Physicochemical, Functional, and In Vitro Fermentation Characteristics of Buckwheat Bran Dietary Fiber Modified by Enzymatic Extrusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Enzymatic Extrusion of BB
2.3. Preparation of BBDF
2.4. Chemical Composition
2.5. Particle Size
2.6. Water Holding Capacity (WHC), Oil Holding Capacity (OHC), and Swelling Capacity (SC)
2.7. Glucose and Cholesterol Absorption Capacity (GAC and CAC)
2.8. Rheological Properties
2.9. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.10. X-Ray Diffraction (XRD)
2.11. Thermo-Gravimetric Analysis (TGA)
2.12. Scanning Electron Microscopy (SEM)
2.13. In Vitro Gastrointestinal Digestion
2.14. In Vitro Fecal Fermentation and Analysis
2.14.1. Donor Recruitment and Fecal Sample Processing
2.14.2. Medium Composition
2.14.3. Gas Production and pH
2.14.4. Short-Chain Fatty Acid (SCFA) Analysis
2.14.5. 16S rDNA Amplicon Sequencing of Gut Microbiota
2.15. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of BBDF
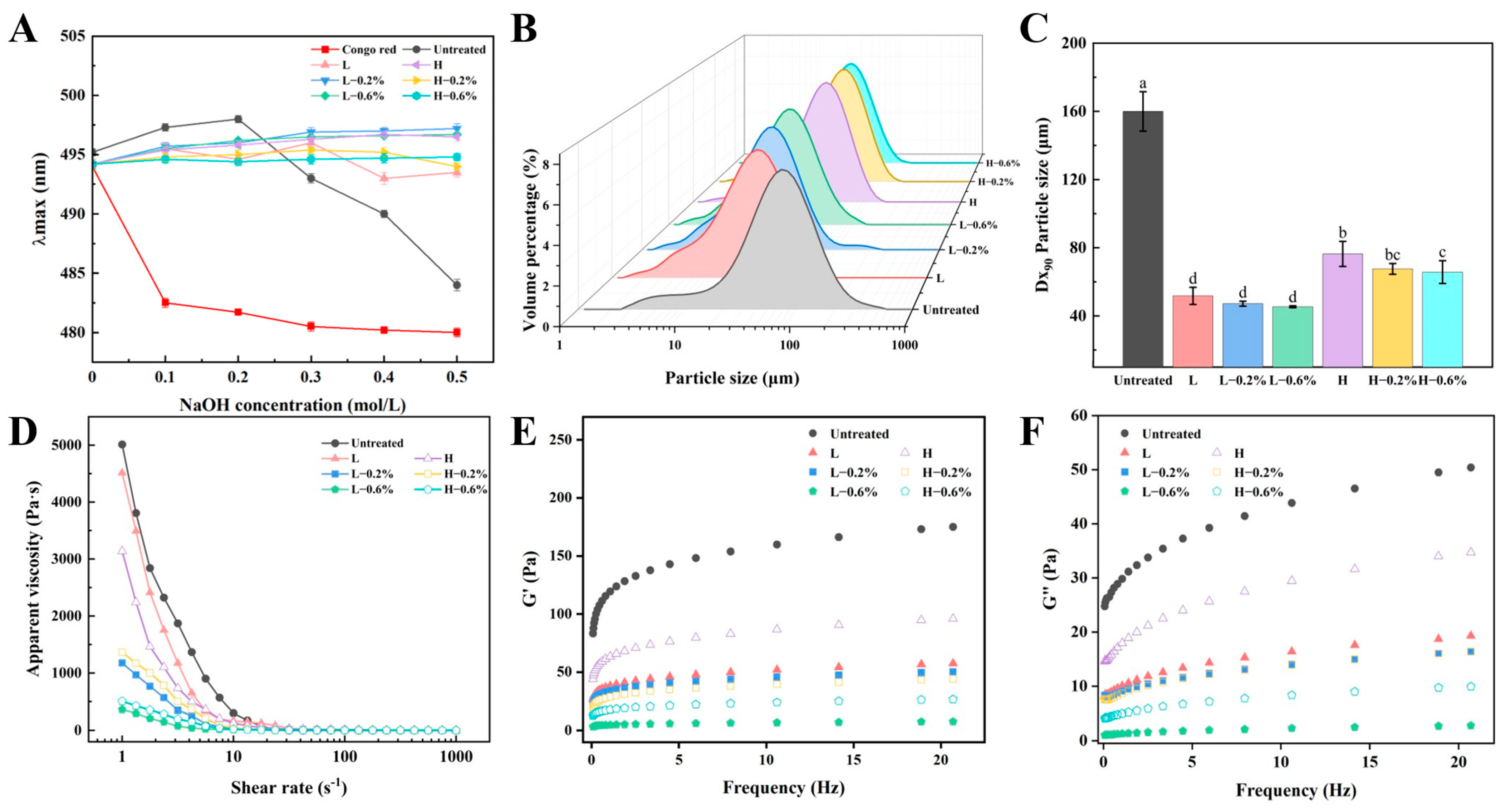
3.2. Particle Size of BBDF
3.3. Rheological Properties of BBDF
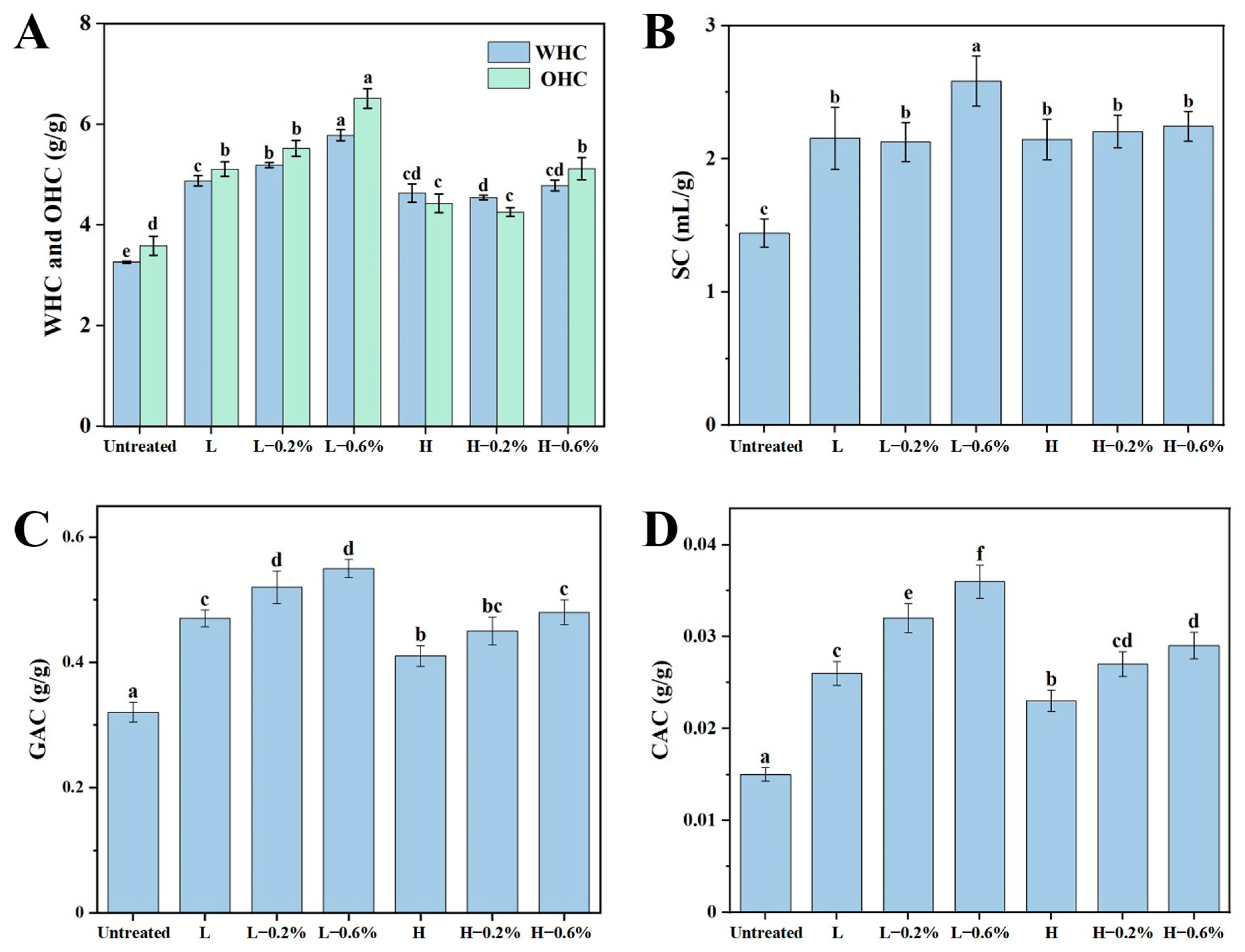
3.4. WHC, OHC, and SC
3.5. GAC and CAC
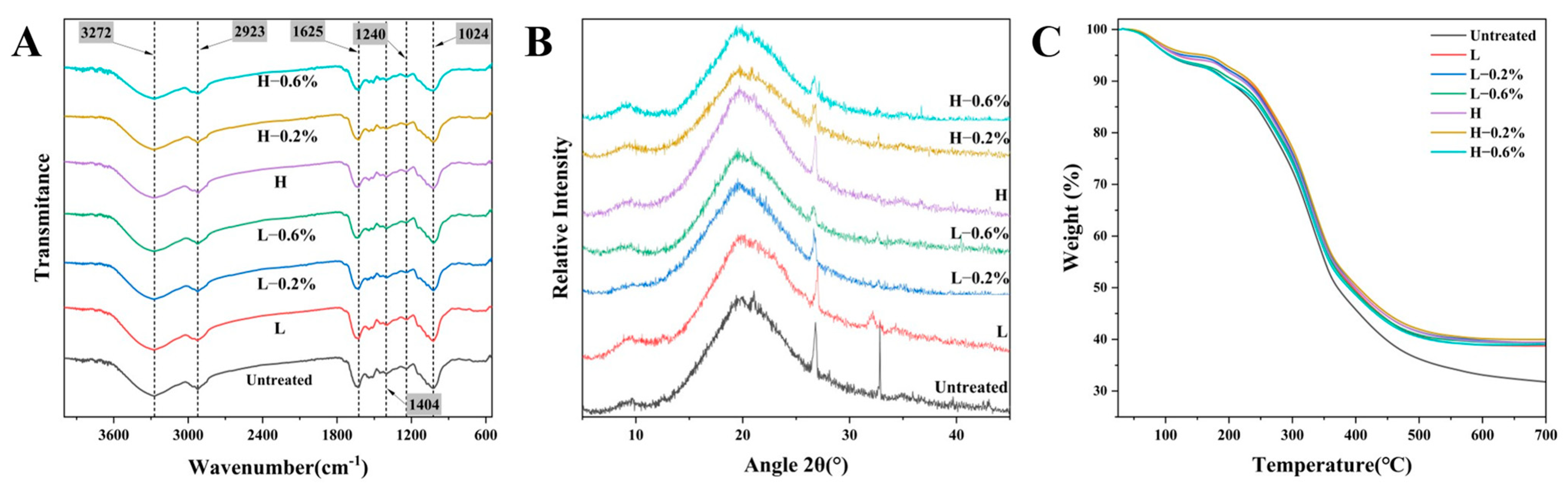
3.6. FT-IR
3.7. XRD
3.8. TGA
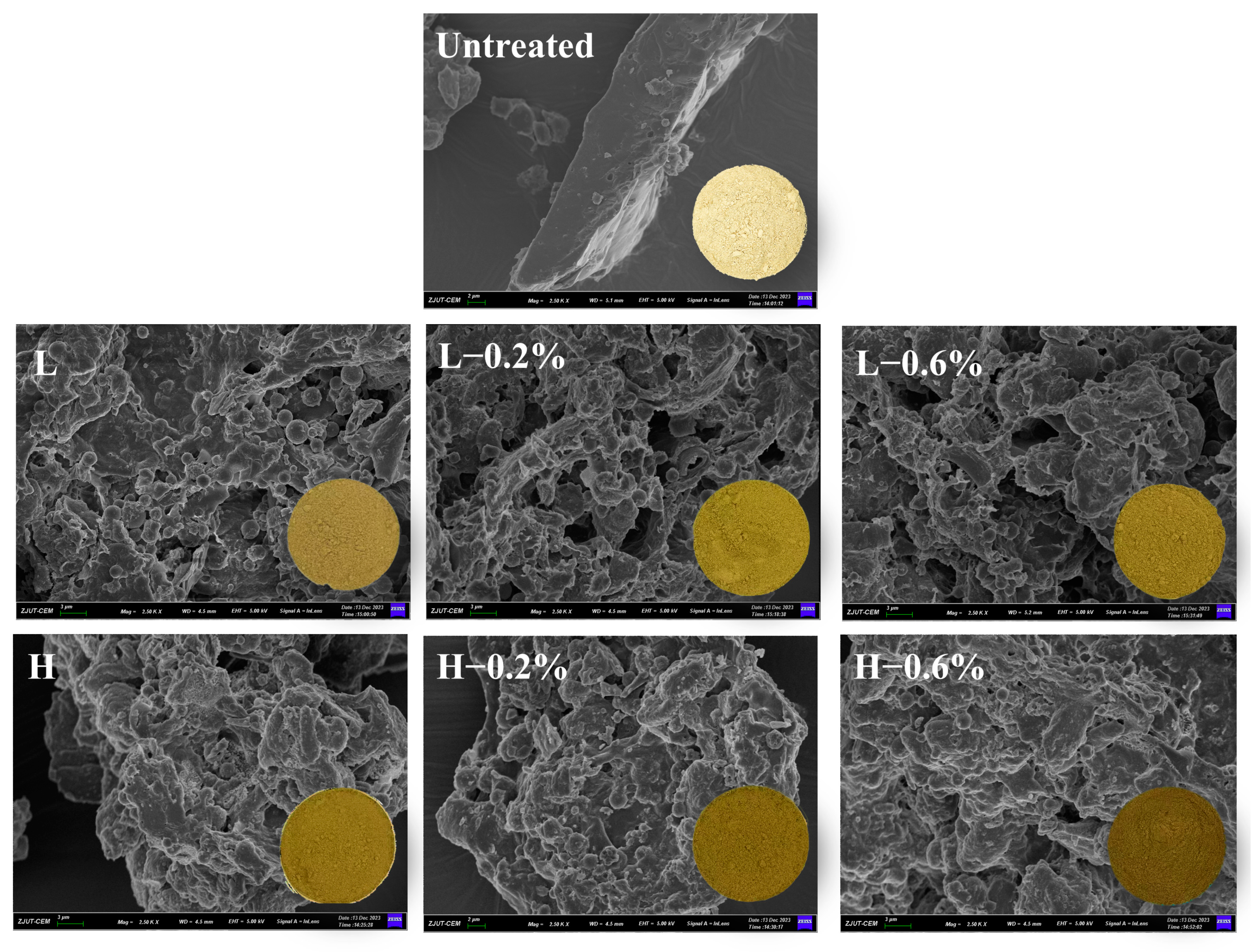
3.9. Morphological Structure
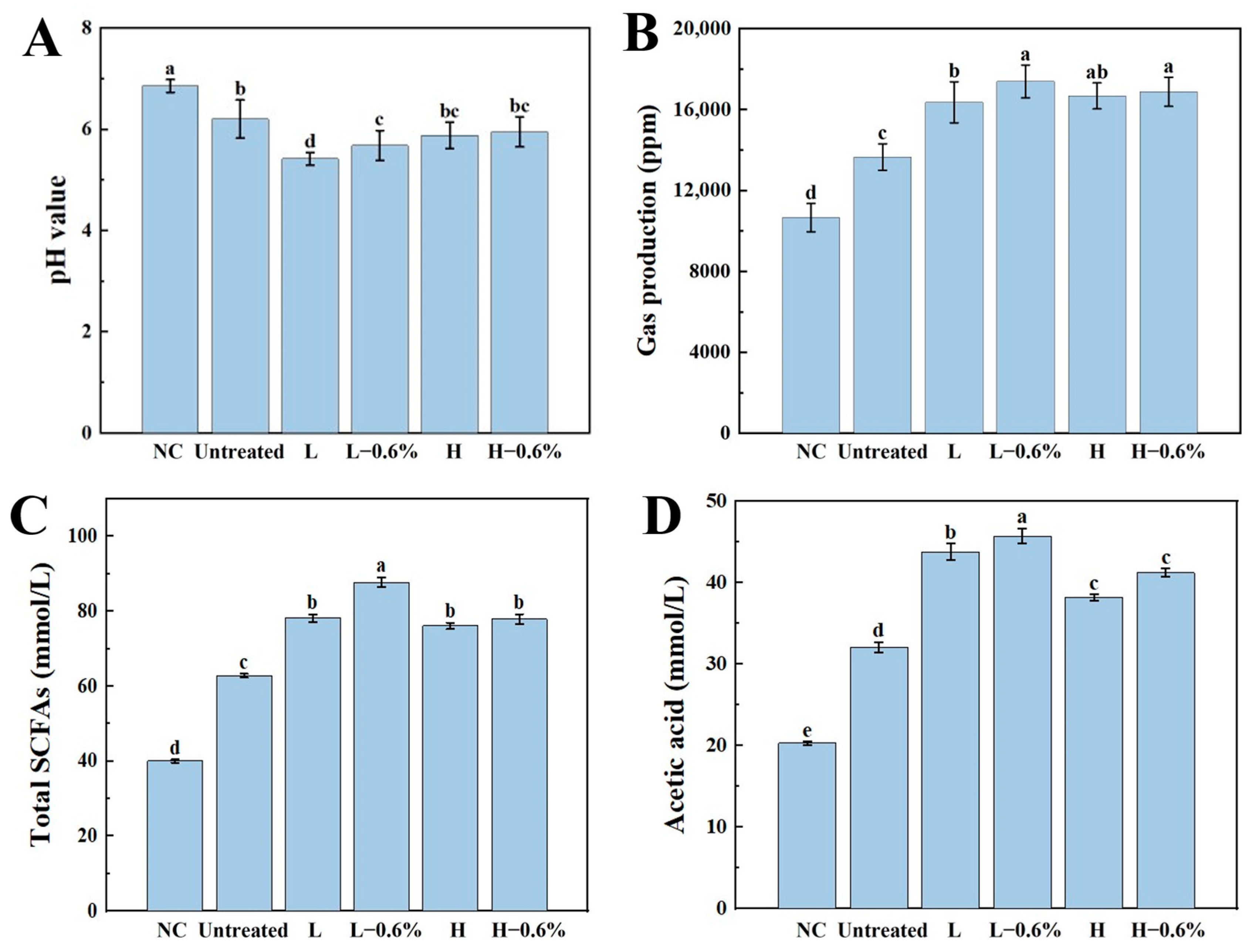
3.10. Gas Production and pH of BBDF
3.11. SCFA Analyses
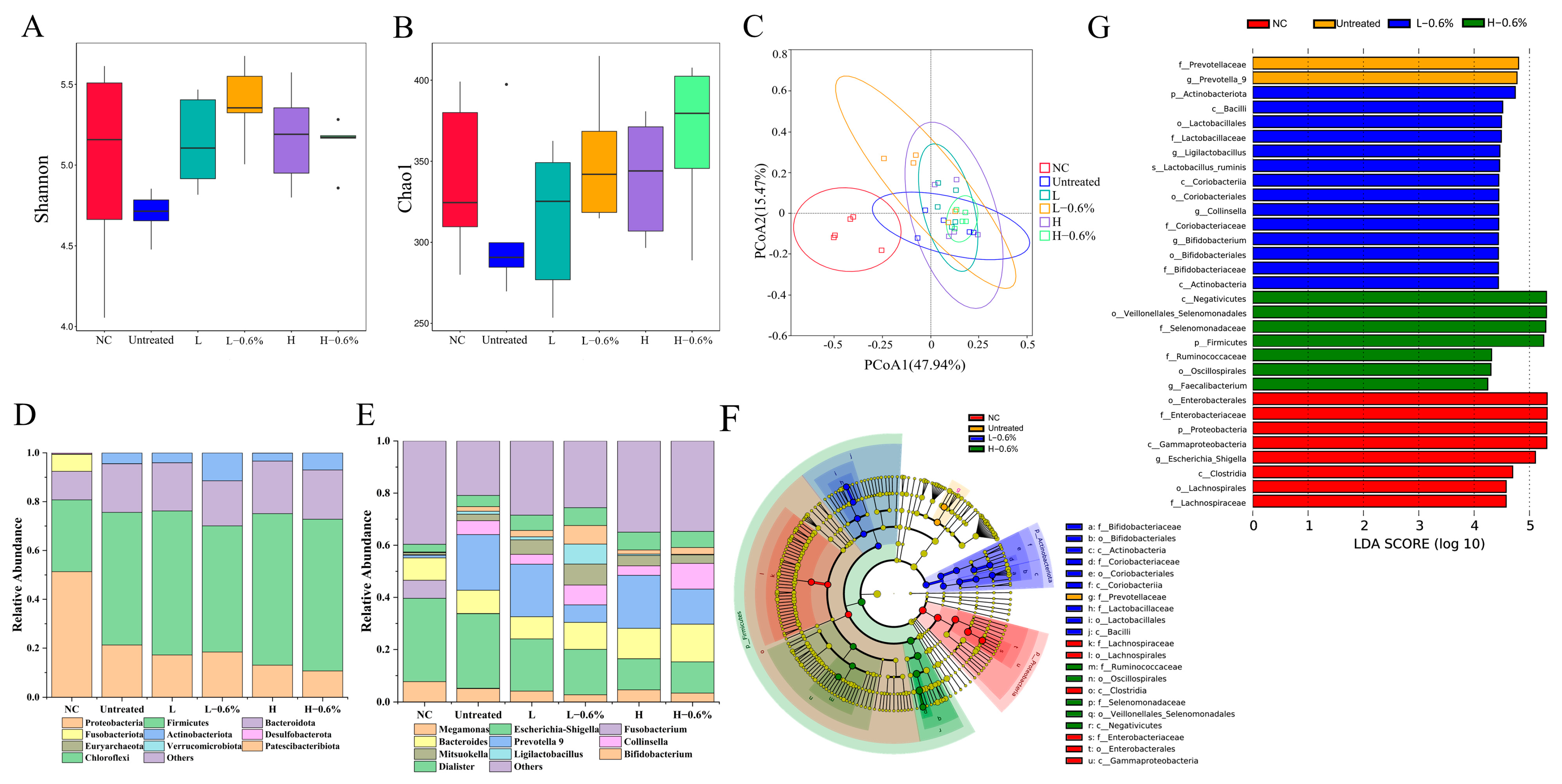
3.12. Fecal Microbiota Change
3.12.1. α-Diversity and β-Diversity
3.12.2. Microbiota Composition Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, Y.Z.; Corke, H.; Wang, D.; Li, W.D. Buckwheat: Overview. In Encyclopedia of Food Grains; Elsevier: Amsterdam, The Netherlands, 2016; pp. 307–315. [Google Scholar]
- Kasar, C.; Thanushree, M.P.; Gupta, S.; Inamdar, A.A. Milled Fractions of Common Buckwheat (Fagopyrum esculentum) from the Himalayan Regions: Grain Characteristics, Functional Properties and Nutrient Composition. J. Food Sci. Technol. 2021, 58, 3871–3881. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Li, J.; Chen, X.; Xu, X. How Lignocellulose Degradation Can Promote the Quality and Function of Dietary Fiber from Bamboo Shoot Residue by Inonotus Obliquus Fermentation. Food Chem. 2024, 451, 139479. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Xie, L.; Peng, G.; Xie, J.; Chen, Y.; Yu, Q. Systematic Review on Modification Methods of Dietary Fiber. Food Hydrocoll. 2021, 119, 106872. [Google Scholar] [CrossRef]
- Alam, M.S.; Kaur, J.; Khaira, H.; Gupta, K. Extrusion and Extruded Products: Changes in Quality Attributes as Affected by Extrusion Process Parameters: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 445–473. [Google Scholar] [CrossRef]
- Nikinmaa, M.; Zehnder-Wyss, O.; Nyström, L.; Sozer, N. Effect of Extrusion Processing Parameters on Structure, Texture and Dietary Fibre Composition of Directly Expanded Wholegrain Oat-Based Matrices. LWT 2023, 184, 114972. [Google Scholar] [CrossRef]
- Xu, E.; Campanella, O.H.; Ye, X.; Jin, Z.; Liu, D.; BeMiller, J.N. Advances in Conversion of Natural Biopolymers: A Reactive Extrusion (REX)–Enzyme-Combined Strategy for Starch/Protein-Based Food Processing. Trends Food Sci. Technol. 2020, 99, 167–180. [Google Scholar] [CrossRef]
- Song, Y.; Su, W.; Mu, Y.C. Modification of Bamboo Shoot Dietary Fiber by Extrusion-Cellulase Technology and Its Properties. Int. J. Food Prop. 2018, 21, 1219–1232. [Google Scholar] [CrossRef]
- Zhou, S.; Tang, X.; Hegyi, F.; Nagy, A.; Takács, K.; Zalán, Z.; Chen, G.; Du, M. In Vitro Digestion and Fermentation Characteristics of Soluble Dietary Fiber from Adlay (Coix Lacryma-Jobi L. Var. Ma-Yuen Staft) Bran Modified by Steam Explosion. Food Res. Int. 2024, 192, 114747. [Google Scholar]
- Fang, F.; He, Y.-X.; Wang, H.-Q.; Zhang, Y.-L.; Zhong, Y.; Hu, X.-T.; Nie, S.-P.; Xie, M.-Y.; Hu, J.-L. Impact of Eight Extruded Starchy Whole Grains on Glycemic Regulation and Fecal Microbiota Modulation. Food Hydrocoll. 2025, 160, 110756. [Google Scholar] [CrossRef]
- Pismag, R.; Pico, J.; Fernández, A.; Hoyos, J.L.; Martinez, M.M. α-Amylase Reactive Extrusion Enhances the Protein Digestibility of Saponin-Free Quinoa Flour While Preserving Its Total Phenolic Content. Innov. Food Sci. Emerg. Technol. 2023, 88, 103448. [Google Scholar] [CrossRef]
- Qiu, C.; Hu, H.; Chen, B.; Lin, Q.; Ji, H.; Jin, Z. Research Progress on the Physicochemical Properties of Starch-Based Foods by Extrusion Processing. Foods 2024, 13, 3677. [Google Scholar] [CrossRef]
- Guan, Z.; Ren, X.; Bian, S.; Xu, E.; Jin, Z.; Jiao, A. Study on the Relationship between the Degradation Degrees of Enzymatically Extruded Glutinous Rice and the Qualities of Fermented Chinese Rice Wine. J. Cereal Sci. 2022, 105, 103476. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Li, L.-Y.; Zhang, T.; Wang, J.-Q.; Huang, X.-J.; Hu, J.-L.; Yin, J.-Y.; Nie, S.-P. Fractionation, Physicochemical and Structural Characterization of Polysaccharides from Barley Water-Soluble Fiber. Food Hydrocoll. 2021, 113, 106539. [Google Scholar] [CrossRef]
- Meng, K.; Wang, Y.; Liu, F.; Zhan, Q.; Zhao, L. Effect of Modifications on Structure, Physicochemical Properties and Lead Ions Adsorption Behavior of Dietary Fiber of Flammulina velutipes. Food Chem. 2025, 464, 141597. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, S.; Li, Y.; Tian, J.; Zhang, C. Structure, Physicochemical Properties and Effects on Nutrients Digestion of Modified Soluble Dietary Fiber Extracted from Sweet Potato Residue. Food Res. Int. 2021, 150, 110761. [Google Scholar] [CrossRef]
- Geng, N.; Wang, H.; Zhang, Y.; Song, J.; Li, Y.; Wu, C. Physicochemical, Structural, and Functional Properties of Microfluidic Modified Dietary Fiber from Fresh Corn Bracts. J. Cereal Sci. 2023, 112, 103731. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Yi, C.; Quan, K.; Lin, B. Chemical Composition, Structure, Physicochemical and Functional Properties of Rice Bran Dietary Fiber Modified by Cellulase Treatment. Food Chem. 2021, 342, 128352. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Zhu, Z.; Song, X.; Yao, J.; Li, Z.; Huang, Z.; Liu, H.; Liu, W.; Dai, F. In Vitro Gastrointestinal Digestion and Fecal Fermentation Behaviors of Pectin from Feijoa (Acca sellowiana) Peel and Its Impact on Gut Microbiota. Food Res. Int. 2024, 197, 115301. [Google Scholar] [CrossRef]
- Cai, M.; Feng, J.; Wang, J.; Chen, P.; Ge, Z.; Liu, W.; Sun, P.; Wu, L.; Wu, J. Characterization of Various Noncovalent Polyphenol–Starch Complexes and Their Prebiotic Activities during In Vitro Digestion and Fermentation. J. Agric. Food Chem. 2024, 72, 2250–2262. [Google Scholar] [CrossRef]
- Qiao, H.; Shao, H.; Zheng, X.; Liu, J.; Liu, J.; Huang, J.; Zhang, C.; Liu, Z.; Wang, J.; Guan, W. Modification of Sweet Potato (Ipomoea Batatas Lam.) Residues Soluble Dietary Fiber Following Twin-Screw Extrusion. Food Chem. 2021, 335, 127522. [Google Scholar] [CrossRef]
- Hassan, M.; Berglund, L.; Hassan, E.; Abou-Zeid, R.; Oksman, K. Effect of Xylanase Pretreatment of Rice Straw Unbleached Soda and Neutral Sulfite Pulps on Isolation of Nanofibers and Their Properties. Cellulose 2018, 25, 2939–2953. [Google Scholar] [CrossRef]
- Zou, X.; Dai, K.; Zhang, M.; Zhang, R.; Jia, X.; Dong, L.; Ma, Q.; Liang, S.; Wang, Z.; Deng, M.; et al. Dietary Fiber from Sweet Potato Residue with Different Processing Methods: Physicochemical, Functional Properties, and Bioactivity in Vitro. LWT 2024, 206, 116581. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Qin, H.; Huang, M.; Liu, S.; Chang, R.; Xi, B.; Mao, J.; Zhang, S. Obtaining Non-Digestible Polysaccharides from Distillers’ Grains of Chinese Baijiu after Extrusion with Enhanced Antioxidation Capability. Int. J. Biol. Macromol. 2023, 243, 124799. [Google Scholar] [CrossRef]
- Ma, Q.; Yu, Y.; Zhou, Z.; Wang, L.; Cao, R. Effects of Different Treatments on Composition, Physicochemical and Biological Properties of Soluble Dietary Fiber in Buckwheat Bran. Food Biosci. 2023, 53, 102517. [Google Scholar] [CrossRef]
- Wang, N.; Wu, L.; Zhang, F.; Kan, J.; Zheng, J. Modifying the Rheological Properties, in Vitro Digestion, and Structure of Rice Starch by Extrusion Assisted Addition with Bamboo Shoot Dietary Fiber. Food Chem. 2022, 375, 131900. [Google Scholar] [CrossRef]
- Li, Y.; Tian, Y.; Deng, L.; Dai, T.; Liu, C.; Chen, J. High Energy Media Mill Modified Pea Dietary Fiber: Physicochemical Property and Its Mechanism in Stabilizing Pea Protein Beverage. Food Hydrocoll. 2024, 147, 109392. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Zhang, Y.; Gao, B.; Niu, Y.; Yu, L.L. The Structural and Functional Characteristics of Soluble Dietary Fibers Modified from Tomato Pomace with Increased Content of Lycopene. Food Chem. 2022, 382, 132333. [Google Scholar] [CrossRef]
- Song, L.; Qi, J.; Liao, J.; Yang, X. Enzymatic and Enzyme-Physical Modification of Citrus Fiber by Xylanase and Planetary Ball Milling Treatment. Food Hydrocoll. 2021, 121, 107015. [Google Scholar] [CrossRef]
- Kaur, S.; Panesar, P.S.; Chopra, H.K. Extraction of Dietary Fiber from Kinnow (Citrus reticulata) Peels Using Sequential Ultrasonic and Enzymatic Treatments and Its Application in Development of Cookies. Food Biosci. 2023, 54, 102891. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Tian, H.; Li, Y.; Shi, P.; Guo, W.; Zhu, Q. Effect of Four Modification Methods on Adsorption Capacities and in Vitro Hypoglycemic Properties of Millet Bran Dietary Fibre. Food Res. Int. 2021, 147, 110565. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Gao, Z.; Zhang, M.; Mu, S.; Cheng, Y.; Qu, K. Effects of Modification Methods on the Structural Characteristics and Functional Properties of Dietary Fiber from Cucumber. Food Chem. X 2024, 24, 101808. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Feng, M.; Chen, Y.; Li, S.; Fang, Z.; Wang, L.; Lin, D.; Zhang, Q.; Liu, Y.; Luo, Y.; et al. Comparison on Structure, Properties and Functions of Pomegranate Peel Soluble Dietary Fiber Extracted by Different Methods. Food Chem. X 2023, 19, 100827. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Yan, H.; Tang, C. Wet Media Planetary Ball Milling Remarkably Improves Functional and Cholesterol-Binding Properties of Okara. Food Hydrocoll. 2021, 111, 106386. [Google Scholar] [CrossRef]
- Ma, Z.-Q.; Zhang, N.; Zhai, X.-T.; Tan, B. Structural, Physicochemical and Functional Properties of Dietary Fiber from Brown Rice Products Treated by Different Processing Techniques. LWT 2023, 182, 114789. [Google Scholar] [CrossRef]
- Ji, R.; Zhang, X.; Liu, C.; Zhang, W.; Han, X.; Zhao, H. Effects of Extraction Methods on the Structure and Functional Properties of Soluble Dietary Fiber from Blue Honeysuckle (Lonicera caerulea L.) Berry. Food Chem. 2024, 431, 137135. [Google Scholar] [CrossRef]
- Li, J.; Fu, J.; Ma, Y.; He, Y.; Fu, R.; Qayum, A.; Jiang, Z.; Wang, L. Low Temperature Extrusion Promotes Transglutaminase Cross-Linking of Whey Protein Isolate and Enhances Its Emulsifying Properties and Water Holding Capacity. Food Hydrocoll. 2022, 125, 107410. [Google Scholar] [CrossRef]
- Fang, F.; He, Y.; Zhao, J.; Zhang, Y.; Chen, C.; He, H.; Wu, Q.; Hu, M.; Nie, S.; Xie, M.; et al. Effects of Boiling and Steaming Process on Dietary Fiber Components and in Vitro Fermentation Characteristics of 9 Kinds of Whole Grains. Food Res. Int. 2023, 164, 112328. [Google Scholar] [CrossRef]
- Tejada-Ortigoza, V.; Garcia-Amezquita, L.E.; Campanella, O.H.; Hamaker, B.R.; Welti-Chanes, J. Extrusion Effect on in Vitro Fecal Fermentation of Fruit Peels Used as Dietary Fiber Sources. LWT 2022, 153, 112569. [Google Scholar] [CrossRef]
- Liang, X.; Liu, M.; Yao, A.; Cui, W.; Wei, Y.; Guo, S.; Duan, J.; Kang, H.; Zhou, X.; Su, S.; et al. In Vitro Fermentation Characteristics and Interaction of Neutral and Acidic Polysaccharides from Lycii Fructus on Human Gut Microbiota. Food Hydrocoll. 2024, 152, 109940. [Google Scholar] [CrossRef]
- Salim, F.; Mizutani, S.; Shiba, S.; Takamaru, H.; Yamada, M.; Nakajima, T.; Yachida, T.; Soga, T.; Saito, Y.; Fukuda, S.; et al. Fusobacterium Species Are Distinctly Associated with Patients with Lynch Syndrome Colorectal Cancer. iScience 2024, 27, 110181. [Google Scholar] [CrossRef] [PubMed]

| Samples * | Untreated | L | L-0.2% | L-0.6% | H | H-0.2% | H-0.6% |
|---|---|---|---|---|---|---|---|
| TDF (g/100 g) | 79.50 ± 3.28 a | 79.21 ± 2.46 a | 80.96 ± 1.99 a | 78.23 ± 2.23 a | 80.32 ± 2.83 a | 79.98 ± 2.73 a | 80.27 ± 3.11 a |
| SDF (g/100 g) | 10.48 ± 0.14 f | 19.94 ± 0.32 e | 23.96 ± 0.58 d | 32.67 ± 0.77 a | 26.22 ± 0.58 c | 28.04 ± 0.45 bc | 29.44 ± 0.75 b |
| IDF (g/100 g) | 69.02 ± 1.78 a | 59.47 ± 1.04 b | 57.01 ± 0.99 c | 45.56 ± 0.97 f | 54.10 ± 1.17 d | 51.94 ± 1.75 e | 50.83 ± 1.15 e |
| IDF composition (g/100 g) | |||||||
| Cellulose | 20.12 ± 0.84 a | 17.92 ± 1.23 b | 11.69 ± 0.70 c | 5.41 ± 0.24 e | 10.51 ± 0.19 c | 7.49 ± 0.07 d | 7.20 ± 0.14 d |
| Hemicellulose | 5.61 ± 0.20 b | 5.60 ± 0.27 b | 4.66 ± 0.22 cd | 3.20 ± 0.05 e | 5.05 ± 0.25 bc | 6.71 ± 0.40 a | 4.12 ± 0.12 d |
| Lignin | 42.53 ± 0.22 a | 34.67 ± 0.41 c | 37.75 ± 1.65 b | 30.04 ± 1.39 d | 35.69 ± 2.08 bc | 34.75 ± 0.16 c | 36.83 ± 0.05 bc |
| Monosaccharide composition (mol %) | |||||||
| Glc:Ara:Gal:Xyl # | 77.85:12.59:5.33:4.23 | 80.47:10.92:4.86:3.74 | 80.98:10.48:4.63:3.67 | 82.82:9.61:4.30:3.27 | 78.60:11.95:5.14:4.31 | 78.84:11.91:4.85:4.30 | 78.91:11.78:4.95:4.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, T.; Yu, Y.; Kong, X.; Wu, W.; Zhang, Z.; Hu, W.; Natallia, K.; Cai, M.; Yang, K.; Sun, P. Physicochemical, Functional, and In Vitro Fermentation Characteristics of Buckwheat Bran Dietary Fiber Modified by Enzymatic Extrusion. Foods 2025, 14, 1300. https://doi.org/10.3390/foods14081300
Bu T, Yu Y, Kong X, Wu W, Zhang Z, Hu W, Natallia K, Cai M, Yang K, Sun P. Physicochemical, Functional, and In Vitro Fermentation Characteristics of Buckwheat Bran Dietary Fiber Modified by Enzymatic Extrusion. Foods. 2025; 14(8):1300. https://doi.org/10.3390/foods14081300
Chicago/Turabian StyleBu, Tingting, Yue Yu, Xiao Kong, Weicheng Wu, Zhiguo Zhang, Weiwei Hu, Komarova Natallia, Ming Cai, Kai Yang, and Peilong Sun. 2025. "Physicochemical, Functional, and In Vitro Fermentation Characteristics of Buckwheat Bran Dietary Fiber Modified by Enzymatic Extrusion" Foods 14, no. 8: 1300. https://doi.org/10.3390/foods14081300
APA StyleBu, T., Yu, Y., Kong, X., Wu, W., Zhang, Z., Hu, W., Natallia, K., Cai, M., Yang, K., & Sun, P. (2025). Physicochemical, Functional, and In Vitro Fermentation Characteristics of Buckwheat Bran Dietary Fiber Modified by Enzymatic Extrusion. Foods, 14(8), 1300. https://doi.org/10.3390/foods14081300








