Optimizing Bay Scallop (Argopecten irradians) Product Quality: Moderate Freezing as an Effective Strategy for Improving Adductor Muscle Gel Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Collection
2.2. Transglutaminase (TGase) Activity of Bay Scallop Adductor
2.3. Preparation of MPs from Bay Scallop Adductor
2.4. Carbonyl Content
2.5. Sulfhydryl Content
2.6. Fourier-Transform Infrared (FTIR) Spectroscopy
2.7. Surface Hydrophobicity
2.8. Fluorescence Intensity
2.9. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis
2.10. Preparation of Heat-Induced Gel from Bay Scallop Adductor
2.11. Textural Properties of Gel from Bay Scallop Adductor
2.12. WHC of Gel from Bay Scallop Adductor
2.13. Whiteness of Gel from Bay Scallop Adductor
2.14. Microstructure of Gel from Bay Scallop Adductor
2.15. Statistical Analysis
3. Results and Discussion
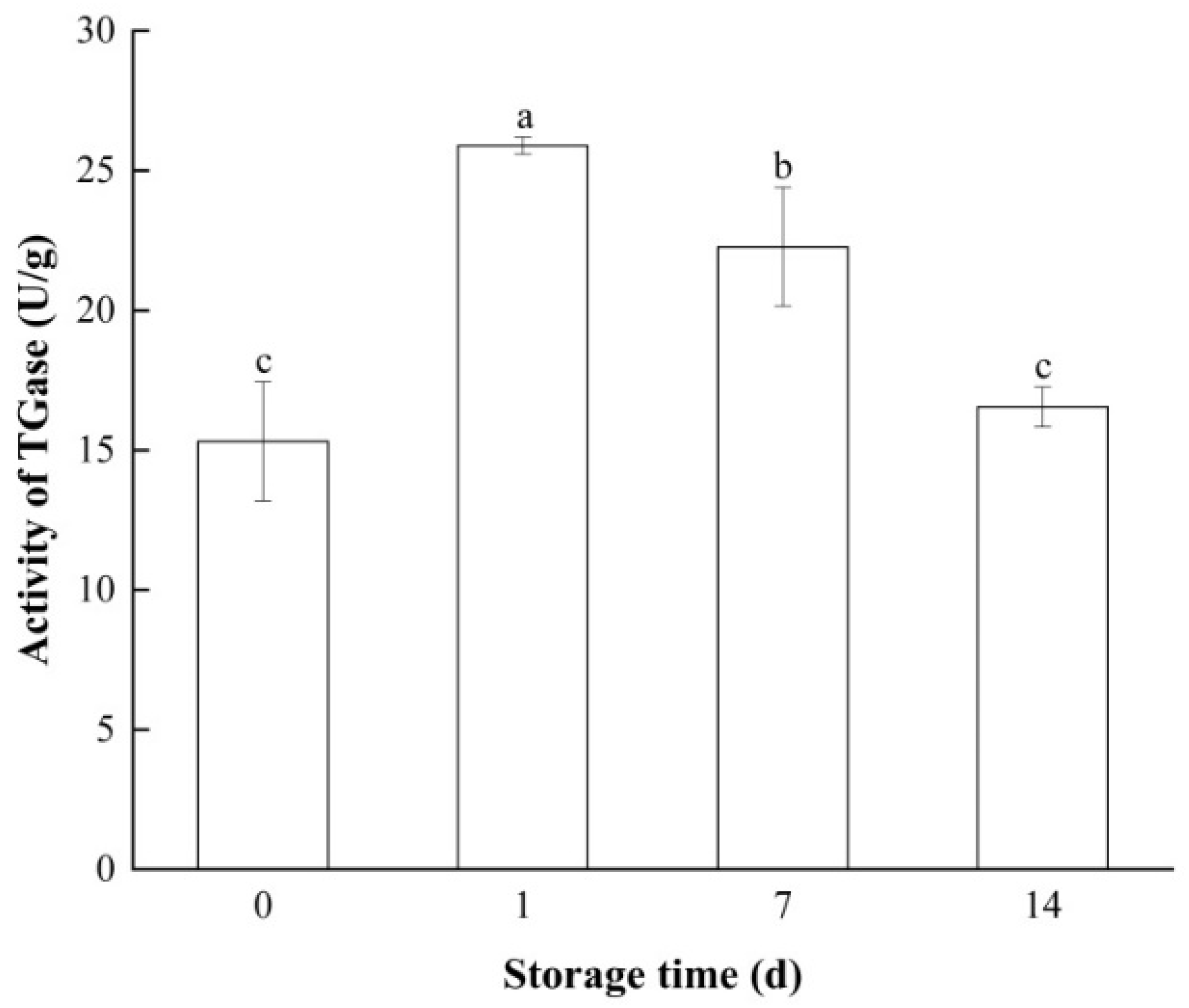
3.1. Changes in TGase Activity of Bay Scallop Adductor
3.2. Changes in Structure and Conformation of Myofibrillar Proteins from Bay Scallop Adductor
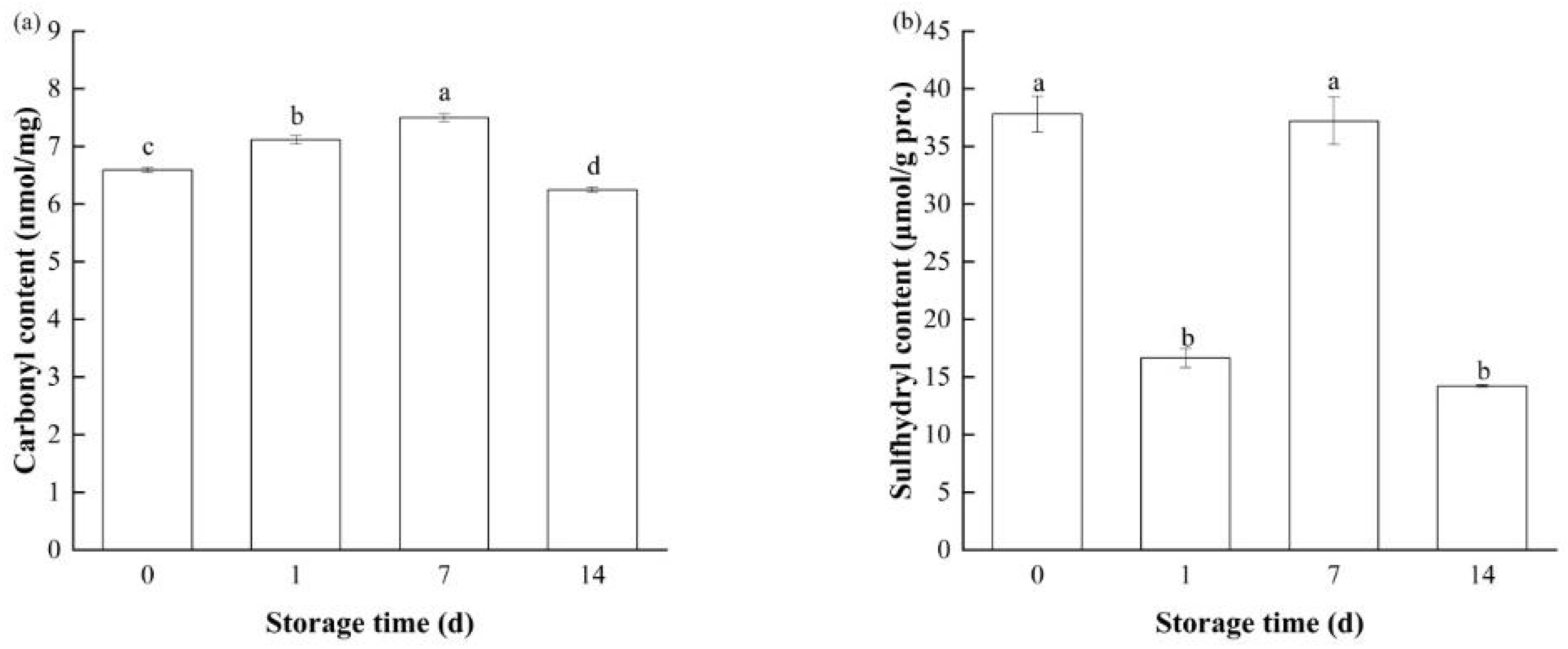
3.2.1. Carbonyl Content
3.2.2. Sulfhydryl Content
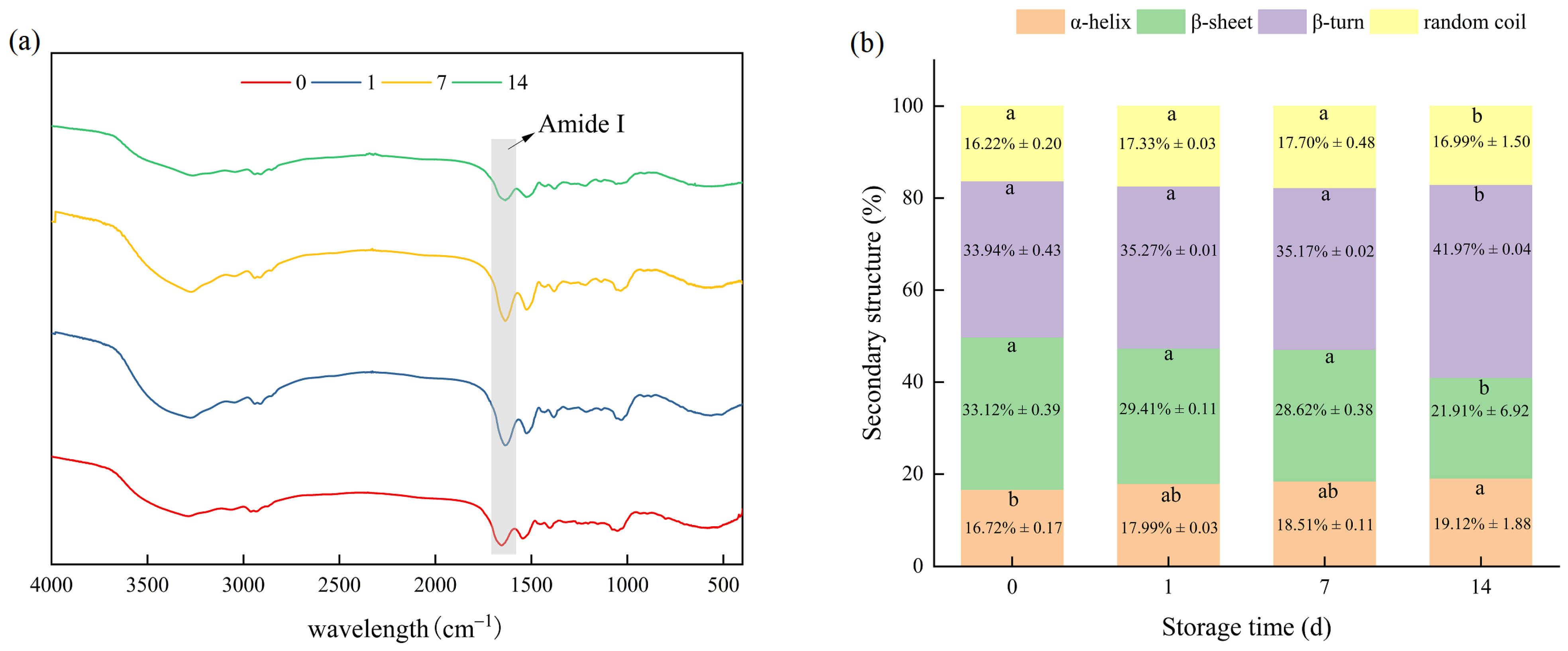
3.2.3. Secondary Structures
3.2.4. Surface Hydrophobicity
3.2.5. Intrinsic Fluorescence Intensity
3.2.6. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis
3.3. Changes in the Properties of Bay Scallop Adductor Gel Caused by Frozen Storage
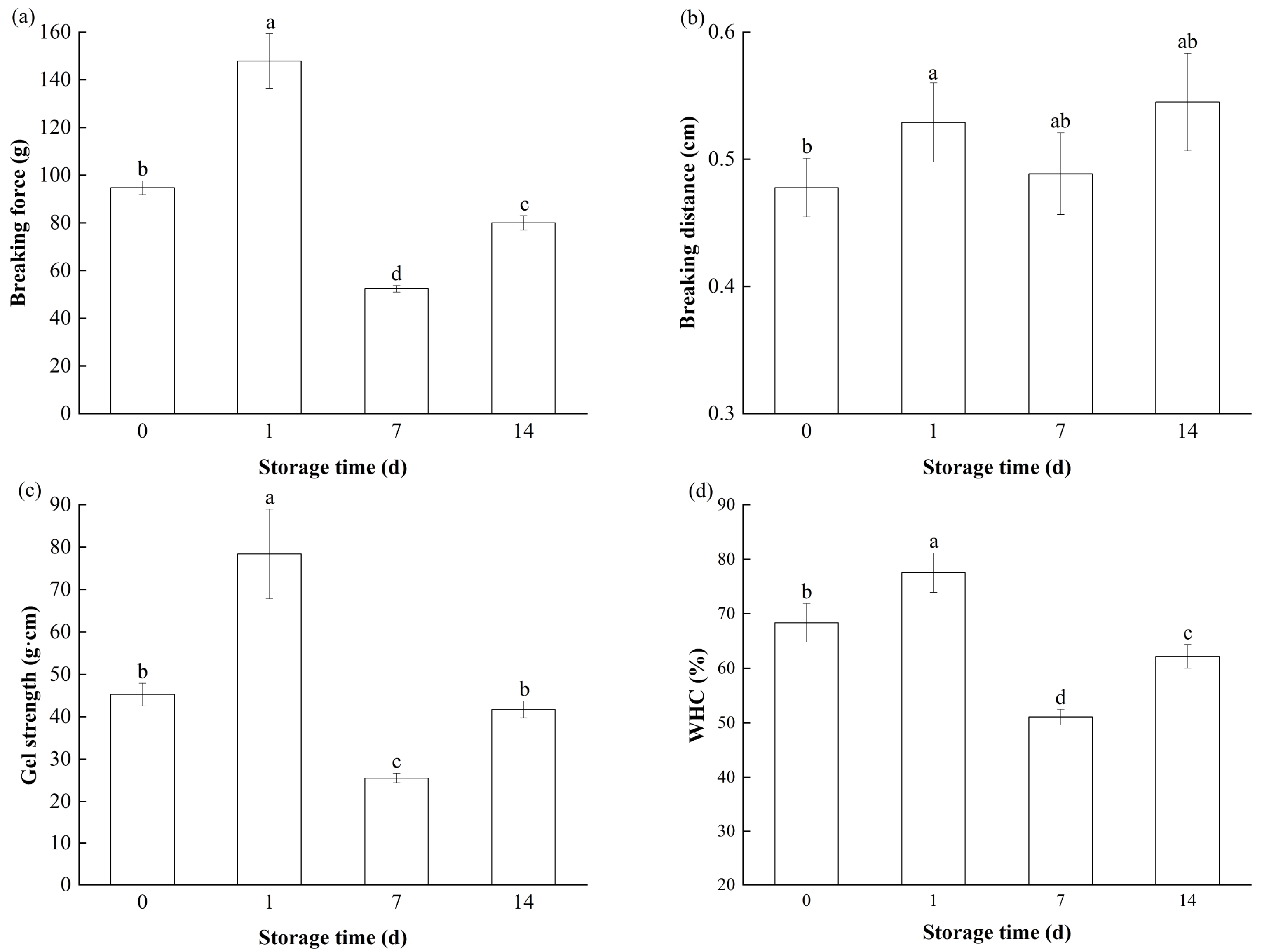
3.3.1. Texture
3.3.2. WHC
3.3.3. Whiteness
3.4. Changes in Microstructure of Gels from Bay Scallop Adductor
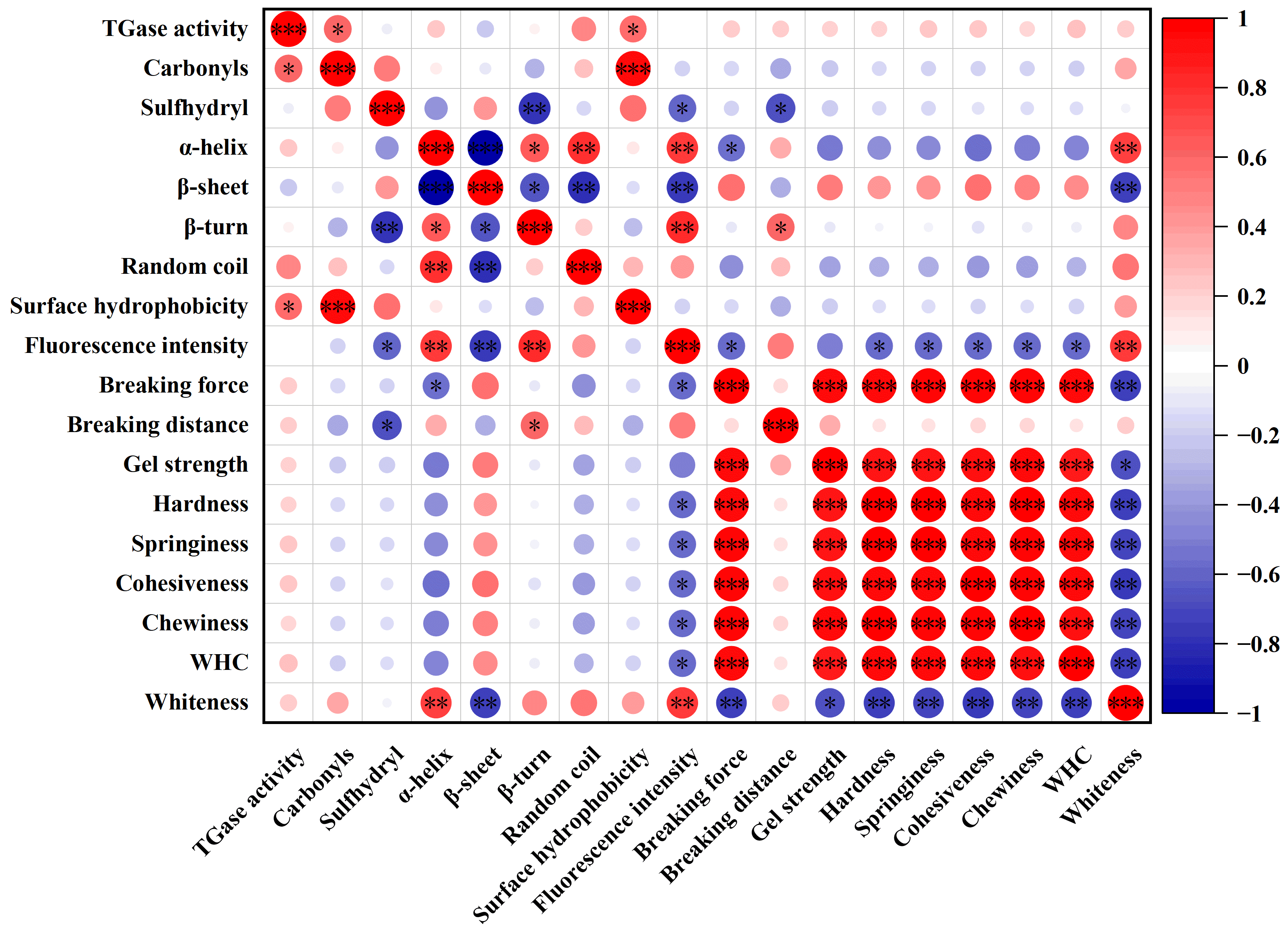
3.5. Correlation of TGase Activity, Protein Structure and Gel Properties of Frozen Bay Scallop Adductor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture: 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Affairs, China Bureau of Fisheries. China Fisheries Yearbook; China Agriculture Press: Beijing, China, 2024. (In Chinese) [Google Scholar]
- Yang, B.; Gao, X.; Zhao, J.; Liu, Y.; Lui, H.; Huang, T.; Chen, C.; Xing, Q. Massive shellfish farming might accelerate coastal acidification: A case study on carbonate system dynamics in a bay scallop (Argopecten irradians) farming area, North Yellow Sea. Sci. Total Environ. 2021, 798, 15. [Google Scholar] [CrossRef] [PubMed]
- Niki, T.; Kato, Y.; Nozawa, H.; Seki, N. Gelation of low salt soluble proteins from scallop adductor muscle in relation to its freshness. Fish. Sci. 2002, 68, 688–693. [Google Scholar] [CrossRef]
- Sui, X.; Zhao, Y.; Zhang, X.; Zhang, Y.; Zhu, L.; Fang, Z.; Shi, Q. Hydrocolloid coating pretreatment makes explosion puffing drying applicable in protein-rich foods—A case study of scallop adductors. Dry. Technol. 2022, 40, 50–64. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Li, X.; Yang, F.; Yu, L.; Li, X.; Huang, J.; Wang, S. Investigation of the cryoprotective mechanism and effect on quality characteristics of surimi during freezing storage by antifreeze peptides. Food Chem. 2022, 371, 131054. [Google Scholar] [CrossRef]
- Liu, B.; Li, D.Y.; Wu, Z.X.; Yang, W.J.; Zhou, D.Y.; Zhu, B.W. Combined effects of ultrasound and antioxidants on the quality maintenance of bay scallop (Argopecten irradians) adductor muscles during cold storage. Ultrason. Sonochem. 2022, 82, 105883. [Google Scholar] [CrossRef]
- Shang, S.; Wang, Y.; Jiang, P.; Fu, B.; Dong, X.; Qi, L. Progress in the application of novel cryoprotectants for the stabilization of myofibrillar proteins. Crit. Rev. Food Sci. Nutr. 2024, 64, 9756–9770. [Google Scholar] [CrossRef]
- Liu, B.; Liao, Y.L.; Jiang, L.L.; Chen, M.M.; Yang, S.B. Effects of ultrasound-assisted immersion freezing on the protein structure, physicochemical properties and muscle quality of the bay scallop (Argopecten irradians) during frozen storage. Foods 2022, 11, 3247. [Google Scholar] [CrossRef]
- Li, D.Y.; Tan, Z.F.; Liu, Z.Q.; Wu, C.; Liu, H.L.; Guo, C.; Zhou, D.Y. Effect of hydroxyl radical induced oxidation on the physicochemical and gelling properties of shrimp myofibrillar protein and its mechanism. Food Chem. 2021, 351, 129344. [Google Scholar] [CrossRef]
- Jun, J.; Jung, M.; Jeong, I.; Kim, D.; Kim, B. Effects of a freeze-thaw-pressing muscle separation on the biochemical quality and self-stability of leg meat from red snow crab (Chionoecetes japonicus). LWT 2019, 99, 276–282. [Google Scholar] [CrossRef]
- Walayat, N.; Liu, J.; Nawaz, A.; Aadil, R.M.; Lopez, P.M.; Lorenzo, J.M. Role of food hydrocolloids as antioxidants along with modern processing techniques on the surimi protein gel textural properties, developments, limitation and future perspectives. Antioxidants 2022, 11, 486. [Google Scholar] [CrossRef]
- Santana, P.; Zilda, D.S.; Huda, N. Physicochemical properties of surimi powder made from threadfin bream (Nemipterus japonicus) with various dryoprotectants added. J. Fundam. Appl. Sci. 2018, 9, 86. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, G.; Wang, J.; Wang, Y.; Jin, G.; Teng, W.; Geng, F.; Cao, J. Myofibrillar protein denaturation/oxidation in freezing-thawing impair the heat-induced gelation: Mechanisms and control technologies. Trends Food Sci. Technol. 2023, 138, 655–670. [Google Scholar] [CrossRef]
- Gao, X.; Li, K.; Xiong, S.; Liu, R. Changes in transglutaminase activity and its contribution to gelation properties of low-salt myosin under ultrasound. Food Bioprocess Technol. 2024, 17, 2253–2264. [Google Scholar] [CrossRef]
- Tang, S.; Feng, G.; Cui, W.; Gao, R.; Bai, F.; Wang, J.; Zhao, Y.; Zeng, M. Effect of α-Tocopherol on the Physicochemical Properties of Sturgeon Surimi during Frozen Storage. Molecules 2019, 24, 710. [Google Scholar] [CrossRef]
- Feng, J.; Wang, J.; Zhang, T.; Liu, Y.; Suo, R.; Ma, Q. Independent and combined effects of ultrasound and transglutaminase on the gel properties and in vitro digestion characteristics of bay scallop (Argopecten irradians) adductor muscle. Curr. Res. Food Sci. 2022, 5, 1185–1194. [Google Scholar] [CrossRef]
- Yan, S.; Liu, X.; Sang, Y.; Tian, G. Gel mechanism analysis of minced scallop (Patinopecten yessoensis) meat modified by three kinds of food colloids. Food Biosci. 2024, 57, 103541. [Google Scholar] [CrossRef]
- Rittenschober, D.; Nowak, V.; Charrondiere, U.R. Review of availability of food composition data for fish and shellfish. Food Chem. 2013, 141, 4303–4310. [Google Scholar] [CrossRef]
- Yang, J.; Yu, X.; Dong, X.; Xu, C. Improvement of surimi gel from frozen-stored silver carp. Gels 2024, 10, 374. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, N.; Yu, W.; Jin, Z.; Jiang, P.; Yu, C.; Dong, X. Effects of microbial transglutaminase on textural, water distribution, and microstructure of frozen-stored longtail southern cod (Patagonotothen ramsayi) fish mince gel. J. Texture Stud. 2022, 53, 844–853. [Google Scholar] [CrossRef]
- Yongsawatdigul, J.; Worratao, A.; Park, J.W. Effect of endogenous transglutaminase on threadfin bream surimi gelation. J. Food Sci. 2002, 67, 3258–3263. [Google Scholar] [CrossRef]
- Yi, S.; Li, Q.; Qiao, C.; Zhang, C.; Wang, W.; Xu, Y.; Mi, H.; Li, X.; Li, J. Myofibrillar protein conformation enhance gel properties of mixed surimi gels with Nemipterus virgatus and Hypophthalmichthys molitrix. Food Hydrocoll. 2020, 106, 105924. [Google Scholar] [CrossRef]
- Xia, X.; Kong, B.; Liu, Q.; Liu, J. Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze-thaw cycles. Meat Sci. 2009, 83, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Wang, Y.; Xia, L.; Lian, H.; Jin, W.; Li, S.; Qiao, F.; Dong, X. Physiochemical and rheological properties of oxidized Japanese seerfish (Scomberomorus niphonius) myofibrillar protein. J. Food Biochem. 2019, 43, e13079. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhao, T.; Li, S.; Wang, Z.; Wen, C.; Wang, H.; Yu, C.; Ji, C. Stability, microstructure, and digestibility of whey protein isolate—Tremella fuciformis polysaccharide complexes. Food Hydrocoll. 2019, 89, 379–385. [Google Scholar] [CrossRef]
- You, J.; Pan, J.; Shen, H.; Luo, Y. Changes in physicochemical properties of bighead carp (Aristichthys mobilis) actomyosin by thermal treatment. Int. J. Food Prop. 2012, 15, 1276–1285. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Jia, S.; Huang, Z.; Luo, Y. Effect of different stunning methods on antioxidant status, in vivo myofibrillar protein oxidation, and the susceptibility to oxidation of silver carp (Hypophthalmichthys molitrix) fillets during 72 h postmortem. Food Chem. 2018, 246, 121–128. [Google Scholar] [CrossRef]
- Liu, B.; Wu, Y.; Liang, Q.Y.; Zheng, H. Effects of high-intensity ultrasound on physicochemical and gel properties of myofibrillar proteins from the bay scallop (Argopecten irradians). Ultrason. Sonochem. 2024, 107, 106935. [Google Scholar] [CrossRef]
- Xie, D.; Tang, Y.; Dong, G. Various factors affecting the gel properties of surimi: A review. J. Texture Stud. 2024, 55, e12847. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, X.; Wei, S.; Zheng, J.; Prakash, S.; Dong, X. Impact of homogenization on the physicochemical properties of the cod protein gel. Food Sci. Technol. 2021, 149, 111841. [Google Scholar] [CrossRef]
- Gao, X.; Yongsawatdigul, J.; Wu, R.; You, J.; Xiong, S.; Du, H.; Liu, R. Effect of ultrasound pre-treatment modes on gelation properties of silver carp surimi. Food Sci. Technol. 2021, 150, 111945. [Google Scholar] [CrossRef]
- Dai, H.; Chen, X.; Peng, L.; Ma, L.; Sun, Y.; Li, L.; Wang, Q.; Zhang, Y. The mechanism of improved myosin gel properties by low dose rosmarinic acid addition during gel formation. Food Hydrocoll. 2020, 106, 105869. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, L.; Gao, P.; Yu, P.; Yang, F.; Yu, D.; Chen, H.; Xia, W. Study on the effect and mechanism of chicken breast on the gel properties of silver carp (Hypophthalmichtys molitrix) surimi. J. Sci. Food Agric. 2024, 104, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, C.; Chen, X.; Li, P.; Ma, F.; Lu, Q. Contribution of Three Ionic Types of Polysaccharides to the Thermal Gelling Properties of Chicken Breast Myosin. J. Agric. Food Chem. 2014, 62, 2655–2662. [Google Scholar] [CrossRef] [PubMed]
- Chanarat, S.; Benjakul, S.; H-Kittikun, A. Comparative study on protein cross-linking and gel enhancing effect of microbial transglutaminase on surimi from different fish. J. Sci. Food Agric. 2012, 92, 844–852. [Google Scholar] [CrossRef]
- An, Y.; You, J.; Xiong, S.; Yin, T. Short-term frozen storage enhances cross-linking that was induced by transglutaminase in surimi gels from silver carp (Hypophthalmichthys molitrix). Food Chem. 2018, 257, 216–222. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Z.Q.; Li, D.Y.; Yu, M.M.; Liu, Y.X.; Qin, L.; Zhou, D.Y.; Shahidi, F.; Zhu, B.W. Action of endogenous proteases on texture deterioration of the bay scallop (Argopecten irradians) adductor muscle during cold storage and its mechanism. Food Chem. 2020, 323, 126790. [Google Scholar] [CrossRef]
- Yang, F.; Jing, D.; Yu, D.; Xia, W.; Jiang, Q.; Xu, Y.; Yu, P. Differential roles of ice crystal, endogenous proteolytic activities and oxidation in softening of obscure pufferfish (Takifugu obscurus) fillets during frozen storage. Food Chem. 2019, 278, 452–459. [Google Scholar] [CrossRef]
- Shi, Y.; Geng, J.T.; Yoshida, Y.; Jiang, J.; Osako, K. Mechanism study of the gel-forming ability of heat-induced gel from Peruvian hake (Merluccius gayi peruanus) surimi. Food Chem. 2023, 413, 135635. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Xie, Q.; Wang, Y.; Yu, J.; Ma, X. Physicochemical and structural changes of myofibrillar proteins in muscle foods during thawing: Occurrence, consequences, evidence, and implications. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3444–3477. [Google Scholar] [CrossRef]
- Tan, M.; Mei, J.; Xie, J. The Formation and Control of Ice Crystal and Its Impact on the Quality of Frozen Aquatic Products: A Review. Crystals 2021, 11, 68. [Google Scholar] [CrossRef]
- Walayat, N.; Xiong, Z.; Xiong, H.; Moreno, H.M.; Niaz, N.; Ahmad, M.N.; Hassan, A.; Nawaz, A.; Ahmad, I.; Wang, P. Cryoprotective effect of egg white proteins and xylooligosaccharides mixture on oxidative and structural changes in myofibrillar proteins of Culter alburnus during frozen storage. Int. J. Biol. Macromol. 2020, 158, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, W.; Wei, H.; Deng, S.; Yu, X.; Huang, T. The mechanisms and applications of cryoprotectants in aquatic products: An overview. Food Chem. 2023, 408, 135202. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhao, Y.; Zhang, Y.; Simpson, B.K.; Chen, S.; Wang, Y.; Li, C.; Wang, D.; Pan, C. Recent advances in the effects of protein oxidation on aquatic products quality: Mechanism and regulation. Int. J. Food Sci. Technol. 2024, 59, 1968–1978. [Google Scholar] [CrossRef]
- Feng, X.; Yu, X.; Yang, Y.; Tang, X. Improving the freeze-thaw stability of fish myofibrils and myofibrillar protein gels: Current methods and future perspectives. Food Hydrocoll. 2023, 144, 109041. [Google Scholar] [CrossRef]
- Wu, D.; Cao, Y.; Yin, T.; Huang, Q. Inhibitive effect of trehalose and sodium pyrophosphate on oxidation and structural changes of myofibrillar proteins in silver carp surimi during frozen storage. Food Res. Int. 2024, 187, 114361. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, J.; Liu, Y.; Zheng, Y.; Pi, R.; Mubango, E.; Tan, Y.; Luo, Y.; Hong, H. Inhibitive effect of cryoprotectants on the oxidative and structural changes in myofibrillar proteins of unwashed mince from silver carp during frozen storage. Food Res. Int. 2022, 161, 111880. [Google Scholar] [CrossRef]
- Xie, J.; Yan, Y.; Pan, Q.; Shi, W.; Gan, J.; Lu, Y.; Tao, N.; Wang, X.; Wang, Y.; Xu, C. Effect of frozen time on Ctenopharyngodon idella surimi: With emphasis on protein denaturation by Tri-step spectroscopy. J. Mol. Struct. 2020, 1217, 128421. [Google Scholar] [CrossRef]
- Tan, M.; Xu, J.; Gao, H.; Yu, Z.; Liang, J.; Mu, D.; Zheng, Z. Effects of combined high hydrostatic pressure and pH-shifting pretreatment on the structure and emulsifying properties of soy protein isolates. J. Food Eng. 2021, 306, 110622. [Google Scholar] [CrossRef]
- Sun, Q.; Kong, B.; Liu, S.; Zheng, O.; Zhang, C. Ultrasonic freezing reduces protein oxidation and myofibrillar gel quality loss of common carp (Cyprinus carpio) during long-time frozen storage. Foods 2021, 10, 629. [Google Scholar] [CrossRef]
- Zhu, W.; Guo, H.; Han, M.; Shan, C.; Bu, Y.; Li, J.; Li, X. Evaluating the effects of nanoparticles combined ultrasonic-microwave thawing on water holding capacity, oxidation, and protein conformation in jumbo squid (Dosidicus gigas) mantles. Food Chem. 2023, 402, 134250. [Google Scholar] [CrossRef]
- Li, F.; Du, X.; Ren, Y.; Kong, B.; Wang, B.; Xia, X.; Bao, Y. Impact of ice structuring protein on myofibrillar protein aggregation behaviour and structural property of quick-frozen patty during frozen storage. Int. J. Biol. Macromol. 2021, 178, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Xu, A.; Chen, Y.; Sun, N.; Zhang, J.; Jia, R.; Huang, T.; Yang, W. Effect of laver powder on textual, rheological properties and water distribution of squid (Dosidicus gigas) surimi gel. J. Texture Stud. 2020, 51, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, L.; Li, Q.; Luo, Y. Comparison of gel properties and biochemical characteristics of myofibrillar protein from bighead carp (Aristichthys nobilis) affected by frozen storage and a hydroxyl radical-generation oxidizing system. Food Chem. 2017, 223, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, J.; Yan, W.; Liu, R.; Yin, T.; You, J.; Du, H.; Xiong, S.; Hu, Y. Physicochemical changes of MTGase cross-linked surimi gels subjected to liquid nitrogen spray freezing. Int. J. Biol. Macromol. 2020, 160, 642–651. [Google Scholar] [CrossRef]
- Bao, Y.; Ertbjerg, P.; Estevez, M.; Yuan, L.; Gao, R. Freezing of meat and aquatic food: Underlying mechanisms and implications on protein oxidation. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5548–5569. [Google Scholar] [CrossRef]
- Zhu, M.; Li, H.; Xing, Y.; Ma, C.; Peng, Z.; Jiao, L.; Kang, Z.; Zhao, S.; Ma, H. Understanding the influence of fluctuated low-temperature combined with high-humidity thawing on gelling properties of pork myofibrillar proteins. Food Chem. 2023, 404, 134238. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Y.; Cao, W.; Zhou, L.; Zhang, C.; Qin, X.; Zheng, H.; Lin, H.; Gao, J. Novel insight into the role of processing stages in nutritional components changes and characteristic flavors formation of noble scallop Chlamys nobilis adductors. Food Chem. 2022, 378, 132049. [Google Scholar] [CrossRef]
- Kono, S.; Kon, M.; Araki, T.; Sagara, Y. Effects of relationships among freezing rate, ice crystal size and color on surface color of frozen salmon fillet. J. Food Eng. 2017, 214, 158–165. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.; Thongkaew, C.; Tanaka, M. Effect of frozen storage on chemical and gel-forming properties of fish commonly used for surimi production in Thailand. Food Hydrocoll. 2005, 19, 197–207. [Google Scholar] [CrossRef]



| Freezing Time (d) | Hardness (N) | Springiness | Chewiness (mJ) | Cohesiveness | Whiteness |
|---|---|---|---|---|---|
| 0 | 12.80 ± 0.70 b | 0.63 ± 0.01 b | 5.22 ± 0.33 b | 0.64 ± 0.01 b | 69.53 ± 0.41 c |
| 1 | 15.08 ± 0.75 a | 0.68 ± 0.01 a | 7.06 ± 0.56 a | 0.68 ± 0.01 a | 71.40 ± 0.26 b |
| 7 | 8.10 ± 0.17 d | 0.42 ± 0.02 d | 1.60 ± 0.18 d | 0.47 ± 0.02 d | 73.41 ± 0.15 a |
| 14 | 10.7 ± 0.45 c | 0.54 ± 0.02 c | 3.29 ± 0.27 c | 0.57 ± 0.02 c | 73.25 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, K.; Lin, Y.; Huang, S.; Fan, X.; Ma, Y.; Li, M.; Zhao, Q. Optimizing Bay Scallop (Argopecten irradians) Product Quality: Moderate Freezing as an Effective Strategy for Improving Adductor Muscle Gel Properties. Foods 2025, 14, 1371. https://doi.org/10.3390/foods14081371
Chang K, Lin Y, Huang S, Fan X, Ma Y, Li M, Zhao Q. Optimizing Bay Scallop (Argopecten irradians) Product Quality: Moderate Freezing as an Effective Strategy for Improving Adductor Muscle Gel Properties. Foods. 2025; 14(8):1371. https://doi.org/10.3390/foods14081371
Chicago/Turabian StyleChang, Kexin, Yufan Lin, Sijia Huang, Xinru Fan, Yongsheng Ma, Meng Li, and Qiancheng Zhao. 2025. "Optimizing Bay Scallop (Argopecten irradians) Product Quality: Moderate Freezing as an Effective Strategy for Improving Adductor Muscle Gel Properties" Foods 14, no. 8: 1371. https://doi.org/10.3390/foods14081371
APA StyleChang, K., Lin, Y., Huang, S., Fan, X., Ma, Y., Li, M., & Zhao, Q. (2025). Optimizing Bay Scallop (Argopecten irradians) Product Quality: Moderate Freezing as an Effective Strategy for Improving Adductor Muscle Gel Properties. Foods, 14(8), 1371. https://doi.org/10.3390/foods14081371






