Possible Genotoxic Effects of Post-Harvest Fungicides Applied on Citrus Peels: Imazalil, Pyrimethanil, Thiabendazole and Their Mixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungicides and Application
2.2. Ames Test
2.3. Comet Assay
2.3.1. Gel Slide Preparation
2.3.2. Cell Culture Preparation
2.4. Statistical Analysis and Methods
3. Results
3.1. Ames Test Results
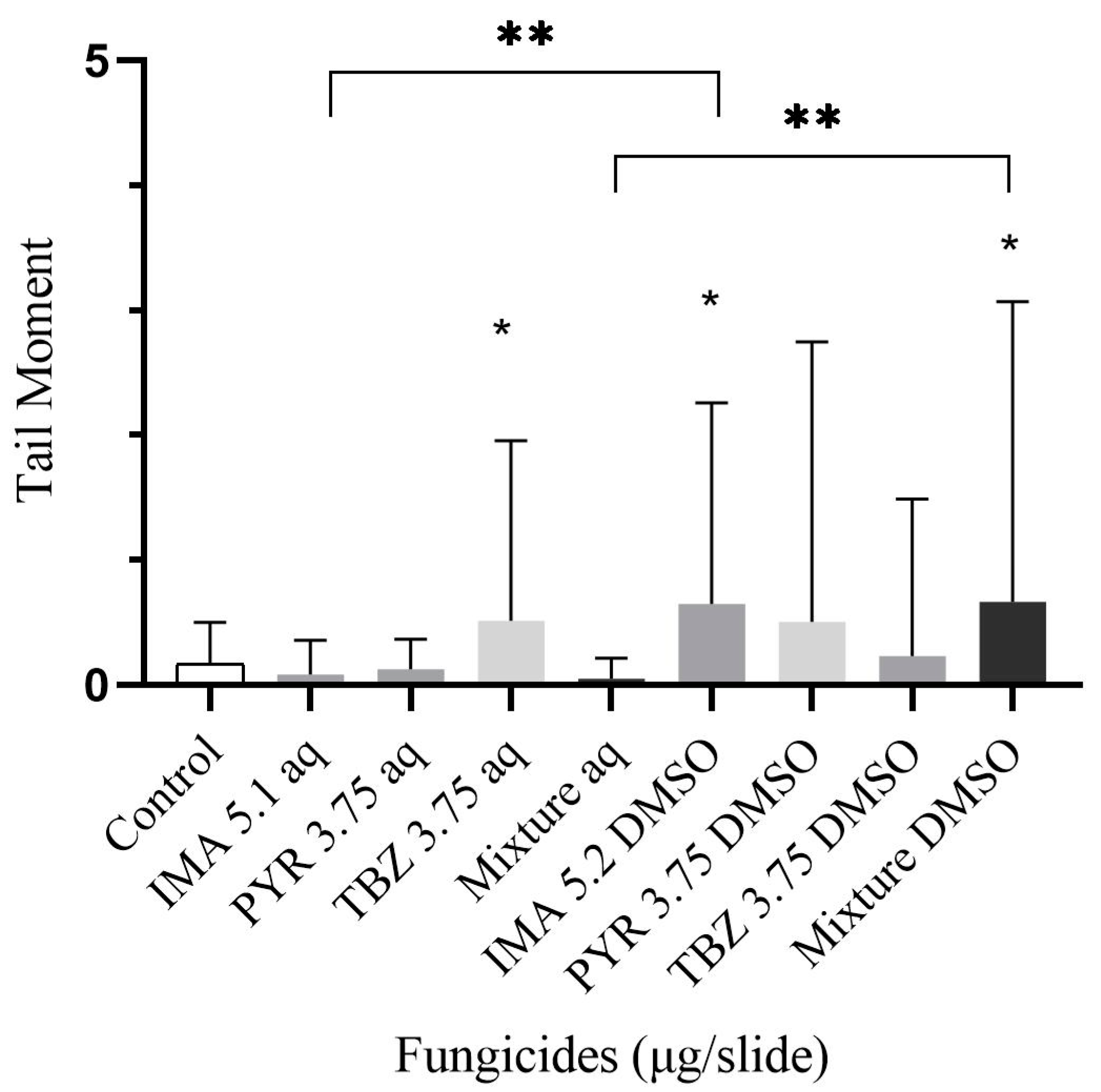
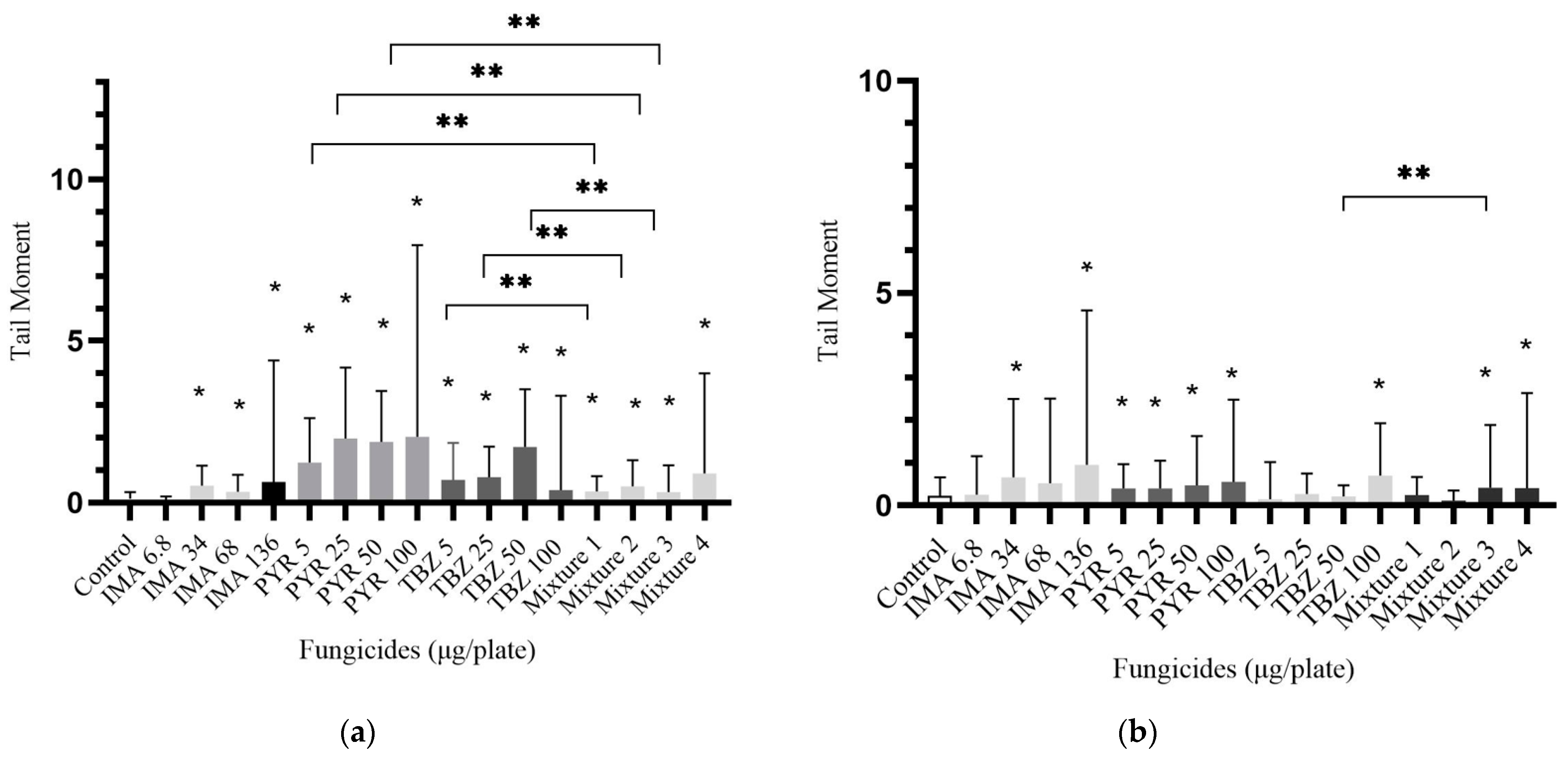
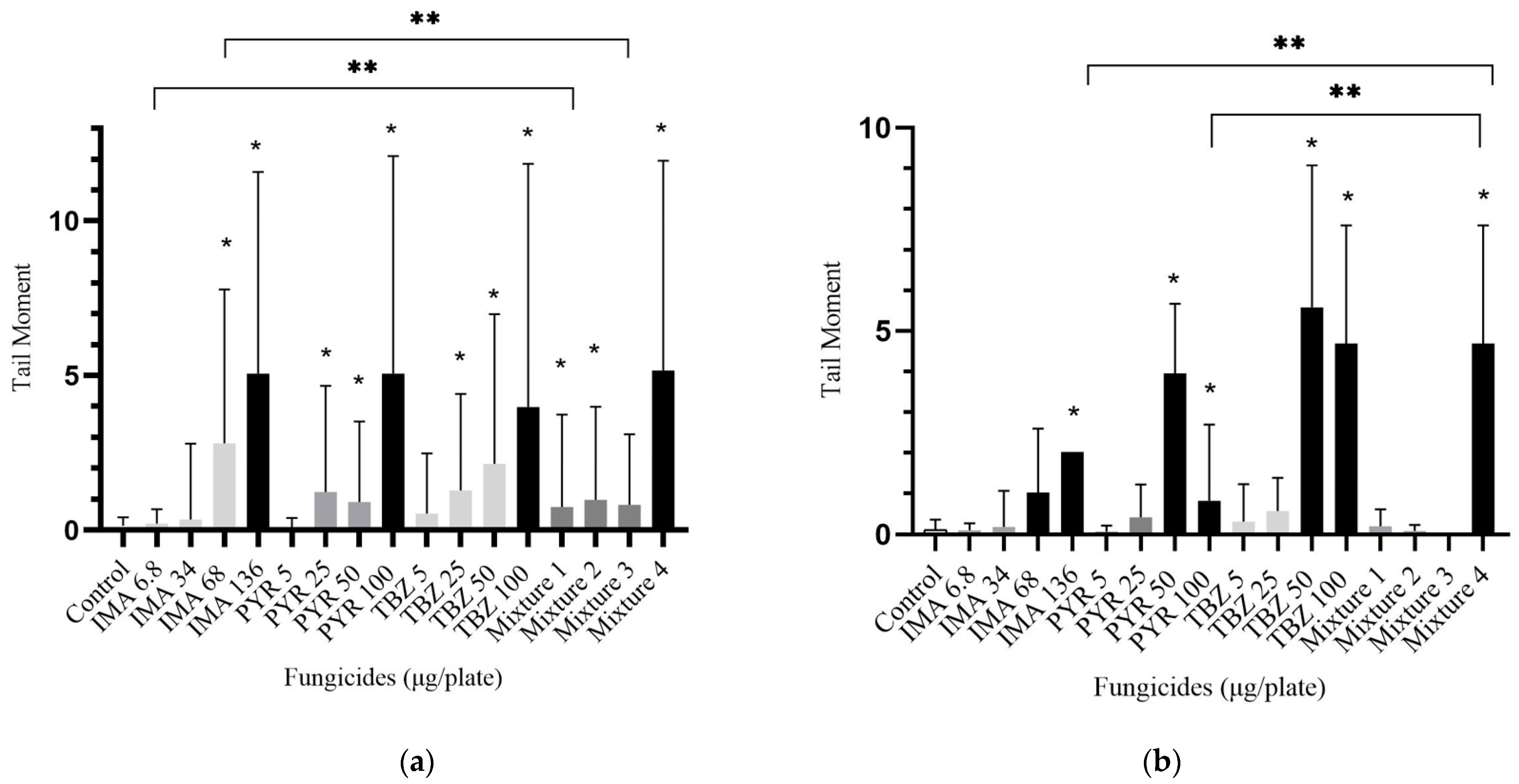
3.2. DNA Lesions Evaluated by the Comet Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serrano-Molina, L.; Hernández-López, M.; Salazar-Piña, D.A.; Bautista-Baños, S.; Ramos-García, M.d.L. The Response of Naturally Based Coatings and Citrus Fungicides to the Development of Four Postharvest Fungi. J. Fungi 2024, 10, 309. [Google Scholar] [CrossRef] [PubMed]
- Altieri, G.; Di Renzo, G.C.; Genovese, F.; Calandra, M.; Strano, M.C. A new method for the postharvest application of imazalil fungicide to citrus fruit. Biosyst. Eng. 2013, 115, 434–443. [Google Scholar] [CrossRef]
- Duan, B.; Wang, Z.; Reymick, O.O.; Zhang, M.; Chen, Y.; Meng, K.; Tao, N. Wax treatment delays the coloration of postharvest citrus fruit by retarding the carotenoid biosynthesis pathway. Sci. Hortic. 2023, 321, 112379. [Google Scholar] [CrossRef]
- Zhang, J.J.; Yang, H. Metabolism and detoxification of pesticides in plants. Sci. Total Environ. 2021, 790, 148034. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Bibi, A.; Anjum, F.; Hussain, S.R.; Ahad, A.; Deen, A.U.; Suleman, M.; Jan, B. Surveillance of pesticide residues in tomato and eggplant and assessment of acute and chronic health risks to the consumers in Pakistan. Environ. Sci. Pollut. Res. Int. 2024, 31, 6385–6397. [Google Scholar] [CrossRef]
- Rahman, A.; Baharlouei, P.; Koh, E.H.Y.; Pirvu, D.G.; Rehmani, R.; Arcos, M.; Puri, S.A. Comprehensive Analysis of Organic Food: Evaluating Nutritional Value and Impact on Human Health. Foods 2024, 13, 208. [Google Scholar] [CrossRef]
- Hashemi, F.; Mogensen, L.; van der Werf, H.M.G.; Cederberg, C.; Knudsen, M.T. Organic food has lower environmental impacts per area unit and similar climate impacts per mass unit compared to conventional. Commun. Earth Environ. 2024, 5, 250. [Google Scholar] [CrossRef]
- Bopp, S.K.; Barouki, R.; Brack, W.; Dalla Costa, S.; Dorne, J.L.C.M.; Drakvik, P.E.; Faust, M.; Karjalainen, T.K.; Kephalopoulos, S.; van Klaveren, J.; et al. Current EU research activities on combined exposure to multiple chemicals. Environ. Int. 2018, 120, 544–562. [Google Scholar] [CrossRef]
- Bhatta, U.K. Alternative Management Approaches of Citrus Diseases Caused by Penicillium digitatum (Green Mold) and Penicillium italicum (Blue Mold). Front. Plant Sci. 2022, 22, 833328. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Imazalil. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/fs_PC-111901_1-Feb-05.pdf (accessed on 1 February 2005).
- Sherif, M.; Makame, K.R.; Östlundh, L.; Paulo, M.S.; Nemmar, A.; Ali, B.R.; Al-Rifai, R.H.; Nagy, K.; Ádám, B. Genotoxicity of Occupational Pesticide Exposures among Agricultural Workers in Arab Countries: A Systematic Review and Meta-Analysis. Toxics 2023, 11, 663. [Google Scholar] [CrossRef]
- Jin, C.; Zeng, Z.; Fu, Z.; Jin, Y. Oral imazalil exposure induces gut microbiota dysbiosis and colonic inflammation in mice. Chemosphere 2016, 160, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Jurak, G.; Bošnir, J.; Đikić, D.; Ćuić, A.M.; Prokurica, I.P.; Racz, A.; Jukić, T.; Stubljar, D.; Starc, A. The Risk Assessment of Pesticide Ingestion with Fruit and Vegetables for Consumer’s Health. Int. J. Food Sci. 2021, 2021, 9990219. [Google Scholar] [CrossRef]
- European Commission. Search Pesticide Residues. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/mrls (accessed on 15 March 2025).
- Aleksić, M.; Stanisavljević, D.; Smiljković, M.; Vasiljević, P.; Stevanović, M.; Soković, M.; Stojković, D. Pyrimethanil: Between efficient fungicide against Aspergillus rot on cherry tomato and cytotoxic agent on human cell lines. Ann. Appl. Biol. 2019, 175, 228–235. [Google Scholar] [CrossRef]
- Giffin, A.; Hoppin, J.A.; Córdoba, L.; Solano-Díaz, K.; Ruepert, C.; Peñaloza-Castañeda, J.; Lindh, C.; Reich, B.J.; van Wendel de Joode, B. Pyrimethanil and chlorpyrifos air concentrations and pregnant women’s urinary metabolites in the Infants’ Environmental Health Study (ISA), Costa Rica. Environ. Int. 2022, 166, 107328. [Google Scholar] [CrossRef] [PubMed]
- Faniband, M.; Ekman, E.; Littorin, M.; Maxe, M.; Larsson, E.; Lindh, C.H. Biomarkers of Exposure to Pyrimethanil After Controlled Human Experiments. J. Anal. Toxicol. 2019, 43, 277–283. [Google Scholar] [CrossRef]
- Lafon, P.A.; Wang, Y.; Arango-Lievano, M.; Torrent, J.; Salvador-Prince, L.; Mansuy, M.; Mestre-Francès, N.; Givalois, L.; Liu, J.; Mercader, J.V.; et al. Fungicide Residues Exposure and β-amyloid Aggregation in a Mouse Model of Alzheimer’s Disease. Environ. Health Perspect. 2020, 128, 17011. [Google Scholar] [CrossRef]
- Rastkari, N.; Ahmadkhaniha, R.; Soleymani, F.; Ravanipour, M. Pesticide residues in drinking water treatment plants and human health risk assessment: A case study from Northern Iran. Environ. Geochem. Health 2024, 46, 68. [Google Scholar] [CrossRef]
- Fujii, T.; Mikuriya, H.; Kamiya, N.; Hiraga, K. Enhancing effect of thiabendazole on urinary bladder carcinogenesis induced by sodium o-phenylphenate in F344 rats. Food Chem. Toxicol. 1986, 24, 207–211. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Thiabendazole and Salts. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/fs_PC-060101_1-May-02.pdf (accessed on 22 March 2025).
- He, J.; Zhu, X.; Xu, K.; Li, Y.; Zhou, J. Network toxicological and molecular docking to investigate the mechanisms of toxicity of agricultural chemical Thiabendazole. Chemosphere 2024, 363, 142711. [Google Scholar] [CrossRef]
- Radulović, J.; Lučić, M.; Nešić, A.; Onjia, A. Multivariate Assessment and Risk Ranking of Pesticide Residues in Citrus Fruits. Foods 2023, 12, 2454. [Google Scholar] [CrossRef] [PubMed]
- Omeroglu, P.Y.; Acoglu Celik, B.; Koc Alibasoglu, E. The Effect of Household Food Processing on Pesticide Residues in Oranges (Citrus sinensis). Foods 2022, 11, 3918. [Google Scholar] [CrossRef] [PubMed]
- Vass, A.; Korpics, E.; Dernovics, M. Follow-up of the fate of imazalil from post-harvest lemon surface treatment to a baking experiment. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2015, 32, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Zhao, Q.; Wang, C.; Cui, Y.; Li, J.; Chen, A.; Liang, G.; Jiao, B. Occurrence, temporal variation, quality and safety assessment of pesticide residues on citrus fruits in China. Chemosphere 2020, 258, 127381. [Google Scholar] [CrossRef]
- Calvaruso, E.; Cammilleri, G.; Pulvirenti, A.; Lo Dico, G.M.; Lo Cascio, G.; Giaccone, V.; Badaco, V.V.; Ciprí, V.; Ciprì, V.; Alessandra, M.M.; et al. Residues of 165 pesticides in citrus fruits using LC-MS/MS: A study of the pesticides distribution from the peel to the pulp. Nat. Prod. Res. 2020, 34, 1–5. [Google Scholar] [CrossRef]
- Tambo, J.A.; Kansiime, M.K.; Alaganthiran, J.R.; Muhammad, D.; Duah, S.A.; Faisal, F.; Khonje, M.G.; Mbugua, F.; Rajendran, G. Consumer pesticide concerns and the choice of fruit and vegetable markets in five low- and middle-income countries. Glob. Food Secur. 2024, 42, 100801. [Google Scholar] [CrossRef]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Zand, A.; Enkhbilguun, S.; Macharia, J.M.; Varajti, K.; Szabó, I.; Gerencsér, G.; Tisza, B.B.; Raposa, B.L.; Gyöngyi, Z.; Varjas, T. Betanin Attenuates Epigenetic Mechanisms and UV-Induced DNA Fragmentation in HaCaT Cells: Implications for Skin Cancer Chemoprevention. Nutrients 2024, 16, 860. [Google Scholar] [CrossRef]
- Gerencsér, G.; Szabó, I.; Szendi, K.; Hanzel, A.; Raposa, B.; Gyöngyi, Z.; Varga, C. Effects of medicinal waters on the UV-sensitivity of human keratinocytes—A comparative pilot study. Int. J. Biometeorol. 2019, 63, 1417–1423. [Google Scholar] [CrossRef]
- Parry, J.M.; Parry, E.M. Genotoxicology Principles and Methods, 1st ed.; Humana Press: New York, NY, USA, 2012; pp. 143–164. [Google Scholar] [CrossRef]
- Szendi, K.; Gyöngyi, Z.; Kontár, Z.; Gerencsér, G.; Berényi, K.; Hanzel, A.; Fekete, J.; Kovács, A.; Varga, C. Mutagenicity and Phthalate Level of Bottled Water Under Different Storage Conditions. Expo. Health 2018, 10, 51–60. [Google Scholar] [CrossRef]
- Erasmus, A.; Lennox, C.L.; Jordaan, H.; Smilanick, J.L.; Lesar, K.; Fourie, P.H. Imazalil residue loading and green mould control in citrus packhouses. Postharvest Biol. Technol. 2011, 62, 193–203. [Google Scholar] [CrossRef]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An Overlooked Pesticide Class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef] [PubMed]
- Ilyushina, N.A.; Egorova, O.V.; Masaltsev, G.V.; Averianova, N.S.; Revazova, Y.A.; Rakitskii, V.N.; Goumenou, M.; Vardavas, A.; Stivaktakis, P.; Tsatsakis, A. Genotoxicity of mixture of imidacloprid, imazalil and tebuconazole. Toxicol. Rep. 2020, 30, 1090–1094. [Google Scholar] [CrossRef]
- Egorova, O.V.; Ilyushina, N.A.; Rakitskii, V.N. Mutagenicity evaluation of pesticide analogs using standard and 6-well miniaturized bacterial reverse mutation tests. Toxicol. In Vitro 2020, 69, 105006. [Google Scholar] [CrossRef]
- Kirkland, D.; Reeve, L.; Gatehouse, D.; Vanparys, P. A core in vitro genotoxicity battery comprising the Ames test plus the in vitro micronucleus test is sufficient to detect rodent carcinogens and in vivo genotoxins. Mutat. Res. 2011, 721, 27–73. [Google Scholar] [CrossRef]
- Watanabe-Akanuma, M.; Ohta, T.; Sasaki, Y.F. A novel genotoxic aspect of thiabendazole as a photomutagen in bacteria and cultured human cells. Toxicol. Lett. 2005, 158, 213–219. [Google Scholar] [CrossRef]
- Đikić, D.; Mojsović-Ćuić, A.; Čupor, I.; Benković, V.; Horvat-Knežević, A.; Lisičić, D.; Oršolić, N. Carbendazim combined with imazalil or cypermethrin potentiate DNA damage in hepatocytes of mice. Hum. Exp. Toxicol. 2012, 31, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Séïde, M.; Marion, M.; Mateescu, M.A.; Averill-Bates, D.A. The fungicide thiabendazole causes apoptosis in rat hepatocytes. Toxicol. In Vitro 2016, 32, 232–239. [Google Scholar] [CrossRef]
- Oskoei, P.; Marçal, R.; Oliveira, H.; Guilherme, S. Hitting two targets with one shot on pesticide genotoxicity assessment—Identifying risk while unveiling ex vivo approach as a throughput tool in gill-breathing animals. J. Hazard. Mater. 2024, 476, 134948. [Google Scholar] [CrossRef]
- Vindas, R.; Ortiz, F.; Ramírez, V.; Cuenca, P. Genotoxicity of three pesticides used in Costa Rican banana plantation. Rev. Biol. Trop. 2004, 52, 601–609. [Google Scholar]
- Nakagawa, Y.; Moore, G.A. Cytotoxic effects of post-harvest fungicides, ortho-phenylphenol, thiabendazole and imazalil, on isolated rat hepatocytes. Life Sci. 1995, 57, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Sişman, T.; Türkez, H. Toxicologic evaluation of imazalil with particular reference to genotoxic and teratogenic potentials. Toxicol. Ind. Health 2010, 26, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.F.; Saga, A.; Akasaka, M.; Yoshida, K.; Nishidate, E.; Su, Y.Q.; Matsusaka, N.; Tsuda, S. In vivo genotoxicity of ortho-phenylphenol, biphenyl, and thiabendazole detected in multiple mouse organs by the alkaline single cell gel electrophoresis assay. Mutat. Res. 1997, 395, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, F.; Arena, M.; Auteri, D.; Leite, S.B.; Binaglia, M.; Castoldi, A.F.; Chiusolo, A.; Colagiorgi, A.; Colas, M.; Crivellente, F.; et al. Peer review of the pesticide risk assessment of the active substance pyrimethanil. EFSA J. 2024, 22, e8998. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Pesticides Functional Classes. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/Functional-Classes (accessed on 16 March 2025).
- Gironi, B.; Kahveci, Z.; McGill, B.; Lechner, B.; Pagliara, S.; Metz, J.; Morresi, A.; Palombo, F.; Sassi, P.; Petrov, P.G. Effect of DMSO on the Mechanical and Structural Properties of Model and Biological Membranes. Biophys. J. 2020, 119, 247–286. [Google Scholar] [CrossRef]
| Concentration | Imazalil | Pyrimethanil | Thiabendazole | Mixture (IMA + PYR + TBZ) |
|---|---|---|---|---|
| Gel slide preparation μg/slide | 5.1 | 3.75 | 3.75 | 1.7 + 1.25 + 1.25 |
| Cell cultures μg/plate | 6.8 | 5 | 5 | 2.27 + 1.67 + 1.67 (Mixture 1) |
| 34 | 25 | 25 | 11.35 + 9.35 + 8.35 (Mixture 2) | |
| 68 | 50 | 50 | 22.7 + 16.7 + 16.7 (Mixture 3) | |
| 126 | 100 | 100 | 45.4 + 33.4 + 33.4 (Mixture 4) |
| Aqueous Samples | Concentration μg/plate | TA98 (S9−) | TA98 (S9+) | TA100 (S9−) | TA100 (S9+) |
|---|---|---|---|---|---|
| Mean ± SD | |||||
| Negative Control | 45 ± 13 | 45 ± 5 | 105.3 ± 5 | 99 ± 1.7 | |
| Positive control | 1206 | 1508 | 1905 | 2132 | |
| Imazalil | 20.4 μg/plate | 65 ± 8.7 | 84.3 ± 6.6 * | 129.6 ± 5.8 * | 124 ± 10.7 |
| Imazalil | 40.8 μg/plate | 53 ± 2 | 111 ± 5.2 * | 156.3 ± 8.9 * | 138 ± 22.6 |
| Pyrimethanil | 15 μg/plate | 46.3 ± 16 | 28 ± 3.6 | 129.3 ± 7 * | 91.6 ± 10 |
| Pyrimethanil | 30 μg/plate | 48.6 ± 13 | 29 ± 5.5 | 111.6 ± 15.9 | 111.3 ± 4.7 * |
| Thiabendazole | 15 μg/plate | 84.6 ± 14 * | 40.3 ± 4.7 | 77.6 ± 19 | 146.3 ± 2.3 * |
| Thiabendazole | 30 μg/plate | 136 ± 29 * | 30.6 ± 3.5 | 120.6 ± 5 * | 177.3 ± 46 * |
| Mixture (IMA + PYR + TBZ) | 6.8 + 5 + 5 μg/plate | 48.3 ± 6 | 30 ±8.8 | 127.5 ± 10.7 | 97.3 ± 9.3 |
| Mixture (IMA + PYR + TBZ) | 13.6 + 10 + 10 μg/plate | 52.3 ± 3 | 29 ± 9.8 | 122 ± 9.6 | 98.3 ± 14 |
| DMSO Samples | Concentration μg/plate | TA98 (S9−) | TA98 (S9+) | TA100 (S9−) | TA100 (S9+) |
|---|---|---|---|---|---|
| Mean ± SD | |||||
| Negative control | 7.3 ± 1.5 | 20.3 ± 2.5 | 85.3 ± 13.4 | 85.3 ± 13.4 | |
| Positive control | 2540 | 762 | 1143 | 1016 | |
| Imazalil | 20.4 μg/plate | 30.3 ± 17.03 | 28.6 ± 8.9 | 116.6 ± 14 * | 112 ±16 |
| Imazalil | 40.8 μg/plate | 41 ± 22.5 * | 10.3 ± 0.5 | 36.3 ± 9.4 | 97 ± 15.6 |
| Pyrimethanil | 15 μg/plate | 15.6 ± 7.2 | 15.3 ± 2 | 123 ± 96.2 | 94.6 ± 16.6 |
| Pyrimethanil | 30 μg/plate | 28 ± 6 * | 15.3 ± 4 | 71 ± 46.9 | 92.6 ± 5.8 |
| Thiabendazole | 15 μg/plate | 21 ± 8.5 | 96 ± 5.2 * | 130.3 ± 8 * | 114 ± 9.1 * |
| Thiabendazole | 30 μg/plate | 212.6 ± 21.7 * | 93.3 ± 7.2 * | 81.6 ± 39.1 | 105 ± 7.9 |
| Mixture (IMA + PYR + TBZ) | 6.8 + 5 + 5 μg/plate | 16 ± 5.3 | 21.6 ± 8.4 | 136.3 ± 12.7 | 107 ± 3 |
| Mixture (IMA + PYR + TBZ) | 13.6 + 10 + 10 μg/plate | 11.6 ± 5.03 | 13.3 ± 1.4 | 42.6 ± 40.1 | 74.3 ± 16.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tisza, B.B.; Járomi, L.; Háhn, J.; Bérczi, B.; Horváth-Sarródi, A.; Gubicskóné Kisbenedek, A.; Gerencsér, G. Possible Genotoxic Effects of Post-Harvest Fungicides Applied on Citrus Peels: Imazalil, Pyrimethanil, Thiabendazole and Their Mixtures. Foods 2025, 14, 1264. https://doi.org/10.3390/foods14071264
Tisza BB, Járomi L, Háhn J, Bérczi B, Horváth-Sarródi A, Gubicskóné Kisbenedek A, Gerencsér G. Possible Genotoxic Effects of Post-Harvest Fungicides Applied on Citrus Peels: Imazalil, Pyrimethanil, Thiabendazole and Their Mixtures. Foods. 2025; 14(7):1264. https://doi.org/10.3390/foods14071264
Chicago/Turabian StyleTisza, Boglárka Bernadett, Luca Járomi, Judit Háhn, Bálint Bérczi, Andrea Horváth-Sarródi, Andrea Gubicskóné Kisbenedek, and Gellért Gerencsér. 2025. "Possible Genotoxic Effects of Post-Harvest Fungicides Applied on Citrus Peels: Imazalil, Pyrimethanil, Thiabendazole and Their Mixtures" Foods 14, no. 7: 1264. https://doi.org/10.3390/foods14071264
APA StyleTisza, B. B., Járomi, L., Háhn, J., Bérczi, B., Horváth-Sarródi, A., Gubicskóné Kisbenedek, A., & Gerencsér, G. (2025). Possible Genotoxic Effects of Post-Harvest Fungicides Applied on Citrus Peels: Imazalil, Pyrimethanil, Thiabendazole and Their Mixtures. Foods, 14(7), 1264. https://doi.org/10.3390/foods14071264







