Changes of Barley Bound Phenolics and Their Characteristics During Simulated Gastrointestinal Digestion and Colonic Fermentation In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction of Barley Bound Phenolics
2.3. Preparation of Hydrolyzed Bound Phenolics and Bound Phenolics-Removed Barley (RBBPs)
2.4. In Vitro Rat Simulated Gastrointestinal Digestion
2.5. In Vitro Colonic Fermentation
2.6. Determination of the Composition and Content of Phenolic Compounds
2.7. Scanning Electron Microscopy (SEM)
2.8. Confocal Laser Scanning Microscopy (CLSM)
2.9. Determination of pH and SCFAs
2.10. Gut Microbiota Analysis
2.11. Statistical Analysis
3. Results and Discussions
3.1. Quantitative Analysis of Digested and Fermented BBPs
3.2. The Microstructural Changes in BBPs During Different Digestion and Colonic Fermentation Stages
3.3. The Changes in the Fluorescence Abundance of Bound Phenolics During Different Digestion and Colonic Fermentation Stages
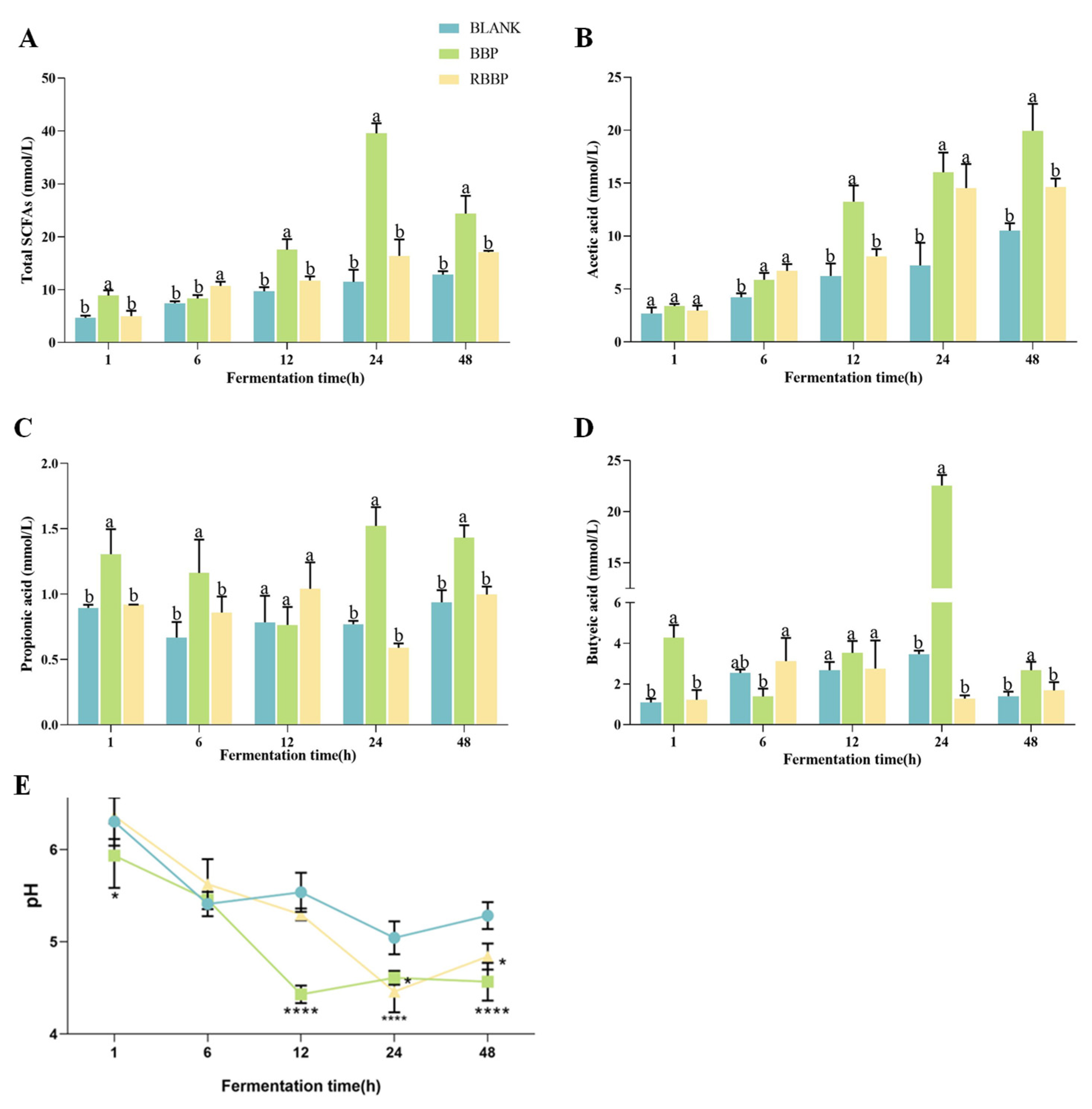
3.4. Effects of BBPs and RBBPs on SCFAs During Fermentation
3.5. Effects of BBPs and RBBPs on pH During Fermentation
3.6. Effects of BBPs and RBBPs Fermentation on Gut Microbiota
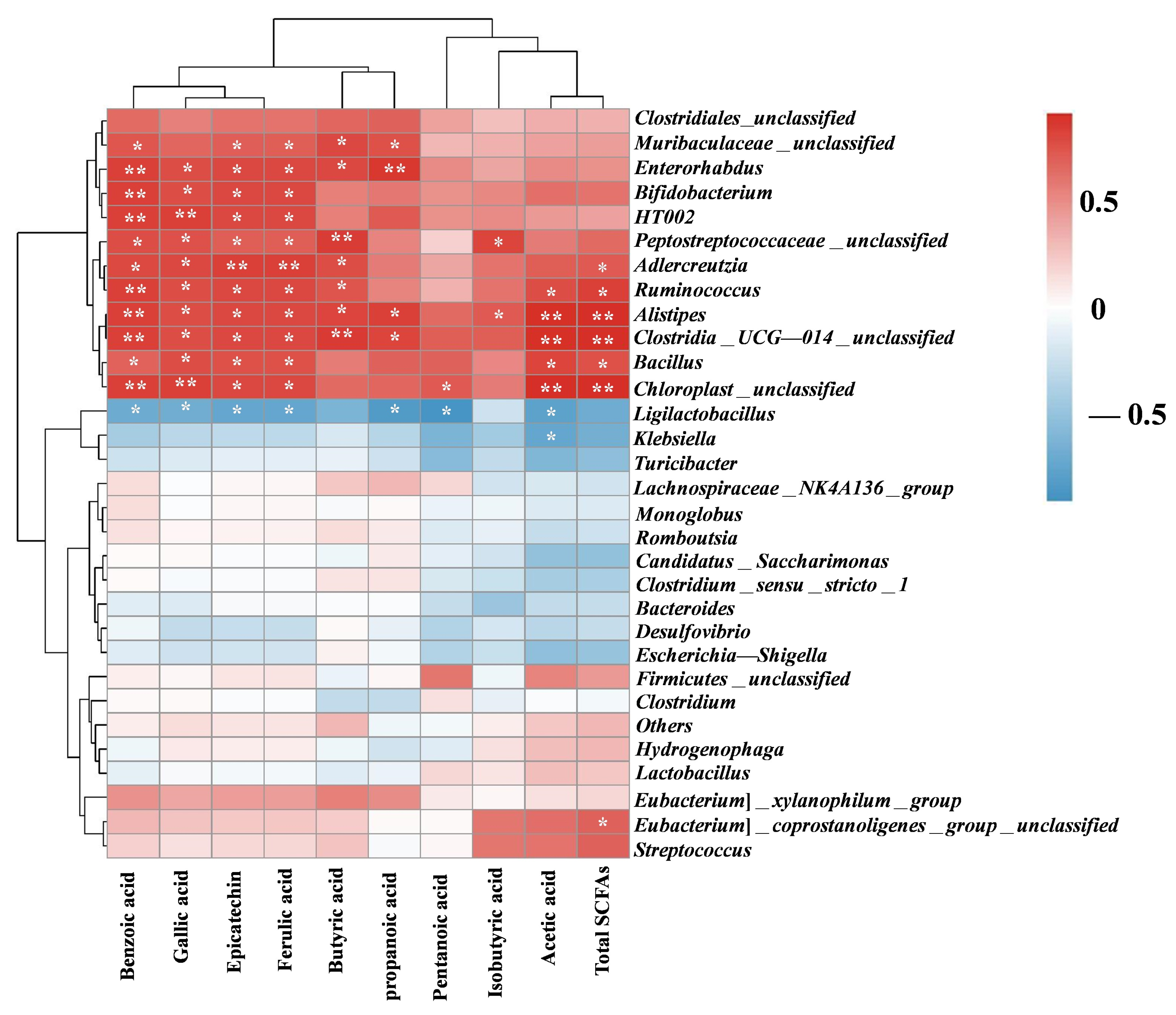
3.7. Correlation Between Phenolics SCFAs and Gut Microbiota
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Zhang, Y.; Li, H.; Deng, Z.; Tsao, R. A review on insoluble-bound phenolics in plant-based food matrix and their contribution to human health with future perspectives. Trends Food Sci. Technol. 2020, 105, 347–362. [Google Scholar] [CrossRef]
- Yan, Z.; Luo, X.; Cong, J.; Zhang, H.; Ma, H.; Duan, Y. Subcritical water extraction, identification and antiproliferation ability on HepG2 of polyphenols from lotus seed epicarp. Ind. Crops Prod. 2019, 129, 472–479. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Wang, T.; Cai, M.; Qian, F.; Sun, Y.; Wang, Y. Green tea polyphenols prevent lipopolysaccharide-induced inflammatory liver injury in mice by inhibiting NLRP3 inflammasome activation. Food Funct. 2019, 10, 3898–3908. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Qu, C.; Bai, J.; Zhao, Y.; Xiao, X. Fermented red rice improved the antioxidant activity, bioaccessibility of polyphenols, and lipid-lowering activity in C. elegans. Food Bioeng. 2024, 3, 160–171. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.; Zhao, Y.; Zhu, Y.; Bai, J.; Fan, S.; Zhu, L.; Song, C.; Xiao, X. Recent Developments in Fermented Cereals on Nutritional Constituents and Potential Health Benefits. Foods 2022, 11, 2243. [Google Scholar] [CrossRef]
- Krawczyk, M.; Burzynska-Pedziwiatr, I.; Wozniak, L.A.; Bukowiecka-Matusiak, M. Impact of Polyphenols on Inflammatory and Oxidative Stress Factors in Diabetes Mellitus: Nutritional Antioxidants and Their Application in Improving Antidiabetic Therapy. Biomolecules 2023, 13, 1402. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Li, S.Y.; Ge, S.H.; Lin, S.L. Review of Distribution, Extraction Methods, and Health Benefits of Bound Phenolics in Food Plants. J. Agric. Food Chem. 2020, 68, 3330–3343. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Ma, H. Effects of pretreatment and type of hydrolysis on the composition, antioxidant potential and HepG2 cytotoxicity of bound polyphenols from Tartary buckwheat (Fagopyrum tataricum L. Gaerth) hulls. Food Res. Int. 2021, 142, 110187. [Google Scholar] [CrossRef]
- Liang, J.J.; Li, H.C.; Han, M.Z.; Gao, Z.P. Polysaccharide-polyphenol interactions: A comprehensive review from food processing to digestion and metabolism. Crit. Rev. Food Sci. 2024, 2368055. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Perez-Perez, L.M.; Huerta-Ocampo, J.Á.; Ruiz-Cruz, S.; Cinco-Moroyoqui, F.J.; Wong-Corral, F.J.; Rascón-Valenzuela, L.A.; Robles-García, M.A.; González-Vega, R.I.; Rosas-Burgos, E.C.; Corella-Madueño, M.A.G.; et al. Evaluation of Quality, Antioxidant Capacity, and Digestibility of Chickpea (Cicer arietinum L. cv Blanoro) Stored under N2 and CO2 Atmospheres. Molecules 2021, 26, 2773. [Google Scholar] [CrossRef]
- Zhang, H.; Troise, A.D.; Qi, Y.; Wu, G.; Zhang, H.; Fogliano, V. Insoluble dietary fibre scavenges reactive carbonyl species under simulated physiological conditions: The key role of fibre-bound polyphenols. Food Chem. 2021, 349, 129018. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Li, X.D.; Bai, J.; Fan, S.T.; Daglia, M.; Li, J.Y.; Ding, Y.W.; Zhang, Y.S.; Zhao, Y.S. Changes in the structural, physicochemical and functional properties and in vitro fecal fermentation characteristics of barley dietary fiber fermented by Lactiplantibacillus plantarum dy-1. Food Funct. 2024, 15, 4276–4291. [Google Scholar] [CrossRef]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wen, T.; Fang, T.; Lao, H.; Zhou, X.; Wei, T.; Luo, Y.; Xie, C.; Huang, Z.; Li, K. A comparative evaluation of the composition and antioxidant activity of free and bound polyphenols in sugarcane tips. Food Chem. 2025, 463, 141510. [Google Scholar] [CrossRef]
- Zhu, Y.; Ba, K.; Li, X.D.; He, Y.F.; Zhang, Y.S.; Ai, L.Z.; Zhang, J.Y.; Zhao, Y.S.; Xiao, X. Comparative analysis of barley dietary fiber fermented with and without Lactiplantibacillus plantarum dy-1 in promoting gut health and regulating hepatic energy metabolism in high-fat diet-induced obese mice. Food Funct. 2025, 16, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Deng, H.; Bai, J.; Zhou, X.H.; Zhao, Y.S.; Zhu, Y.; McClements, D.J.; Xiao, X.; Sun, Q.C. Health-promoting properties of barley: A review of nutrient and nutraceutical composition, functionality, bioprocessing, and health benefits. Crit. Rev. Food Sci. Nutr. 2023, 63, 1155–1169. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Tong, X.M.; Wu, X.M.; Bai, J.; Fan, S.T.; Zhu, Y.; Zhang, J.Y.; Xiao, X. Metabolomics Reveal the Regulatory Effect of Polysaccharides from Fermented Barley Bran Extract on Lipid Accumulation in HepG2 Cells. Metabolites 2023, 13, 223. [Google Scholar] [CrossRef]
- Gade, A.; Kumar, M.S. Gut microbial metabolites of dietary polyphenols and their potential role in human health and diseases. J. Physiol. Biochem. 2023, 79, 695–718. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Currenti, W.; Micek, A.; Falzone, L.; Libra, M.; Giampieri, F.; Forbes-Hernandez, T.Y.; Quiles, J.L.; Battino, M.; et al. The Effect of Dietary Polyphenols on Vascular Health and Hypertension: Current Evidence and Mechanisms of Action. Nutrients 2022, 14, 545. [Google Scholar] [CrossRef]
- Wang, T.; He, F.; Chen, G. Improving bioaccessibility and bioavailability of phenolic compounds in cereal grains through processing technologies: A concise review. J. Funct. Foods 2014, 7, 101–111. [Google Scholar] [CrossRef]
- Hou, C.; Zhao, L.; Ji, M.; Yu, J.; Di, Y.; Liu, Q.; Zhang, Z.; Sun, L.; Liu, X.; Wang, Y. Liberated bioactive bound phenolics during in vitro gastrointestinal digestion and colonic fermentation boost the prebiotic effects of triticale insoluble dietary fiber. Food Chem. 2024, 457, 140124. [Google Scholar] [CrossRef]
- Pan, R.; Yuan, J.; Bai, J.; Zhang, J.; Zhang, J.; Gu, Y.; Xia, S.; Qu, M.; Liu, Q.; Dong, Y.; et al. Antiobesity Effect of Lactiplantibacillus plantarum Fermented Barley Extracts via the Interactions with Gut Microbiota of the Obese Adult Humans. J. Food Biochem. 2023, 2023, 5521789. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, Y.; Chen, Q.; Fu, X.; Huang, Q.; Bin, Z.; Dong, H.; Li, C. Exploring the synergistic benefits of insoluble dietary fiber and bound phenolics: Unveiling the role of bound phenolics in enhancing bioactivities of insoluble dietary fiber. Trends Food Sci. Technol. 2024, 149, 104554. [Google Scholar] [CrossRef]
- Cheng, Z.; Wu, B.; Bai, J.; Fan, S.; Daglia, M.; Li, J.; Zhao, Y.; He, Y.; Zhu, L.; Xiao, X. Heterologous expression and enzymatic characteristics of sulfatase from Lactobacillus plantarum dy-1. Food Funct. 2024, 15, 5439–5449. [Google Scholar] [CrossRef]
- Xue, H.; Sang, Y.; Gao, Y.; Zeng, Y.; Liao, J.; Tan, J. Research Progress on Absorption, Metabolism, and Biological Activities of Anthocyanins in Berries: A Review. Antioxidants 2022, 12, 3. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Regolo, L.; Alvarez-Suarez, J.M.; Navarro-Hortal, M.D.; Xiao, J.B.; Quiles, J.L.; Battino, M.; Giampieri, F. The reciprocal interaction between polyphenols and other dietary compounds: Impact on bioavailability, antioxidant capacity and other physico-chemical and nutritional parameters. Food Chem. 2022, 375, 131904. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Dong, L.; Jia, X.; Liu, L.; Ma, Y.; Huang, F.; Zhang, R. Phytochemical Profile, Bioactivity, and Prebiotic Potential of Bound Phenolics Released from Rice Bran Dietary Fiber during in Vitro Gastrointestinal Digestion and Colonic Fermentation. J. Agric. Food Chem. 2019, 67, 12796–12805. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, S.; Xie, J.; Chen, Y.; Dong, R.; Zhang, X.; Liu, S.; Xie, J.; Hu, X.; Yu, Q. Antioxidant, α-amylase and α-glucosidase inhibitory activities of bound polyphenols extracted from mung bean skin dietary fiber. LWT 2020, 132, 109943. [Google Scholar] [CrossRef]
- Jia, W.; Dong, X.Y.; Shi, L.; Chu, X.G. Discrimination of Milk from Different Animal Species by a Foodomics Approach Based on High-Resolution Mass Spectrometry. J. Agric. Food Chem. 2020, 68, 6638–6645. [Google Scholar] [CrossRef]
- Jin, X.; Ru, Y.; Zhang, X.; Kan, H.; Xiang, P.; He, X.; Sun, J.; He, X.; Wang, Z. The influence of in vitro gastrointestinal digestion and fecal fermentation on the flowers of Juglans regia: Changes in the active compounds and bioactivities. Front. Nutr. 2022, 9, 1014085. [Google Scholar] [CrossRef]
- Dong, R.; Liu, S.; Zheng, Y.; Zhang, X.; He, Z.; Wang, Z.; Wang, Y.; Xie, J.; Chen, Y.; Yu, Q. Release and metabolism of bound polyphenols from carrot dietary fiber and their potential activity in vitro digestion and colonic fermentation. Food Funct. 2020, 11, 6652–6665. [Google Scholar] [CrossRef]
- Swallah, M.S.; Fu, H.; Sun, H.; Affoh, R.; Yu, H. The Impact of Polyphenol on General Nutrient Metabolism in the Monogastric Gastrointestinal Tract. J. Food Qual. 2020, 2020, 5952834. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, T.; Zhang, Y.; Chen, Y.; Ge, X.; Sui, W.; Zhu, Q.; Geng, J.; Zhang, M. Release of bound polyphenols from wheat bran soluble dietary fiber during simulated gastrointestinal digestion and colonic fermentation in vitro. Food Chem. 2023, 402, 134111. [Google Scholar] [CrossRef]
- Garzón, A.G.; Van de Velde, F.; Drago, S.R. Gastrointestinal and colonic in vitro bioaccessibility of γ-aminobutiric acid (GABA) and phenolic compounds from novel fermented sorghum food. LWT 2020, 130, 109664. [Google Scholar] [CrossRef]
- Tamargo, A.; Cueva, C.; Alvarez, M.D.; Herranz, B.; Moreno-Arribas, M.V.; Laguna, L. Physical effects of dietary fibre on simulated luminal flow, studied by in vitro dynamic gastrointestinal digestion and fermentation. Food Funct. 2019, 10, 3452–3465. [Google Scholar] [CrossRef]
- Wei, X.; Wang, J.; Wang, Y.; Zhao, Y.; Long, Y.; Tan, B.; Li, Q.X.; Dong, Z.; Wan, X. Dietary fiber and polyphenols from whole grains: Effects on the gut and health improvements. Food Funct. 2024, 15, 4682–4702. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit. Rev. Food Sci. Nutr. 2017, 58, 2531–2548. [Google Scholar] [CrossRef]
- Xing, T.; Yu, S.; Zhen, F.; Kong, X.; Sun, Y. Anaerobic fermentation of hybrid Pennisetum mixed with fruit and vegetable wastes to produce volatile fatty acids. RSC Adv. 2020, 10, 33261–33267. [Google Scholar] [CrossRef]
- Qi, J.; Xia, C.; Zhang, Y.; Ding, R.; Zhang, Y.; Cao, W.; Duan, C.; Yao, Z.; Qin, H.; Ye, Y.; et al. Impact of high-fat diet on ovarian epigenetics: Insights from altered intestinal butyric acid levels. Heliyon 2024, 10, e33170. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Liu, W.; Yang, X.; Zhu, L.; Wu, G.; Zhang, H. Methylglyoxal scavenging capacity of fiber-bound polyphenols from highland barley during colonic fermentation and its modulation on methylglyoxal-interfered gut microbiota. Food Chem. 2024, 434, 137409. [Google Scholar] [CrossRef]
- Zahid, H.F.; Ali, A.; Legione, A.R.; Ranadheera, C.S.; Fang, Z.; Dunshea, F.R.; Ajlouni, S. Probiotic Yoghurt Enriched with Mango Peel Powder: Biotransformation of Phenolics and Modulation of Metabolomic Outputs after In Vitro Digestion and Colonic Fermentation. Int. J. Mol. Sci. 2023, 24, 8560. [Google Scholar] [CrossRef] [PubMed]
- Klostermann, C.E.; Endika, M.F.; Kouzounis, D.; Buwalda, P.L.; de Vos, P.; Zoetendal, E.G.; Bitter, J.H.; Schols, H.A. Presence of digestible starch impacts in vitro fermentation of resistant starch. Food Funct. 2024, 15, 223–235. [Google Scholar] [CrossRef]
- Roupar, D.; González, A.; Martins, J.T.; Gonçalves, D.A.; Teixeira, J.A.; Botelho, C.; Nobre, C. Modulation of Designed Gut Bacterial Communities by Prebiotics and the Impact of Their Metabolites on Intestinal Cells. Foods 2023, 12, 4216. [Google Scholar] [CrossRef] [PubMed]
- Marathe, S.J.; Snider, M.A.; Flores-Torres, A.S.; Dubin, P.J.; Samarasinghe, A.E. Human matters in asthma: Considering the microbiome in pulmonary health. Front. Pharmacol. 2022, 13, 1020133. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Zhao, S.; Ma, X.; Zhang, Y.; Zhang, M.; Zeng, Y.; Zhao, J.; Liu, Z.; Liu, H. Effects of dietary supplementation of gallic tanninc acid on growth, intestinal digestive enzymes activity, innate immunity, morphology, and microbial composition of Cyprinus carpio. Aquacult. Int. 2024, 32, 3815–3833. [Google Scholar] [CrossRef]
- Xie, J.; Sun, N.; Huang, H.; Xie, J.; Chen, Y.; Hu, X.; Hu, X.; Dong, R.; Yu, Q. Catabolism of polyphenols released from mung bean coat and its effects on gut microbiota during in vitro simulated digestion and colonic fermentation. Food Chem. 2022, 396, 133719. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, P.; Chen, S.; Xu, H.; Zhang, Y.; Chen, H.; Zhang, J.; Sun, H. Electroacupuncture and human iPSC-derived small extracellular vesicles regulate the gut microbiota in ischemic stroke via the brain-gut axis. Front. Immunol. 2023, 14, 1107559. [Google Scholar] [CrossRef]
- Feng, S.; Liu, Y.; Xu, J.; Fan, J.; Li, J.; Wu, Z.; Sun, Y.; Xiong, W. Three Strains of Lactobacillus Derived from Piglets Alleviated Intestinal Oxidative Stress Induced by Diquat through Extracellular Vesicles. Nutrients 2023, 15, 4198. [Google Scholar] [CrossRef]
- Nie, Q.; Sun, Y.; Li, M.; Zuo, S.; Chen, C.; Lin, Q.; Nie, S. Targeted modification of gut microbiota and related metabolites via dietary fiber. Carbohydr. Polym. 2023, 316, 120986. [Google Scholar] [CrossRef]
- Cao, M.; Wang, W.; Zhang, L.; Liu, G.; Zhou, X.; Li, B.; Shi, Y.; Zhu, Z.; Zhang, J. Epidemic and molecular characterization of fluoroquinolone-resistant Shigella dysenteriae 1 isolates from calves with diarrhea. BMC Microbiol. 2021, 21, 6. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, W.H.; Yang, F.W.; Cheng, Y.L.; Guo, Y.H.; Yao, W.R.; Zhao, Y.; Qian, H. The combination of microbiome and metabolome to analyze the cross-cooperation mechanism of Echinacea purpurea polysaccharide with the gut microbiota in vitro and in vivo. Food Funct. 2022, 13, 10069–10082. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Shen, G.X. Impact of anthocyanin component and metabolite of Saskatoon berry on gut microbiome and relationship with fecal short chain fatty acids in diet-induced insulin resistant mice. J. Nutr. Biochem. 2023, 111, 109201. [Google Scholar] [CrossRef]
- Walsh, C.; Owens, R.A.; Bottacini, F.; Lane, J.A.; van Sinderen, D.; Hickey, R.M. HMO-primed bifidobacteria exhibit enhanced ability to adhere to intestinal epithelial cells. Front. Microbiol. 2023, 14, 1232173. [Google Scholar] [CrossRef]

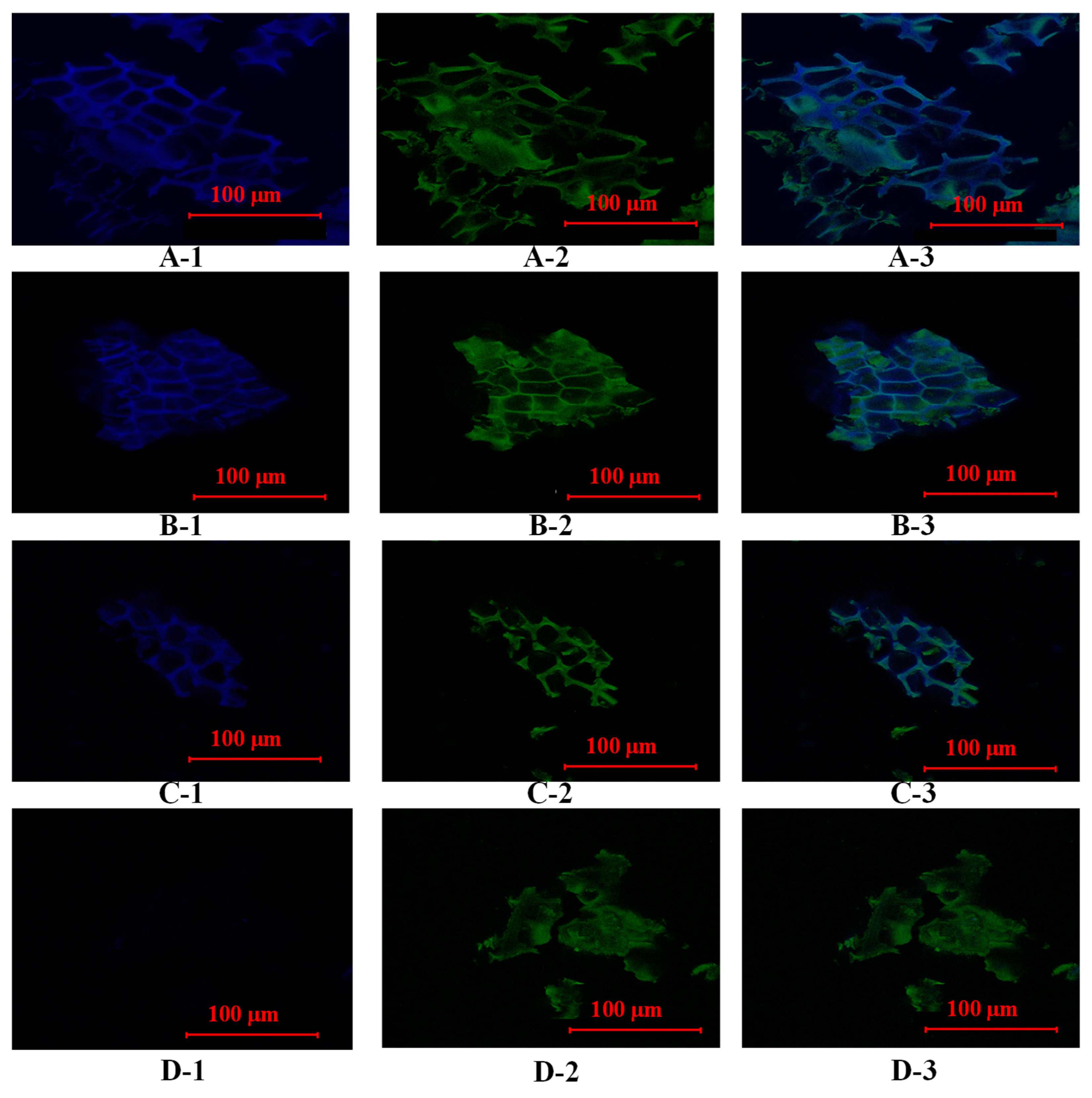


| Compounds | Undigested BBPs | Gastric Digestion | Intestinal Digestion |
|---|---|---|---|
| Caffeic acid | 372.00 ± 12.25 a | 0.32 ± 0.01 b | 0.41 ± 0.01 b |
| Catechin | 195.00 ± 7.35 a | 1.22 ± 0.01 b | 1.99 ± 0.01 b |
| Chlorogenic acid | 657.00 ± 22.05 a | 0.82 ± 0.02 b | 1.52 ± 0.02 b |
| Syringic acid | 2654.00 ± 89.41 a | 1.75 ± 0.02 b | 3.28 ± 0.07 b |
| 2,4-dihydroxybenzoic acid | 582.00 ± 19.6 a | 0.26 ± 0.01 b | 0.54 ± 0.01 b |
| Salicylic acid | 945.00 ± 31.84 a | 0.47 ± 0.01 b | 1.09 ± 0.02 b |
| Ferulic acid | 270.00 ± 9.8 a | 3.23 ± 0.01 c | 11.93 ± 0.16 b |
| Coumaric acid | 999.00 ± 34.29 a | 1.04 ± 0.01 c | 4.33 ± 0.05 b |
| Epicatechin | 384.00 ± 12.25 a | 1.79 ± 0.01 c | 8.19 ± 0.11 c |
| Protocatechuic acid | 192.00 ± 7.35 a | 0.10 ± 0.01 c | 0.48 ± 0.01 b |
| Gallic acid | 3524.00 ± 118.8 a | 2.61 ± 0.00 c | 13.01 ± 0.3 b |
| Compounds | Colonic Fermentation | ||||
|---|---|---|---|---|---|
| 1 h | 6 h | 12 h | 24 h | 48 h | |
| Caffeic acid | 0.58 ± 0.03 a | 0.30 ± 0.00 b | 0.29 ± 0.01 b | 0.22 ± 0.00 c | 0.13 ± 0.00 d |
| Catechin | 4.38 ± 0.09 a | 4.01 ± 0.12 b | 2.55 ± 0.04 c | 1.65 ± 0.03 d | 0.99 ± 0.03 e |
| Chlorogenic acid | 3.23 ± 0.04 a | 2.73 ± 0.05 b | 2.30 ± 0.05 c | 1.93 ± 0.06 d | 1.59 ± 0.05 e |
| Syringic acid | 7.95 ± 0.18 a | 3.43 ± 0.06 b | 2.83 ± 0.12 c | 1.70 ± 0.05 d | 0.90 ± 0.01 e |
| 2,4-dihydroxybenzoic acid | 1.51 ± 0.02 e | 2.64 ± 0.03 d | 3.98 ± 0.09 c | 5.48 ± 0.12 b | 10.36 ± 0.20 a |
| Salicylic acid | 3.49 ± 0.05 e | 7.41 ± 0.05 d | 7.94 ± 0.26 c | 9.98 ± 0.09 b | 12.65 ± 0.24 a |
| Ferulic acid | 38.58 ± 0.53 e | 54.65 ± 0.28 d | 74.64 ± 0.53 c | 98.13 ± 0.65 b | 154.06 ± 3.60 a |
| Coumaric acid | 12.55 ± 0.06 e | 15.44 ± 0.21 d | 19.2 ± 0.48 c | 27.93 ± 0.50 b | 47.3 ± 0.85 a |
| Epicatechin | 23.78 ± 0.67 d | 50.32 ± 1.73 c | 52.87 ± 0.69 c | 63.48 ± 0.75 b | 124.54 ± 3.85 a |
| Protocatechuic acid | 1.67 ± 0.04 d | 2.44 ± 0.07 c | 2.51 ± 0.10 c | 3.23 ± 0.00 b | 6.93 ± 0.16 a |
| Gallic acid | 45.32 ± 1.49 e | 72.46 ± 0.20 d | 88.51 ± 1.08 c | 110.03 ± 1.06 b | 193.98 ± 2.21 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Leng, F.; Fan, S.; Ding, Y.; Chen, T.; Zhou, H.; Xiao, X. Changes of Barley Bound Phenolics and Their Characteristics During Simulated Gastrointestinal Digestion and Colonic Fermentation In Vitro. Foods 2025, 14, 1114. https://doi.org/10.3390/foods14071114
Zhao Y, Leng F, Fan S, Ding Y, Chen T, Zhou H, Xiao X. Changes of Barley Bound Phenolics and Their Characteristics During Simulated Gastrointestinal Digestion and Colonic Fermentation In Vitro. Foods. 2025; 14(7):1114. https://doi.org/10.3390/foods14071114
Chicago/Turabian StyleZhao, Yansheng, Fei Leng, Songtao Fan, Yiwei Ding, Tong Chen, Hongbin Zhou, and Xiang Xiao. 2025. "Changes of Barley Bound Phenolics and Their Characteristics During Simulated Gastrointestinal Digestion and Colonic Fermentation In Vitro" Foods 14, no. 7: 1114. https://doi.org/10.3390/foods14071114
APA StyleZhao, Y., Leng, F., Fan, S., Ding, Y., Chen, T., Zhou, H., & Xiao, X. (2025). Changes of Barley Bound Phenolics and Their Characteristics During Simulated Gastrointestinal Digestion and Colonic Fermentation In Vitro. Foods, 14(7), 1114. https://doi.org/10.3390/foods14071114







