Effect of Glycosidase Production by Rhodotorula mucilaginosa on the Release of Flavor Compounds in Fermented White Radish
Abstract
1. Introduction
2. Materials and Methods
2.1. Genomics Analysis
2.2. Preparation of Fermentation Medium
2.3. Determination of Physical and Chemical Properties of Fermented Radish Juice
2.3.1. Yeast Growth Performance Monitoring
2.3.2. pH, Total Soluble Solid, and Sugar Determination
2.3.3. Soluble Protein
2.4. Determination of Glycosidase Activity
2.5. Proteome Analysis
2.6. Transcriptome Analysis
2.7. Effects of Commercial Glycosidase and Yeast Fermentation on the Flavor Characteristics of Radish Juice
2.8. Statistical Analysis
3. Results and Discussion
3.1. Identification of Glycosidase-Encoding Genes in Yeast
3.2. Fermentation Performance of Yeast in Radish Juice
3.3. Glycosidase Activity
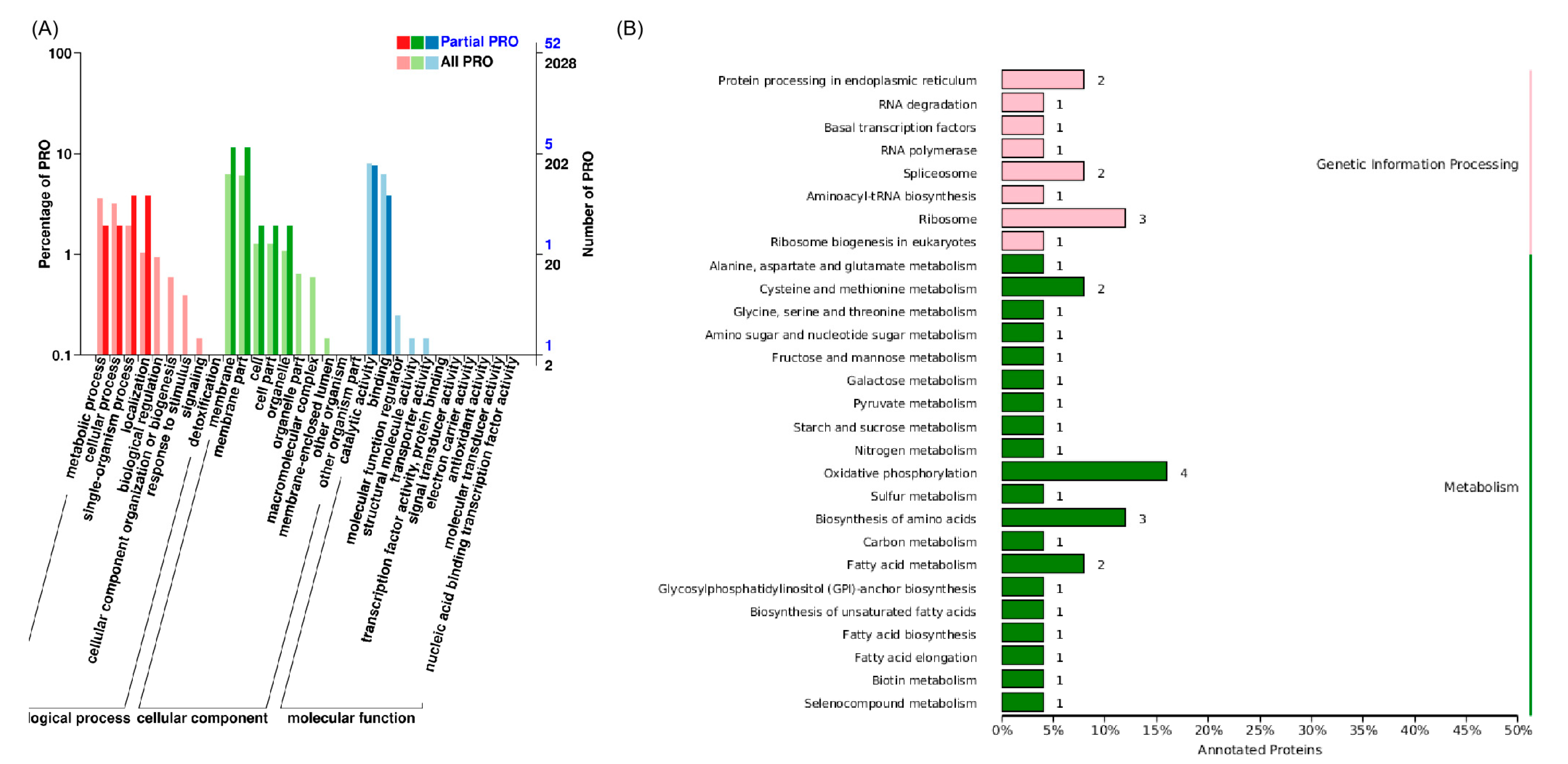
3.4. Proteome Analysis
3.5. Transcriptome Analysis
3.6. Identification of Aroma Compounds Released by Glycosidase Produced by Yeast
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BglX | β-glucosidase gene |
| malL | α-glucosidase maltase gene |
| HPP | high-pressure processing |
| YPD | yeast extract peptone dextrose |
| TSS | total soluble solid |
| DEPs | differentially expressed proteins |
| DEGs | differentially expressed genes |
| OAV | odor activity value |
| ANOVA | analysis of variance |
| CAZy | carbohydrate-active enzyme |
| GHs | glycoside hydrolases |
| manA | mannose-6-phosphate isomerase |
| sacA | sucrose-6-phosphate hydrolase |
| FDR | false discovery rate |
References
- Guo, Q.; Chen, P.; Chen, X. Bioactive peptides derived from fermented foods: Preparation and biological activities. J. Funct. Foods 2023, 101, 105422. [Google Scholar] [CrossRef]
- Kim, J.; Choi, K.-B.; Park, J.H.; Kim, K.H. Metabolite profile changes and increased antioxidative and antiinflammatory activities of mixed vegetables after fermentation by Lactobacillus plantarum. PLoS ONE 2019, 14, e0217180. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, M.; Zheng, J.; Ganzle, M.G. Bacillus species in food fermentations: An underappreciated group of organisms for safe use in food fermentations. Curr. Opin. Food Sci. 2023, 50, 101007. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, Y.; Xiao, L.; Qu, L.; Zhang, X.; Wei, Y. Advancing Insights into Probiotics during Vegetable Fermentation. Foods 2023, 12, 3789. [Google Scholar] [CrossRef]
- Wieczorek, M.N.; Drabinska, N. Flavour Generation during Lactic Acid Fermentation of Brassica Vegetables-Literature Review. Appl. Sci. 2022, 12, 5598. [Google Scholar] [CrossRef]
- Michlmayr, H.; Kneifel, W. β-Glucosidase activities of lactic acid bacteria: Mechanisms, impact on fermented food and human health. Fems Microbiol. Lett. 2014, 352, 1–10. [Google Scholar] [CrossRef]
- Li, K.; Gao, N.; Tang, J.; Ma, H.; Jiang, J.; Duan, Y.; Li, Z. A Study on the Formation of Flavor Substances by Bacterial Diversity in the Fermentation Process of Canned Bamboo Shoots in Clear Water. Foods 2023, 12, 3478. [Google Scholar] [CrossRef]
- Fang, X.; Dong, Y.; Xie, Y.; Wang, L.; Wang, J.; Liu, Y.; Zhao, L.; Cao, F. Effects of -glucosidase and -rhamnosidase on the Contents of Flavonoids, Ginkgolides, and Aroma Components in Ginkgo Tea Drink. Molecules 2019, 24, 2009. [Google Scholar] [CrossRef]
- Song, H.; Wang, X.; Li, A.; Liu, J.; Tao, Y.-S. Profiling terpene glycosides from ecolly, cabernet gernischet, and muscat hamburg grapes by ultra performance liquid chromatography-quadrupole time-of-flight mass spectrometry. J. Food Sci. 2020, 85, 2032–2040. [Google Scholar] [CrossRef]
- Li, M.; Xiao, Y.; Zhong, K.; Wu, Y.; Gao, H. Delving into the Biotransformation Characteristics and Mechanism of Steamed Green Tea Fermented by Aspergillus niger PW-2 Based on Metabolomic and Proteomic Approaches. Foods 2022, 11, 865. [Google Scholar] [CrossRef]
- Chigo-Hernandez, M.M.; DuBois, A.; Tomasino, E. Aroma Perception of Rose Oxide, Linalool and α-Terpineol Combinations in Gewurztraminer Wine. Fermentation 2022, 8, 30. [Google Scholar] [CrossRef]
- Renchinkhand, G.; Magsar, U.; Bae, H.C.; Choi, S.-H.; Nam, M.S. Identification of β-Glucosidase Activity of Lentilactobacillus buchneri URN103L and Its Potential to Convert Ginsenoside Rb1 from Panax ginseng. Foods 2022, 11, 529. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-C.; Li, A.-H.; Dizy, M.; Ullah, N.; Sun, W.-X.; Tao, Y.-S. Evaluation of aroma enhancement for “Ecolly” dry white wines by mixed inoculation of selected Rhodotorula mucilaginosa and Saccharomyces cerevisiae. Food Chem. 2017, 228, 550–559. [Google Scholar] [CrossRef]
- Wu, L.; Wen, Y.; Chen, W.; Yan, T.; Tian, X.; Zhou, S. Simultaneously deleting ADH2 and THI3 genes of Saccharomyces cerevisiae for reducing the yield of acetaldehyde and fusel alcohols. FEMS Microbiol. Lett. 2021, 368, fnab094. [Google Scholar] [CrossRef]
- Waymark, C.; Hill, A.E. The Influence of Yeast Strain on Whisky New Make Spirit Aroma. Fermentation 2021, 7, 311. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Y.; Jiang, L.; Deng, F. Determination of fungal community diversity in fresh and traditional Chinese fermented pepper by pyrosequencing. Microbiol. Lett. 2016, 363, fnw273. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Y.; Wang, T.; Chu, C.; Yi, J.; Liu, Z. Enhancing fermented vegetable flavor with Lactobacillus plantarum and Rhodotorula mucilaginosa. Food Res. Int. 2025, 200, 115500. [Google Scholar] [CrossRef]
- Singh, S.; Chaturvedi, S.; Syed, N.; Rastogi, D.; Kumar, P.; Sharma, P.K.; Kumar, D.; Sahoo, D.; Srivastava, N.; Nannaware, A.D.; et al. Production of fatty acids from distilled aromatic waste biomass using oleaginous yeast. Biomass Bioenergy 2024, 185, 107213. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Cheng, P.; Yu, G. Rhodotorula mucilaginosa-alternative sources of natural carotenoids, lipids, and enzymes for industrial use. Heliyon 2022, 8, e11505. [Google Scholar] [CrossRef]
- Hou, R.; Liu, S.; Lv, N.; Cui, B.; Wei, Y.; Long, F.; Fu, H.; Wang, Y. Nutritional- and flavor-enhancing effects of biomass production by Rhodotorula spp. on cantaloupe juice. Food Biosci. 2024, 59, 103866. [Google Scholar] [CrossRef]
- Wang, R.; Zeng, Y.; Liang, J.; Zhang, H.; Yi, J.; Liu, Z. Effect of Rhodotorula mucilaginosa inoculation on the aroma development of a fermented vegetables simulated system. Food Res. Int. 2024, 179, 113941. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, W.; Guo, C.; Hu, X.; Yi, J. Role of pectin characteristics in orange juice stabilization: Effect of high-pressure processing in combination with centrifugation pretreatments. Int. J. Biol. Macromol. 2022, 215, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Shang, Z.; Zhang, S.; Li, M.; Zhang, X.; Ren, H.; Hu, X.; Yi, J. Dynamic analysis of flavor properties and microbial communities in Chinese pickled chili pepper (Capsicum frutescens L.): A typical industrial-scale natural fermentation process. Food Res. Int. 2022, 153, 110952. [Google Scholar] [CrossRef]
- Jelinek-Kelly, S.; Akiyama, T.; Saunier, B.; Tkacz, J.S.; Herscovics, A. Characterization of a specific alpha-mannosidase involved in oligosaccharide processing in Saccharomyces cerevisiae. J. Biol. Chem. 1985, 260, 2253–2257. [Google Scholar]
- Strahsburger, E.; de Lopez Lacey, A.M.; Marotti, I.; DiGioia, D.; Biavati, B.; Dinelli, G. In vivo assay to identify bacteria with β-glucosidase activity. Electron. J. Biotechnol. 2017, 30, 83–87. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Alazzeh, A.Y.; Awaisheh, S.S.; Song, D.; Shahbazi, A.; AbuGhazaleh, A.A. Enhancement of α- and β-Galactosidase Activity in Lactobacillus reuteri by Different Metal Ions. Biol. Trace Elem. Res. 2010, 136, 106–116. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Zhao, N.; Lai, H.; Wang, Y.; Huang, Y.; Shi, Q.; He, W.; Zhu, S.; Li, Y.; Zhu, Y.; Li, H.; et al. Assessment of biogenic amine and nitrite production in low-salt Paocai during fermentation as affected by reused brine and fresh brine. Food Biosci. 2021, 41, 100958. [Google Scholar] [CrossRef]
- Shang, Z.; Ye, Z.; Li, M.; Ren, H.; Cai, S.; Hu, X.; Yi, J. Dynamics of microbial communities, flavor, and physicochemical properties of pickled chayote during an industrial-scale natural fermentation: Correlation between microorganisms and metabolites. Food Chem. 2022, 377, 132004. [Google Scholar] [CrossRef]
- Ells, T.C.; Hansen, L.T. Increased Thermal and Osmotic Stress Resistance in Listeria monocytogenes 568 Grown in the Presence of Trehalose due to Inactivation of the Phosphotrehalase-Encoding Gene treA. Appl. Environ. Microbiol. 2011, 77, 6841–6851. [Google Scholar] [CrossRef] [PubMed]
- Damian-Almazo, J.Y.; Lopez-Munguia, A.; Soberon-Mainero, X.; Saab-Rincon, G. Role of the phenylalanine 260 residue in defining product profile and alcoholytic activity of the α-amylase AmyA from Thermotoga maritima. Biologia 2008, 63, 1035–1043. [Google Scholar] [CrossRef]
- Pinu, F.R.; Edwards, P.J.B.; Gardner, R.C.; Villas-Boas, S.G. Nitrogen and carbon assimilation by Saccharomyces cerevisiae during Sauvignon blanc juice fermentation. Fems Yeast Res. 2014, 14, 1206–1222. [Google Scholar] [CrossRef] [PubMed]
- Satora, P.; Skotniczny, M.; Strnad, S.; Piechowicz, W. Chemical composition and sensory quality of sauerkraut produced from different cabbage varieties. LWT-Food Sci. Technol. 2021, 141, 111088. [Google Scholar] [CrossRef]
- Sun, N.; Liu, X.; Wang, X.; Shi, H.; Zhang, H.; Li, L.; Na, W.; Guan, Q. Optimization of fermentation conditions for the production of acidophilic β-glucosidase by Trichoderma reesei S12 from mangrove soil. Biotechnol. Biotechnol. Equip. 2021, 35, 1838–1849. [Google Scholar] [CrossRef]
- Yin, J.; Tao, Y.; Sun, W.; Chen, S. Effect of aroma enhancement for dry white wine by selected non-Saccharomyces extracellular enzymes. Trans. Chin. Soc. Agric. Eng. 2020, 36, 278–286. [Google Scholar] [CrossRef]
- Van den Ende, W. Multifunctional fructans and raffinose family oligosaccharides. Front. Plant Sci. 2013, 4, 247. [Google Scholar] [CrossRef]
- Kajiura, H.; Koiwa, H.; Nakazawa, Y.; Okazawa, A.; Kobayashi, A.; Seki, T.; Fujiyama, K. Two Arabidopsis thaliana Golgi α-mannosidase I enzymes are responsible for plant N-glycan maturation. Glycobiology 2010, 20, 235–247. [Google Scholar] [CrossRef]
- Lombard, V.; Ramulu, H.G.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef]
- Zhang, M.; Zhuo, X.; Wang, J.; Yang, C.; Powell, C.A.; Chen, R. Phosphomannose isomerase affects the key enzymes of glycolysis and sucrose metabolism in transgenic sugarcane overexpressing the manA gene. Mol. Breed. 2015, 35, 100. [Google Scholar] [CrossRef]
- Melanie, H.; Susilowati, A.; Maryati, Y. Fermented Inulin Hydrolysate by Bifidobacterium breve as Cholesterol Binder in Functional Food Application. In Proceedings of the 2nd International Symposium on Applied Chemistry (ISAC), Tangerang, Indonesia, 2–3 October 2016; Volume 1803. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, R.; Sirisena, S.; Gan, R.; Fang, Z. Beta-glucosidase activity of wine yeasts and its impacts on wine volatiles and phenolics: A mini-review. Food Microbiol. 2021, 100, 103859. [Google Scholar]
- Watanabe, K.; Miyake, K.; Suzuki, Y. Identification of catalytic and substrate-binding site residues in Bacillus cereus ATCC7064 oligo-1,6-glucosidase. Biosci. Biotechnol. Biochem. 2001, 65, 2058–2064. [Google Scholar] [CrossRef]
- Ngo, L.; Weimer, J.; Sui, L.; Pickens, T.; Stourman, N.V. Periplasmic beta-glucosidase BglX from E. coli demonstrates greater activity towards galactose-containing substrates. Int. J. Biochem. Mol. Biol. 2023, 14, 76–86. [Google Scholar]




| NR Hit | Gene Name | Family | Family Description |
|---|---|---|---|
| KWU45371.1 | bglX | GH3 | beta-glucosidase |
| TKA50830.1 | MNS1_2 | GH47 | alpha-mannosidase |
| TKA53735.1 | sacA | GH32 | fructan beta-(2,1)-fructosidase |
| KWU44931.1 | manB | GH2 | beta-galactosidase; beta-mannosidase |
| TKA58479.1 | treA | GH37 | alpha, alpha-trehalase |
| KWU41236.1 | malL | GH13_40 | GH13’s subfamily |
| TKA52136.1 | amyA | GH13_5 | GH13’s subfamily |
| TKA57373.1 | MNS3 | GH47 | alpha-mannosidase |
| TKA50408.1 | SAE1 | GH5_8 | GH5’s subfamily |
| Gene ID | Gene Name | L24-Count | L72-Count | L24-FPKM | L72-FPKM | log2FC | Regulated | KEGG_ Annotation |
|---|---|---|---|---|---|---|---|---|
| Gene0092 | bglX | 482 | 1443 | 4.79 | 12.39 | 1.11 | up | K05349 beta-glucosidase [EC:3.2.1.21] |
| Gene0077 | bglX | 291 | 816 | 2.95 | 7.17 | 1.17 | up | K05349 beta-glucosidase [EC:3.2.1.21] |
| Gene0853 | malL | 343 | 1791 | 4.55 | 20.52 | 2.26 | up | K01182 oligo-1,6-glucosidase [EC:3.2.1.10] |
| Code | CAS | Compounds | Concentrations (uL/L) | Description | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WRI 0 | WRI 24 | WRI 72 | GAL 24 | GAL 72 | GLU 24 | GLU 72 | MAN 24 | MAN 72 | ||||
| 1 | 1576-95-0 | (Z)-2-pentenol | - | 34.88 ±3.71 b | - | 231.23 ±16.38 a | 197.14 ±17.99 a | 231.16 ±12.64 a | 196.08 ±33.79 a | 181.34 ±9.98 a | 202.56 ±6.69 a | green scent |
| 2 | 122-78-1 | Hyacinthin | - | 38.17 ±4.14 b | 30.08 ±10.42 b | - | 33.55 ±0.52 b | - | 27.68 ±2.72 b | 228.24 ±1.27 a | 234.54 ±5.42 a | floral, honey |
| 3 | 689-67-8 | Geranylacetone | - | 13.27 ±0.64 a | 12.45 ±0.22 a | 21.32 ±0.85 a | 22.66 ±0.4 a | 17.34 ±1.51 a | 19.63 ±2.05 a | 5.93 ±0.72 a | 4.72 ±0.46 a | fruity |
| 4 | 112-53-8 | 1-Dodecanol | - | 6.76 ±2.03 c | 9.40 ±0.94 b | 19.08 ±1.83 b | 19.00 ±3.48 b | 16.67 ±4.82 b | 21.64 ±3.98 b | 5.94 ±1.21 c | 60.51 ±6.88 a | soapy, sweet |
| 5 | 623-42-7 | methyl butanoate | + | + | - | + | + | + | + | + | + | fruity |
| 6 | 868-57-5 | methyl 2-methylbutanoate | + | + | + | + | + | + | + | + | + | fruity |
| 7 | 626-89-1 | 4-methyl-1-pentanol | + | + | + | + | + | + | + | + | + | fruity, ethereal |
| 8 | 3658-80-8 | dimethyl trisulfide | + | + | - | + | + | + | + | + | + | sulfuric, cabbage-like |
| 9 | 104-76-7 | 2-ethyl-1-hexanol | + | + | + | + | + | + | + | + | + | fruity, ethereal |
| 10 | 124-19-6 | nonanal | + | + | + | + | + | + | + | + | + | citrus-like, soapy |
| 11 | 91-16-7 | 1,2-Dimethoxybenzene | + | + | + | + | + | + | + | - | - | vanilla |
| 12 | 143-08-8 | 1-nonanol | + | + | - | + | + | + | + | + | + | soapy, fruity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Wang, R.; Wang, Y.; Wang, Y.; Wang, T.; Chu, C.; Cai, S.; Yi, J.; Liu, Z. Effect of Glycosidase Production by Rhodotorula mucilaginosa on the Release of Flavor Compounds in Fermented White Radish. Foods 2025, 14, 1263. https://doi.org/10.3390/foods14071263
Zhang H, Wang R, Wang Y, Wang Y, Wang T, Chu C, Cai S, Yi J, Liu Z. Effect of Glycosidase Production by Rhodotorula mucilaginosa on the Release of Flavor Compounds in Fermented White Radish. Foods. 2025; 14(7):1263. https://doi.org/10.3390/foods14071263
Chicago/Turabian StyleZhang, Huixin, Rui Wang, Yaoying Wang, Yanfei Wang, Tao Wang, Chuanqi Chu, Shengbao Cai, Junjie Yi, and Zhijia Liu. 2025. "Effect of Glycosidase Production by Rhodotorula mucilaginosa on the Release of Flavor Compounds in Fermented White Radish" Foods 14, no. 7: 1263. https://doi.org/10.3390/foods14071263
APA StyleZhang, H., Wang, R., Wang, Y., Wang, Y., Wang, T., Chu, C., Cai, S., Yi, J., & Liu, Z. (2025). Effect of Glycosidase Production by Rhodotorula mucilaginosa on the Release of Flavor Compounds in Fermented White Radish. Foods, 14(7), 1263. https://doi.org/10.3390/foods14071263







