Effects of Hot-Air Drying Temperatures on Quality and Volatile Flavor Components of Cooked Antarctic krill (Euphausia superba)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Antarctic krill Preparation and Drying Conditions
2.3. Sensory Evaluation
2.4. Moisture Ratio (MR) and Water Loss Ratio (WLR) Detection
2.5. Color Detection
2.6. Low-Field Nuclear Magnetic Resonance (LF-NMR) Measurement
2.7. Lipid Oxidation of Dried Antarctic krill
2.8. Fatty Acids Composition Analysis
2.9. Gas Chromatography-Ino Mobility Spectrometry (GC-IMS) Analysis
2.10. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.11. Statistical Analysis
3. Results
3.1. Sensory Evaluation of Cooked Antarctic krill Treated at Various Hot-Air Drying Temperatures
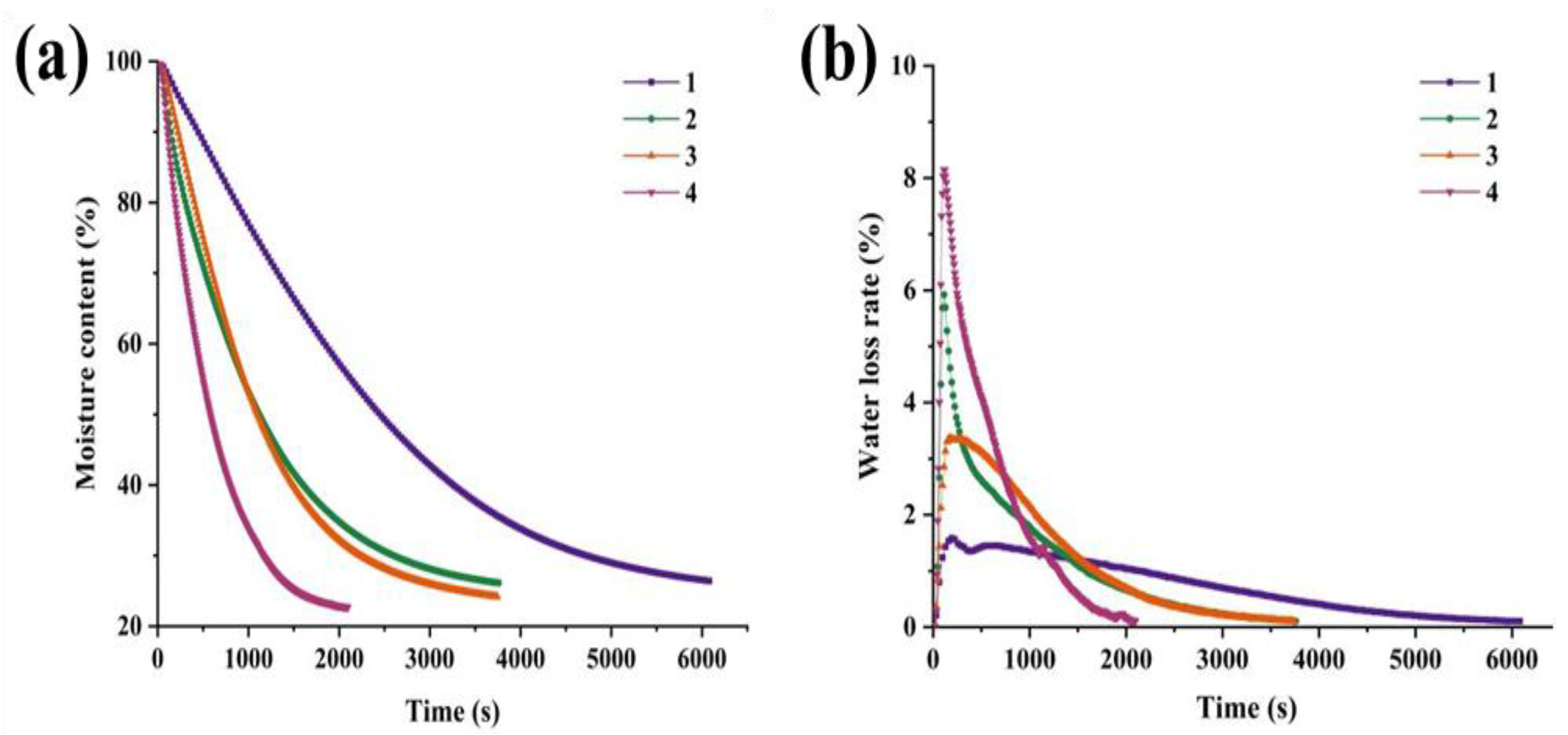
3.2. Moisture Content (WC) and Water Loss Ratio (WLR) Analysis
3.3. Water Distribution
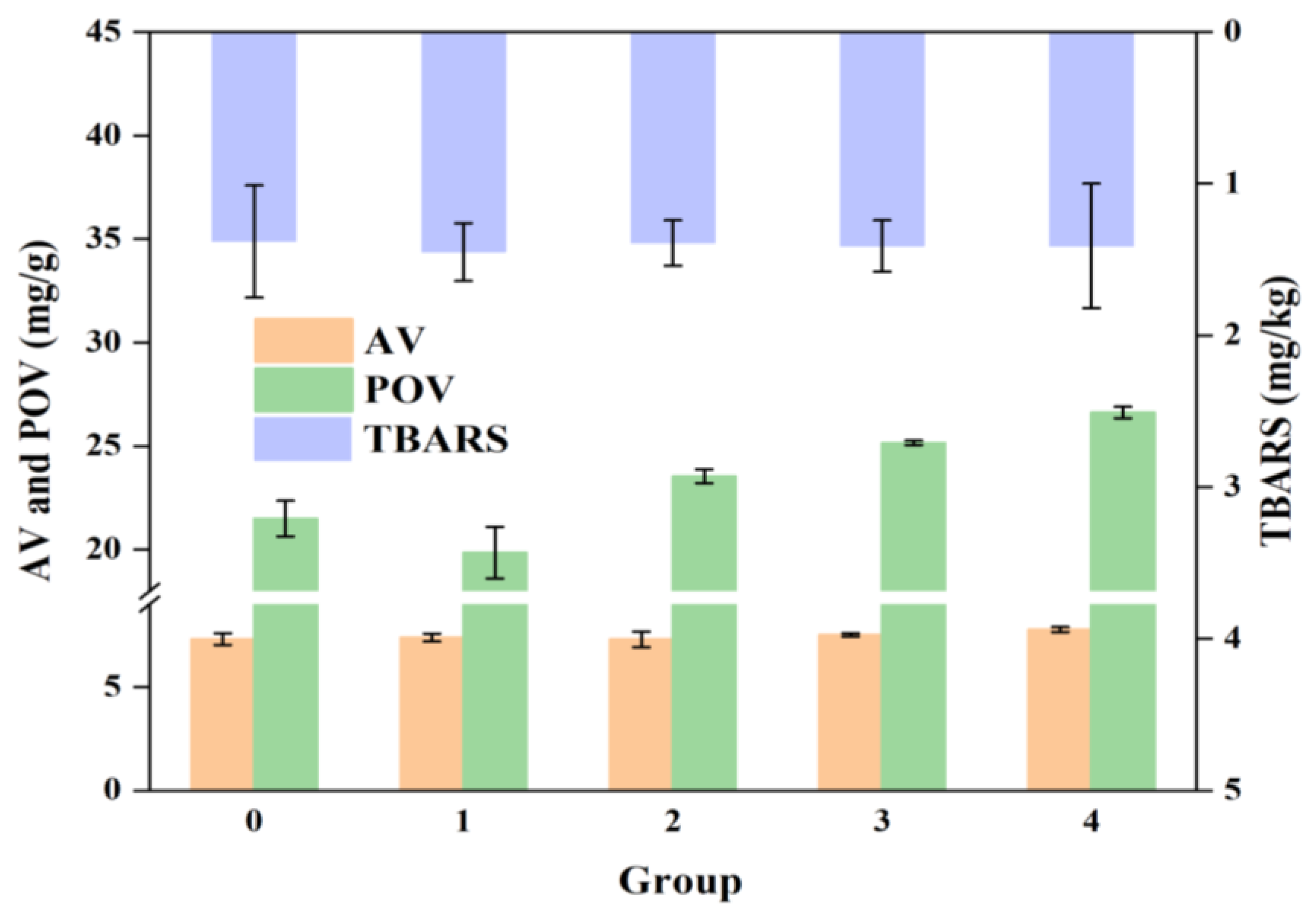
3.4. Lipid Oxidation Analysis
3.5. Color Changes
3.6. GC-MS Analysis
3.7. GC-IMS Analysis
3.8. Fatty Acids Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavan, E.L.; Belcher, A.; Atkinson, A.; Hill, S.L.; Kawaguchi, S.; McCormack, S.; Meyer, B.; Nicol, S.; Ratnarajah, L.; Schmidt, K.; et al. The importance of Antarctic krill in biogeochemical cycles. Nat. Commun. 2019, 10, 4742. [Google Scholar] [CrossRef]
- Schaafsma, F.L.; David, C.L.; Kohlbach, D.; Ehrlich, J.; Castellani, G.; Lange, B.A.; Vortkamp, M.; Meijboom, A.; Fortuna-Wünsch, A.; Immerz, A.; et al. Allometric relationships of ecologically important Antarctic and Arctic zooplankton and fish species. Polar Biol. 2022, 45, 203–224. [Google Scholar] [CrossRef]
- Sun, P.; Lin, J.; Ren, X.; Zhang, B.; Liu, J.; Zhao, Y.; Li, D. Effect of Heating on Protein Denaturation, Water State, Microstructure, and Textural Properties of Antarctic Krill (Euphausia superba) Meat. Food Bioprocess Technol. 2022, 15, 2313–2326. [Google Scholar] [CrossRef]
- Chi, H.; Zhang, Y.; Zhao, L.; Lin, N.; Kang, W. Quality analysis and shelf-life prediction of antarctic krill (Euphausia superba) sauce based on kinetic model and back propagation neural network model. J. Food Process. Preserv. 2024, 2024, 4506851. [Google Scholar] [CrossRef]
- Suzuki, T.; Shibata, N. The utilization of Antarctic krill for human food. Food Rev. Int. 1990, 6, 119–147. [Google Scholar] [CrossRef]
- Xie, D.; Gong, M.; Wei, W.; Jin, J.; Wang, X.; Wang, X.; Jin, Q. Antarctic Krill (Euphausia superba) Oil: A Comprehensive Review of Chemical Composition, Extraction Technologies, Health Benefits, and Current Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 514–534. [Google Scholar] [CrossRef]
- Berge, K.; Musa-Veloso, K.; Harwood, M.; Hoem, N.; Burri, L. Krill oil supplementation lowers serum triglycerides without increasing low-density lipoprotein cholesterol in adults with borderline high or high triglyceride levels. Nutr. Res. 2014, 34, 126–133. [Google Scholar] [CrossRef]
- Yang, X.; Shi, Y.; Cai, Y.; Chi, H. Quality changes and safety evaluation of ready-to-eat roasted antarctic krill (Euphausia superba) during storage at room temperature (25 °C). J. Ocean. Univ. China 2023, 22, 235–241. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, S.; Yang, L.; Wei, B.; Guo, Q. Prevention of the Quality Degradation of Antarctic Krill (Euphausia superba) Meal through Two-Stage Drying. Foods 2024, 13, 1706. [Google Scholar] [CrossRef]
- Nicol, S.; Foster, J.; Kawaguchi, S. The fishery for Antarctic krill–recent developments. Fish Fish. 2012, 13, 30–40. [Google Scholar] [CrossRef]
- Fikry, M.; Benjakul, S.; Al-Ghamdi, S.; Tagrida, M.; Prodpran, T. Evaluating Kinetics of Convection Drying and Microstructure Characteristics of Asian Seabass Fish Skin without and with Ultrasound Pretreatment. Foods 2023, 12, 3024. [Google Scholar] [CrossRef]
- Lee, S.; Han, S.; Jo, K.; Jung, S. The impacts of freeze-drying-induced stresses on the quality of meat and aquatic products: Mechanisms and potential solutions to acquire high-quality products. Food Chem. 2024, 459, 140437. [Google Scholar] [CrossRef]
- Bai, J.; Fan, Y.; Zhu, L.; Wang, Y.; Hou, H. Characteristic flavor of Antarctic krill (Euphausia superba) and white shrimp (Penaeus vannamei) induced by thermal treatment. Food Chem. 2022, 378, 132074. [Google Scholar] [CrossRef]
- Zeng, J.; Song, Y.; Fan, X.; Luo, J.; Song, J.; Xu, J.; Xue, C. Effect of lipid oxidation on quality attributes and control technologies in dried aquatic animal products: A critical review. Crit. Rev. Food Sci. 2024, 64, 10397–10418. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Mujumdar, A.S.; Tang, J.; Miao, S.; Wang, Y. Recent developments in high-quality drying of vegetables, fruits, and aquatic products. Crit. Rev. Food Sci. 2017, 57, 1239–1255. [Google Scholar] [CrossRef]
- Sun, P.; Lin, S.; Li, X.; Li, D. Different stages of flavor variations among canned Antarctic krill (Euphausia superba): Based on GC-IMS and PLS-DA. Food Chem. 2024, 459, 140465. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Fu, Z.; Wang, P.; Yu, D.; Zhao, L.; Li, L.; Liu, Y.; Zheng, J. Process Optimization for Antarctic Krill (Euphausia superba) Sauce Based on Back Propagation Neural Network Combined with Genetic Algorithm. Appl. Sci. 2024, 14, 7337. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Poblete, J.; Rojas-Carmona, R.; Uribe, E.; Pastén, A.; Goñi, M.G. Vacuum drying of Chilean papaya (Vasconcellea pubescens) fruit pulp: Effect of drying temperature on kinetics and quality parameters. J. Food Sci. Technol. 2021, 58, 3482–3492. [Google Scholar] [CrossRef]
- Zhang, K.; Li, J.; Tan, Z.; Yu, X.; Wang, S.; Zhou, D.; Li, D. Investigation of Soy Protein Isolate-Konjac Glucomannan Sodium Salt Hydrogel: Molecular Docking, Microstructure, Rheological Properties, and 3D Printing Characteristics. Food Bioprocess Technol. 2024, 18, 3313–3328. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Cao, R.; Liu, Q.; Su, D.; Zhang, Y.; Yu, Y. The role of ultraviolet radiation in the flavor formation during drying processing of Pacific saury (Cololabis saira). J. Sci. Food Agric. 2024, 104, 8099–8108. [Google Scholar] [CrossRef]
- Ghani, M.A.; Barril, C.; Bedgood, D.R.; Prenzler, P.D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef]
- Nava, V.; Turco, V.L.; Licata, P.; Panayotova, V.; Peycheva, K.; Fazio, F.; Rando, R.; Di Bella, G.; Potortì, A.G. Determination of Fatty Acid Profile in Processed Fish and Shellfish Foods. Foods 2023, 12, 2631. [Google Scholar] [CrossRef]
- Miao, X.; Li, S.; Liu, Y.; Li, J.; Dong, X.; Du, M.; Jiang, P. The dynamic changes of flavor characteristics of sea cucumber (Apostichopus japonicus) during puffing revealed by GC-MS combined with HS-GC-IMS. Food Chem. X 2024, 23, 101709. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, Y.; Huang, J.; Fu, B.; Wang, K.; Xu, Z. Analysis of volatile flavor compounds in Antarctic krill paste with different processing methods based on GC-IMS. Food Sci. Nutr. 2024, 12, 8353–8363. [Google Scholar] [CrossRef]
- Lacalle-Bergeron, L.; Portolés, T.; Sales, C.; Carmen Corell, M.; Domínguez, F.; Beltrán, J.; Vicente Sancho, J.; Hernández, F. Gas chromatography-mass spectrometry based untargeted volatolomics for smoked seafood classification. Food Res. Int. 2020, 137, 109698. [Google Scholar] [CrossRef]
- Sarpong, F.; Zhou, C.; Bai, J.; Amenorfe, L.P.; Golly, M.K.; Ma, H. Modeling of drying and ameliorative effects of relative humidity (RH) against β-carotene degradation and color of carrot (Daucus carota var.) slices. Food Sci. Biotechnol. 2018, 28, 75–85. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhao, G.X.; Suo, S.K.; Wang, Y.M.; Chi, C.F.; Wang, B. Purification, Identification, Activity Evaluation, and Stability of Antioxidant Peptides from Alcalase Hydrolysate of Antarctic Krill (Euphausia superba) Proteins. Mar. Drugs 2021, 19, 347. [Google Scholar] [CrossRef]
- Benjamin, D.C.; Kristjánsdóttir, S.; Gudmundsdóttir, A. Increasing the thermal stability of euphauserase. A cold-active and multifunctional serineprotease from Antarctic krill. Eur. J. Biochem. 2021, 268, 127–131. [Google Scholar] [CrossRef]
- Hu, Y.; Zeng, X.; Jiang, K.; Luo, Y.; Quan, Z.; Li, J.; Ma, Y.; Guo, X.; Zhou, D.; Zhu, B. Effect of non-enzymatic browning on oysters during hot air drying process: Color and chemical changes and insights into mechanisms. Food Chem. 2024, 454, 139758. [Google Scholar] [CrossRef]
- Liu, Y.; Cong, P.; Li, B.; Song, Y.; Liu, Y.; Xu, J.; Xue, C. Effect of thermal processing towards lipid oxidation and non-enzymatic browning reactions of Antarctic krill (Euphausia superba) meal. J. Sci. Food Agric. 2018, 98, 5257–5268. [Google Scholar] [CrossRef] [PubMed]
- Abel, N.; Rotabakk, B.T.; Lerfall, J. Mild processing of seafood-A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 340–370. [Google Scholar] [CrossRef]
- Kontominas, M.G.; Badeka, A.V.; Kosma, I.S.; Nathanailides, C.I. Recent Developments in Seafood Packaging Technologies. Foods 2021, 10, 940. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Shen, L.; Shi, X.; Deng, Y.; Qiao, Y.; Wu, W.; Xiong, G.; Li, X.; Ding, A.; Shi, L. Characterization of flavor perception and characteristic aroma of traditional dry-cured fish by flavor omics combined with multivariate statistics. LWT 2023, 173, 114240. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Yanagita, T.; Xue, C.; Zhang, T.; Wang, Y. Trimethylamine N-Oxide in Aquatic Foods. J. Agric. Food Chem. 2024, 72, 14498–14520. [Google Scholar] [CrossRef]
- Song, G.; Zeng, M.; Chen, S.; Lyu, Z.; Jiang, N.; Wang, D.; Yuan, T.; Li, L.; Mei, G.; Shen, Q.; et al. Exploring molecular mechanisms underlying changes in lipid fingerprinting of salmon (Salmo salar) during air frying integrating machine learning-guided REIMS and lipidomics analysis. Food Chem. 2024, 460, 140770. [Google Scholar] [CrossRef] [PubMed]
- Valeur, J.; Landfald, B.; Berstad, A.; Raa, J. Trimethylamine N-Oxide in Seafood: Rotten or Forgotten? J. Am. Coll. Cardiol. 2016, 68, 2916–2917. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Chen, L.; Liu, T. Dandelion polysaccharide suppresses lipid oxidation in Antarctic krill (Euphausia superba). Int. J. Biol. Macromol. 2019, 133, 1164–1167. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.H.; Wang, X.; Tian, H.; Wang, L.; Zhou, D.Y.; Qin, L. Compensatory effect of lipase on the flavor of lightly-salted large yellow croaker: Integration of flavoromics and lipidomics. Food Biosci. 2024, 59, 103907. [Google Scholar] [CrossRef]
- Wang, R.; Sun, X.; Dong, G.; Guo, C.; Yin, F.; Liu, H.; Song, L.; Zhou, D. Influence of lipid oxidation on the digestive efficiency of Antarctic krill oil: Insights from a simulated gastrointestinal digestion model. Food Funct. 2024, 15, 10190–10199. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, F.; Luo, Y.; Zeng, X.; Pei, X.; Zhao, G.; Zhang, M.; Zhou, D.; Yin, F. Effects of Tea Polyphenol and Its Combination with Other Antioxidants Added during the Extraction Process on Oxidative Stability of Antarctic Krill (Euphausia superba) Oil. Foods 2022, 11, 3768. [Google Scholar] [CrossRef]
- Xu, M.; Liu, Q.; Ni, X.; Chen, C.; Deng, X.; Fang, Y.; Wang, X.; Shen, Q.; Yu, R. Lipidomics reveals the effect of hot-air drying on the quality characteristics and lipid oxidation of Tai lake whitebait (Neosalanx taihuensis Chen). LWT 2024, 197, 115942. [Google Scholar] [CrossRef]


| Groups | Appearance | Smells | Texture | Total Scores |
|---|---|---|---|---|
| 0 | 8.50 ± 0.05a | 3.53 ± 0.47d | 8.05 ± 0.17a | 6.94 ± 0.16b |
| 1 | 8.73 ± 0.08a | 7.05 ± 0.39c | 8.05 ± 0.11a | 8.09 ± 0.09a |
| 2 | 8.00 ± 0.41b | 7.80 ± 0.25b | 8.28 ± 0.13a | 8.00 ± 0.29a |
| 3 | 4.45 ± 0.15c | 8.45 ± 0.05a | 8.40 ± 0.14a | 6.44 ± 0.10c |
| 4 | 3.93 ± 0.23d | 8.58 ± 0.22a | 5.40 ± 0.74b | 5.62 ± 0.28d |
| Color | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| L* | 56.26 ± 6.08a | 59.06 ± 6.20a | 60.16 ± 4.53a | 58.20 ± 3.51a | 58.51 ± 3.61a |
| a* | 15.71 ± 2.50ab | 16.63 ± 2.90a | 17.00 ± 2.20ab | 15.31 ± 2.10ab | 14.45 ± 2.51b |
| b* | 27.07 ± 2.64a | 26.85 ± 2.95a | 29.33 ± 4.04a | 25.39 ± 2.72a | 22.32 ± 4.56b |
| 0 | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| n-Hexane | 25.46 ± 1.47a | 31.75 ± 4.57a | 27.50 ± 4.75a | 40.70 ± 7.06a | 45.96 ± 6.61a |
| Furan, tetrahydro-3-methyl | 49.18 ± 18.62a | 37.56 ± 29.85ab | 19.19 ± 6.01b | 14.12 ± 9.01b | 15.65 ± 9.39b |
| Furan, tetrahydro-2-methyl | 2.46 ± 2.11a | 2.51 ± 2.78a | 2.64 ± 3.50a | 6.00 ± 5.32a | 0.75 ± 0.12a |
| Methylamine, N, N-dimethyl | 1.45 ± 0.61b | 0.82 ± 0.11b | 1.17 ± 0.27b | 0.93 ± 0.92b | 7.31 ± 3.76a |
| Ethanamine, N-methyl- | 0.02 ± 0.02a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.03 ± 0.02a | 0.05 ± 0.04a |
| 1-Butanol | 0.04 ± 0.03a | 0.01 ± 0.01b | 0.01 ± 0.01b | 0.02 ± 0.01ab | 0.05 ± 0.02ab |
| 3-Pentanone, 2-methyl- | 0.03 ± 0.01ab | 0.02 ± 0.01b | 0.04 ± 0.01ab | 0.18 ± 0.16a | 0.18 ± 0.10a |
| (E)-2-Butenal | 0.01 ± 0.00b | 0.04 ± 0.00b | 0.10 ± 0.03b | 0.73 ± 0.55a | 0.81 ± 0.13a |
| Cyclopentasiloxane, decamethyl- | 0.03 ± 0.03a | 0.07 ± 0.06a | 0.06 ± 0.04a | 0.03 ± 0.03a | 0.00 ± 0.00a |
| 2-Propen-1-ol | 0.01 ± 0.01a | 0.01 ± 0.01a | 0.02 ± 0.01a | 0.02 ± 0.02a | 0.03 ± 0.03a |
| (E)-2-Pentenal | 0.01 ± 0.00b | 0.02 ± 0.01b | 0.01 ± 0.00b | 0.07 ± 0.04a | 0.05 ± 0.02ab |
| 2-Ethyl-trans-2-butenal | 0.01 ± 0.01b | 0.07 ± 0.03b | 0.17 ± 0.05b | 2.09 ± 2.09a | 0.96 ± 0.74ab |
| Cyclopentanol | 0.06 ± 0.06a | 0.25 ± 0.25a | 0.48 ± 0.47a | 0.58 ± 0.08a | 0.01 ± 0.01a |
| Heptanal | 0.01 ± 0.01a | 0.02 ± 0.01a | 0.06 ± 0.02a | 0.07 ± 0.06a | 0.07 ± 0.07a |
| Dodecane | 0.00 ± 0.00a | 0.01 ± 0.01a | 0.03 ± 0.03a | 0.04 ± 0.06a | 0.06 ± 0.06a |
| (E)-2-Hexenal | 0.01 ± 0.00b | 0.01 ± 0.01b | 0.02 ± 0.01ab | 0.02 ± 0.02ab | 0.04 ± 0.01a |
| 1,6-Cyclodecadiene | 0.01 ± 0.01a | 0.01 ± 0.01a | 0.02 ± 0.01a | 0.02 ± 0.02a | 0.06 ± 0.06a |
| (E,Z)-3,6-Nonadien-1-ol | 0.01 ± 0.01a | 0.01 ± 0.01a | 0.04 ± 0.01a | 0.02 ± 0.02a | 0.07 ± 0.09a |
| 1-Pentanol | 0.01 ± 0.00c | 0.02 ± 0.00c | 0.03 ± 0.00b | 0.07 ± 0.01a | 0.07 ± 0.01a |
| Styrene | 0.05 ± 0.05a | 0.06 ± 0.04a | 0.06 ± 0.01a | 0.04 ± 0.03a | 0.04 ± 0.03a |
| Dicyclopropylmethanol, chlorodifluoroacetate | 0.00 ± 0.00a | 0.01 ± 0.01a | 0.03 ± 0.01a | 0.01 ± 0.01a | 0.07 ± 0.09a |
| 1-Penten-3-ol, 4-methyl- | 0.01 ± 0.00b | 0.02 ± 0.00ab | 0.03 ± 0.00ab | 0.04 ± 0.01a | 0.03 ± 0.01ab |
| Cyclohexasiloxane, dodecamethyl- | 0.03 ± 0.03a | 0.10 ± 0.08a | 0.10 ± 0.06a | 0.07 ± 0.07a | 0.03 ± 0.03a |
| (Z)-2-Penten-1-ol | 0.05 ± 0.02c | 0.11 ± 0.01bc | 0.18 ± 0.02b | 0.43 ± 0.10a | 0.54 ± 0.08a |
| Pyrazine, 2,5-dimethyl- | 0.00 ± 0.00b | 0.01 ± 0.01b | 0.02 ± 0.01b | 0.03 ± 0.02b | 0.10 ± 0.02a |
| 1-Hepten-6-one, 2-methyl- | 0.01 ± 0.01a | 0.02 ± 0.01a | 0.03 ± 0.01a | 0.03 ± 0.03a | 0.05 ± 0.05a |
| 1,2-Butanediol | 0.00 ± 0.00b | 0.01 ± 0.00b | 0.02 ± 0.00b | 0.06 ± 0.03a | 0.07 ± 0.03a |
| Cyclohexane, 1-ethyl-2-methyl-, trans- | 0.00 ± 0.00b | 0.00 ± 0.00b | 0.01 ± 0.00ab | 0.05 ± 0.05a | 0.03 ± 0.02ab |
| Pyrazine, 2-ethyl-5-methyl- | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.01 ± 0.01a | 0.02 ± 0.01a | 0.03 ± 0.03a |
| Pyrazine, trimethyl- | 0.00 ± 0.00b | 0.00 ± 0.00b | 0.00 ± 0.00b | 0.01 ± 0.01b | 0.10 ± 0.05a |
| alpha.-Hydroxyisobutyric acid, acetate | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.01 ± 0.00a | 0.03 ± 0.03a | 0.05 ± 0.07a |
| 1-Octen-3-ol | 0.01 ± 0.01a | 0.03 ± 0.02a | 0.04 ± 0.02a | 0.07 ± 0.07a | 0.06 ± 0.08a |
| 2-Hexene, 3,5,5-trimethyl- | 0.04 ± 0.04a | 0.15 ± 0.09a | 0.20 ± 0.11a | 0.26 ± 0.26a | 0.25 ± 0.16a |
| 1-Hexanol, 2-ethyl- | 0.06 ± 0.03ab | 0.03 ± 0.03b | 0.04 ± 0.03b | 0.14 ± 0.08ab | 0.22 ± 0.07a |
| (E,E)-2,4-Heptadienal | 0.00 ± 0.00b | 0.00 ± 0.00b | 0.00 ± 0.00b | 0.07 ± 0.05a | 0.06 ± 0.05a |
| Isovanillin | 0.02 ± 0.02a | 0.05 ± 0.04a | 0.06 ± 0.04a | 0.04 ± 0.04a | 0.03 ± 0.03a |
| Pyrrole | 0.02 ± 0.00d | 0.08 ± 0.02c | 0.12 ± 0.01ab | 0.13 ± 0.01bc | 0.20 ± 0.03a |
| Benzaldehyde | 0.00 ± 0.00b | 0.02 ± 0.02b | 0.01 ± 0.01b | 0.05 ± 0.05ab | 0.18 ± 0.12a |
| 3,5-Octadien-2-one | 0.01 ± 0.01b | 0.01 ± 0.01b | 0.00 ± 0.00b | 0.17 ± 0.11a | 0.30 ± 0.17a |
| Cyclohexene, 3-ethenyl- | 0.02 ± 0.01c | 0.03 ± 0.01c | 0.05 ± 0.00c | 0.11 ± 0.02b | 0.21 ± 0.07c |
| Cyclohexanone, 4-methyl- | 0.00 ± 0.00c | 0.01 ± 0.00c | 0.02 ± 0.00c | 0.08 ± 0.01b | 0.18 ± 0.05a |
| 2(5H)-Furanone, 5-ethyl- | 0.00 ± 0.00b | 0.01 ± 0.01b | 0.02 ± 0.00b | 0.08 ± 0.01a | 0.13 ± 0.07a |
| 3-Dodecyne | 0.02 ± 0.00b | 0.02 ± 0.01b | 0.03 ± 0.00b | 0.05 ± 0.01b | 0.09 ± 0.03a |
| 2-Vinylfuran | 0.01 ± 0.00d | 0.02 ± 0.00a | 0.02 ± 0.00ab | 0.02 ± 0.00bc | 0.02 ± 0.00c |
| 2,4-Di-tert-butylphenol | 0.03 ± 0.00a | 0.03 ± 0.01a | 0.03 ± 0.01a | 0.03 ± 0.00a | 0.03 ± 0.01a |
| 0 | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| C4:0 | 0.15 ± 0.10a | 0.24 ± 0.03a | 0.01 ± 0.00a | 0.11 ± 0.11a | 0.00 ± 0.00a |
| C6:0 | 0.07 ± 0.02a | 0.07 ± 0.01a | 0.03 ± 0.02a | 0.06 ± 0.03a | 0.04 ± 0.04a |
| C8:0 | 0.03 ± 0.02a | 0.02 ± 0.00a | 0.02 ± 0.00a | 0.01 ± 0.01a | 0.02 ± 0.00a |
| C10:0 | 0.01 ± 0.00a | 0.01 ± 0.00a | 0.00 ± 0.00a | 0.01 ± 0.00a | 0.00 ± 0.00a |
| C12:0 | 0.06 ± 0.00a | 0.06 ± 0.00a | 0.05 ± 0.00a | 0.06 ± 0.01a | 0.05 ± 0.00a |
| C14:0 | 2.82 ± 0.14ab | 3.06 ± 0.08ab | 2.52 ± 0.16c | 3.10 ± 0.29a | 2.61 ± 0.16c |
| C14:1 | 0.04 ± 0.00a | 0.04 ± 0.00a | 0.04 ± 0.00a | 0.08 ± 0.03a | 0.04 ± 0.00a |
| C15:0 | 0.09 ± 0.00a | 0.09 ± 0.00a | 0.08 ± 0.00a | 0.09 ± 0.03a | 0.08 ± 0.00a |
| C16:0 | 5.23 ± 0.23a | 5.28 ± 0.26a | 5.11 ± 0.18a | 3.93 ± 1.61a | 5.04 ± 0.17a |
| C16:1 | 0.10 ± 0.00a | 0.09 ± 0.00b | 0.09 ± 0.00b | 0.10 ± 0.02a | 0.08 ± 0.00b |
| C17:0 | 0.41 ± 0.02a | 0.40 ± 0.01a | 0.44 ± 0.01a | 0.30 ± 0.19a | 0.39 ± 0.01a |
| C18:0 | 0.30 ± 0.02a | 0.32 ± 0.01a | 0.32 ± 0.01a | 0.77 ± 0.60a | 0.32 ± 0.01a |
| C18:1n9t | 2.53 ± 0.15a | 2.76 ± 0.04a | 2.63 ± 0.09a | 3.00 ± 0.65a | 2.66 ± 0.07a |
| C18:2n6t | 0.02 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.02 ± 0.00a | 0.00 ± 0.00a |
| C18:2n6c | 0.46 ± 0.03a | 0.45 ± 0.01a | 0.48 ± 0.02a | 0.38 ± 0.13a | 0.44 ± 0.01a |
| C18:3n3 | 0.17 ± 0.01a | 0.15 ± 0.00a | 0.15 ± 0.02a | 0.11 ± 0.07a | 0.13 ± 0.03a |
| C20:1 | 0.16 ± 0.01a | 0.17 ± 0.00a | 0.11 ± 0.02a | 0.14 ± 0.07a | 0.14 ± 0.02a |
| C20:5n3 (EPA) | 3.05 ± 0.17a | 3.17 ± 0.04a | 3.34 ± 0.11a | 3.43 ± 0.14a | 3.14 ± 0.07a |
| C22:1n9 | 0.13 ± 0.00a | 0.13 ± 0.00a | 0.09 ± 0.01b | 0.14 ± 0.02a | 0.11 ± 0.01b |
| C20:4n6 | 0.05 ± 0.00a | 0.06 ± 0.00a | 0.00 ± 0.00a | 0.06 ± 0.01a | 0.03 ± 0.00a |
| C23:0 | 0.09 ± 0.00a | 0.10 ± 0.00a | 0.08 ± 0.01a | 0.09 ± 0.00a | 0.07 ± 0.01a |
| C24:0 | 0.07 ± 0.00a | 0.05 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| C22:6n3 (DHA) | 1.38 ± 0.11ab | 1.33 ± 0.01ab | 1.51 ± 0.02a | 1.48 ± 0.24b | 1.37 ± 0.01ab |
| EPA + DHA | 4.43 ± 0.28 | 4.50 ± 0.05 | 4.85 ± 0.13 | 4.91 ± 0.38 | 4.51 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Yu, D.; Wang, P.; Liu, Y.; Zheng, H.; Sun, L.; Zheng, J.; Chi, H. Effects of Hot-Air Drying Temperatures on Quality and Volatile Flavor Components of Cooked Antarctic krill (Euphausia superba). Foods 2025, 14, 1221. https://doi.org/10.3390/foods14071221
Zhang R, Yu D, Wang P, Liu Y, Zheng H, Sun L, Zheng J, Chi H. Effects of Hot-Air Drying Temperatures on Quality and Volatile Flavor Components of Cooked Antarctic krill (Euphausia superba). Foods. 2025; 14(7):1221. https://doi.org/10.3390/foods14071221
Chicago/Turabian StyleZhang, Ruxin, Di Yu, Peng Wang, Yujun Liu, Hanfeng Zheng, Lechang Sun, Jie Zheng, and Hai Chi. 2025. "Effects of Hot-Air Drying Temperatures on Quality and Volatile Flavor Components of Cooked Antarctic krill (Euphausia superba)" Foods 14, no. 7: 1221. https://doi.org/10.3390/foods14071221
APA StyleZhang, R., Yu, D., Wang, P., Liu, Y., Zheng, H., Sun, L., Zheng, J., & Chi, H. (2025). Effects of Hot-Air Drying Temperatures on Quality and Volatile Flavor Components of Cooked Antarctic krill (Euphausia superba). Foods, 14(7), 1221. https://doi.org/10.3390/foods14071221





