classification for Specific Hazardous Properties in Certain Materials and Articles Intended to Come into Contact with Food, Amending Regulation (EU) No 10/2011, Amending Regulation (EC) No 1895/2005 and Repealing Regulation (EU) 2018/213; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- European Commission. Questions and Answers (Q&A) Concerning the Risk Management Approach for Bisphenol A (BPA) and Other Bisphenols in Food Contact Materials (FCMs); European Commission: Brussels, Belgium, 2023; pp. 1–9. [Google Scholar]
- Mahmudiono, T.; Fakhri, Y.; Adiban, M.; Sarafraz, M.; Mohamadi, S. Concentration of Potential Toxic Elements in Canned Tuna Fish: Systematic Review and Health Risk Assessment. Int. J. Environ. Health Res. 2024, 34, 2619–2637. [Google Scholar] [CrossRef]
- Rúbies, A.; Muñoz, E.; Gibert, D.; Cortés-Francisco, N.; Granados, M.; Caixach, J.; Centrich, F. New Method for the Analysis of Lipophilic Marine Biotoxins in Fresh and Canned Bivalves by Liquid Chromatography Coupled to High Resolution Mass Spectrometry: A Quick, Easy, Cheap, Efficient, Rugged, Safe Approach. J. Chromatogr. A 2015, 1386, 62–73. [Google Scholar] [CrossRef]
- Diaz-Basantes, M.F.; Nacimba-Aguirre, D.; Conesa, J.A.; Fullana, A. Presence of Microplastics in Commercial Canned Tuna. Food Chem. 2022, 385, 132721. [Google Scholar] [CrossRef] [PubMed]
- Aksun Tümerkan, E.T. Detection of Parvalbumin Fish Allergen in Canned Tuna by Real-Time PCR Driven by Tuna Species and Can-Filling Medium. Molecules 2022, 27, 5674. [Google Scholar] [CrossRef] [PubMed]
- DG MARE. Evaluation of the Marketing Standards Framework for Fishery and Aquaculture Products. Specific Contract No. 5 under Framework Contract EASME/EMFF/2016/029. Final Report; DG MARE: Brussels, Belgium, 2019; pp. 1–104. [Google Scholar]
- Bavinck, M.; Ahern, M.; Hapke, H.M.; Johnson, D.S.; Kjellevold, M.; Kolding, J.; Overå, R.; Schut, T.; Franz, N. Small Fish for Food Security and Nutrition; FAO Fisheries and Aquaculture Technical Paper No. 694; FAO: Rome, Italiy, 2023; ISBN 978-92-5-137910-3. [Google Scholar]
- Ali, A.; Wei, S.; Ali, A.; Khan, I.; Sun, Q.; Xia, Q.; Wang, Z.; Han, Z.; Liu, Y.; Liu, S. Research Progress on Nutritional Value, Preservation and Processing of Fish—A Review. Foods 2022, 11, 3669. [Google Scholar] [CrossRef] [PubMed]
- Tavares, J.; Martins, A.; Fidalgo, L.G.; Lima, V.; Amaral, R.A.; Pinto, C.A.; Silva, A.M.; Saraiva, J.A. Fresh Fish Degradation and Advances in Preservation Using Physical Emerging Technologies. Foods 2021, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Prego, R.; Trigo, M.; Martínez, B.; Aubourg, S.P. Effect of Previous Frozen Storage, Canning Process and Packing Medium on the Fatty Acid Composition of Canned Mackerel. Mar. Drugs 2022, 20, 666. [Google Scholar] [CrossRef]
- Campos, C.A.; Gliemmo, M.F.; Aubourg, S.P.; Velázquez, J.B. Novel Technologies for the Preservation of Chilled Aquatic Food Products. In Novel Technologies in Food Science: Their Impact on Products, Consumer Trends and the Environment; McElhatton, A., do Amaral Sobral, P.J., Eds.; Springer: New York, NY, USA, 2012; pp. 299–323. ISBN 978-1-4419-7880-6. [Google Scholar]
- Fardella, M.; Ramírez, C.; Caballero, E.; Sánchez, E.; Pinto, M.; Núñez, H.; Valencia, P.; Almonacid, S.; Simpson, R. Variable Retort Temperature Profiles (VRTPs) and Retortable Pouches as Tools to Minimize Furan Formation in Thermally Processed Food. Foods 2021, 10, 2205. [Google Scholar] [CrossRef]
- Simpson, R.; Jiménez, D.; Almonacid, S.; Nuñez, H.; Pinto, M.; Ramírez, C.; Vega-Castro, O.; Fuentes, L.; Angulo, A. Assessment and Outlook of Variable Retort Temperature Profiles for the Thermal Processing of Packaged Foods: Plant Productivity, Product Quality, and Energy Consumption. J. Food Eng. 2020, 275, 109839. [Google Scholar] [CrossRef]
- Barbosa, R.G.; Trigo, M.; Fett, R.; Aubourg, S.P. Impact of a Packing Medium with Alga Bifurcaria Bifurcata Extract on Canned Atlantic Mackerel (Scomber Scombrus) Quality. J. Sci. Food Agric. 2018, 98, 3462–3467. [Google Scholar] [CrossRef] [PubMed]
- Lahamy, A.A.E.; Mohamed, H.R. Changes in Fish Quality During Canning Process and Storage Period of Canned Fish Products: Review Article. Glob. J. Nutr. Food Sci. 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Malga, J.M.; Trigo, M.; Martínez, B.; Aubourg, S.P. Preservative Effect on Canned Mackerel (Scomber colias) Lipids by Addition of Octopus (Octopus vulgaris) Cooking Liquor in the Packaging Medium. Molecules 2022, 27, 739. [Google Scholar] [CrossRef] [PubMed]
- Reblová, Z.; Aubourg, S.P.; Pokorný, J. The Effect of Different Freshness of Raw Material on Lipid Quality and Sensory Acceptance of Canned Sardines. Foods 2022, 11, 1987. [Google Scholar] [CrossRef] [PubMed]
- Prego, R.; Fidalgo, L.G.; Saraiva, J.A.; Vázquez, M.; Aubourg, S.P. Impact of Prior High-Pressure Processing on Lipid Damage and Volatile Amines Formation in Mackerel Muscle Subjected to Frozen Storage and Canning. LWT 2021, 135, 109957. [Google Scholar] [CrossRef]
- Domiszewski, Z. Effect of Sterilization on True Retention Rate of Eicosapentaenoic and Docosahexaenoic Acid Content in Mackerel (Scomber scombrus), Herring (Clupea Harengus), and Sprat (Sprattus sprattus) Canned Products. J. Food Process. Preserv. 2021, 45, e15461. [Google Scholar] [CrossRef]
- Mohan, C.O.; Remya, S.; Murthy, L.N.; Ravishankar, C.N.; Asok Kumar, K. Effect of Filling Medium on Cooking Time and Quality of Canned Yellowfin Tuna (Thunnus albacares). Food Control 2015, 50, 320–327. [Google Scholar] [CrossRef]
- Vinagre, J.; Rodríguez, A.; Larraín, M.A.; Aubourg, S.P. Chemical Composition and Quality Loss during Technological Treatment in Coho Salmon (Oncorhynchus kisutch). Food Res. Int. 2011, 44, 1–13. [Google Scholar] [CrossRef]
- Naseri, M.; Rezaei, M. Lipid Changes During Long-Term Storage of Canned Sprat. J. Aquat. Food Prod. Technol. 2012, 21, 48–58. [Google Scholar] [CrossRef]
- Gómez-Limia, L.; Cobas, N.; Franco, I.; Martínez-Suárez, S. Fatty Acid Profiles and Lipid Quality Indices in Canned European Eels: Effects of Processing Steps, Filling Medium and Storage. Food Res. Int. 2020, 136, 109601. [Google Scholar] [CrossRef]
- Dantas, N.M.; De Oliveira, V.S.; Sampaio, G.R.; Chrysostomo, Y.S.K.; Chávez, D.W.H.; Gamallo, O.D.; Sawaya, A.C.H.F.; Torres, E.A.F.D.S.; Saldanha, T. Lipid Profile and High Contents of Cholesterol Oxidation Products (COPs) in Different Commercial Brands of Canned Tuna. Food Chem. 2021, 352, 129334. [Google Scholar] [CrossRef] [PubMed]
- Samarajeewa, U. Emerging Challenges in Maintaining Marine Food-Fish Availability and Food Safety. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4734–4757. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs; European Commission: Brussels, Belgium, 2005. [Google Scholar]
- Mercogliano, R.; Santonicola, S. Scombroid Fish Poisoning: Factors Influencing the Production of Histamine in Tuna Supply Chain. A Review. LWT 2019, 114, 108374. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EU) No 1019/2013 of 23 October 2013 Amending Annex I to Regulation (EC) No 2073/2005 as Regards Histamine in Fishery productstext with EEA Relevance; European Union: Brussels, Belgium, 2013. [Google Scholar]
- FAO. WHO Revision of the Code of Practice for Fish and Fishery Products (Cxc_52_3002) and Revisions of the Section on Sampling, Examination and Analyses Related to Histamine Food Safety; FAO: Rome, Italy, 2018; pp. 1–26. [Google Scholar]
- European Union. Commission Regulation (EU) 2018/213 of 12 February 2018 on the Use of Bisphenol A in Varnishes and Coatings Intended to Come into Contact with Food and Amending Regulation (EU) No 10 /2011 as Regards the Use of That Substance in Plastic Food Contact Materials; European Union: Brussels, Belgium, 2018. [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids). Scientific Opinion on the Risks to Public Health Related to the Presence of Bisphenol A (BPA) in Foodstuffs: Executive Summary. EFSA J. 2015, 13, 1–23. [Google Scholar] [CrossRef]
- European Commission. Commission Directive 2002/16/EC of 20 February 2002 on the Use of Certain. Epoxy Derivatives in Materials and Articles Intended to Come into Contact with Foodstuffs; European Commission: Brussels, Belgium, 2002. [Google Scholar]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) Related to 2,2-Bis(4-Hydroxyphenyl)Propane Bis(2,3-Epoxypropyl)Ether (Bisphenol A Diglycidyl Ether, BADGE). REF. No 13510 and 39700. EFSA J. 2004, 86, 1–4. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 1895/2005 of 18 November 2005 on the Restriction of Use of Certain Epoxy Derivatives in Materials and Articles Intended to Come into Contact with Food; European Commission: Brussels, Belgium, 2005. [Google Scholar]
- European Union. Commission Regulation (EU) No 835/2011 of 19 August 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Polycyclic Aromatic Hydrocarbons in Foodstuffstext with EEA Relevance; European Union: Brussels, Belgium, 2011. [Google Scholar]
- BfR. Epoxide Resin Coatings of Cans: Substance Transfer to Oil-Containing Foods Possible. 2016. Available online: https://www.bfr.bund.de/cm/349/epoxide-resin-coatings-of-cans-substance-transfer-to-oil-containing-foods-possible.pdf (accessed on 13 February 2024).
- Biedermann, S.; Zurfluh, M.; Grob, K.; Vedani, A.; Brüschweiler, B.J. Migration of Cyclo-diBA from Coatings into Canned Food: Method of Analysis, Concentration Determined in a Survey and in Silico Hazard Profiling. Food Chem. Toxicol. 2013, 58, 107–115. [Google Scholar] [CrossRef] [PubMed]
- EFSA Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Food Contact Materials on a Request from European Commission on Safety of Aluminium from Dietary Intake. EFSA J. 2008, 754, 1–34.
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Statement on Tolerable Weekly Intake for Cadmium. EFS2 2011, 9, 1975. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the Risk for Public Health Related to the Presence of Mercury and Methylmercury in Food. EFS2 2012, 10, 2985. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; European Commission: Brussels, Belgium, 2006. [Google Scholar]
- FAO. WHO General Standard for Contaminants and Toxins in Food and Feed. CXS 193-1995; FAO: Rome, Italy, 2023. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants: Seventy-Fourth [74th] Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series 966; FAO/WHO: Rome, Italy, 2011; Available online: https://iris.who.int/bitstream/handle/10665/44788/WHO_TRS_966_eng.pdf (accessed on 31 January 2024).
- European Union. Commission Regulation (EU) 2022/2388 of 7 December 2022 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Perfluoroalkyl Substances in Certain Foodstuffs; European Union: Brussels, Belgium, 2022. [Google Scholar]
- Elbayoumi, Z.H.; Dawod, E.E.; Shawish, R.R. Occurrence and Control of Biogenic Amines in Fresh Fish and Products of Fish. J. Adv. Vet. Res. 2023, 13, 936–940. [Google Scholar]
- Nagy, N.; Kirrella, G.A.K.; Moustafa, N.Y.; Abdallah, R. Quality Assessment of Some Imported and Local Canned Tuna Sold in Kafrelsheikh, Egypt. J. Adv. Vet. Res. 2023, 13, 377–383. [Google Scholar]
- Harmoko, H.; Kartasasmita, R.E.; Munawar, H.; Rakhmawati, A.; Budiawan, B. Determination of Histamine in Different Compositions of Commercially Canned Fish in Indonesia by Modified QuEChERS and LC-MS/MS. J. Food Compos. Anal. 2022, 105, 104256. [Google Scholar] [CrossRef]
- Crobu, L.; Mudadu, A.G.; Melillo, R.; Pais, G.L.; Meloni, D. Qualitative Determination of Histamine in Canned Yellowfin Tuna (Thunnus Albacares) Marketed in Sardinia (Italy) by Rapid Screening Methods. Ital J Food Saf. 2021, 10, 9379. [Google Scholar] [CrossRef] [PubMed]
- Lo Magro, S.; Summa, S.; Iammarino, M.; D’Antini, P.; Marchesani, G.; Chiaravalle, A.; Muscarella, M. A 5-Years (2015–2019) Control Activity of an EU Laboratory: Contamination of Histamine in Fish Products and Exposure Assessment. Appl. Sci. 2020, 10, 8693. [Google Scholar] [CrossRef]
- Peivasteh-Roudsari, L.; Rahmani, A.; Shariatifar, N.; Tajdar-Oranj, B.; Mazaheri, M.; Sadighara, P.; Khaneghah, A.M. Occurrence of Histamine in Canned Fish Samples (Tuna, Sardine, Kilka, and Mackerel) from Markets in Tehran. J. Food Prot. 2020, 83, 136–141. [Google Scholar] [CrossRef]
- Weremfo, A.; Eduafo, M.K.; Gyimah, H.A.; Abassah-Oppong, S. Monitoring the Levels of Biogenic Amines in Canned Fish Products Marketed in Ghana. J. Food Qual. 2020, 2020, 2684235. [Google Scholar] [CrossRef]
- Pavlović, M.; Ivanović, S.; Pavlović, I.; Rokvić, N.; Radosavljević, V.; Vasilev, D. Histamine Levels in Fish Samples Collected from Serbian Market in 2018. Food Feed Res. 2019, 46, 37–43. [Google Scholar] [CrossRef]
- El Hariri, O.; Bouchriti, N.; Bengueddour, R. Risk Assessment of Histamine in Chilled, Frozen, Canned and Semi-Preserved Fish in Morocco; Implementation of Risk Ranger and Recommendations to Risk Managers. Foods 2018, 7, 157. [Google Scholar] [CrossRef]
- Rahmani, J.; Miri, A.; Mohseni-Bandpei, A.; Fakhri, Y.; Bjørklund, G.; Keramati, H.; Moradi, B.; Amanidaz, N.; Shariatifar, N.; Khaneghah, A.M. Contamination and Prevalence of Histamine in Canned Tuna from Iran: A Systematic Review, Meta-Analysis, and Health Risk Assessment. J. Food Prot. 2018, 81, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Yesudhason, P.; Al-Zidjali, M.; Al-Zidjali, A.; Al-Busaidi, M.; Al-Waili, A.; Al-Mazrooei, N.; Al-Habsi, S. Histamine Levels in Commercially Important Fresh and Processed Fish of Oman with Reference to International Standards. Food Chem. 2013, 140, 777–783. [Google Scholar] [CrossRef]
- Silva, T.M.; Sabaini, P.S.; Evangelista, W.P.; Gloria, M.B.A. Occurrence of Histamine in Brazilian Fresh and Canned Tuna. Food Control 2011, 22, 323–327. [Google Scholar] [CrossRef]
- Li, C.; Vrdoljak, G.; Moezzi, B. Sampling and Analysis of Histamine in Fish Products from Local Northern California Markets. Food Nutr. J. 2018, 8, 2575–7091. [Google Scholar] [CrossRef]
- Sadeghi, N.; Behzad, M.; Jannat, B.; Oveisi, M.R.; Hajimahmoodi, M.; Mozafazri, M. Determination of Histamine in Canned Tuna Fish Available in Tehran Market by ELISA Method. J. Food Saf. Hyg. 2020, 5, 46–50. [Google Scholar] [CrossRef]
- Er, B.; Demirhan, B.; Bas, S.Y.; Yentur, G.; Oktem, A.B. Determination of Histamine Levels in Canned Tuna Fish. Bulg. J. Agric. Sci. 2014, 20, 834–838. [Google Scholar]
- Kuehn, A.; Swoboda, I.; Arumugam, K.; Hilger, C.; Hentges, F. Fish Allergens at a Glance: Variable Allergenicity of Parvalbumins, the Major Fish Allergens. Front. Immunol. 2014, 5, 179. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Taylor, S.L.; Baumert, J.; Lopata, A.L.; Lee, N.A. Effects of Thermal Treatment on the Immunoreactivity and Quantification of Parvalbumin from Southern Hemisphere Fish Species with Two Anti-Parvalbumin Antibodies. Food Control 2021, 121, 107675. [Google Scholar] [CrossRef]
- Blickem, E.R.; Bell, J.W.; Baumgartel, D.M.; Debeer, J. Review and Analysis of Tuna Recalls in the United States, 2002 through 2020. J. Food Prot. 2022, 85, 60–72. [Google Scholar] [CrossRef]
- Taki, A.C.; Ruethers, T.; Nugraha, R.; Karnaneedi, S.; Williamson, N.A.; Nie, S.; Leeming, M.G.; Mehr, S.S.; Campbell, D.E.; Lopata, A.L. Thermostable Allergens in Canned Fish: Evaluating Risks for Fish Allergy. Allergy 2023, 78, 3221–3234. [Google Scholar] [CrossRef]
- Pecoraro, L.; Infante, S.; Fuentes-Aparicio, V.; Cabrera-Freitag, P.; Antonucci, N.; Alvarez-Perea, A. IgE-mediated Fish Allergy in Pediatric Age: Does Canned Tuna Have a Chance for Tolerance? Pediatr. Allergy Immunol. 2021, 32, 1114–1117. [Google Scholar] [CrossRef]
- Nielsen, L.T.; Hansen, P.J.; Krock, B.; Vismann, B. Accumulation, Transformation and Breakdown of DSP Toxins from the Toxic Dinoflagellate Dinophysis Acuta in Blue Mussels, Mytilus Edulis. Toxicon 2016, 117, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Leite, I.D.P.; Sdiri, K.; Taylor, A.; Viallon, J.; Gharbia, H.B.; Mafra Júnior, L.L.; Swarzenski, P.; Oberhaensli, F.; Darius, H.T.; Chinain, M.; et al. Experimental Evidence of Ciguatoxin Accumulation and Depuration in Carnivorous Lionfish. Toxins 2021, 13, 564. [Google Scholar] [CrossRef]
- European Economic Community, E.E.C. Council Directive of 15 July 1991 Laying down the Health Conditions for the Production and the Placing on the Market of Live Bivalve Molluscs. Off. J. Eur. Union 1991, 268, 1–14. [Google Scholar]
- European Commission. Directive 2004/41/EC of the European Parliament and of the Council of 21 April 2004 Repealing Certain Directives Concerning Food Hygiene and Health Conditions for the Production and Placing on the Market of Certain Products of Animal Origin Intended for Human Consumption and Amending Council Directives 89/662/EEC and 92/118/EEC and Council Decision 95/408/EC; European Commission: Brussels, Belgium, 2004. [Google Scholar]
- Blanco, J.; Arévalo, F.; Correa, J.; Porro, M.C.; Cabado, A.G.; Vieites, J.M.; Moroño, A. Effect of the Industrial Canning on the Toxicity of Mussels Contaminated with Diarrhetic Shellfish Poisoning (DSP) Toxins. Toxicon 2016, 112, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, I.; Alfonso, A.; Antelo, A.; Alvarez, M.; Botana, L. Evaluation of the Impact of Mild Steaming and Heat Treatment on the Concentration of Okadaic Acid, Dinophysistoxin-2 and Dinophysistoxin-3 in Mussels. Toxins 2016, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- García, C.; Oyaneder-Terrazas, J.; Contreras, C.; Del Campo, M.; Torres, R.; Contreras, H.R. Determination of the Toxic Variability of Lipophilic Biotoxins in Marine Bivalve and Gastropod Tissues Treated with an Industrial Canning Process. Food Addit. Contam. Part A 2016, 33, 1711–1727. [Google Scholar] [CrossRef] [PubMed]
- LaKind, J.S. Can Coatings for Foods and Beverages: Issues and Options. Int. J. Technol. Policy Manag. 2013, 13, 80–95. [Google Scholar] [CrossRef]
- Toptancı, İ.; Kıralan, M.; Ketenoglu, O.; Ramadan, M.F. Monitoring of Bisphenol A Diglycidyl Ether (BADGE) and Some Derivatives in Fish Products in the Turkey Market. Environ. Sci. Pollut. Res. 2022, 29, 52788–52795. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.C.; Fernandes, J.O. Assessment of Bisphenol A and Bisphenol B in Canned Vegetables and Fruits by Gas Chromatography–Mass Spectrometry after QuEChERS and Dispersive Liquid–Liquid Microextraction. Food Control 2013, 33, 549–555. [Google Scholar] [CrossRef]
- Beausoleil, C.; Emond, C.; Cravedi, J.-P.; Antignac, J.-P.; Applanat, M.; Appenzeller, B.R.; Beaudouin, R.; Belzunces, L.P.; Canivenc-Lavier, M.-C.; Chevalier, N.; et al. Regulatory Identification of BPA as an Endocrine Disruptor: Context and Methodology. Mol. Cell. Endocrinol. 2018, 475, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Ohore, O.E.; Zhang, S. Endocrine Disrupting Effects of Bisphenol A Exposure and Recent Advances on Its Removal by Water Treatment Systems. A Review. Sci. Afr. 2019, 5, e00135. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, H.; Fei, X.; Synder, S.A.; Fang, M.; Liu, M. A Comprehensive Review on the Analytical Method, Occurrence, Transformation and Toxicity of a Reactive Pollutant: BADGE. Environ. Int. 2021, 155, 106701. [Google Scholar] [CrossRef]
- Catenza, C.J.; Farooq, A.; Shubear, N.S.; Donkor, K.K. A Targeted Review on Fate, Occurrence, Risk and Health Implications of Bisphenol Analogues. Chemosphere 2021, 268, 129273. [Google Scholar] [CrossRef]
- Geens, T.; Aerts, D.; Berthot, C.; Bourguignon, J.-P.; Goeyens, L.; Lecomte, P.; Maghuin-Rogister, G.; Pironnet, A.-M.; Pussemier, L.; Scippo, M.-L.; et al. A Review of Dietary and Non-Dietary Exposure to Bisphenol-A. Food Chem. Toxicol. 2012, 50, 3725–3740. [Google Scholar] [CrossRef]
- Toptancı, İ. Risk Assessment of Bisphenol Related Compounds in Canned Convenience Foods, Olives, Olive Oil, and Canned Soft Drinks in Turkey. Environ. Sci. Pollut. Res. 2023, 30, 54177–54192. [Google Scholar] [CrossRef]
- Gálvez-Ontiveros, Y.; Moscoso-Ruiz, I.; Rodrigo, L.; Aguilera, M.; Rivas, A.; Zafra-Gómez, A. Presence of Parabens and Bisphenols in Food Commonly Consumed in Spain. Foods 2021, 10, 92. [Google Scholar] [CrossRef]
- Repossi, A.; Farabegoli, F.; Gazzotti, T.; Zironi, E.; Pagliuca, G. Bisphenol A in Edible Part of Seafood. Ital. J. Food Saf. 2016, 5, 5666. [Google Scholar] [CrossRef] [PubMed]
- Al Ghoul, L.; Abiad, M.G.; Jammoul, A.; Matta, J.; El Darra, N. Zinc, Aluminium, Tin and Bis-Phenol a in Canned Tuna Fish Commercialized in Lebanon and Its Human Health Risk Assessment. Heliyon 2020, 6, e04995. [Google Scholar] [CrossRef] [PubMed]
- Lestido-Cardama, A.; Sendón, R.; Bustos, J.; Santillana, M.I.; Paseiro Losada, P.; Rodríguez Bernaldo De Quirós, A. Multi-Analyte Method for the Quantification of Bisphenol Related Compounds in Canned Food Samples and Exposure Assessment of the Spanish Adult Population. Food Packag. Shelf Life 2021, 28, 100671. [Google Scholar] [CrossRef]
- Maragou, N.C.; Thomaidis, N.S.; Theodoridis, G.A.; Lampi, E.N.; Koupparis, M.A. Determination of Bisphenol A in Canned Food by Microwave Assisted Extraction, Molecularly Imprinted Polymer-Solid Phase Extraction and Liquid Chromatography-Mass Spectrometry. J. Chromatogr. B 2020, 1137, 121938. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, H.; Mostafa, A.; Alqarni, A.M.; Almohamed, Y.; Abualrahi, D.; Hussein, D.; Alghamdi, M. Simultaneous Determination of Bisphenol A and Its Analogues in Foodstuff Using UPLC-MS/MS and Assessment of Their Health Risk in Adult Population. J. Food Compos. Anal. 2022, 110, 104549. [Google Scholar] [CrossRef]
- Osman, M.A.; Mahmoud, G.I.; Elgammal, M.H.; Hasan, R.S. Studying of Bisphenol A Levels in Some Canned Food, Feed and Baby Bottles in Egyptian Markets. Fresenius Environ. Bull. 2018, 27, 9374–9381. [Google Scholar]
- Morshdy, A.E.; Hussein, M.; Darwish, W.; Yousef, R.; Tharwat, A. Residual Contents of Selected Heavy Metals in Commercial Canned Fish in Egypt: Dietary Intakes and Health Risk Assessment. Slov. Vet. Res. 2021, 58, 101–107. [Google Scholar] [CrossRef]
- Inan-Eroglu, E.; Ayaz, A. Is Aluminum Exposure a Risk Factor for Neurological Disorders? J. Res. Med Sci. 2018, 23, 51. [Google Scholar] [CrossRef] [PubMed]
- Mol, S. Levels of Heavy Metals in Canned Bonito, Sardines, and Mackerel Produced in Turkey. Biol. Trace Element Res. 2011, 143, 974–982. [Google Scholar] [CrossRef]
- Kosker, A.R.; Gundogdu, S.; Esatbeyoglu, T.; Ayas, D.; Ozogul, F. Metal Levels of Canned Fish Sold in Türkiye: Health Risk Assessment. Front. Nutr. 2023, 10, 1255857. [Google Scholar] [CrossRef] [PubMed]
- de Lima, N.V.; Melo, E.S.D.P.; Arakaki, D.G.; Tschinkel, P.F.S.; De Souza, I.D.; Ulbrecht, M.O.D.O.; Mendes Dos Reis, F.J.; Rosa, A.C.G.; Rosa, R.H.; Aragão Do Nascimento, V. Data on Metals, Nonmetal, and Metalloid in the Samples of the Canned Tuna and Canned Sardines Sold in Brazil. Data Brief 2021, 35, 106865. [Google Scholar] [CrossRef] [PubMed]
- Ababneh, F.A.; Al-Momani, I.F. Levels of Mercury, Cadmium, Lead and Other Selected Elements in Canned Tuna Fish Commercialised in Jordan. Int. J. Environ. Anal. Chem. 2013, 93, 755–766. [Google Scholar] [CrossRef]
- Massadeh, A.M.; Al-Massaedh, A.A.T.; Kharibeh, S. Determination of Selected Elements in Canned Food Sold in Jordan Markets. Environ. Sci. Pollut. Res. 2018, 25, 3501–3509. [Google Scholar] [CrossRef] [PubMed]
- Sadighara, P.; Mofid, V.; Mahmudiono, T.; Rahmani, A.; Tajdar-Oranj, B.; Peivasteh-Roudsari, L.; Farhangfar, A.; Moradi, M.; Fakhri, Y. Concentration of Heavy Metals in Canned Tuna Fish and Probabilistic Health Risk Assessment in Iran. Int. J. Environ. Anal. Chem. 2022, 104, 1719–1729. [Google Scholar] [CrossRef]
- Sobhanardakani, S. Tuna Fish and Common Kilka: Health Risk Assessment of Metal Pollution through Consumption of Canned Fish in Iran. J. Consum. Prot. Food Saf. 2017, 12, 157–163. [Google Scholar] [CrossRef]
- Mansouri, B.; Azadi, N.A.; Albrycht, M.; Binkowski, L.J.; Błaszczyk, M.; Hamesadeghi, U.; Rahmani, R.; Maleki, A.; Majnoni, F. Metal Risk Assessment Study of Canned Fish Available on the Iranian Market. Biol. Trace Element Res. 2021, 199, 3470–3477. [Google Scholar] [CrossRef]
- Núñez, R.; García, M.Á.; Alonso, J.; Melgar, M.J. Arsenic, Cadmium and Lead in Fresh and Processed Tuna Marketed in Galicia (NW Spain): Risk Assessment of Dietary Exposure. Sci. Total Environ. 2018, 627, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Popovic, A.R.; Djinovic-Stojanovic, J.M.; Djordjevic, D.S.; Relic, D.J.; Vranic, D.V.; Milijasevic, M.P.; Pezo, L.L. Levels of Toxic Elements in Canned Fish from the Serbian Markets and Their Health Risks Assessment. J. Food Compos. Anal. 2018, 67, 70–76. [Google Scholar] [CrossRef]
- Novakov, N.J.; Mihaljev, Ž.A.; Kartalović, B.D.; Blagojević, B.J.; Petrović, J.M.; Ćirković, M.A.; Rogan, D.R. Heavy Metals and PAHs in Canned Fish Supplies on the Serbian Market. Food Addit. Contam. Part B 2017, 10, 208–215. [Google Scholar] [CrossRef]
- Andayesh, S.; Hadiani, M.R.; Mousavi, Z.; Shoeibi, S. Lead, Cadmium, Arsenic and Mercury in Canned Tuna Fish Marketed in Tehran, Iran. Food Addit. Contam. Part B 2015, 8, 93–98. [Google Scholar] [CrossRef]
- de Mello Lazarini, T.E.; Milani, R.F.; Yamashita, D.M.; Saron, E.S.; Morgano, M.A. Canned Sardines Commercialized in Brazil: Packaging and Inorganic Contaminants Evaluation. Food Packag. Shelf Life 2019, 21, 100372. [Google Scholar] [CrossRef]
- Rahimi, E.; Hajisalehi, M.; Kazemeini, H.R.; Chakeri, A.; Derakhshesh, M.; Mirdamadi, M.; Ebadi, A.G.; Rezvani, A.; Kashkahi, M.F. Analysis and Determination of Mercury, Cadmium and Lead in Canned Tuna Fish Marketed in Iran. Afr. J. Biotechnol. 2010, 9, 4938–4941. [Google Scholar]
- Huang, W.; Huang, Y.; Chen, Y.; Tan, W.; Wu, K. A Comprehensive Review of the Human Body Burden of Persistent Organic Pollutants (POPs) and Associated Health Effects in an e-Waste Recycling Area in China. Discov. Environ. 2023, 1, 13. [Google Scholar] [CrossRef]
- Singh, L.; Agarwal, T. Polycyclic Aromatic Hydrocarbons in Diet: Concern for Public Health. Trends Food Sci. Technol. 2018, 79, 160–170. [Google Scholar] [CrossRef]
- Benson, N.U.; Anake, W.U.; Adedapo, A.E.; Fred-Ahmadu, O.H.; Eke, K.P. Polycyclic Aromatic Hydrocarbons in Imported Sardinops Sagax: Levels and Health Risk Assessments through Dietary Exposure in Nigeria. J. Food Compos. Anal. 2017, 57, 109–116. [Google Scholar] [CrossRef]
- Zachara, A.; Gałkowska, D.; Juszczak, L. Contamination of Smoked Meat and Fish Products from Polish Market with Polycyclic Aromatic Hydrocarbons. Food Control 2017, 80, 45–51. [Google Scholar] [CrossRef]
- Drabova, L.; Pulkrabova, J.; Kalachova, K.; Tomaniova, M.; Kocourek, V.; Hajslova, J. Polycyclic Aromatic Hydrocarbons and Halogenated Persistent Organic Pollutants in Canned Fish and Seafood Products: Smoked versus Non-Smoked Products. Food Addit. Contam. Part A 2013, 30, 515–527. [Google Scholar] [CrossRef] [PubMed]
- El Morsy, F.A.M.; El-Sadaawy, M.M.; Ahdy, H.H.H.; Abdel-Fattah, L.M.; El-Sikaily, A.M.; Khaled, A.; Tayel, F.M.T. Potential Human Health Risks from Toxic Metals, Polycyclic Aromatic Hydrocarbons, Polychlorinated Biphenyls, and Organochlorine Pesticides via Canned Fish Consumption: Estimation of Target Hazard Quotients. J. Environ. Sci. Health Part A 2013, 48, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Iwegbue, C.M.A.; Overah, L.C.; Tesi, G.O.; Bassey, F.I.; Martincigh, B.S. Polycyclic Aromatic Hydrocarbon Profiles of Some Brands of Canned Fish in the Nigerian Market. Hum. Ecol. Risk Assess. Int. J. 2015, 21, 157–168. [Google Scholar] [CrossRef]
- Johnson, L.L.; Anulacion, B.F.; Arkoosh, M.R.; Burrows, D.G.; Da Silva, D.A.M.; Dietrich, J.P.; Myers, M.S.; Spromberg, J.; Ylitalo, G.M. Effects of Legacy Persistent Organic Pollutants (POPs) in Fish—Current and Future Challenges. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 33, pp. 53–140. ISBN 978-0-12-398254-4. [Google Scholar]
- Afolabi, O.L.; Iwegbue, C.M.A.; Obi, G.; Tesi, G.O.; Nwajei, G.E.; Martincigh, B.S. Polychlorinated Biphenyls and Polychlorinated Dibenzo-p-Dioxins and Furans in Imported Canned Fish in Nigeria and Risk Assessment. Food Addit. Contam. Part B 2023, 16, 32–41. [Google Scholar] [CrossRef]
- Vali Mohammadi, F.; Qajarbeygi, P.; Shariatifar, N.; Mahmoudi, R.; Arabameri, M. Measurement of Polychlorinated Biphenyls in Different High Consumption Canned Foods, Using the QuEChERS/GC-MS Method. Food Chem. X 2023, 20, 100957. [Google Scholar] [CrossRef] [PubMed]
- Hrádková, P.; Poustka, J.; Hloušková, V.; Pulkrabová, J.; Tomaniová, M.; Hajšlová, J. Perfluorinated Compounds: Occurrence of Emerging Food Contaminants in Canned Fish and Seafood Products. Czech J. Food Sci. 2010, 28, 333–342. [Google Scholar] [CrossRef]
- TFA in Water: Dirty PFAS Legacy Under the Radar. Available online: https://www.pan-europe.info/resources/reports/2024/05/tfa-water-dirty-pfas-legacy-under-radar (accessed on 3 October 2024).
- Pye, C.; Crews, C. Furan in Canned Sardines and Other Fish. Food Addit. Contam. Part B 2014, 7, 43–45. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM) Presence of Microplastics and Nanoplastics in Food, with Particular Focus on Seafood. EFSA J. 2016, 14, e04501. [CrossRef]
- Lopes, C.; Ambrosino, A.C.; Figueiredo, C.; Caetano, M.; Santos, M.M.; Garrido, S.; Raimundo, J. Microplastic Distribution in Different Tissues of Small Pelagic Fish of the Northeast Atlantic Ocean. Sci. Total Environ. 2023, 901, 166050. [Google Scholar] [CrossRef] [PubMed]
- Nalbone, L.; Cincotta, F.; Giarratana, F.; Ziino, G.; Panebianco, A. Microplastics in Fresh and Processed Mussels Sampled from Fish Shops and Large Retail Chains in Italy. Food Control 2021, 125, 108003. [Google Scholar] [CrossRef]
- Di Giacinto, F.; Di Renzo, L.; Mascilongo, G.; Notarstefano, V.; Gioacchini, G.; Giorgini, E.; Bogdanović, T.; Petričević, S.; Listeš, E.; Brkljača, M.; et al. Detection of Microplastics, Polymers and Additives in Edible Muscle of Swordfish (Xiphias gladius) and Bluefin Tuna (Thunnus thynnus) Caught in the Mediterranean Sea. J. Sea Res. 2023, 192, 102359. [Google Scholar] [CrossRef]
- Gündoğdu, S.; Köşker, A.R. Microplastic Contamination in Canned Fish Sold in Türkiye. PeerJ 2023, 11, e14627. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Dobaradaran, S.; Nabipour, I.; Tajbakhsh, S.; Darabi, A.H.; Spitz, J. Abundance, Composition, and Potential Intake of Microplastics in Canned Fish. Mar. Pollut. Bull. 2020, 160, 111633. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Larat, V.; Karbalaei, S.; Salamatinia, B. Microplastic and Mesoplastic Contamination in Canned Sardines and Sprats. Sci. Total Environ. 2018, 612, 1380–1386. [Google Scholar] [CrossRef]
- Carlucci, D.; Nocella, G.; De Devitiis, B.; Viscecchia, R.; Bimbo, F.; Nardone, G. Consumer Purchasing Behaviour towards Fish and Seafood Products. Patterns and Insights from a Sample of International Studies. Appetite 2015, 84, 212–227. [Google Scholar] [CrossRef]
- Gouvêa, F.D.J.; De Oliveira, V.S.; Mariano, B.J.; Takenaka, N.A.R.; Gamallo, O.D.; Da Silva Ferreira, M.; Saldanha, T. Natural Antioxidants as Strategy to Minimize the Presence of Lipid Oxidation Products in Canned Fish: Research Progress, Current Trends and Future Perspectives. Food Res. Int. 2023, 173, 113314. [Google Scholar] [CrossRef] [PubMed]
- Aubourg, S.P.; Trigo, M.; Martínez, B.; Rodríguez, A. Effect of Prior Chilling Period and Alga-Extract Packaging on the Quality of a Canned Underutilised Fish Species. Foods 2020, 9, 1333. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.F.; Soares, C.; Machado, S.; Oliva-Teles, M.T.; Correia, M.; João Ramalhosa, M.; Carvalho, A.; Domingues, V.F.; Antunes, F.; Morais, S.; et al. Development of New Canned Chub Mackerel Products Incorporating Edible Seaweeds—Influence on the Minerals and Trace Elements Composition. Molecules 2020, 25, 1133. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.G.; Trigo, M.; Campos, C.A.; Aubourg, S.P. Preservative Effect of Algae Extracts on Lipid Composition and Rancidity Development in Brine-Canned Atlantic Chub Mackerel (Scomber colias). Eur. J. Lipid Sci. Technol. 2019, 121, 1900129. [Google Scholar] [CrossRef]
- Shulgin, Y.P.; Lazhentseva, L.Y.; Shulgina, L.V.; Kalenik, T.K.; Matveeva, V.A.; Piekoszewski, W. Quality Improvement of Canned Fish with the Use of Cinnamon Oil Extract. Int. J. Food Eng. 2017, 13, 20160430. [Google Scholar] [CrossRef]
- Circuncisão, A.R.; Catarino, M.D.; Cardoso, S.M.; Silva, A.M.S. Minerals from Macroalgae Origin: Health Benefits and Risks for Consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef] [PubMed]
- Afonso, N.C.; Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Brown Macroalgae as Valuable Food Ingredients. Antioxidants 2019, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- National Food Institute, Technical University of Denmark, Denmark; Sá Monteiro, M.; Sloth, J.; Holdt, S.; Hansen, M. Analysis and Risk Assessment of Seaweed. EFS2 2019, 17, e170915. [Google Scholar] [CrossRef]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Saljoughian, S.; Amid, M.; Greiner, R. Application of Seaweeds to Develop New Food Products with Enhanced Shelf-Life, Quality and Health-Related Beneficial Properties. Food Res. Int. 2017, 99, 1066–1083. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Camarena-Gómez, M.T.; Piiparinen, J.; Spilling, K.; Yang, B. European Union Legislation on Macroalgae Products. Aquac. Int. 2021, 29, 487–509. [Google Scholar] [CrossRef]
| Hazard Type | Hazard Sub-Type | Geographical Range | SML/MPLs | TDI | References |
| Biological | Histamine | Europe | 200* | - | [35,37] |
| Histamine | FAO/WHO | 200 | - | [38] | |
| Chemical | BPA | Europe | 0.05 | 0.002 | [10,39,40] |
| Σ (BADGE, HD) | 9 | 150 | [41,42] | ||
| Σ (BADGE, CD) | 1 | - | [43] | ||
| BPS | 0.05 | - | [44] | ||
| CdB | 0.05 | 1.5 | [45,46] | ||
| Al | Europe | 5 | 142.9 | [47,48,49,50] | |
| Cd | 0.1–0.25 | 0.36 | |||
| Hg | 0.3–1 | 0.57 | |||
| Pb | 0.3 | - | |||
| Sn | 200 | - | [51] | ||
| Al | FAO/WHO | - | 285.7 | [52,53] | |
| Hg | 0.5–1 | 0.57 | |||
| Pb | 0.3 | - | |||
| Sn | 250 | 2 | |||
| PAH4 | Europe | 0.03 | [44] | ||
| BaP | 0.005 | ||||
| Σ dioxins (WHO-PCDD/F-TEQ) | 0.0000035 | [50] | |||
| Σ dioxins and dioxin-like PCBs (WHO-PCDD/F-PCB-TEQ) | 0.0000065–0.00001 | ||||
| Σ (PCB28, PCB52, PCB101, PCB138, PCB153, and PCB180 (ICES-6)) | 0.075–0.300 | 0.00001 | |||
| PFOS | 0.002–0.035 | [54] | |||
| PFOA | 0.0003–0.008 | ||||
| PFNA | 0.0007–0.008 | ||||
| PFHxS | 0.0003–0.0015 | ||||
| Σ (PFOS, PFOA, PFNA, and PFHxS) | 0.0017–0.045 |
| Origin | Species (Filling Medium) | BPA (n) | BPA Analogs | BADGE and Derivatives | CdB (n) | References | ||
|---|---|---|---|---|---|---|---|---|
| BPS (n) | Othersa (n) | Σ[BADGE; HDb] (n) | Σ[CDc] (n) | |||||
| European countries | Tuna (oil) | <LODa–0.409 (30) | <LOD*–0.19 (30) | <LOD*–0.07 (30) | <LOD*–0.84 (49) | <LOD*–0.93 (49) | <LOQ*–0.67 (28) | [83,90,91,94,95] |
| Tuna (water/brine) | <LOD*–0.042 (11) | <LOD* (6) | <LOD* (7) | <LOD*–0.51 (10) | 1.03 (10) | 0.06–0.34 (7) | ||
| Non-European countries | Tuna (n.s.) | 0.061–0.200 (274_SF#) | - | - | - | - | - | [93,96,97] |
| Tuna (oil) | 0.197–0.198 (200_LF##) | - | - | - | - | - | ||
| Tuna (water/brine) | 0.197 (74_LF##) | - | - | - | - | - | ||
| Fish, squid, and shrimp (n.s.) | 0.078 (4) | - | 0.02 (4) | - | - | - | ||
| Origin | Species (Filling Medium) | BPA | BPA Analogs | BADGE and Derivatives | CdB | References | ||
|---|---|---|---|---|---|---|---|---|
| BPS | Othersa | Σ[BADGE; HDb] | Σ[CDc] | |||||
| European countries | Tuna (oil) | 0.005–0.009 | - | 0.020 | 0.015 | 0.020 | 0.005 | [93,94] |
| Tuna (water/brine) | 0.046 | - | 0.028 | 0.553 | 0.028 | 0.239 | ||
| Sardines (oil) | 0.009 | - | 0.036 | 0.027 | 0.036 | 0.055 | ||
| Clams (water/brine) | 0.005 | - | 0.020 | 0.015 | 0.020 | 0.005 | ||
| Mussels (pickled) | 0.035 | - | 0.140 | 0.269 | 0.140 | 0.066 | ||
| Non-European countries | Tuna (n.s.) | 0.006 | - | - | - | - | - | [93] |
| Species (n) | Filling Medium | Frequency of Occurrence (%) | Number of Identified MPs | Type of Plastics | References |
|---|---|---|---|---|---|
| Tuna (14) | Oil | 100 | 1–12 | POF, PAN, PMAME, PA, PET, and PP. | [131] |
| Tuna (4) | Water/Brine | 100 | 3–4 | ||
| Skipjack tuna (5) | Oil | 100 | 1–6 | ||
| Salmon (3) | Oil | 100 | 2–6 | ||
| Longtail tuna (20) | Oil | 60–100 | 2–3 | PET, PS, PP, PS-PP, PS-PET, Nylon, PVC, and LDPE. | [132] |
| Longtail tuna (5) | Water/Brine | 80 | 4–5 | ||
| Yellowfin tuna (20) | Oil | 40–100 | 1–3 | ||
| Mackerel (5) | Oil | 100 | 3–3 | ||
| Sprat (9 brands) | Oil | 22 | 0–1 | PP, PET, PE, and PVC. | [133] |
| Sardine (12 brands) | Oil | 0 | 0 | - |
| Species | Ingredient Tested | Quantities Tested | Effects | Reference |
|---|---|---|---|---|
| Mackerel | Aqueous extract of Fucus spiralis (ratio: 0.28 of lyophilised alga/5 mL of extract) | 5, 15, or 30 mL of extract + 35, 25, or 10 mL of distilled water + 40 mL of brine solution (4% w/v) | - Free fatty acid content decreased. - Increased peroxide retention. - Reduced fluorescent compounds. | [136] |
| Mackerel | Dehydrated: Ascophyllum nodosum Fucus spiralis Saccorhiza polyschides Chondrus crispus Porphyra sp. Ulva sp. (ratio: 2 g dw of seaweed/60 g fw of fish) | C. crispus and F. spiralis were: - added in the canning step (trial A) - boiled with the fish for 20 min and removed after boiling; added new portion in the canning step (trial B) | - Product from trial B was the preferred sensory option. | [137] |
| Mackerel | Aqueous extracts (brine—aqueous, 2% NaCl medium) of Fucus spiralis + Ulva lactuca (ratio: 0.56 g of extracted alga/10 mL extract) | 10 or 30 mL of each alga extract + 30 or 10 mL of distilled water + 40 mL of brine solution (4% w/v) | - Loss of lipids after canning inhibited. - Breakdown of fatty acids and peroxides prevented. - Formation of fluorescent compounds reduced. | [138] |
| Mackerel | Aqueous extract (water) of Bifurcaria bifurcata (ratio: 0.625 g of extracted alga/5 mL extract) | 5, 10, 25, and 50 mL of alga extract + completed with distilled water | - Inhibitory effect on lipid oxidation development and color parameters. | [23] |
| Herring Salmon Mackerel | Cinnamon oil extract (which contains a set of fat-soluble substances with a distinct antimicrobial and enzymatic inhibition activity), instead of vegetable/soybean oil | Extract added to the cans: 15% of the net weight | - Cinnamon oil extract: histamine content < 35 mg/kg. - Control (with soybean oil): accumulated histamine ≥ 50 mg/kg. | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pais-Costa, A.J.; Marques, A.; Oliveira, H.; Gonçalves, A.; Camacho, C.; Augusto, H.C.; Nunes, M.L. New Perspectives on Canned Fish Quality and Safety on the Road to Sustainability. Foods 2025, 14, 99. https://doi.org/10.3390/foods14010099
Pais-Costa AJ, Marques A, Oliveira H, Gonçalves A, Camacho C, Augusto HC, Nunes ML. New Perspectives on Canned Fish Quality and Safety on the Road to Sustainability. Foods. 2025; 14(1):99. https://doi.org/10.3390/foods14010099
Chicago/Turabian StylePais-Costa, Antónia Juliana, António Marques, Helena Oliveira, Amparo Gonçalves, Carolina Camacho, Helga Coelho Augusto, and Maria Leonor Nunes. 2025. "New Perspectives on Canned Fish Quality and Safety on the Road to Sustainability" Foods 14, no. 1: 99. https://doi.org/10.3390/foods14010099
APA StylePais-Costa, A. J., Marques, A., Oliveira, H., Gonçalves, A., Camacho, C., Augusto, H. C., & Nunes, M. L. (2025). New Perspectives on Canned Fish Quality and Safety on the Road to Sustainability. Foods, 14(1), 99. https://doi.org/10.3390/foods14010099







