Gastrodia elata, Polygonatum sibiricum, and Poria cocos as a Functional Food Formula: Cognitive Enhancement via Modulation of Hippocampal Neuroinflammation and Neuroprotection in Sleep-Restricted Mice
Abstract
:1. Introduction
2. Materials and Methods
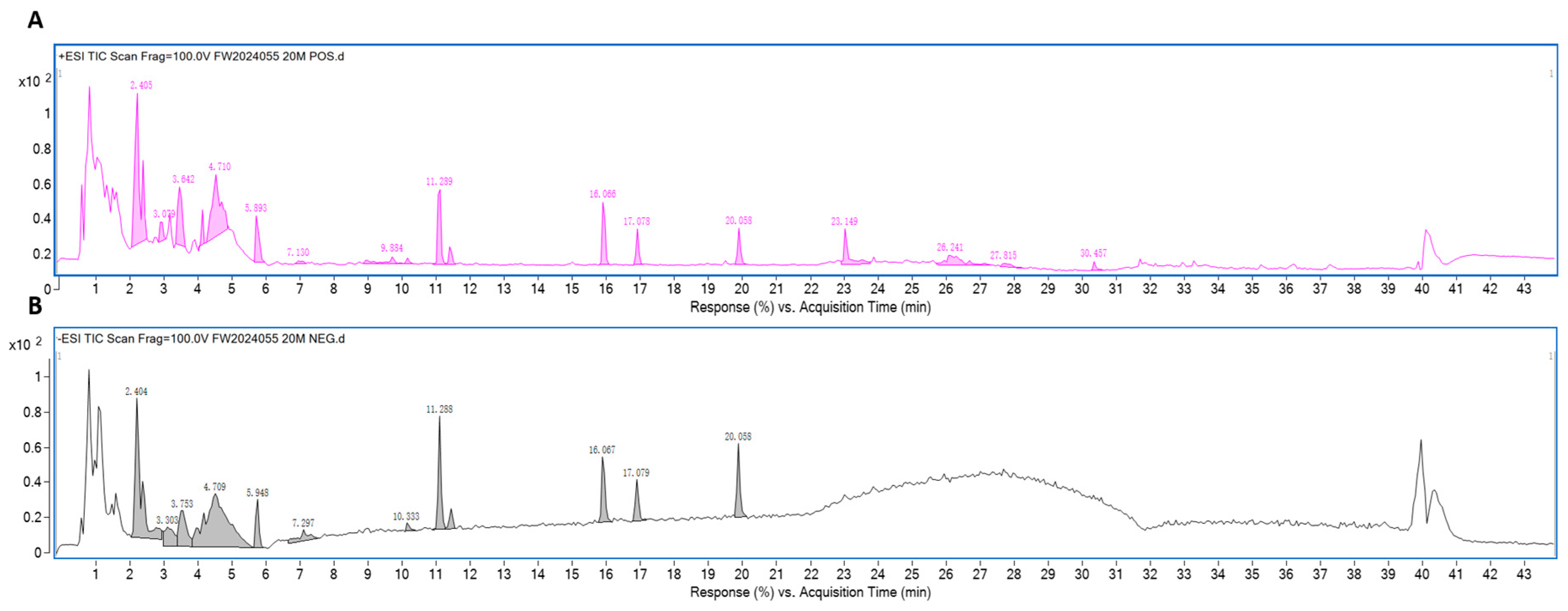
2.1. Preparation and Analysis of CGEF
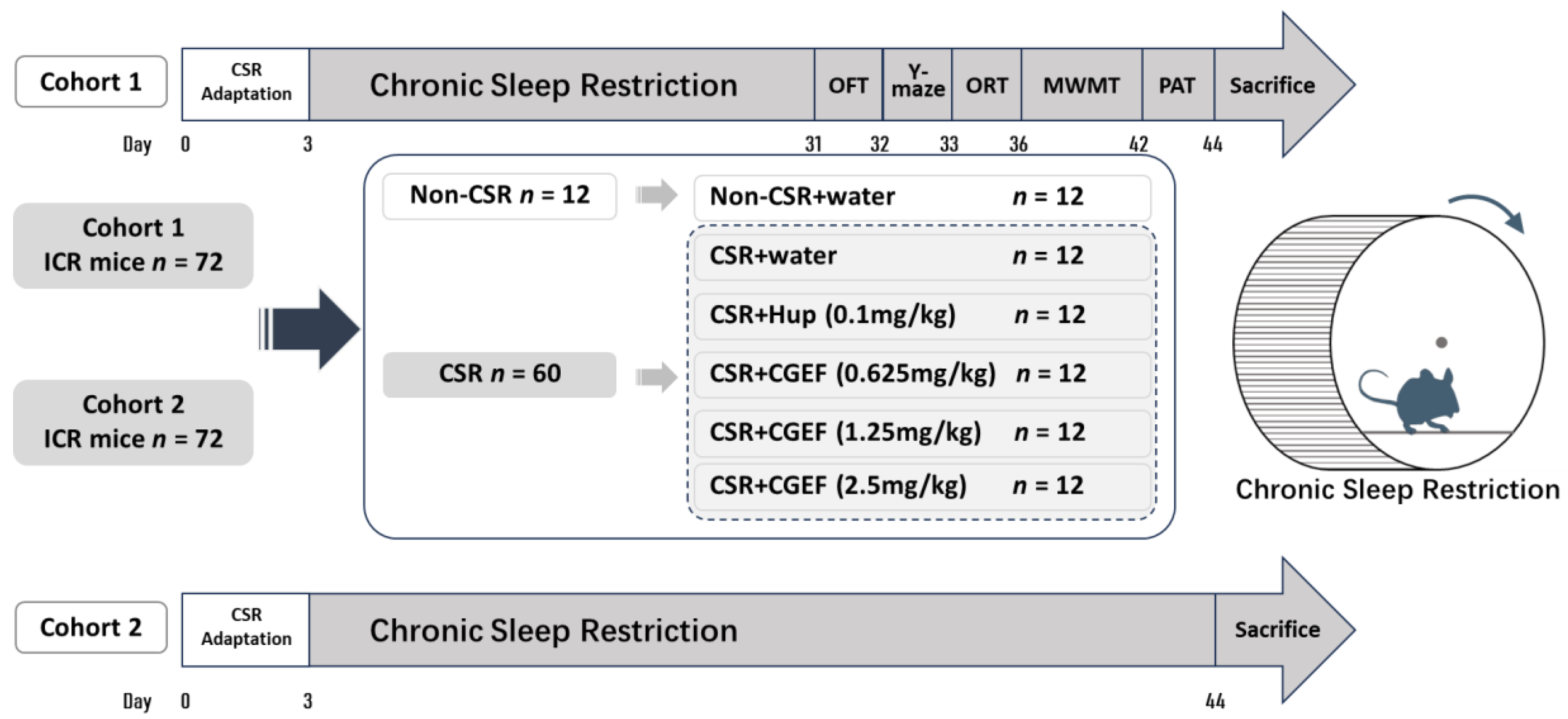
2.2. Animals and Experimental Groups
2.3. Chronic Sleep Restriction (CSR)
2.4. Behavioral Tests
2.4.1. Open Field Test (OFT)
2.4.2. Y-Maze Test
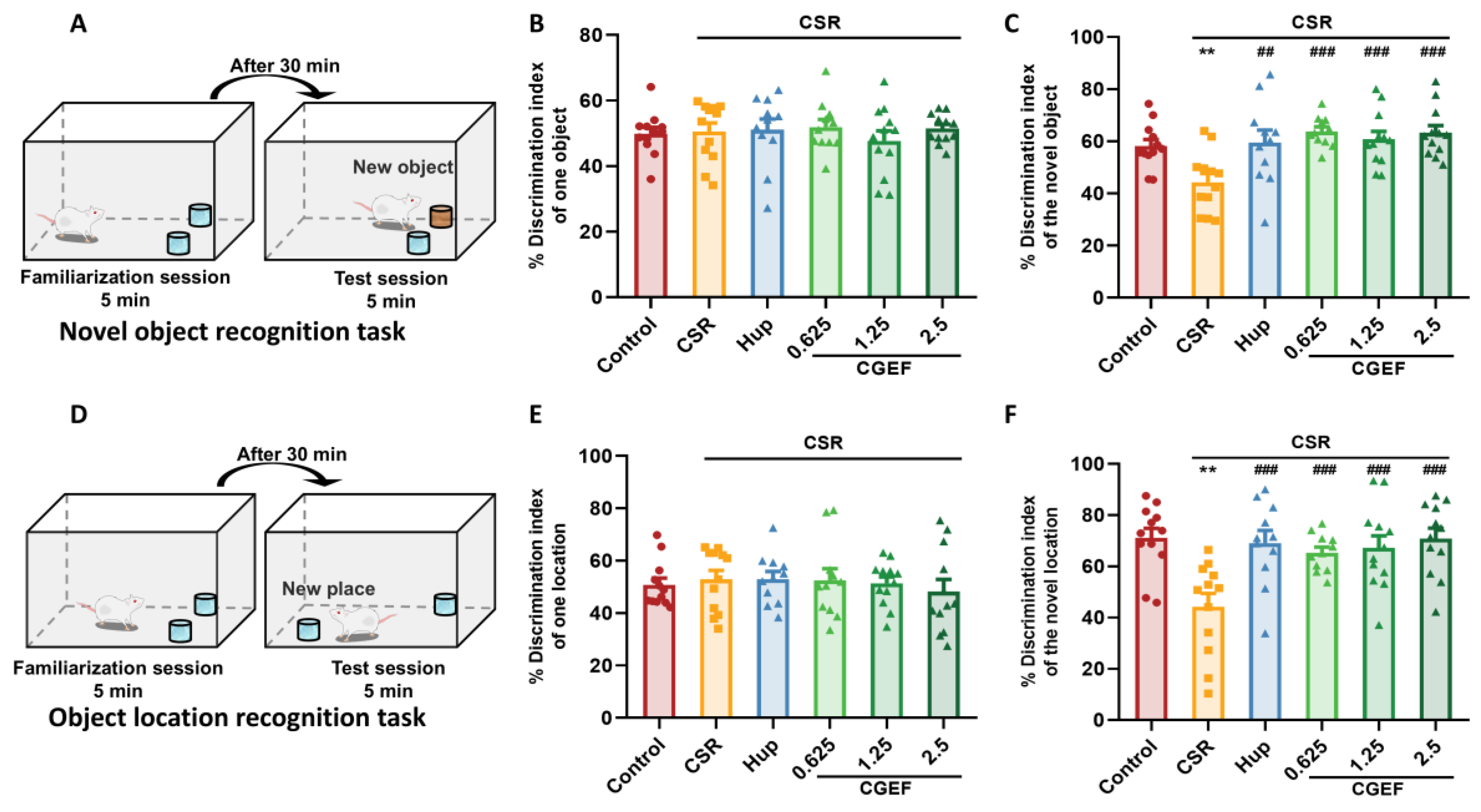
2.4.3. Object Recognition Test
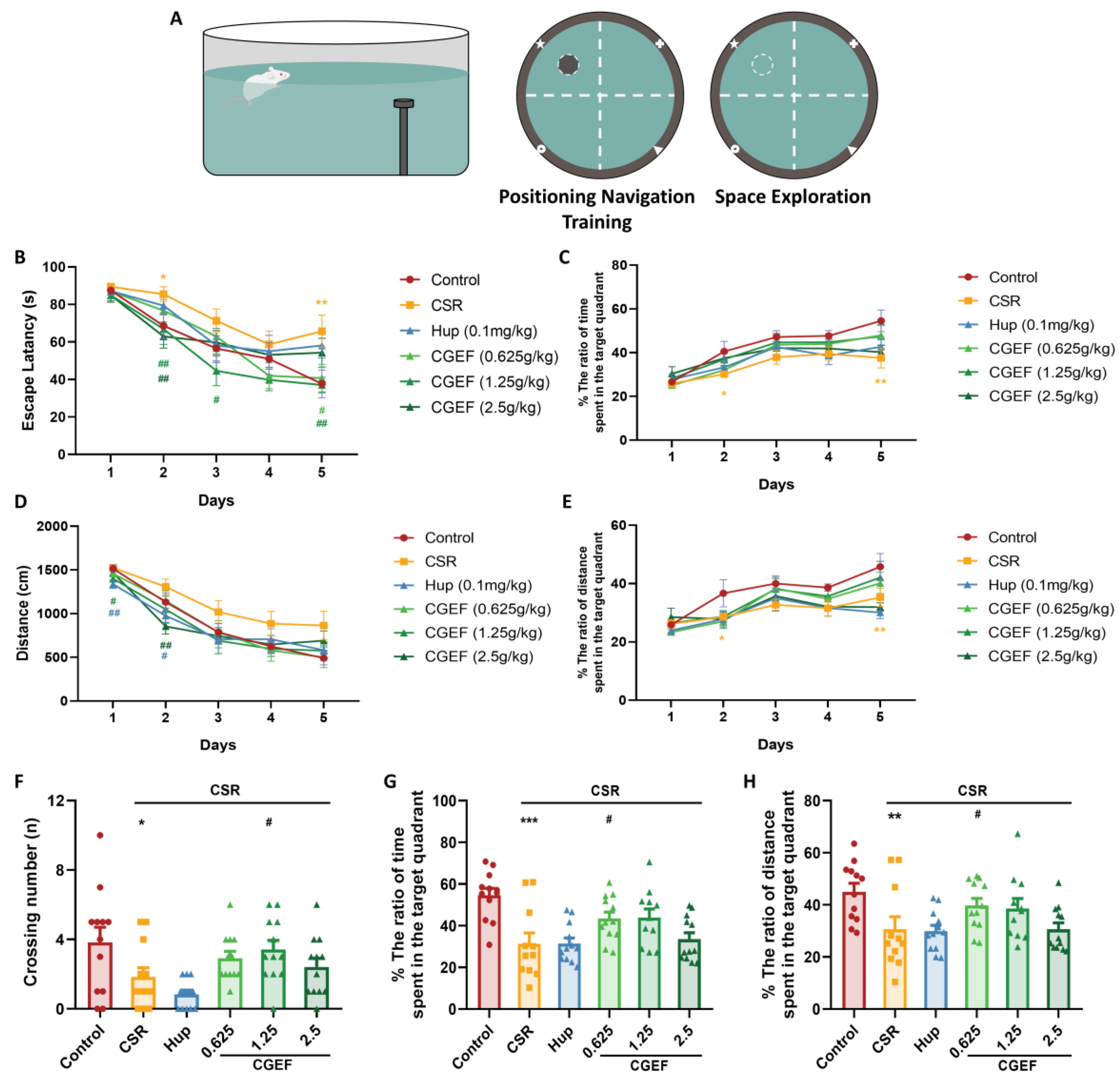
2.4.4. Morris Water Maze Test (MWMT)
2.4.5. Passive Avoidance Test (PAT)
2.5. Biochemical Analysis
2.5.1. Preparation of Serum and Brain Tissue Samples
2.5.2. Determination of Biochemical Parameters
2.5.3. Western Blotting Analysis
2.5.4. Neurotransmitter Detection
2.6. Statistical Analysis
3. Result
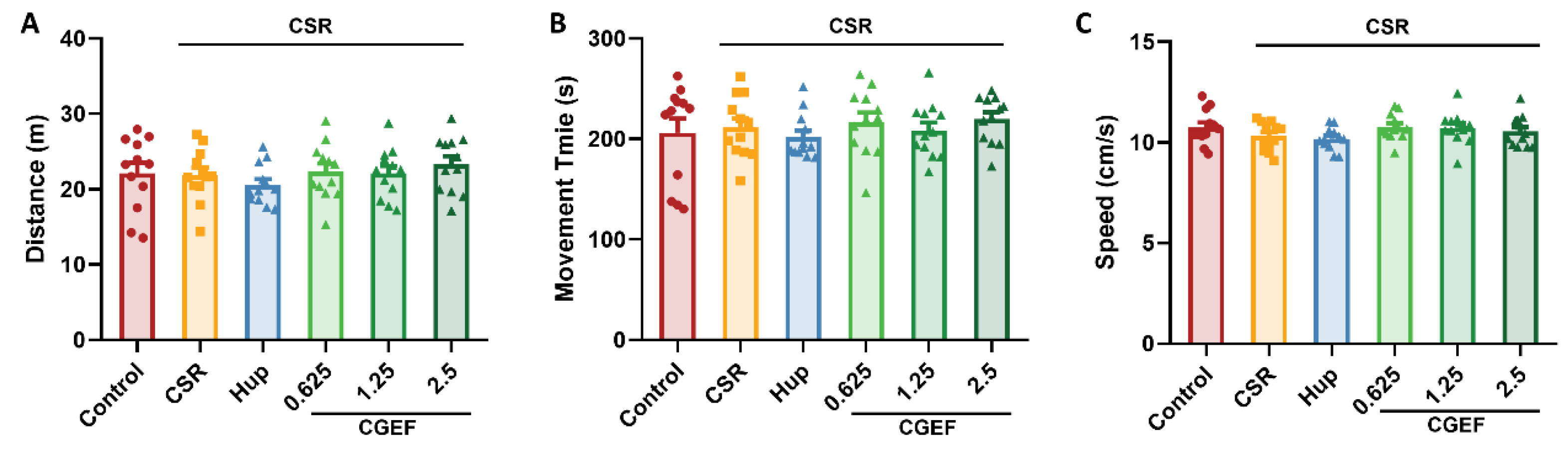
3.1. Effect of CGEF on Locomotor Activity in Mice with CSR
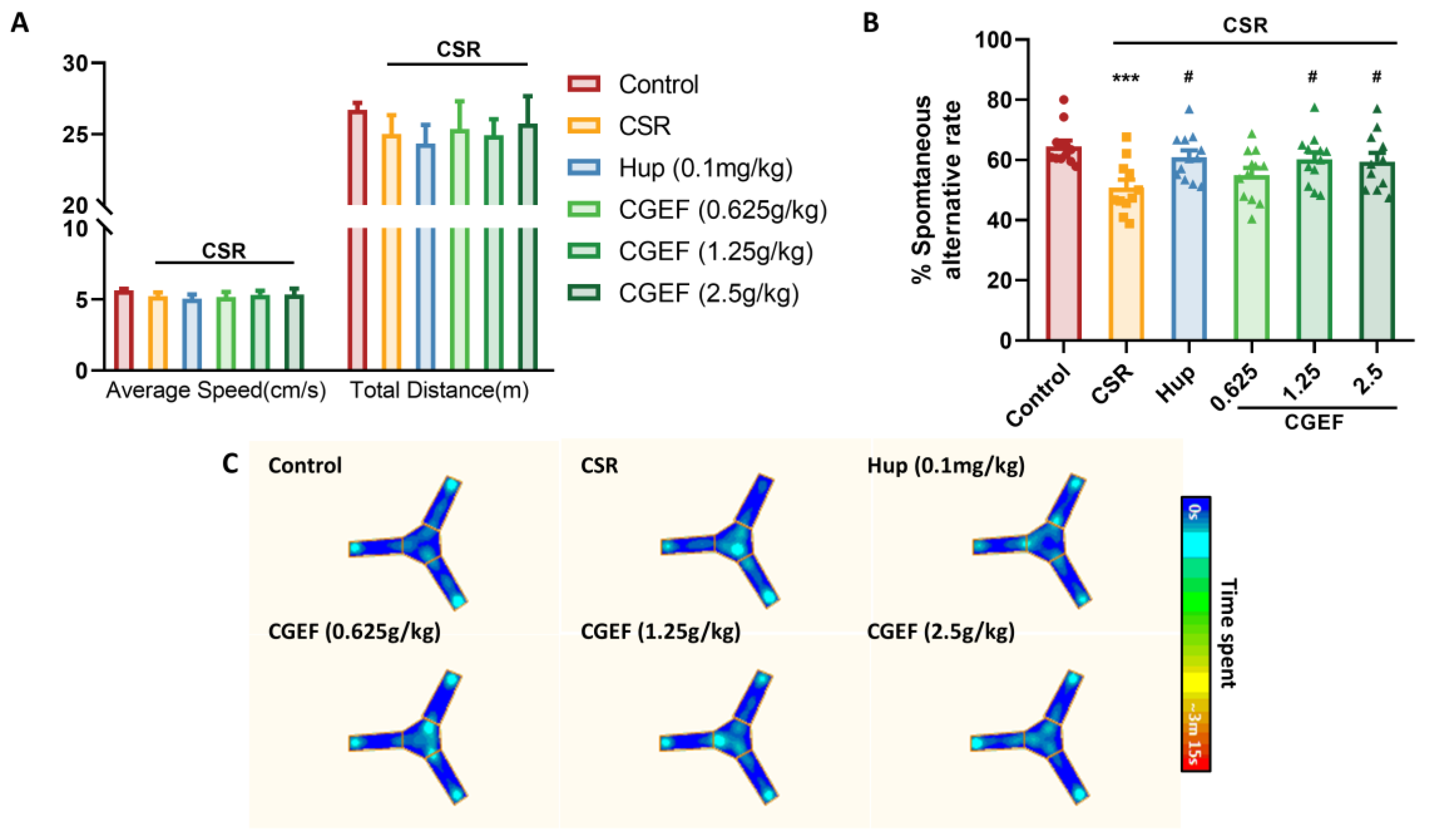
3.2. Effect of CGEF on the Y-Maze Test
3.3. Effect of CGEF on Object Recognition Test
3.4. Effect of CGEF on the Morris Water Maze Test
3.5. Effect of CGEF on Passive Avoidance Test
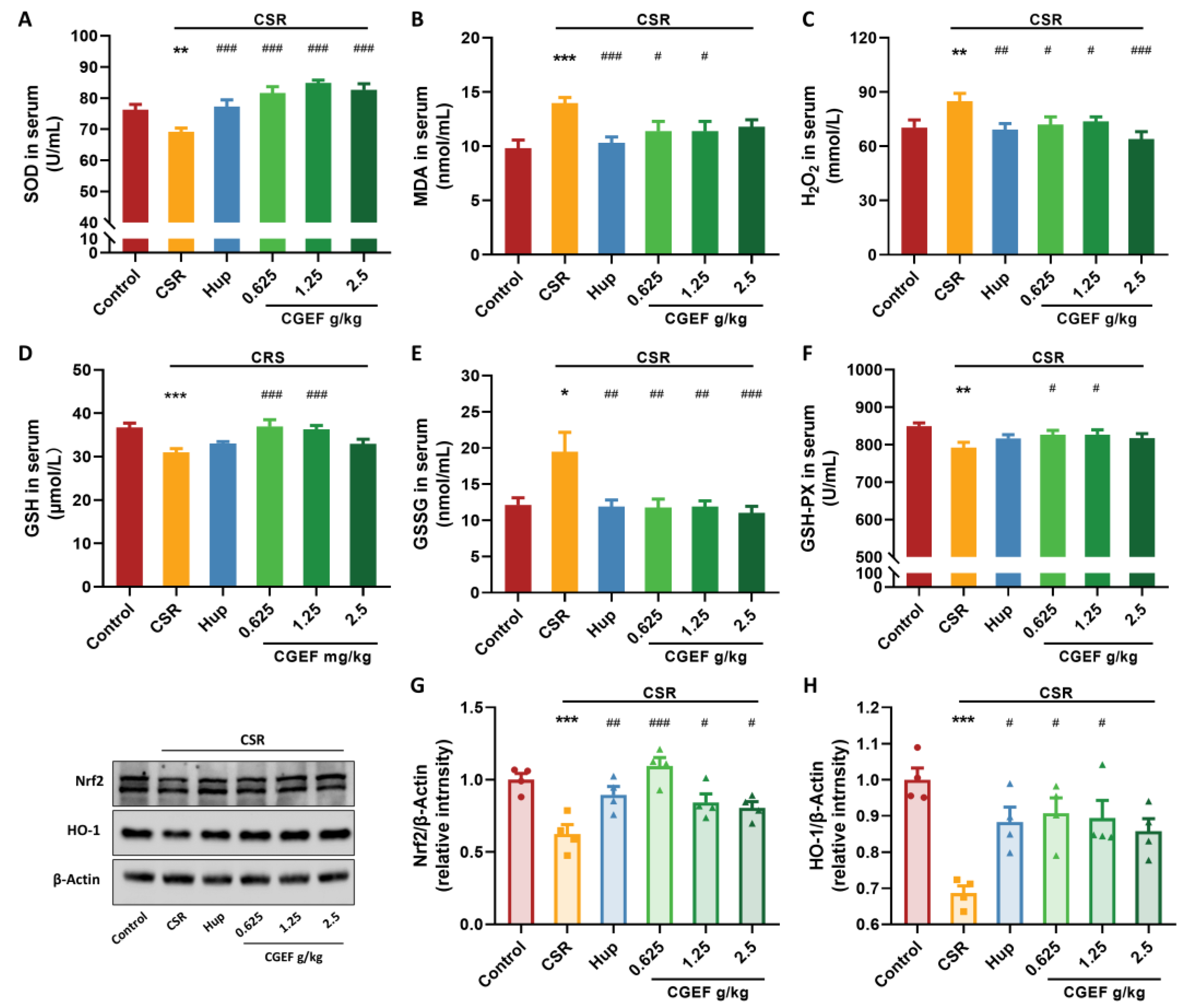
3.6. Effect of CGEF on Oxidative Stress Markers and Nrf2/HO-1 Pathway
3.7. Effect of CGEF on Inflammatory Cytokine Levels in Serum and Hippocampus
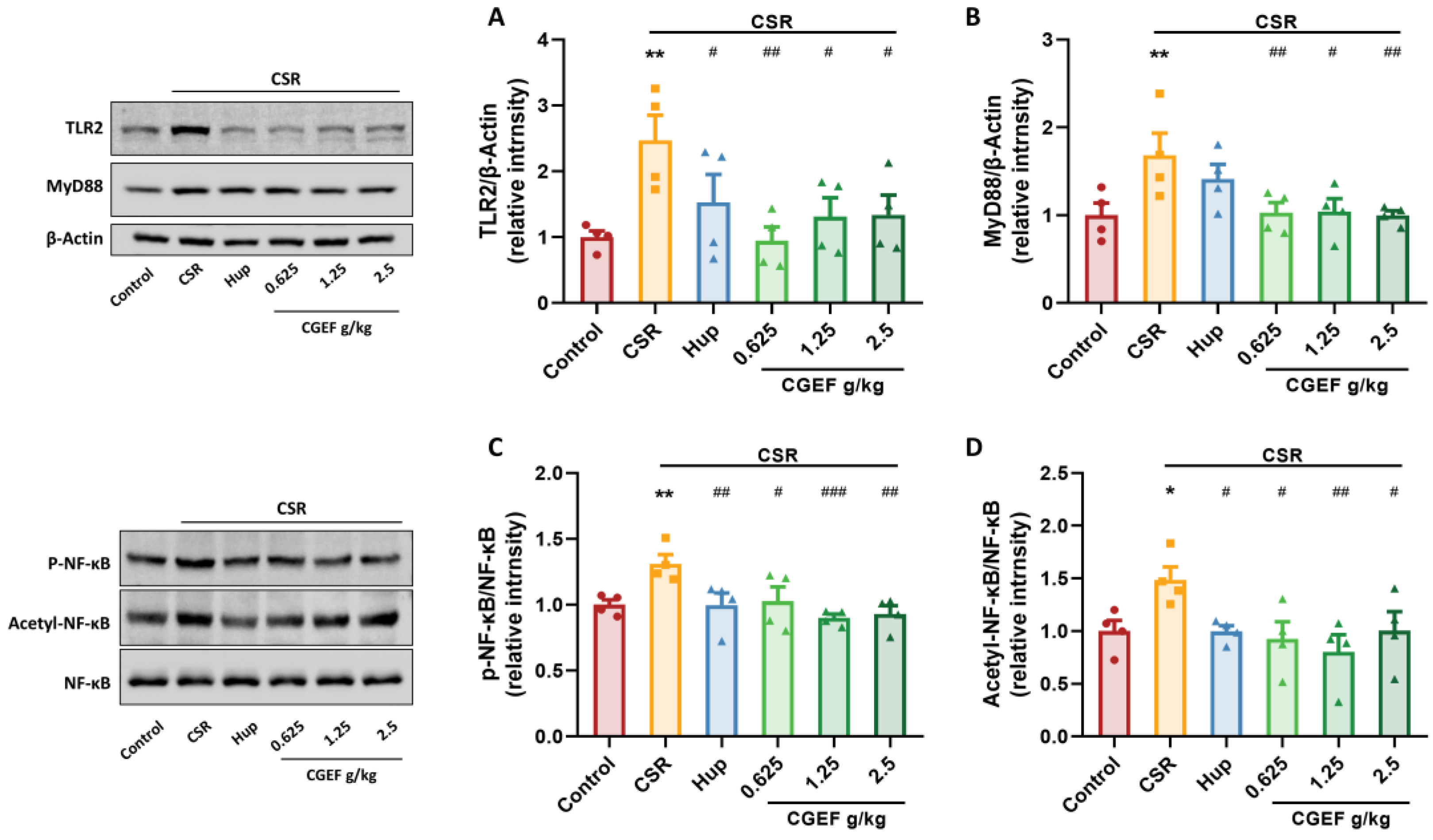
3.8. Effect of CGEF on the TLR2/MyD88/NF-κB Pathway in the Hippocampus of Mice with CSR
3.9. Effect of CGEF on Apoptosis Protein Expression in the Hippocampus
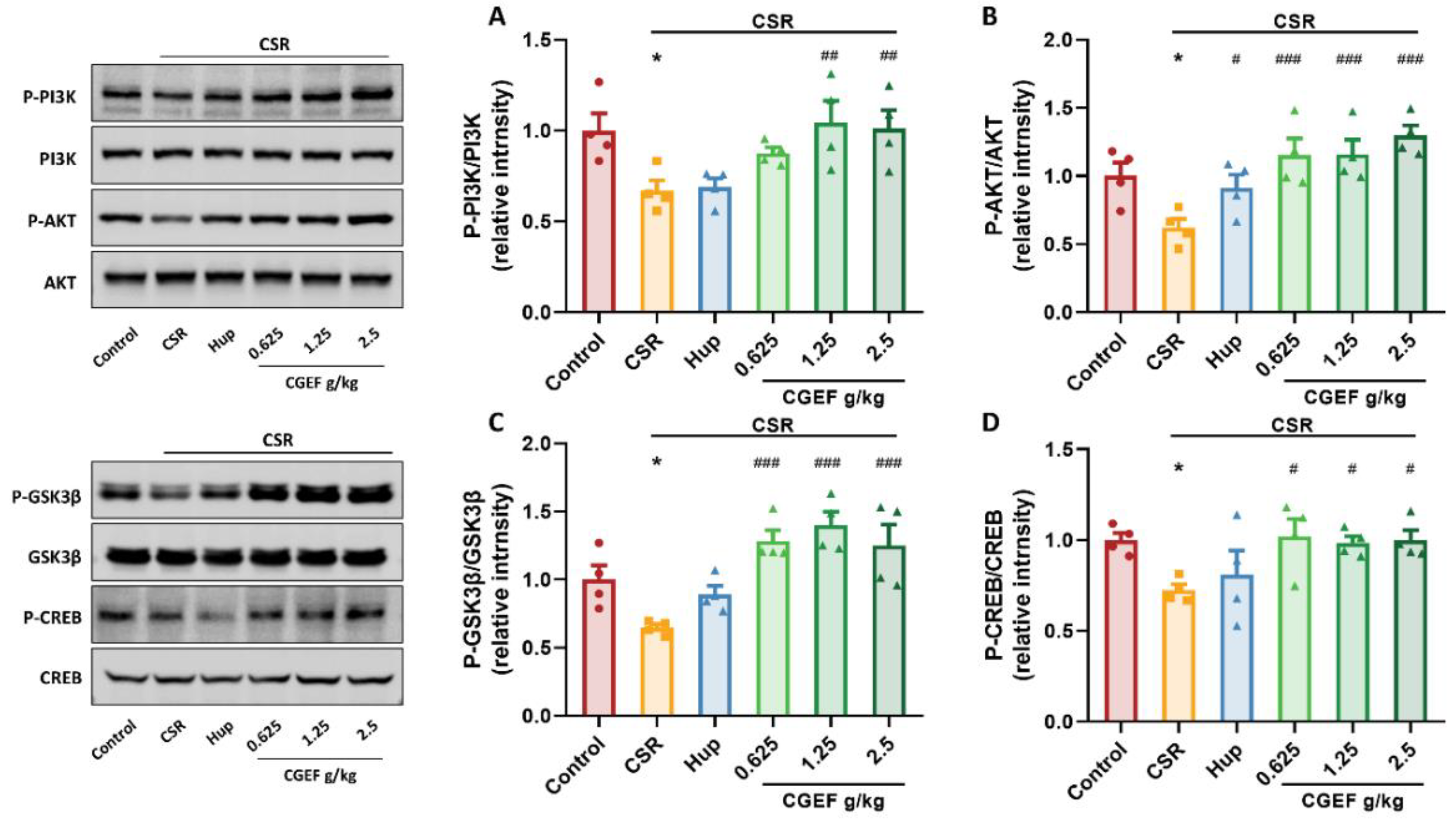
3.10. Effect of CGEF on PI3K/AKT/GSK3β and CREB Phosphorylation in the Hippocampus
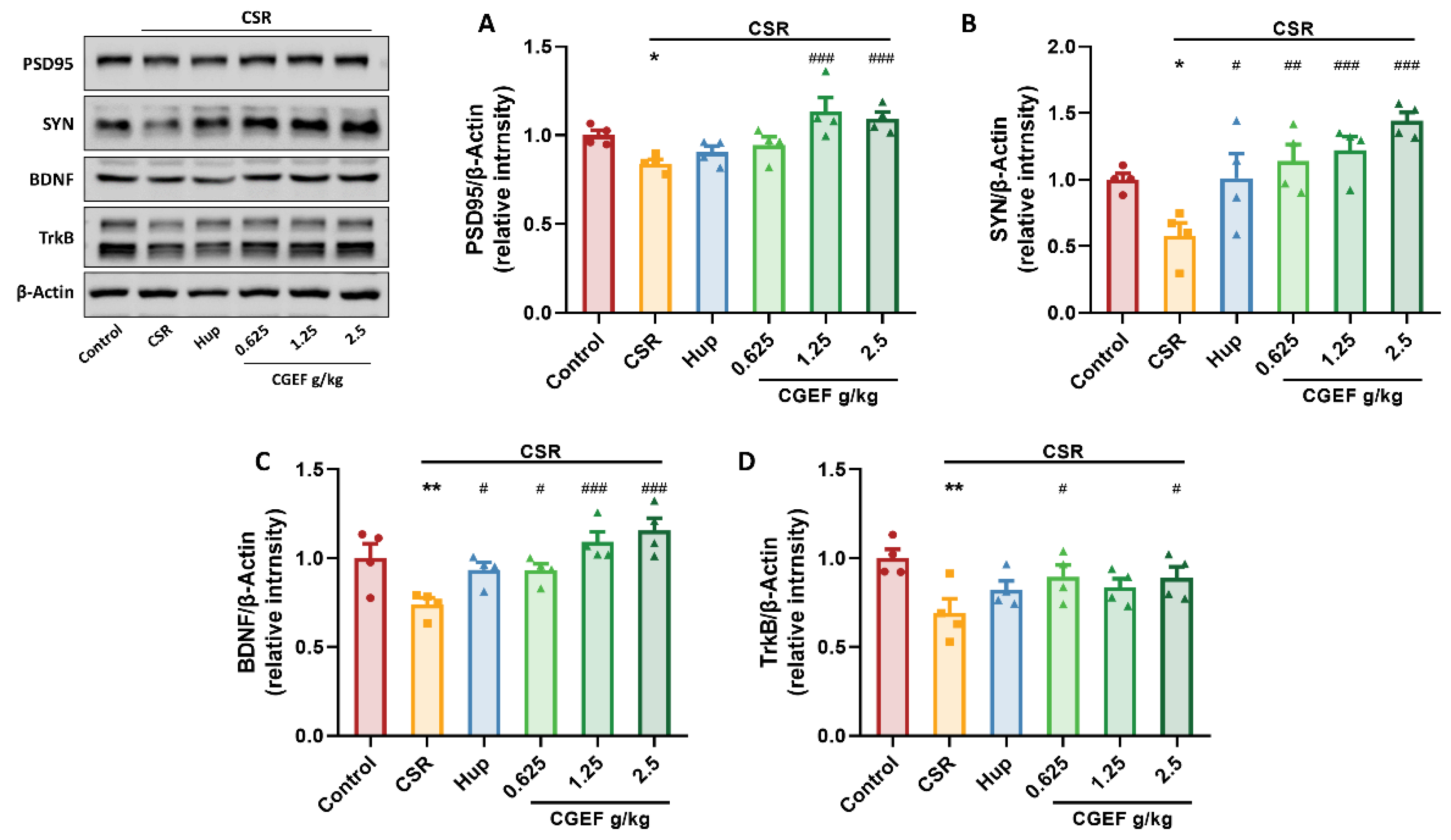
3.11. Effect of CGEF on Synaptic Plasticity and Neurotrophic Factor Expression in the Hippocampus
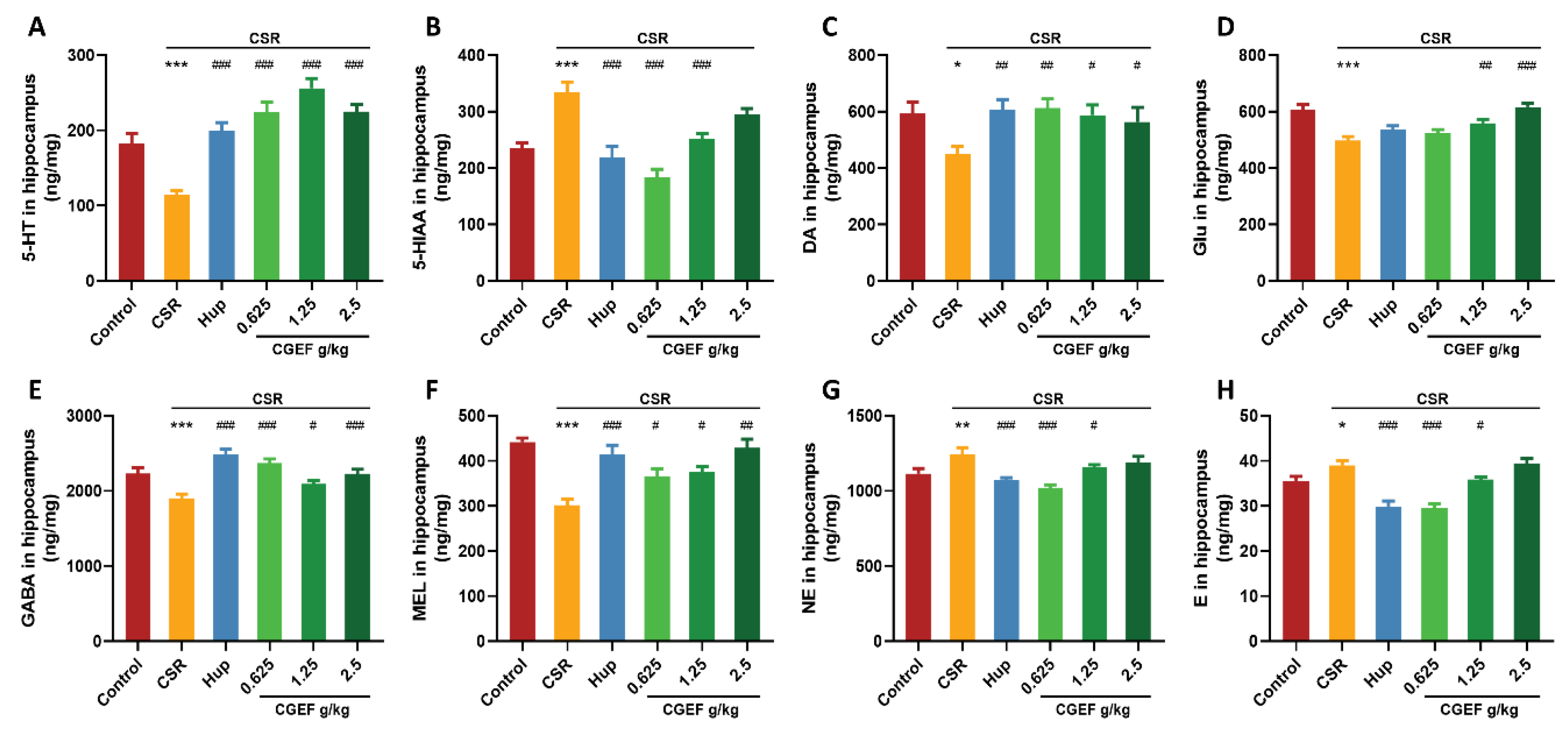
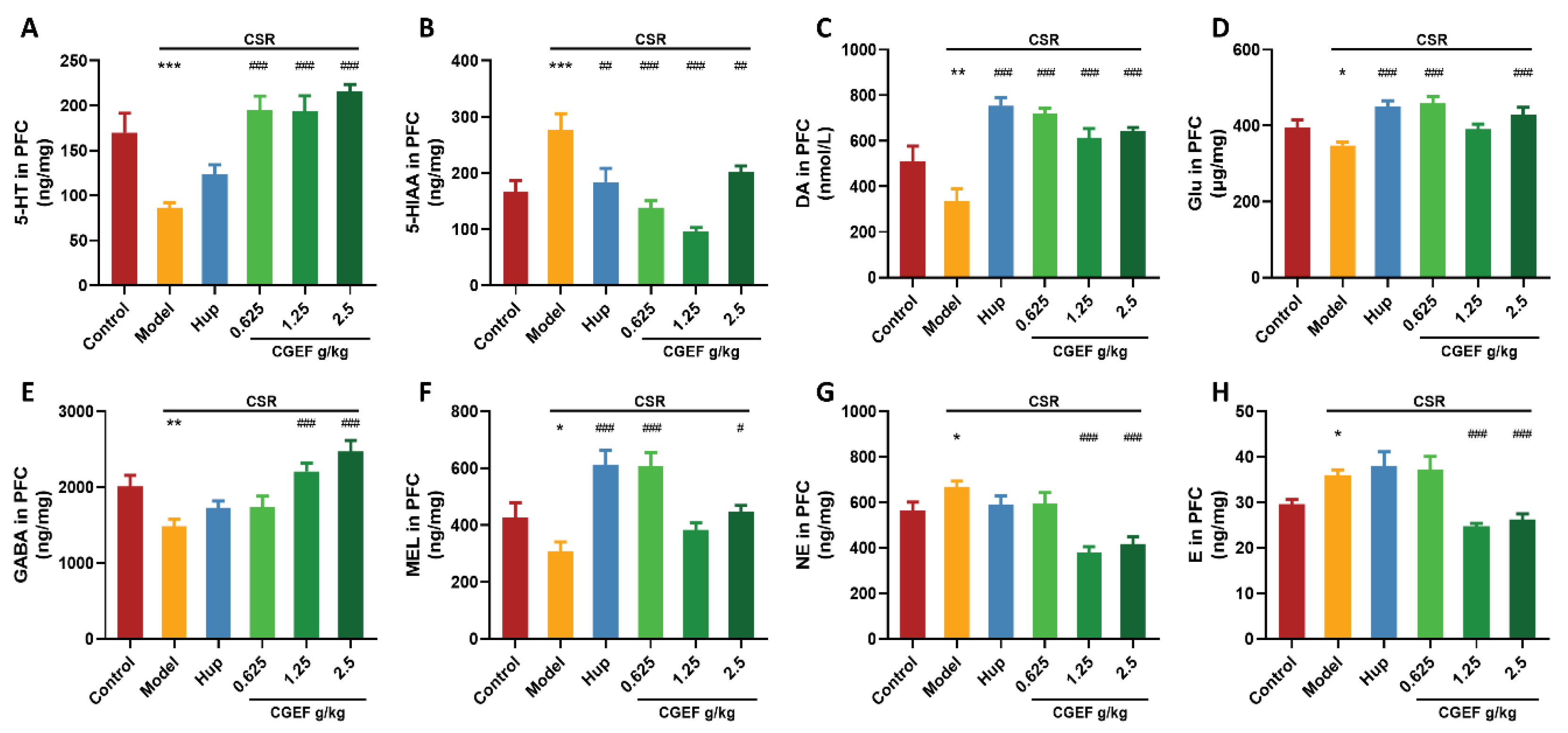
3.12. Effect of CGEF on Neurotransmitter Levels in the Hippocampus and PFC
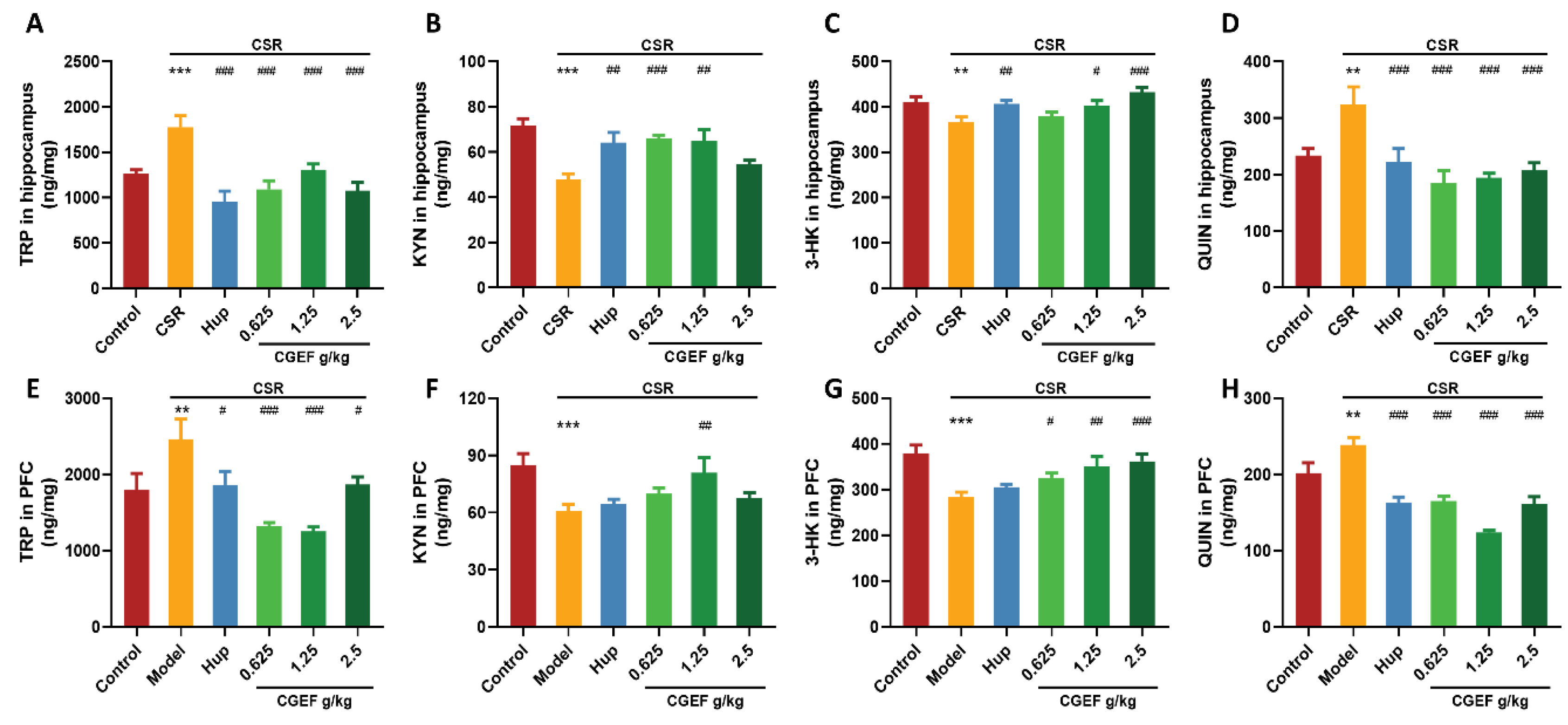
3.13. Effect of CSR and CGEF on Tryptophan Metabolism in the Hippocampus and Prefrontal Cortex
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-HK | 3-Hydroxykynurenine |
| 5-HIAA | 5-Hydroxyindoleacetic acid |
| 5-HT | 5-Hydroxytryptamine (Serotonin) |
| BCL2 | B-cell lymphoma 2 |
| BAX | BCL2-associated X protein |
| BDNF | Brain-derived neurotrophic factor |
| CREB | cAMP-responsive element-binding protein |
| CSR | Chronic sleep restriction |
| DA | Dopamine |
| E | Epinephrine |
| GABA | Gamma-aminobutyric acid |
| Glu | Glutamate |
| HO-1 | Heme oxygenase-1 |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| KYN | Kynurenine |
| MEL | Melatonin |
| MyD88 | Myeloid differentiation factor 88 |
| NE | Norepinephrine |
| NF-κB | Nuclear factor-kappa B |
| Nf2 | Nuclear factor erythroid 2-related factor 2 |
| NORT | Novel object recognition experiment |
| OLRT | Object location recognition experiment |
| OFT | Open Field Test |
| PAT | Passive Avoidance Test |
| PI3K | Phosphoinositide 3-kinase |
| PSD95 | Postsynaptic Density Protein-95 |
| AKT | Protein kinase B |
| GSK3β | Glycogen synthase kinase 3 beta |
| QUIN | Quinolinic acid |
| SYN | Synaptophysin |
| TrkB | Tropomyosin receptor kinase B |
| TLR2 | Toll-like receptor 2 |
| TNFα | Tumor Necrosis Factor alpha |
| TRP | Tryptophan |
References
- Dzierzewski, J.M.; Sabet, S.M.; Ghose, S.M.; Perez, E.; Soto, P.; Ravyts, S.G.; Dautovich, N.D. Lifestyle Factors and Sleep Health across the Lifespan. Int. J. Environ. Res. Public Health 2021, 18, 6626. [Google Scholar] [CrossRef]
- Hetland, J.; Bakker, A.B.; Espevik, R.; Olsen, O.K. Daily work pressure and task performance: The moderating role of recovery and sleep. Front. Psychol. 2022, 13, 857318. [Google Scholar] [CrossRef]
- Fjell, A.M.; Walhovd, K.B. Individual sleep need is flexible and dynamically related to cognitive function. Nat. Hum. Behav. 2024, 8, 422–430. [Google Scholar] [PubMed]
- Leng, Y.; Knutson, K.; Carnethon, M.R.; Yaffe, K. Association Between Sleep Quantity and Quality in Early Adulthood with Cognitive Function in Midlife. Neurology 2024, 102, e208056. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.C.; Aung, T. Sleep deprivation and its association with diseases—A review. Sleep Med. 2021, 77, 192–204. [Google Scholar] [PubMed]
- Ye, Z.; Lai, H.; Ning, J.; Liu, J.; Huang, J.; Yang, S.; Jin, J.; Liu, Y.; Liu, J.; Zhao, H.; et al. Traditional Chinese medicine for insomnia: Recommendation mapping of the global clinical guidelines. J. Ethnopharmacol. 2024, 322, 117601. [Google Scholar]
- Esmaeili, M.; Ajami, M.; Barati, M.; Javanmardi, F.; Houshiarrad, A.; Mousavi Khaneghah, A. The significance and potential of functional food ingredients for control appetite and food intake. Food Sci. Nutr. 2022, 10, 1602–1612. [Google Scholar] [CrossRef]
- Hasnieza Mohd Rosli, N.; Mastura Yahya, H.; Shahar, S.; Wahida Ibrahim, F.; Fadilah Rajab, N. Alzheimer’s Disease and Functional Foods: An Insight on Neuroprotective Effect of its Combination. Pak. J. Biol. Sci. 2020, 23, 575–589. [Google Scholar]
- Topolska, K.; Florkiewicz, A.; Filipiak-Florkiewicz, A. Functional Food-Consumer Motivations and Expectations. Int. J. Environ. Res. Public Health 2021, 18, 5327. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, A.; Sharma, S.; Kant, A.; Sevda, S.; Taherzadeh, M.J.; Garlapati, V.K. Functional foods as a formulation ingredients in beverages: Technological advancements and constraints. Bioengineered 2021, 12, 11055–11075. [Google Scholar]
- Jo, K.; Jeon, S.; Ahn, C.-W.; Han, S.H.; Suh, H.J. Changes in Drosophila melanogaster Sleep-Wake Behavior Due to Lotus (Nelumbo nucifera) Seed and Hwang Jeong (Polygonatum sibiricum) Extracts. Prev. Nutr. Food Sci. 2017, 22, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, H.; Yao, C.; Sun, X.; He, Q.; Choudharyc, M.I.; Chen, S.; Liu, X.; Jiang, N. Fresh Gastrodia elata Blume alleviates simulated weightlessness-induced cognitive impairment by regulating inflammatory and apoptosis-related pathways. Front. Pharmacol. 2023, 14, 1173920. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Ban, W.; Yang, Z. Gastrodin, a Promising Natural Small Molecule for the Treatment of Central Nervous System Disorders, and Its Recent Progress in Synthesis, Pharmacology and Pharmacokinetics. Int. J. Mol. Sci. 2024, 25, 9540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ni, L.; Hu, S.; Yue, B.; Chen, X.; Yuan, D.; Wang, T.; Zhou, Z. Polygonatum sibiricum ameliorated cognitive impairment of naturally aging rats through BDNF-TrkB signaling pathway. J. Food Biochem. 2022, 46, e14510. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, X.; Dai, H.; Luo, S.; Zhang, X. Polygonatum sibiricum polysaccharide regulation of gut microbiota: A viable approach to alleviate cognitive impairment. Int. J. Biol. Macromol. 2024, 277 Pt 3, 134494. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Deng, J.; Gao, S.; Qiu, J.; He, J.; Yang, T.; Tan, N.; Cheng, S.; Song, Z. Research on the anti-oxidant and anti-aging effects of Polygonatum kingianum saponins in Caenorhabditis elegans. Heliyon 2024, 10, e35556. [Google Scholar] [CrossRef]
- Lv, Q.; Di, X.; Bian, B.; Li, K.; Guo, J. Neuroprotective Effects of Poria cocos (Agaricomycetes) Essential Oil on Aβ1-40-Induced Learning and Memory Deficit in Rats. Int. J. Med. Mushrooms 2022, 24, 73–82. [Google Scholar] [CrossRef]
- Liu, X.; Yu, X.; Xu, X.; Zhang, X.; Zhang, X. The protective effects of Poria cocos-derived polysaccharide CMP33 against IBD in mice and its molecular mechanism. Food Funct. 2018, 9, 5936–5949. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, H.; Wang, S.; Xiang, Z.; Kong, H.; Xue, Q.; He, M.; Yu, X.; Li, Y.; Sun, D.; et al. A review on the advances in the extraction methods and structure elucidation of Poria cocos polysaccharide and its pharmacological activities and drug carrier applications. Int. J. Biol. Macromol. 2022, 217, 536–551. [Google Scholar] [CrossRef]
- Yongyan, Y. A Brief History of the Development of Modern Traditional Chinese Ophthalmology (1840–1949). Chin. J. Tradit. Chin. Ophthalmol. 1997, 7, 47–48. [Google Scholar]
- Lu, C.; Wei, Z.; Jiang, N.; Chen, Y.; Wang, Y.; Li, S.; Wang, Q.; Fan, B.; Liu, X.; Wang, F. Soy isoflavones protects against cognitive deficits induced by chronic sleep deprivation via alleviating oxidative stress and suppressing neuroinflammation. Phytother. Res. 2022, 36, 2072–2080. [Google Scholar]
- Lv, J.; Lu, C.; Jiang, N.; Wang, H.; Huang, H.; Chen, Y.; Li, Y.; Liu, X. Protective effect of ginsenoside Rh2 on scopolamine-induced memory deficits through regulation of cholinergic transmission, oxidative stress and the ERK-CREB-BDNF signaling pathway. Phytother. Res. 2021, 35, 337–345. [Google Scholar] [PubMed]
- Wang, L.-S.; Zhang, M.-D.; Tao, X.; Zhou, Y.-F.; Liu, X.-M.; Pan, R.-L.; Liao, Y.-H.; Chang, Q. LC-MS/MS-based quantification of tryptophan metabolites and neurotransmitters in the serum and brain of mice. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1112, 24–32. [Google Scholar] [PubMed]
- Deurveilher, S.; Bush, J.E.; Rusak, B.; Eskes, G.A.; Semba, K. Psychomotor vigilance task performance during and following chronic sleep restriction in rats. Sleep 2015, 38, 515–528. [Google Scholar]
- Simpson, S.M.; Hickey, A.J.; Baker, G.B.; Reynolds, J.N.; Beninger, R.J. The antidepressant phenelzine enhances memory in the double Y-maze and increases GABA levels in the hippocampus and frontal cortex of rats. Pharmacol. Biochem. Behav. 2012, 102, 109–117. [Google Scholar]
- Wang, H.; Lv, J.; Jiang, N.; Huang, H.; Wang, Q.; Liu, X. Ginsenoside Re protects against chronic restraint stress-induced cognitive deficits through regulation of NLRP3 and Nrf2 pathways in mice. Phytother. Res. 2021, 35, 2523–2535. [Google Scholar] [PubMed]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar]
- Jabbarpour, Z.; Shahidi, S.; Saidijam, M.; Sarihi, A.; Hassanzadeh, T.; Esmaeili, R. Effect of tempol on the passive avoidance and novel object recognition task in diabetic rats. Brain Res. Bull. 2014, 101, 51–56. [Google Scholar]
- Tobaldini, E.; Costantino, G.; Solbiati, M.; Cogliati, C.; Kara, T.; Nobili, L.; Montano, N. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci. Biobehav. Rev. 2017, 74 Pt B, 321–329. [Google Scholar]
- Song, L.; Li, H.; Fu, X.; Cen, M.; Wu, J. Association of the Oxidative Balance Score and Cognitive Function and the Mediating Role of Oxidative Stress: Evidence from the National Health and Nutrition Examination Survey (NHANES) 2011–2014. J. Nutr. 2023, 153, 1974–1983. [Google Scholar]
- Alzoubi, K.H.; Khabour, O.F.; Rashid, B.A.; Damaj, I.M.; Salah, H.A. The neuroprotective effect of vitamin E on chronic sleep deprivation-induced memory impairment: The role of oxidative stress. Behav. Brain Res. 2012, 226, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-Z.; Liu, K.; Wu, X.-M.; Shi, C.-N.; He, Q.-L.; Wu, H.-P.; Yang, J.-J.; Yao, H.; Ji, M.-H. Oxidative Stress-mediated Loss of Hippocampal Parvalbumin Interneurons Contributes to Memory Precision Decline After Acute Sleep Deprivation. Mol. Neurobiol. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, L.; Bonfili, L.; de Vivo, L.; Eleuteri, A.M.; Bellesi, M. Probiotics Supplementation Attenuates Inflammation and Oxidative Stress Induced by Chronic Sleep Restriction. Nutrients 2023, 15, 1518. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Aspects Med. 2011, 32, 234–246. [Google Scholar] [CrossRef]
- Jayasinghe, A.M.K.; Kirindage, K.G.I.S.; Fernando, I.P.S.; Han, E.J.; Oh, G.-W.; Jung, W.-K.; Ahn, G. Fucoidan Isolated from Sargassum confusum Suppresses Inflammatory Responses and Oxidative Stress in TNF-α/IFN-γ-Stimulated HaCaT Keratinocytes by Activating Nrf2/HO-1 Signaling Pathway. Mar. Drugs 2022, 20, 117. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, L.; Chen, Y.; Li, W.; Liu, Y.; Yi, F.; Zhou, Y.; Tan, H. Butylphthalide alleviates sleep deprivation-induced cognitive deficit by regulating Nrf2/HO-1 pathway. Sleep Med. 2022, 100, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Nakano-Kobayashi, A.; Canela, A.; Yoshihara, T.; Hagiwara, M. Astrocyte-targeting therapy rescues cognitive impairment caused by neuroinflammation via the Nrf2 pathway. Proc. Natl. Acad. Sci. USA 2023, 120, e2303809120. [Google Scholar] [CrossRef]
- Fullagar, H.H.K.; Skorski, S.; Duffield, R.; Hammes, D.; Coutts, A.J.; Meyer, T. Sleep and athletic performance: The effects of sleep loss on exercise performance, and physiological and cognitive responses to exercise. Sports Med. 2015, 45, 161–186. [Google Scholar] [CrossRef] [PubMed]
- Kincheski, G.C.; Valentim, I.S.; Clarke, J.R.; Cozachenco, D.; Castelo-Branco, M.T.L.; Ramos-Lobo, A.M.; Rumjanek, V.M.B.D.; Donato, J.; De Felice, F.G.; Ferreira, S.T. Chronic sleep restriction promotes brain inflammation and synapse loss, and potentiates memory impairment induced by amyloid-β oligomers in mice. Brain Behav. Immun. 2017, 64, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Shan, W.; Zheng, Y.; Pan, L.; Zuo, Z. Toll-like receptor 2 activation and up-regulation by high mobility group box-1 contribute to post-operative neuroinflammation and cognitive dysfunction in mice. J. Neurochem. 2021, 158, 328–341. [Google Scholar] [CrossRef]
- Yueh, M.-F.; Chen, S.; Nguyen, N.; Tukey, R.H. Developmental onset of bilirubin-induced neurotoxicity involves Toll-like receptor 2-dependent signaling in humanized UDP-glucuronosyltransferase1 mice. J. Biol. Chem. 2014, 289, 4699–4709. [Google Scholar] [CrossRef]
- Dutta, D.; Jana, M.; Majumder, M.; Mondal, S.; Roy, A.; Pahan, K. Selective targeting of the TLR2/MyD88/NF-κB pathway reduces α-synuclein spreading in vitro and in vivo. Nat. Commun. 2021, 12, 5382. [Google Scholar]
- Napetschnig, J.; Wu, H. Molecular basis of NF-κB signaling. Annu. Rev. Biophys. 2013, 42, 443–468. [Google Scholar] [CrossRef] [PubMed]
- Bakunina, N.; Pariante, C.M.; Zunszain, P.A. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 2015, 144, 365–373. [Google Scholar] [PubMed]
- Xie, G.; Huang, X.; Li, H.; Wang, P.; Huang, P. Caffeine-related effects on cognitive performance: Roles of apoptosis in rat hippocampus following sleep deprivation. Biochem. Biophys. Res. Commun. 2021, 534, 632–638. [Google Scholar] [PubMed]
- Krajewska, M.; You, Z.; Rong, J.; Kress, C.; Huang, X.; Yang, J.; Kyoda, T.; Leyva, R.; Banares, S.; Hu, Y.; et al. Neuronal deletion of caspase 8 protects against brain injury in mouse models of controlled cortical impact and kainic acid-induced excitotoxicity. PLoS ONE 2011, 6, e24341. [Google Scholar]
- Chen, P.; Li, X.; Zhang, Y.; Wang, H.; Yu, Y.; Wu, C.; Jia, L.; Zhang, J. Oudemansiella radicata polysaccharides alleviated LPS-induced liver damage via regulating TLR4/NF-κB and Bax/Bcl-2 signaling pathways. Int. J. Biol. Macromol. 2024, 282, 137370. [Google Scholar]
- Su, W.Y.; Tian, L.Y.; Guo, L.P.; Huang, L.Q.; Gao, W.Y. PI3K signaling-regulated metabolic reprogramming: From mechanism to application. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188952. [Google Scholar]
- Vaidya, R.J.; Ray, R.M.; Johnson, L.R. Akt-mediated GSK-3beta inhibition prevents migration of polyamine-depleted intestinal epithelial cells via Rac1. Cell. Mol. Life Sci. 2006, 63, 2871–2879. [Google Scholar]
- Li, Z.-H.; Cheng, L.; Wen, C.; Ding, L.; You, Q.-Y.; Zhang, S.-B. Activation of CNR1/PI3K/AKT Pathway by Tanshinone IIA Protects Hippocampal Neurons and Ameliorates Sleep Deprivation-Induced Cognitive Dysfunction in Rats. Front. Pharmacol. 2022, 13, 823732. [Google Scholar]
- Nelson, C.D.; Kim, M.J.; Hsin, H.; Chen, Y.; Sheng, M. Phosphorylation of threonine-19 of PSD-95 by GSK-3β is required for PSD-95 mobilization and long-term depression. J. Neurosci. 2013, 33, 12122–12135. [Google Scholar]
- Jaworski, T.; Banach-Kasper, E.; Gralec, K. GSK-3β at the Intersection of Neuronal Plasticity and Neurodegeneration. Neural Plast. 2019, 2019, 4209475. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, L.; Kordes, S.; Reinhardt, P.; Glatza, M.; Baumann, M.; Drexler, H.C.A.; Menninger, S.; Zischinsky, G.; Eickhoff, J.; Fröb, C.; et al. Dual Inhibition of GSK3β and CDK5 Protects the Cytoskeleton of Neurons from Neuroinflammatory-Mediated Degeneration in vitro and in vivo. Stem Cell Rep. 2019, 12, 502–517. [Google Scholar]
- Grimes, C.A.; Jope, R.S. CREB DNA binding activity is inhibited by glycogen synthase kinase-3 beta and facilitated by lithium. J. Neurochem. 2001, 78, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Benito, E.; Barco, A. CREB’s control of intrinsic and synaptic plasticity: Implications for CREB-dependent memory models. Trends Neurosci. 2010, 33, 230–240. [Google Scholar]
- Wang, Y.; Liang, J.; Xu, B.; Yang, J.; Wu, Z.; Cheng, L. TrkB/BDNF signaling pathway and its small molecular agonists in CNS injury. Life Sci. 2024, 336, 122282. [Google Scholar] [CrossRef]
- Jin, W. Regulation of BDNF-TrkB Signaling and Potential Therapeutic Strategies for Parkinson’s Disease. J. Clin. Med. 2020, 9, 257. [Google Scholar] [CrossRef]
- Lv, X.; Li, D.; Ouyang, T.; Zhang, Z.; Liu, Z.; Li, Z.; Liu, M.; He, Y.; Zhong, Y.; Li, Y.; et al. Resolvin D1 combined with exercise rehabilitation alleviates neurological injury in mice with intracranial hemorrhage via the BDNF/TrkB/PI3K/AKT pathway. Sci. Rep. 2024, 14, 31447. [Google Scholar]
- Andreska, T.; Lüningschrör, P.; Sendtner, M. Regulation of TrkB cell surface expression-a mechanism for modulation of neuronal responsiveness to brain-derived neurotrophic factor. Cell Tissue Res. 2020, 382, 5–14. [Google Scholar]
- Siegel, J.M. The neurotransmitters of sleep. J. Clin. Psychiatry 2004, 65 (Suppl. S16), 4–7. [Google Scholar]
- Yang, Z.; Zou, Y.; Wang, L. Neurotransmitters in Prevention and Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 3841. [Google Scholar] [CrossRef]
- Wu, X.-R.; Zhu, X.-N.; Pan, Y.-B.; Gu, X.; Liu, X.-D.; Chen, S.; Zhang, Y.; Xu, T.-L.; Xu, N.-J.; Sun, S. Amygdala neuronal dyshomeostasis via 5-HT receptors mediates mood and cognitive defects in Alzheimer’s disease. Aging Cell 2024, 23, e14187. [Google Scholar] [PubMed]
- Borwick, C.; Lal, R.; Lim, L.W.; Stagg, C.J.; Aquili, L. Dopamine depletion effects on cognitive flexibility as modulated by tDCS of the dlPFC. Brain Stimul. 2020, 13, 105–108. [Google Scholar] [PubMed]
- Sood, A.; Preeti, K.; Fernandes, V.; Khatri, D.K.; Singh, S.B. Glia: A major player in glutamate-GABA dysregulation-mediated neurodegeneration. J. Neurosci. Res. 2021, 99, 3148–3189. [Google Scholar]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- da Silva Rocha-Lopes, J.; Machado, R.B.; Suchecki, D. Chronic REM Sleep Restriction in Juvenile Male Rats Induces Anxiety-Like Behavior and Alters Monoamine Systems in the Amygdala and Hippocampus. Mol. Neurobiol. 2018, 55, 2884–2896. [Google Scholar] [PubMed]
- Hirotsu, C.; Tufik, S.; Andersen, M.L. Interactions between sleep, stress, and metabolism: From physiological to pathological conditions. Sleep Sci. 2015, 8, 143–152. [Google Scholar]
- Maddison, D.C.; Giorgini, F. The kynurenine pathway and neurodegenerative disease. Semin. Cell Dev. Biol. 2015, 40, 134–141. [Google Scholar] [CrossRef]
- Fu, X.; Yan, S.; Hu, Z.; Sheng, W.; Li, W.; Kuang, S.; Feng, X.; Liu, L.; Zhang, W.; He, Q. Guhan Yangsheng Jing mitigates hippocampal neuronal pyroptotic injury and manifies learning and memory capabilities in sleep deprived mice via the NLRP3/Caspase1/GSDMD signaling pathway. J. Ethnopharmacol. 2024, 326, 117972. [Google Scholar] [CrossRef]
- Bhat, A.; Pires, A.S.; Tan, V.; Babu Chidambaram, S.; Guillemin, G.J. Effects of Sleep Deprivation on the Tryptophan Metabolism. Int. J. Tryptophan Res. 2020, 13, 1178646920970902. [Google Scholar]
- Hestad, K.; Alexander, J.; Rootwelt, H.; Aaseth, J.O. The Role of Tryptophan Dysmetabolism and Quinolinic Acid in Depressive and Neurodegenerative Diseases. Biomolecules 2022, 12, 998. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, F.; Li, X.; Xu, Y.; Liu, X.; Barkat, M.Q.; Choudhary, M.I.; Chang, Q.; Jiang, N. Gastrodia elata, Polygonatum sibiricum, and Poria cocos as a Functional Food Formula: Cognitive Enhancement via Modulation of Hippocampal Neuroinflammation and Neuroprotection in Sleep-Restricted Mice. Foods 2025, 14, 1103. https://doi.org/10.3390/foods14071103
Zhang Y, Chen F, Li X, Xu Y, Liu X, Barkat MQ, Choudhary MI, Chang Q, Jiang N. Gastrodia elata, Polygonatum sibiricum, and Poria cocos as a Functional Food Formula: Cognitive Enhancement via Modulation of Hippocampal Neuroinflammation and Neuroprotection in Sleep-Restricted Mice. Foods. 2025; 14(7):1103. https://doi.org/10.3390/foods14071103
Chicago/Turabian StyleZhang, Yiwen, Fang Chen, Xueyan Li, Yanfei Xu, Xinmin Liu, Muhammad Qasim Barkat, Muhammad Iqbal Choudhary, Qi Chang, and Ning Jiang. 2025. "Gastrodia elata, Polygonatum sibiricum, and Poria cocos as a Functional Food Formula: Cognitive Enhancement via Modulation of Hippocampal Neuroinflammation and Neuroprotection in Sleep-Restricted Mice" Foods 14, no. 7: 1103. https://doi.org/10.3390/foods14071103
APA StyleZhang, Y., Chen, F., Li, X., Xu, Y., Liu, X., Barkat, M. Q., Choudhary, M. I., Chang, Q., & Jiang, N. (2025). Gastrodia elata, Polygonatum sibiricum, and Poria cocos as a Functional Food Formula: Cognitive Enhancement via Modulation of Hippocampal Neuroinflammation and Neuroprotection in Sleep-Restricted Mice. Foods, 14(7), 1103. https://doi.org/10.3390/foods14071103






