Thai Cannabis sativa Leaves as a Functional Ingredient for Quality Improvement and Lactic Acid Bacterial Growth Enhancement in Kombucha
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Chemical Reagents
2.2. Analysis of Antioxidant Properties and Proximate Composition of Plant Materials
2.2.1. Antioxidant Properties of Plant Materials
2.2.2. Proximate Analysis of Plant Materials
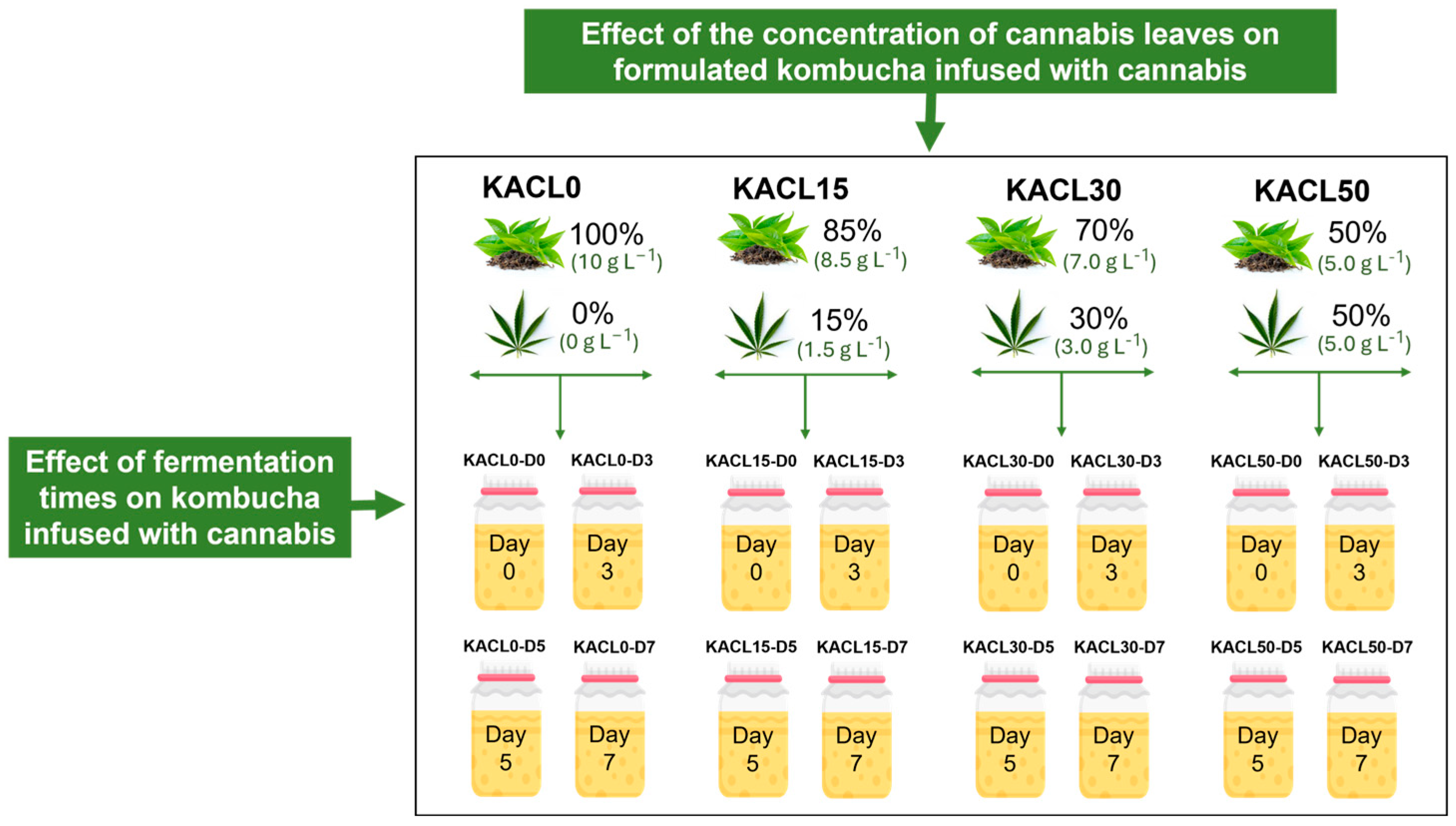
2.3. Preparation of Cannabis Leaf-Infused Kombucha
2.4. Effect of Cannabis Leaves Concentration and Fermentation Time on the Changes in Microbial, Physicochemical, and Antioxidant Properties of the Fermented Kombucha
2.4.1. Microbiological Analysis
2.4.2. pH Value and Total Acidity
2.4.3. Total Soluble Solids and Turbidity
2.4.4. Total Phenolic Content and Antioxidant Capacity
2.5. Microbiota Diversity Using Metagenomic Analysis
2.6. Volatile Compounds Using HS-SPME-GC-MS Method
2.7. Statistical Analysis
3. Results
3.1. Antioxidant Properties and Proximate Compositions of Plant Materials
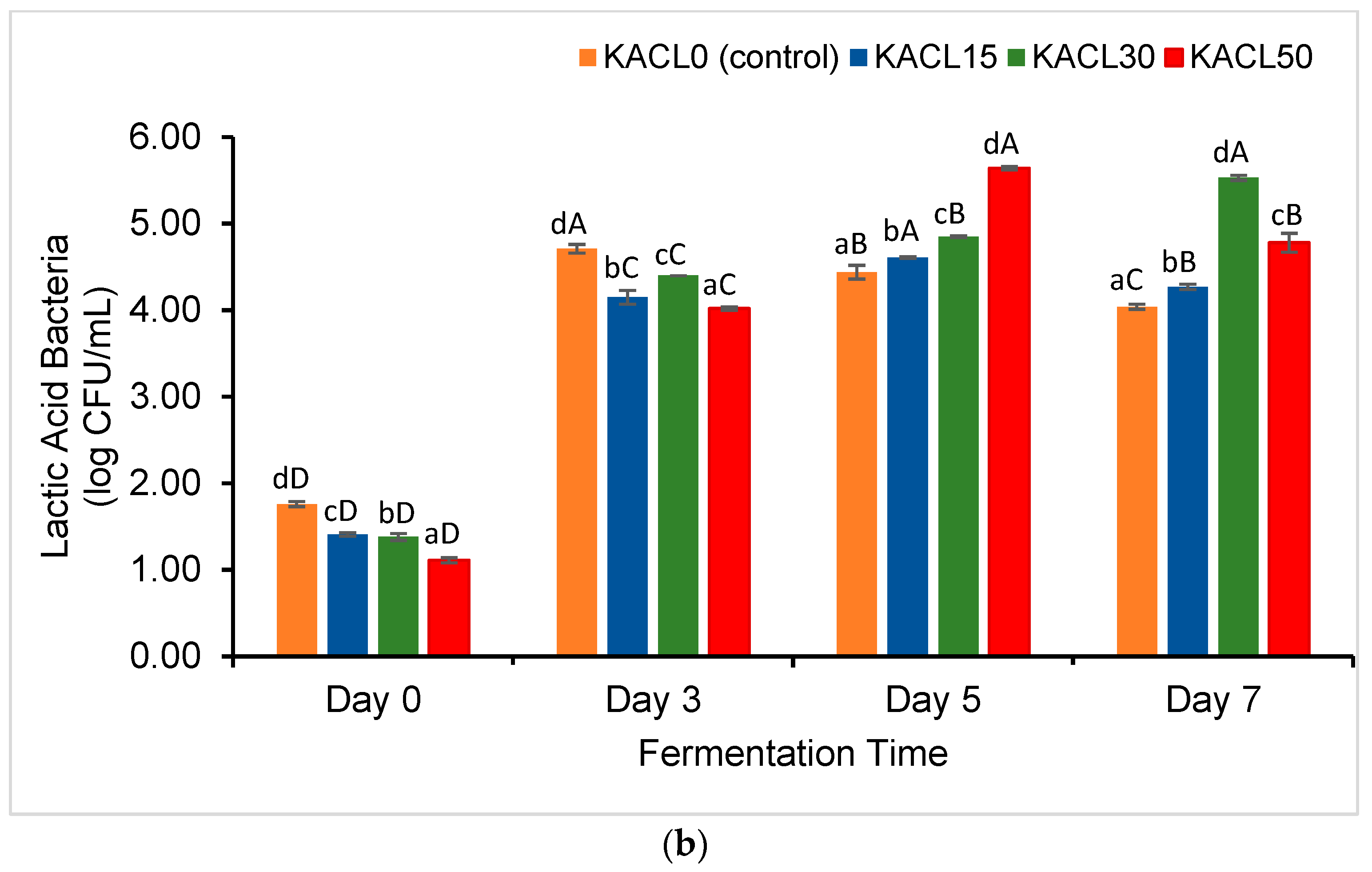
3.2. Effect of the Concentration of Cannabis Leaves and Fermentation Time on the Growth of Lactic Acid Bacteria (LAB) and Total Yeast and Mold (TYM) of the Fermented Kombucha
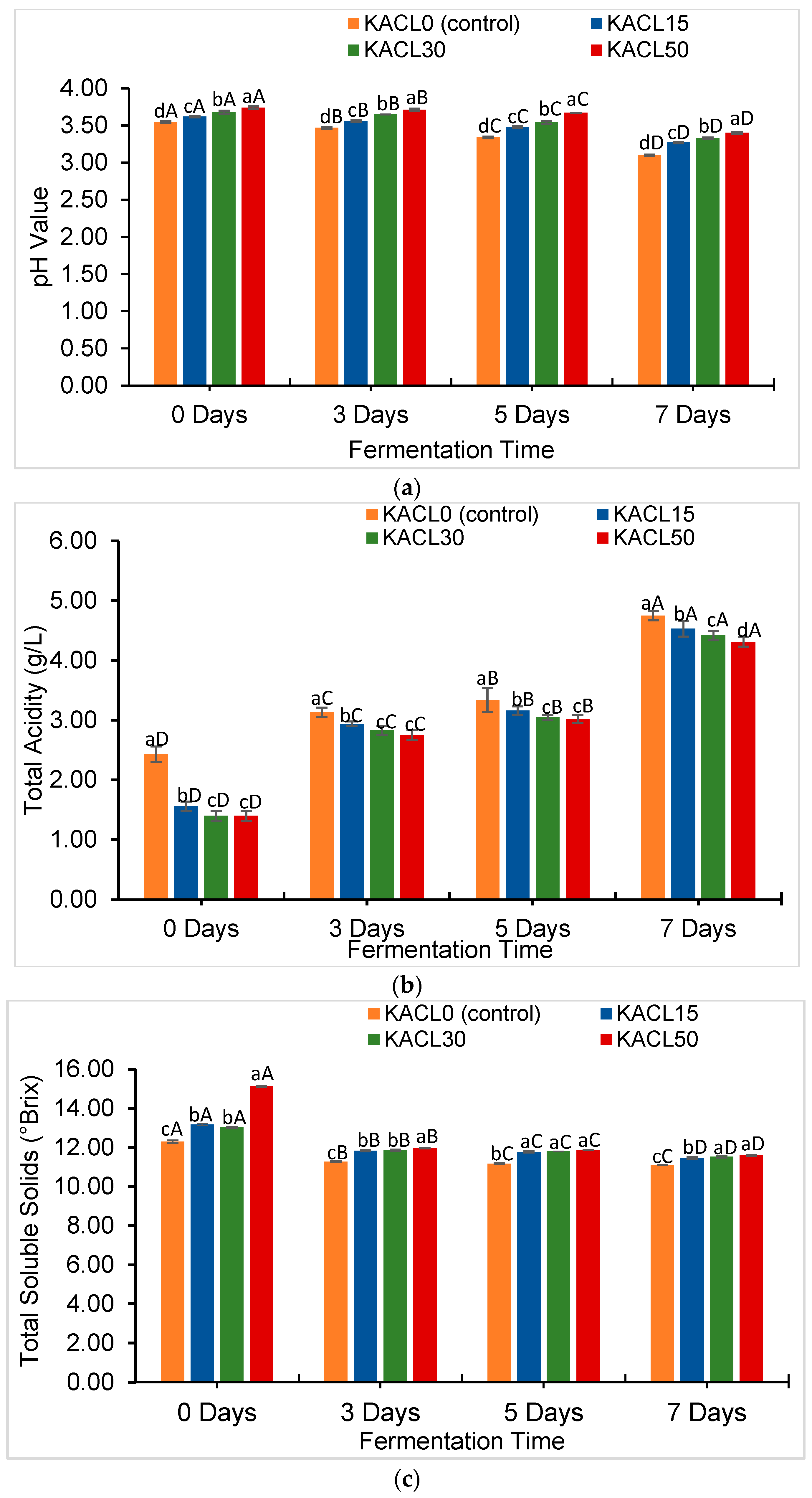
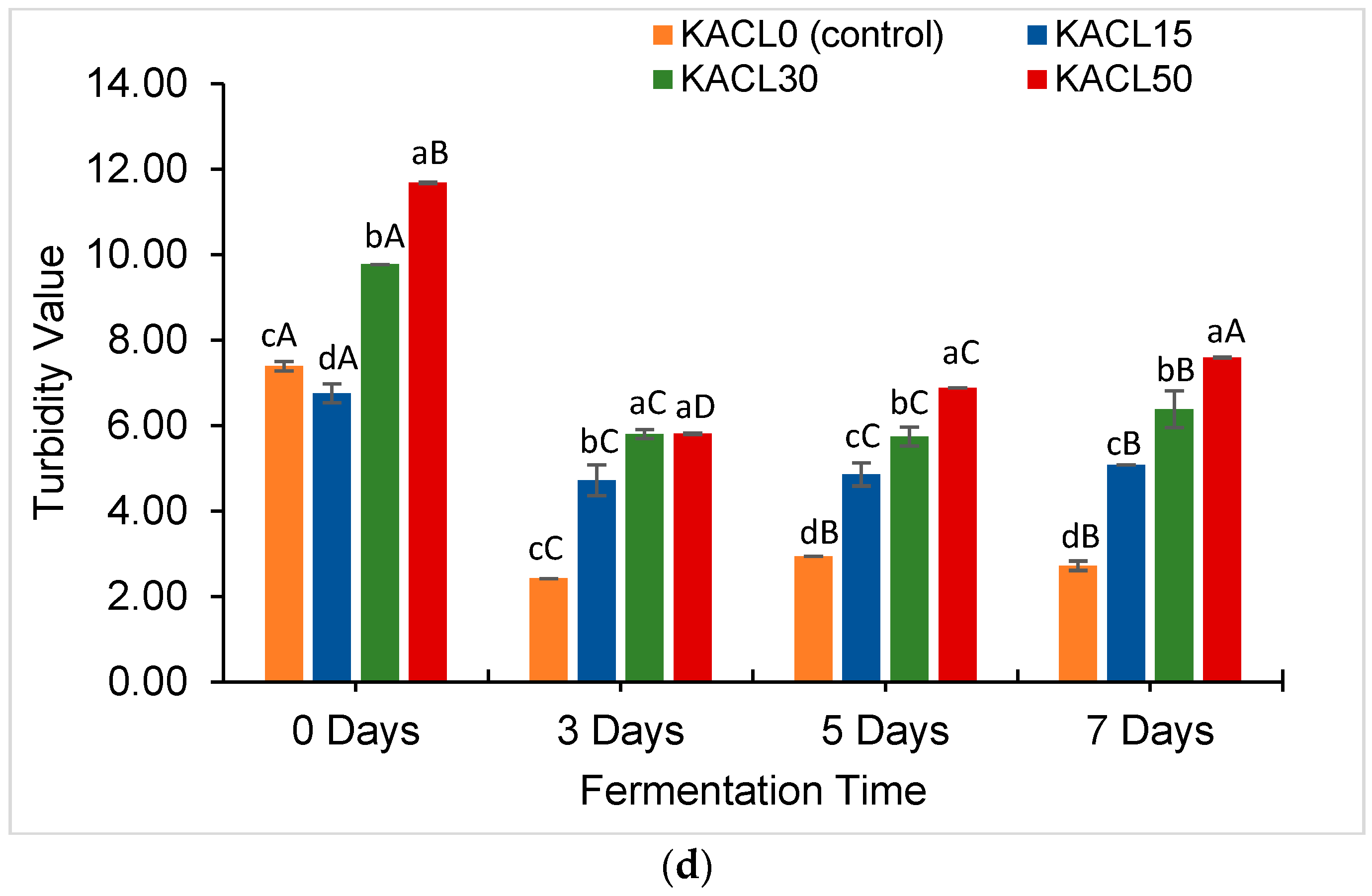
3.3. Effect of the Concentration of Cannabis Leaves and Fermentation Time on Quality Characteristics Changes in the Fermented Kombucha
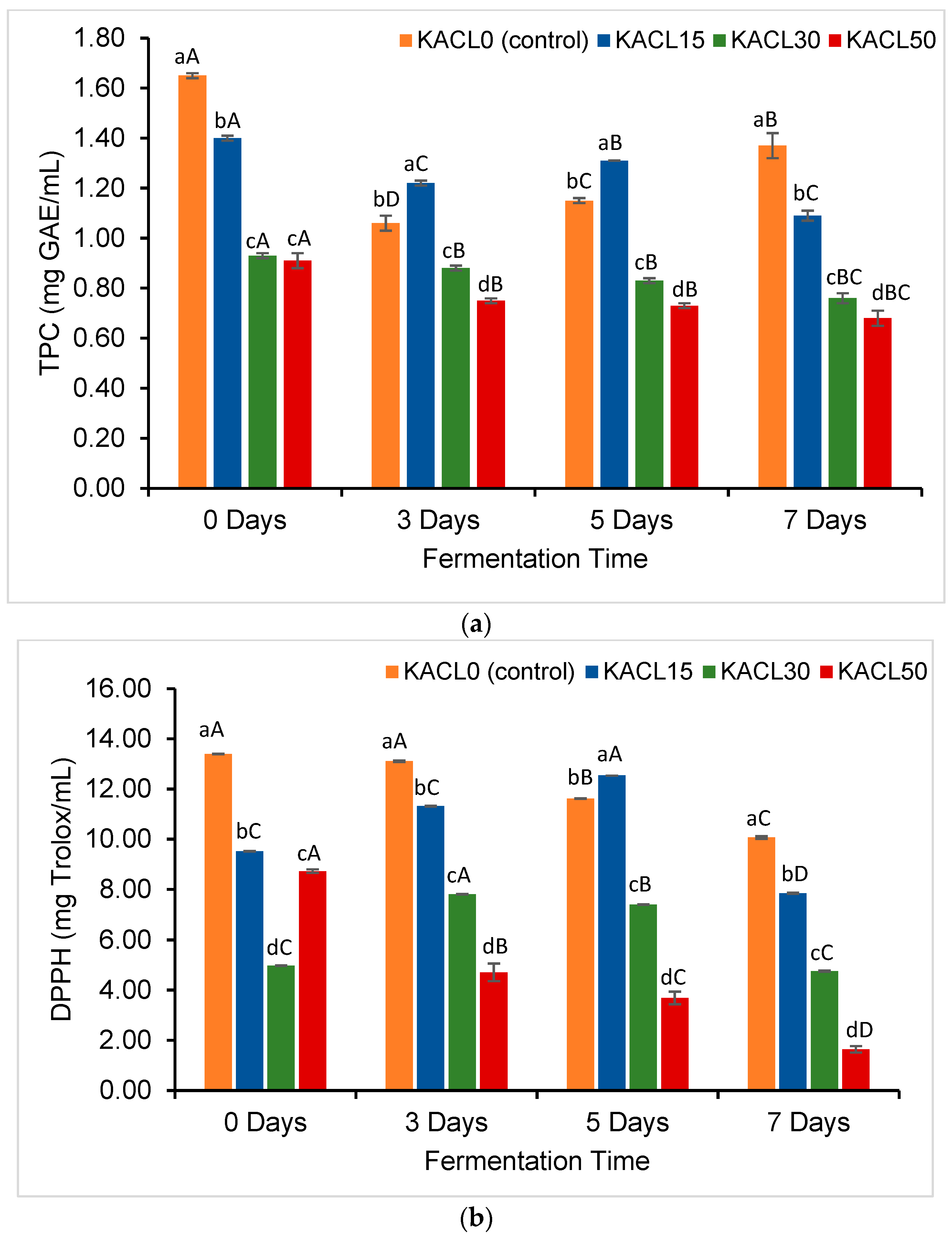
3.4. Effect of the Concentration of Cannabis Leaves and Fermentation Time on Total Phenolic Content and Antioxidant Properties of the Fermented Kombucha
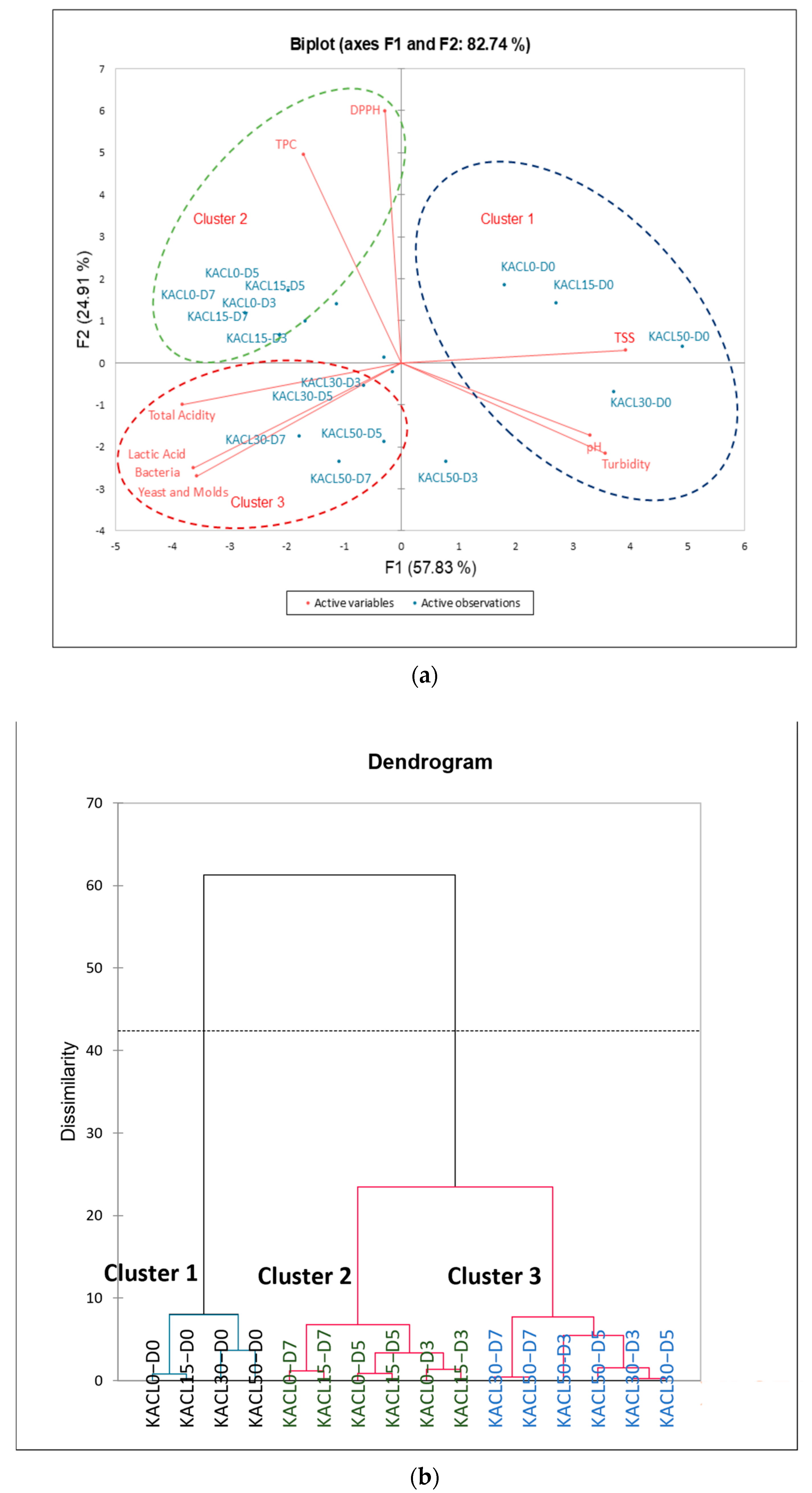
3.5. PCA and HCA Clustering of Kombucha Regarding the Quality Characteristics
3.6. Effect of the Concentration of Cannabis Leaves on Microbial Communities and Diversity Changes in the Final Fermented Kombucha
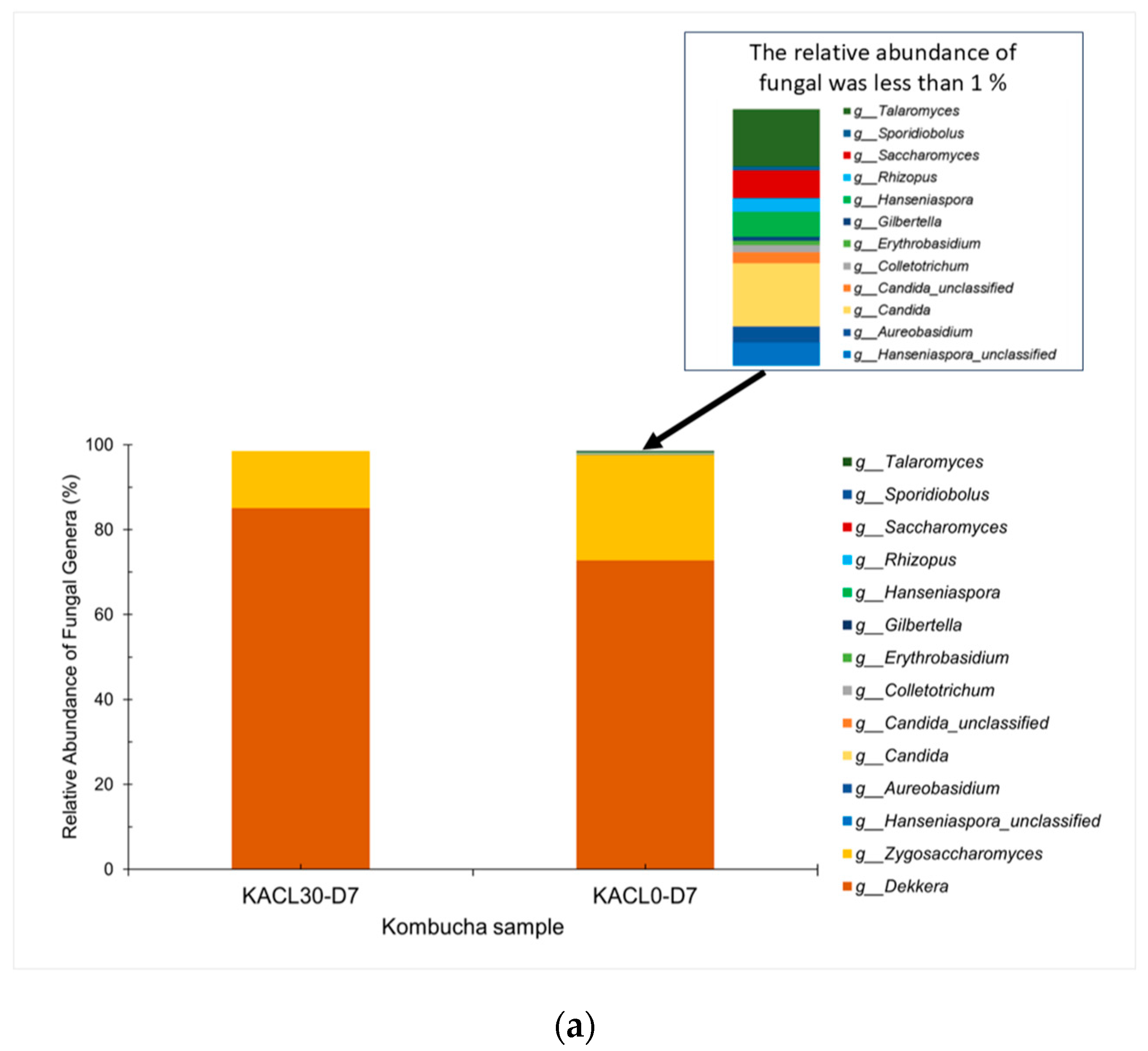
3.6.1. Fungal Communities and Diversity of Cannabis-Infused Kombucha: Effects on Fermentation and Quality Changes
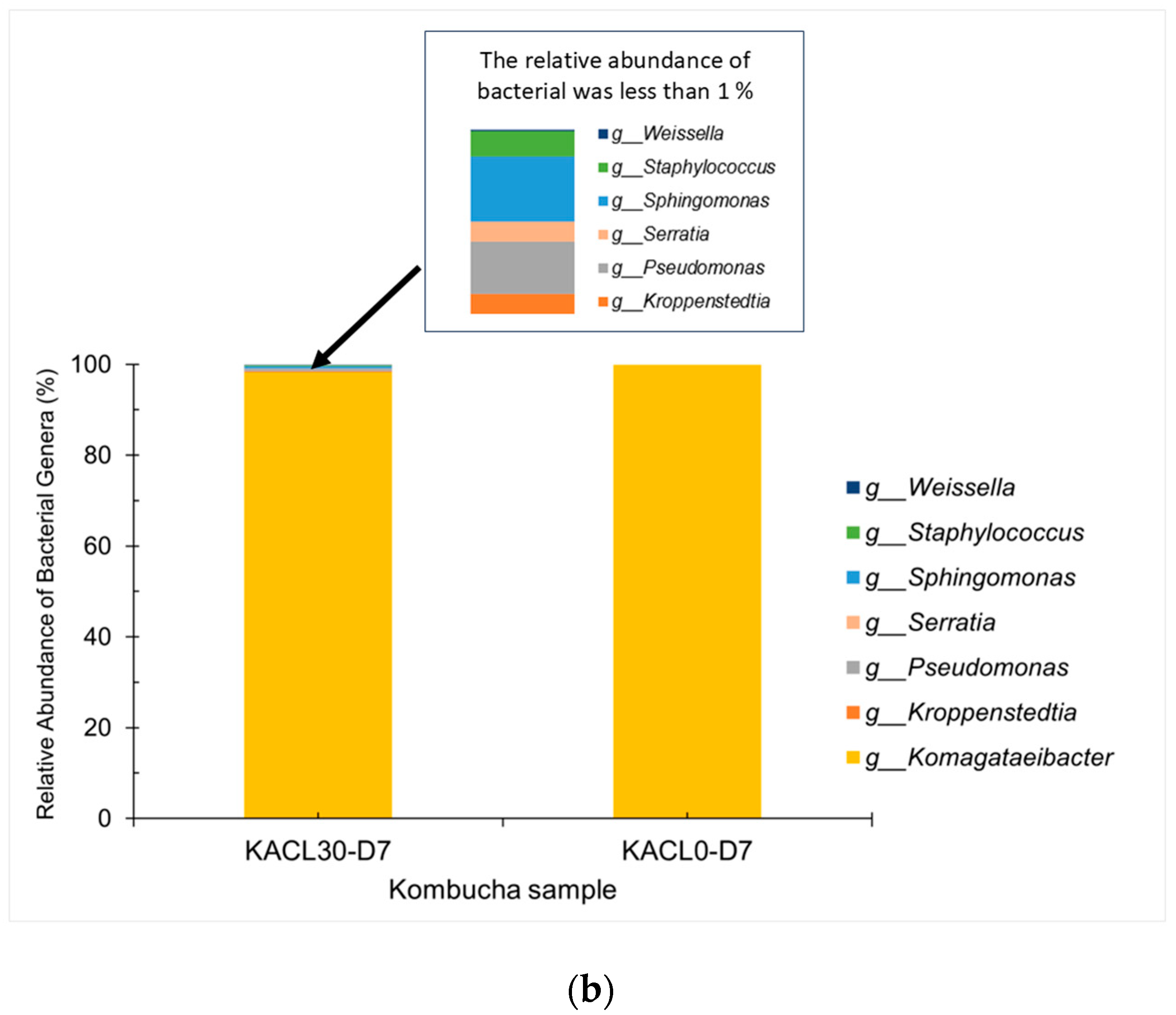
3.6.2. Bacterial Communities and Diversity of Cannabis-Infused Kombucha: Effects on Fermentation and Quality Changes
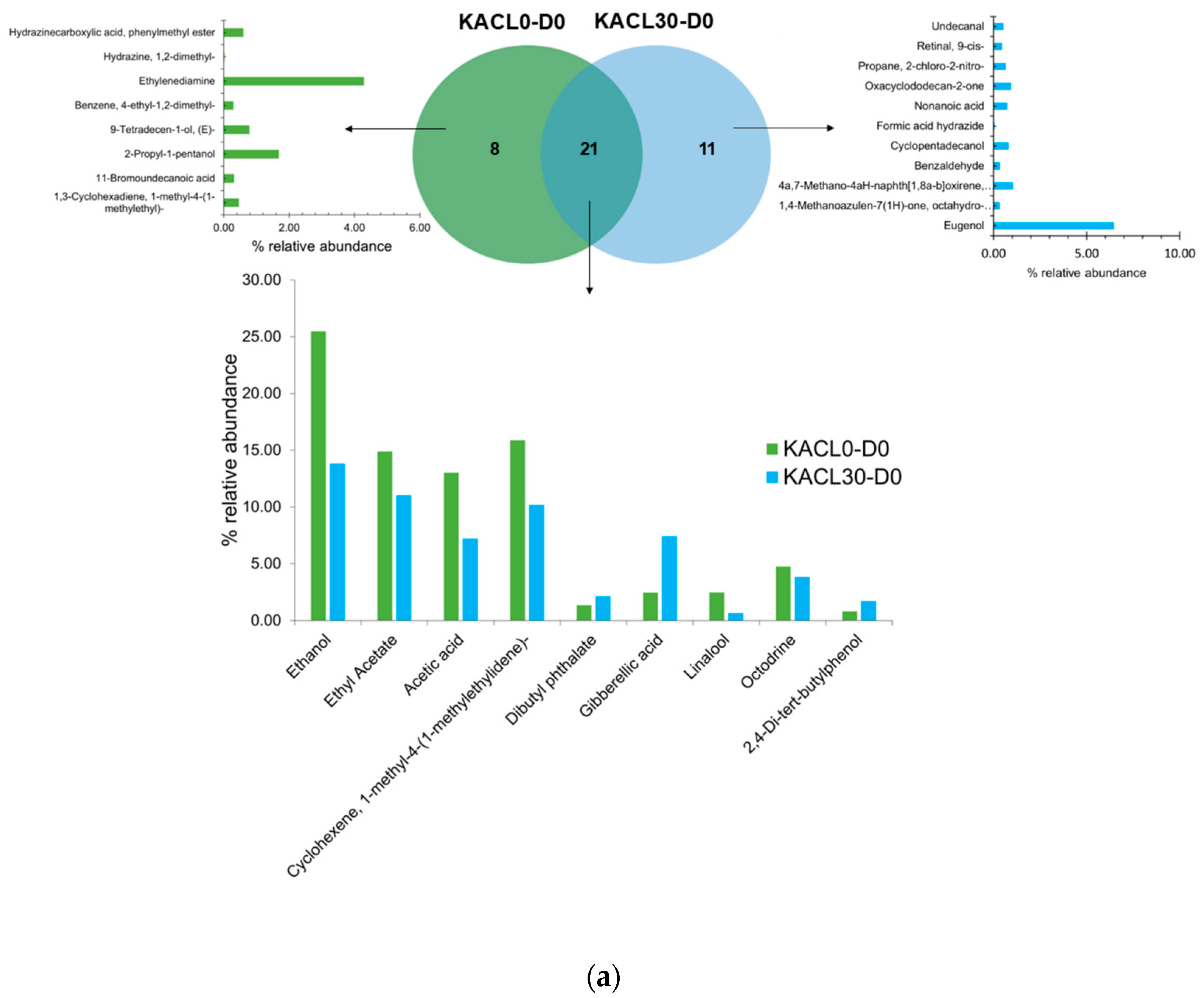
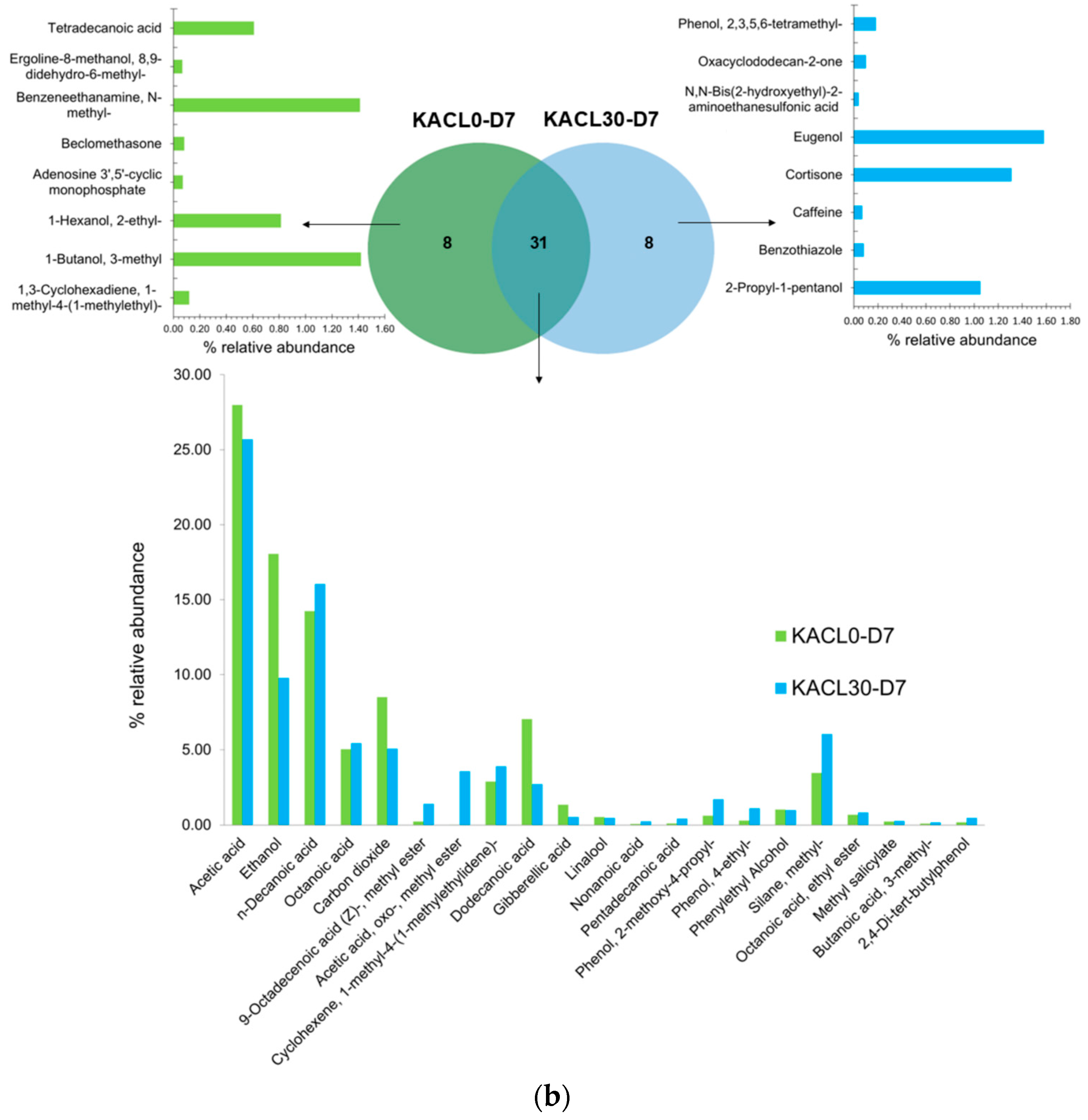
3.7. Effect of Microbiota Communities on Volatile Compounds Changes in the Fermented Kombucha Infused with Cannabis Leaves
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.; Hur, S.; Lim, J.; Jin, K.; Yang, T.-H.; Keehm, I.-S.; Kim, S.W.; Kim, T.; Kim, D. Enhancement of the phenolic compounds and antioxidant activities of Kombucha prepared using specific bacterial and yeast. Food Biosci. 2023, 56, 103431. [Google Scholar] [CrossRef]
- Khazi, M.I.; Liaqat, F.; Liu, X.; Yilin, Y.; Zhu, D. Fermentation, functional analysis and biological activities of turmeric kombucha. J. Sci. Food Agric. 2024, 104, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Lavasani, P.S.; Motevaseli, E.; Sanikhani, N.S.; Modarressi, M.H. Komagataeibacter xylinus as a novel probiotic candidate with high glucose conversion rate properties. Heliyon 2019, 5, e01571. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Rutherfurd-Markwick, K.; Liu, N.; Zhang, X.-X.; Mutukumira, A.N. Evaluation of the probiotic potential of yeast isolated from kombucha in New Zealand. Curr. Res. Food Sci. 2024, 8, 100711. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y.; Zhang, B.; Tian, H.; Liang, Y.; Dang, H.; Zhao, Y. Dynamic Changes in Microbial Communities, Physicochemical Properties, and Flavor of Kombucha Made from Fu-Brick Tea. Foods 2023, 12, 4242. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, J.; Lu, J.; Chen, D.; Song, S.; Wang, H.; Sun, M.; Feng, T. Revealing the influence of microbiota on the flavor of kombucha during natural fermentation process by metagenomic and GC-MS analysis. Food Res. Int. 2023, 169, 112909. [Google Scholar] [CrossRef]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha tea fermentation: Microbial and biochemical dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef]
- Barbosa, C.D.; Trovatti Uetanabaro, A.P.; Rodrigues Santos, W.C.; Caetano, R.G.; Albano, H.; Kato, R.; Cosenza, G.P.; Azeredo, A.; Góes-Neto, A.; Rosa, C.A.; et al. Microbial-physicochemical integrated analysis of kombucha fermentation. LWT 2021, 148, 111788. [Google Scholar] [CrossRef]
- Phung, L.T.; Kitwetcharoen, H.; Chamnipa, N.; Boonchot, N.; Thanonkeo, S.; Tippayawat, P.; Klanrit, P.; Yamada, M.; Thanonkeo, P. Changes in the chemical compositions and biological properties of kombucha beverages made from black teas and pineapple peels and cores. Sci. Rep. 2023, 13, 7859. [Google Scholar] [CrossRef]
- Rahmani, R.; Beaufort, S.; Villarreal-Soto, S.A.; Taillandier, P.; Bouajila, J.; Debouba, M. Kombucha fermentation of African mustard (Brassica tournefortii) leaves: Chemical composition and bioactivity. Food Biosci. 2019, 30, 100414. [Google Scholar] [CrossRef]
- Grassi, A.; Cristani, C.; Palla, M.; Di Giorgi, R.; Giovannetti, M.; Agnolucci, M. Storage time and temperature affect microbial dynamics of yeasts and acetic acid bacteria in a kombucha beverage. Int. J. Food Microbiol. 2022, 382, 109934. [Google Scholar] [CrossRef]
- Zhang, J.; Van Mullem, J.; Dias, D.R.; Schwan, R.F. The chemistry and sensory characteristics of new herbal tea-based kombuchas. J. Food Sci. 2021, 86, 740–748. [Google Scholar] [CrossRef]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef]
- Frolova, Y.; Vorobyeva, V.; Vorobyeva, I.; Sarkisyan, V.; Malinkin, A.; Isakov, V.; Kochetkova, A. Development of Fermented Kombucha Tea Beverage Enriched with Inulin and B Vitamins. Fermentation 2023, 9, 552. [Google Scholar] [CrossRef]
- Silva, K.A.; Uekane, T.M.; de Miranda, J.F.; Ruiz, L.F.; da Motta, J.C.B.; Silva, C.B.; de Souza Pitangui, N.; Gonzalez, A.G.M.; Fernandes, F.F.; Lima, A.R. Kombucha beverage from non-conventional edible plant infusion and green tea: Characterization, toxicity, antioxidant activities and antimicrobial properties. Biocatal. Agric. Biotechnol. 2021, 34, 102032. [Google Scholar] [CrossRef]
- Miglioranza, M.V.; Lodi, K.Z.; Minello, L.; Aver, I.; Magrini, F.E.; Paesi, S.; Branco, C.S. Innovative applications based on agro-industrial residues of pitahaya for improving antioxidant and biological performance in Kombuchas. Food Biosci. 2024, 61, 104780. [Google Scholar] [CrossRef]
- Xu, C.; Zhou, S.; Zhang, J.; Bu, D.; Zang, C.; Fan, R.; Wang, J.; Guo, T.; Han, R.; Yang, Y. Dynamic changes in microbial communities and volatile compounds in kombucha fermentation using Flos sophorae and Elm fruits, compared to black and green tea. Food Res. Int. 2024, 197, 115233. [Google Scholar] [CrossRef]
- Coton, M.; Pawtowski, A.; Taminiau, B.; Burgaud, G.; Deniel, F.; Coulloumme-Labarthe, L.; Fall, A.; Daube, G.; Coton, E. Unraveling microbial ecology of industrial-scale Kombucha fermentations by metabarcoding and culture-based methods. FEMS Microbiol. Ecol. 2017, 93, fix048. [Google Scholar] [CrossRef]
- Sales, A.L.; Cunha, S.C.; Morgado, J.; Cruz, A.; Santos, T.F.; Ferreira, I.M.; Fernandes, J.O.; Miguel, M.A.L.; Farah, A. Volatile, Microbial, and Sensory Profiles and Consumer Acceptance of Coffee Cascara Kombuchas. Foods 2023, 12, 2710. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Xu, Q.; Wang, Y.; Wang, S.; Zou, Y.; Yang, Z.; Yuan, L. Addition of lactic acid bacteria modulates microbial community and promotes the flavor profiles of Kombucha. Food Biosci. 2024, 60, 104340. [Google Scholar] [CrossRef]
- Rasera, G.B.; Ohara, A.; de Castro, R.J.S. Innovative and emerging applications of cannabis in food and beverage products: From an illicit drug to a potential ingredient for health promotion. Trends Food Sci. Technol. 2021, 115, 31–41. [Google Scholar] [CrossRef]
- Zandani, G.; Anavi-Cohen, S.; Assa-Glazer, T.; Gorelick, J.; Nyska, A.; Sela, N.; Bernstein, N.; Madar, Z. Cannabis Extract Effects on Metabolic Parameters and Gut Microbiota Composition in a Mice Model of NAFLD and Obesity. Evid.-Based Complement. Altern. Med. 2022, 2022, 7964018. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, A.; Zafar, U.; Ahmed, W.; Shabbir, M.A.; Sameen, A.; Sahar, A.; Bhat, Z.F.; Kowalczewski, P.; Jarzębski, M.; Aadil, R.M. Applications of Cannabis sativa L. in Food and Its Therapeutic Potential: From a Prohibited Drug to a Nutritional Supplement. Molecules 2021, 26, 7699. [Google Scholar] [CrossRef] [PubMed]
- Naim-Feil, E.; Elkins, A.C.; Malmberg, M.M.; Ram, D.; Tran, J.; Spangenberg, G.C.; Rochfort, S.J.; Cogan, N.O.I. The Cannabis Plant as a Complex System: Interrelationships between Cannabinoid Compositions, Morphological, Physiological and Phenological Traits. Plants 2023, 12, 493. [Google Scholar] [CrossRef]
- Department of Medical Sciences, Rajamangala University of Technology Isan. Cannabis sativa L. (Hang Kra Rog Phu Phan ST1). 2022. Available online: https://www.doa.go.th/pvp/wp-content/uploads/2021/07/AnnoDOA_Public241.pdf (accessed on 26 February 2025).
- Jangchud, K.; Kantrong, H.; Tepsongkroh, B.; Jangchud, A.; Sangchum, K.; Prinyawiwatkul, W. Antioxidant activity, structural and physical properties of soy-based textured vegetable protein with added cannabis leaves as affected by extrusion process. Int. J. Food Sci. Technol. 2024, 59, 7276–7287. [Google Scholar] [CrossRef]
- Tran, T.; Steyer, D.; Verdier, F.; Martin, A.; Alexandre, H.; Grandvalet, C.; Tourdot-Maréchal, R. Field Investigation of Flavored Kombucha’s Shelf Life Unveils High Sensitivity of Microbial Dynamics Towards Assimilable Nitrogen. Food Bioprocess Technol. 2024, 18, 370–391. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J.; et al. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 7. [Google Scholar] [CrossRef]
- MINTEL. Mintel’s Cannabis and CBD Market Research Combines the Latest Market Intelligence, Industry Insights; MINTEL: London, UK, 2023. [Google Scholar]
- Ministry of Public Health. Thai Herbal Pharmacopoeia 2021; Department of Medical Sciences, Ministry of Public Health: Nonthaburi, Thailand, 2021.
- Tongkasee, P.; Srithat, D.; Sriyasak, P.; Jitcharerntham, A.; Piwgern, T.; Khoontawad, J.; Insuwan, W. Formulation and Phytochemical Profile of a Product Prototype Infused with Cannabis sativa Leaves. Trends Sci. 2023, 20, 5835. [Google Scholar] [CrossRef]
- Tongsai, S.; Jangchud, K.; Jangchud, A.; Tepsongkroh, B.; Boonbumrung, S.; Prinyawiwatkul, W. Relationship between sensory and chemical properties of Assam green teas under different pan-firing and rolling time conditions. Int. J. Food Sci. Technol. 2022, 57, 3116–3127. [Google Scholar] [CrossRef]
- Chemists, A.O.A. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithershbug, MD, USA, 2003. [Google Scholar]
- Wang, B.; Rutherfurd-Markwick, K.; Zhang, X.-X.; Mutukumira, A.N. Isolation and characterisation of dominant acetic acid bacteria and yeast isolated from Kombucha samples at point of sale in New Zealand. Curr. Res. Food Sci. 2022, 5, 835–844. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, J.; Chen, L.; Ban, Z.; Zheng, Y.; Jiang, Y.; Jiang, Y.; Li, X. Effects of Fruit Storage Temperature and Time on Cloud Stability of Not from Concentrated Apple Juice. Foods 2022, 11, 2568. [Google Scholar] [CrossRef] [PubMed]
- Mok, K.; Suratanon, N.; Roytrakul, S.; Charoenlappanit, S.; Patumcharoenpol, P.; Chatchatee, P.; Vongsangnak, W.; Nakphaichit, M. ITS2 Sequencing and Targeted Meta-Proteomics of Infant Gut Mycobiome Reveal the Functional Role of Rhodotorula sp. During Atopic Dermatitis Manifestation. J. Fungi 2021, 7, 748. [Google Scholar] [CrossRef] [PubMed]
- Mok, K.; Poolsawat, T.; Somnuk, S.; Wanikorn, B.; Patumcharoenpol, P.; Nitisinprasert, S.; Vongsangnak, W.; Nakphaichit, M. Preliminary characterization of gut mycobiome enterotypes reveals the correlation trends between host metabolic parameter and diet: A case study in the Thai Cohort. Sci. Rep. 2024, 14, 5805. [Google Scholar] [CrossRef] [PubMed]
- Abarenkov, K.; Henrik Nilsson, R.; Larsson, K.H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; Pennanen, T.; et al. The UNITE database for molecular identification of fungi—Recent updates and future perspectives. New Phytol. 2010, 186, 281–285. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014, 42, D633–D642. [Google Scholar] [CrossRef]
- Wang, Z.; Ahmad, W.; Zhu, A.; Geng, W.; Kang, W.; Ouyang, Q.; Chen, Q. Identification of volatile compounds and metabolic pathway during ultrasound-assisted kombucha fermentation by HS-SPME-GC/MS combined with metabolomic analysis. Ultrason. Sonochem. 2023, 94, 106339. [Google Scholar] [CrossRef]
- He, J.; Xu, L.; Yang, L.; Wang, X. Epigallocatechin Gallate Is the Most Effective Catechin Against Antioxidant Stress via Hydrogen Peroxide and Radical Scavenging Activity. Med. Sci. Monit. 2018, 24, 8198–8206. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, X.; Li, Y.; Chen, J.; Chen, X. Microbial interactions and dynamic changes of volatile flavor compounds during the fermentation of traditional kombucha. Food Chem. 2024, 430, 137060. [Google Scholar] [CrossRef]
- Hutkins, R.W. Microbiology and Technology of Fermented Foods. In Microbiology and Technology of Fermented Foods; Blackwell Publishing: Hoboken, NJ, USA, 2006; pp. 29–216. [Google Scholar]
- Zhang, T.; Jeong, C.H.; Cheng, W.N.; Bae, H.; Seo, H.G.; Petriello, M.C.; Han, S.G. Moringa extract enhances the fermentative, textural, and bioactive properties of yogurt. LWT 2019, 101, 276–284. [Google Scholar] [CrossRef]
- May, A.; Narayanan, S.; Alcock, J.; Varsani, A.; Maley, C.; Aktipis, A. Kombucha: A novel model system for cooperation and conflict in a complex multi-species microbial ecosystem. PeerJ 2019, 7, e7565. [Google Scholar] [CrossRef]
- Landis, E.A.; Fogarty, E.; Edwards, J.C.; Popa, O.; Eren, A.M.; Wolfe, B.E. Microbial Diversity and Interaction Specificity in Kombucha Tea Fermentations. mSystems 2022, 7, e0015722. [Google Scholar] [CrossRef] [PubMed]
- Neffe-Skocińska, K.; Sionek, B.; Ścibisz, I.; Kołożyn-Krajewska, D. Acid contents and the effect of fermentation condition of Kombucha tea beverages on physicochemical, microbiological and sensory properties. CyTA-J. Food 2017, 15, 601–607. [Google Scholar] [CrossRef]
- Majid, A.A.; Suroto, D.A.; Utami, T.; Rahayu, E.S. Probiotic potential of kombucha drink from butterfly pea (Clitoria ternatea L.) flower with the addition of Lactiplantibacillus plantarum subsp. plantarum Dad-13. Biocatal. Agric. Biotechnol. 2023, 51, 102776. [Google Scholar] [CrossRef]
- Hooi, S.; Dwiyanto, J.; Chong, C.; Toh, K.; Lee, J.; Lim, J.; Tan, G. The microbial composition and functional roles of different kombucha products in Singapore. CyTA-J. Food 2023, 21, 269–274. [Google Scholar] [CrossRef]
- Nissen, L.; di Carlo, E.; Gianotti, A. Prebiotic potential of hemp blended drinks fermented by probiotics. Food Res. Int. 2020, 131, 109029. [Google Scholar] [CrossRef]
- Tran, T.; Romanet, R.; Roullier-Gall, C.; Verdier, F.; Martin, A.; Schmitt-Kopplin, P.; Alexandre, H.; Grandvalet, C.; Tourdot-Maréchal, R. Non-Targeted Metabolomic Analysis of the Kombucha Production Process. Metabolites 2022, 12, 160. [Google Scholar] [CrossRef]
- Gaggìa, F.; Baffoni, L.; Galiano, M.; Nielsen, D.S.; Jakobsen, R.R.; Castro-Mejía, J.L.; Bosi, S.; Truzzi, F.; Musumeci, F.; Dinelli, G. Kombucha beverage from green, black and rooibos teas: A comparative study looking at microbiology, chemistry and antioxidant activity. Nutrients 2018, 11, 1. [Google Scholar] [CrossRef]
- Gaudreau, H.; Champagne, C.P.; Remondetto, G.E.; Bazinet, L.; Subirade, M. Effect of catechins on the growth of oxygen-sensitive probiotic bacteria. Food Res. Int. 2013, 53, 751–757. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Taillandier, P. Understanding kombucha tea fermentation: A review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef]
- Harrison, K.; Curtin, C. Microbial Composition of SCOBY Starter Cultures Used by Commercial Kombucha Brewers in North America. Microorganisms 2021, 9, 1060. [Google Scholar] [CrossRef]
- Arıkan, M.; Mitchell, A.L.; Finn, R.D.; Gürel, F. Microbial composition of Kombucha determined using amplicon sequencing and shotgun metagenomics. J. Food Sci. 2020, 85, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Sarmiento, W.; Peña-Ocaña, B.A.; Lam-Gutiérrez, A.; Guzmán-Albores, J.M.; Jasso-Chávez, R.; Ruíz-Valdiviezo, V.M. Microbial community structure, physicochemical characteristics and predictive functionalities of the Mexican tepache fermented beverage. Microbiol. Res. 2022, 260, 127045. [Google Scholar] [CrossRef] [PubMed]
- Ohwofasa, A.; Dhami, M.; Winefield, C.; On, S.L.W. Elevated abundance of Komagataeibacter results in a lower pH in kombucha production; insights from microbiomic and chemical analyses. Curr. Res. Food Sci. 2024, 8, 100694. [Google Scholar] [CrossRef] [PubMed]
- Lakra, A.K.; Domdi, L.; Hanjon, G.; Tilwani, Y.M.; Arul, V. Some probiotic potential of Weissella confusa MD1 and Weissella cibaria MD2 isolated from fermented batter. LWT 2020, 125, 109261. [Google Scholar] [CrossRef]
- Wongsurawat, T.; Sutheeworapong, S.; Jenjaroenpun, P.; Charoensiddhi, S.; Khoiri, A.N.; Topanurak, S.; Sutthikornchai, C.; Jintaridth, P. Microbiome analysis of thai traditional fermented soybeans reveals short-chain fatty acid-associated bacterial taxa. Sci. Rep. 2023, 13, 7573. [Google Scholar] [CrossRef]
- Venegas, C.A.; Saona, L.A.; Urbina, K.; Quintrel, P.; Peña, T.A.; Mardones, W.; Cubillos, F.A. Addition of Saccharomyces eubayanus to SCOBY fermentations modulates the chemical and volatile compound profiles in kombucha. Food Microbiol. 2023, 116, 104357. [Google Scholar] [CrossRef]
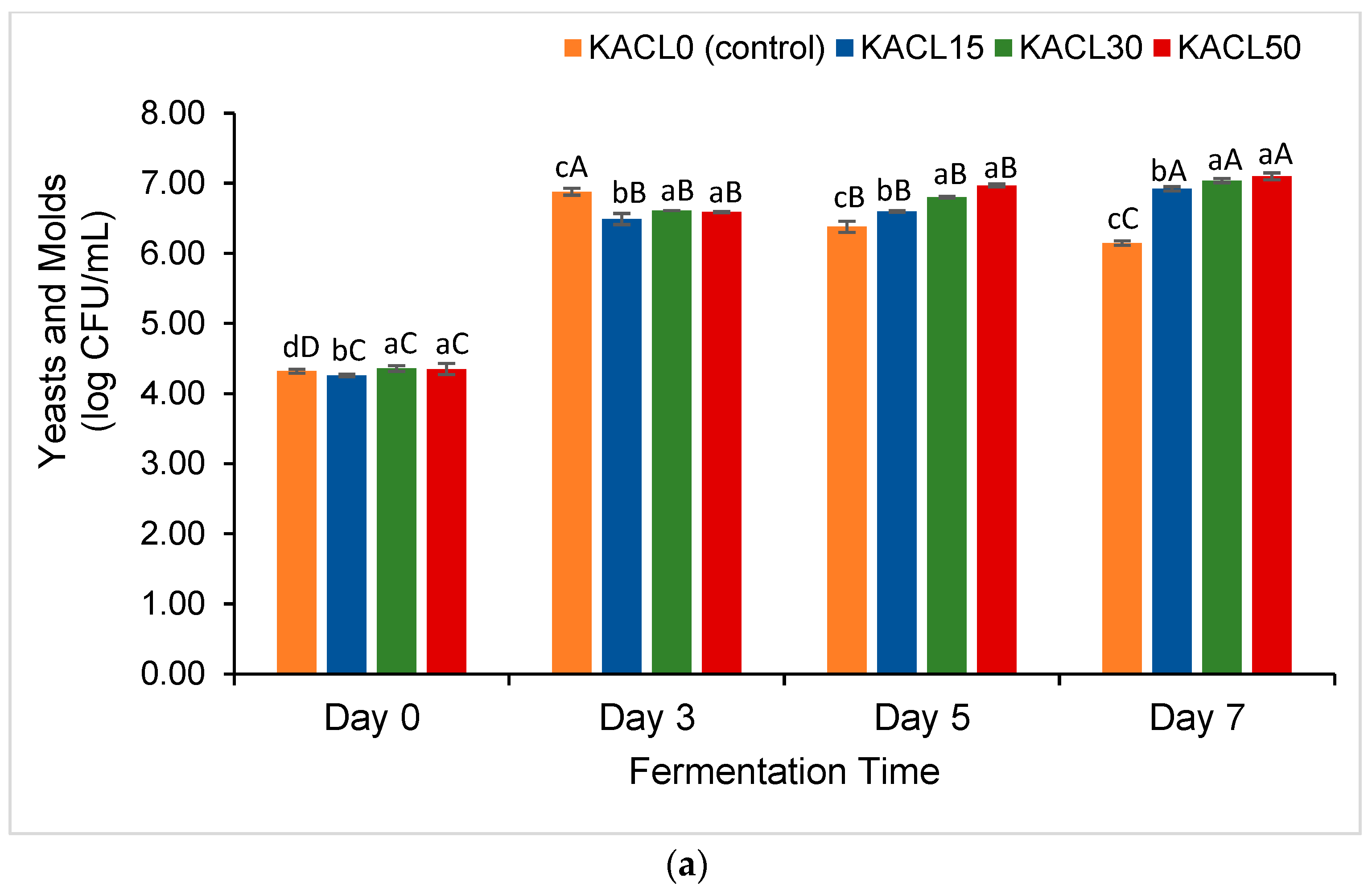
| Parameters | Assam Green Tea Leaves | Cannabis Leaves |
|---|---|---|
| TPC (mg GAE/mL extract) | 1.08 ± 0.01 a | 0.10 ± 0.00 b |
| DPPH (mg Trolox/mL extract) | 21.28 ± 1.06 a | 0.63 ± 0.01 b |
| Ash (g/100 g) | 6.23 ± 0.10 b | 14.79 ± 0.20 a |
| Moisture (g/100 g) | 2.98 ± 0.04 b | 9.96 ± 0.11 a |
| Crude fat (g/100 g) | 0.45 ± 0.02 b | 4.09 ± 0.12 a |
| Crude protein (g/100 g) | 21.68 ± 0.68 b | 23.10 ± 0.17 a |
| Crude fiber (g/100 g) | 21.27 ± 0.58 a | 16.92 ± 1.20 b |
| Carbohydrates (g/100 g) | 47.39 ± 0.68 a | 31.14 ± 1.57 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
A’yuni, Q.; Mok, K.; Nakphaichit, M.; Jangchud, K.; Pirak, T. Thai Cannabis sativa Leaves as a Functional Ingredient for Quality Improvement and Lactic Acid Bacterial Growth Enhancement in Kombucha. Foods 2025, 14, 942. https://doi.org/10.3390/foods14060942
A’yuni Q, Mok K, Nakphaichit M, Jangchud K, Pirak T. Thai Cannabis sativa Leaves as a Functional Ingredient for Quality Improvement and Lactic Acid Bacterial Growth Enhancement in Kombucha. Foods. 2025; 14(6):942. https://doi.org/10.3390/foods14060942
Chicago/Turabian StyleA’yuni, Qurrata, Kevin Mok, Massalin Nakphaichit, Kamolwan Jangchud, and Tantawan Pirak. 2025. "Thai Cannabis sativa Leaves as a Functional Ingredient for Quality Improvement and Lactic Acid Bacterial Growth Enhancement in Kombucha" Foods 14, no. 6: 942. https://doi.org/10.3390/foods14060942
APA StyleA’yuni, Q., Mok, K., Nakphaichit, M., Jangchud, K., & Pirak, T. (2025). Thai Cannabis sativa Leaves as a Functional Ingredient for Quality Improvement and Lactic Acid Bacterial Growth Enhancement in Kombucha. Foods, 14(6), 942. https://doi.org/10.3390/foods14060942






