Physiological Benefits, Applications, and Future Directions of β-Hydroxy-β-Methylbutyrate (HMB) in Food and Health Industries
Abstract
1. Introduction
2. The Safety of HMB
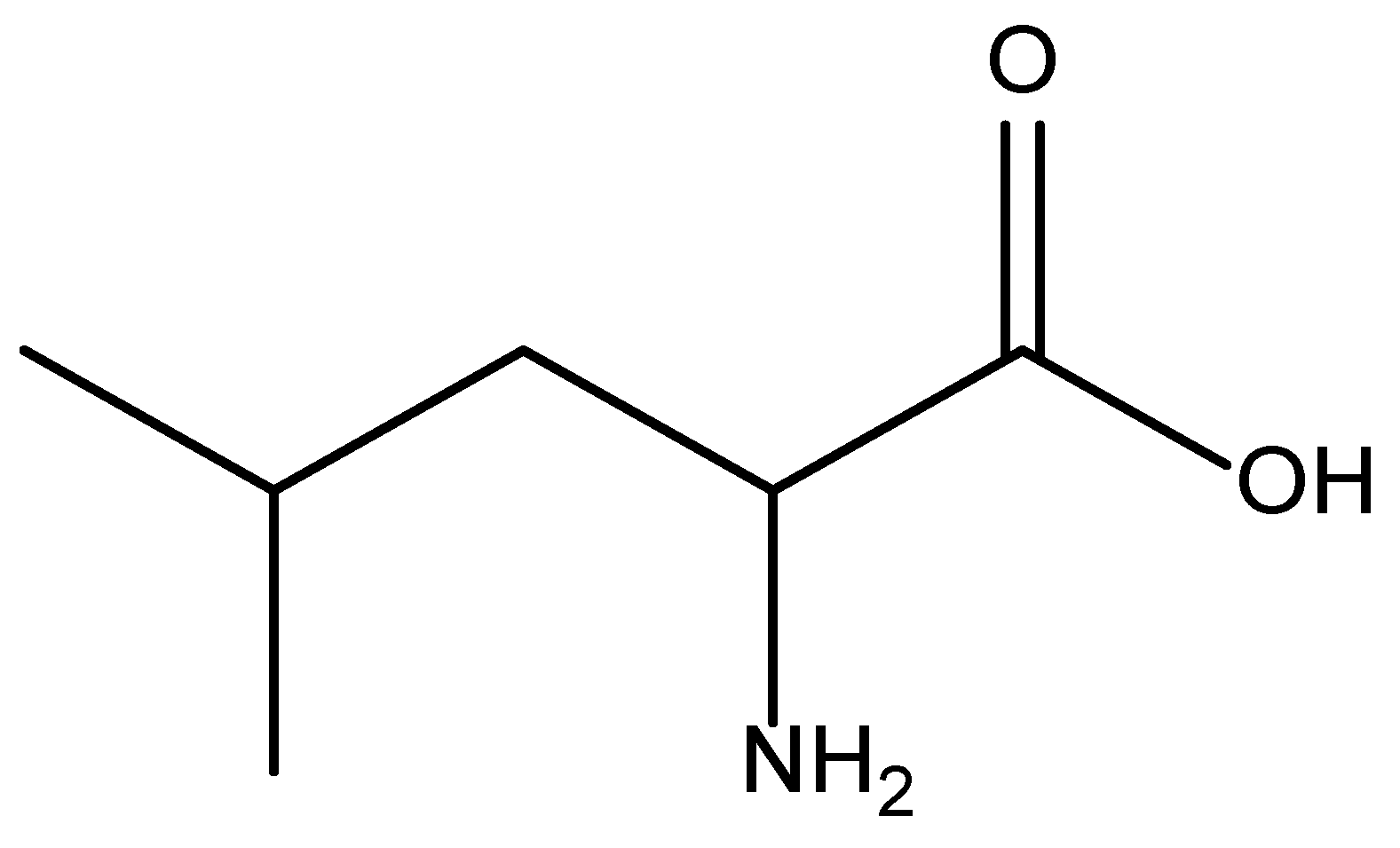
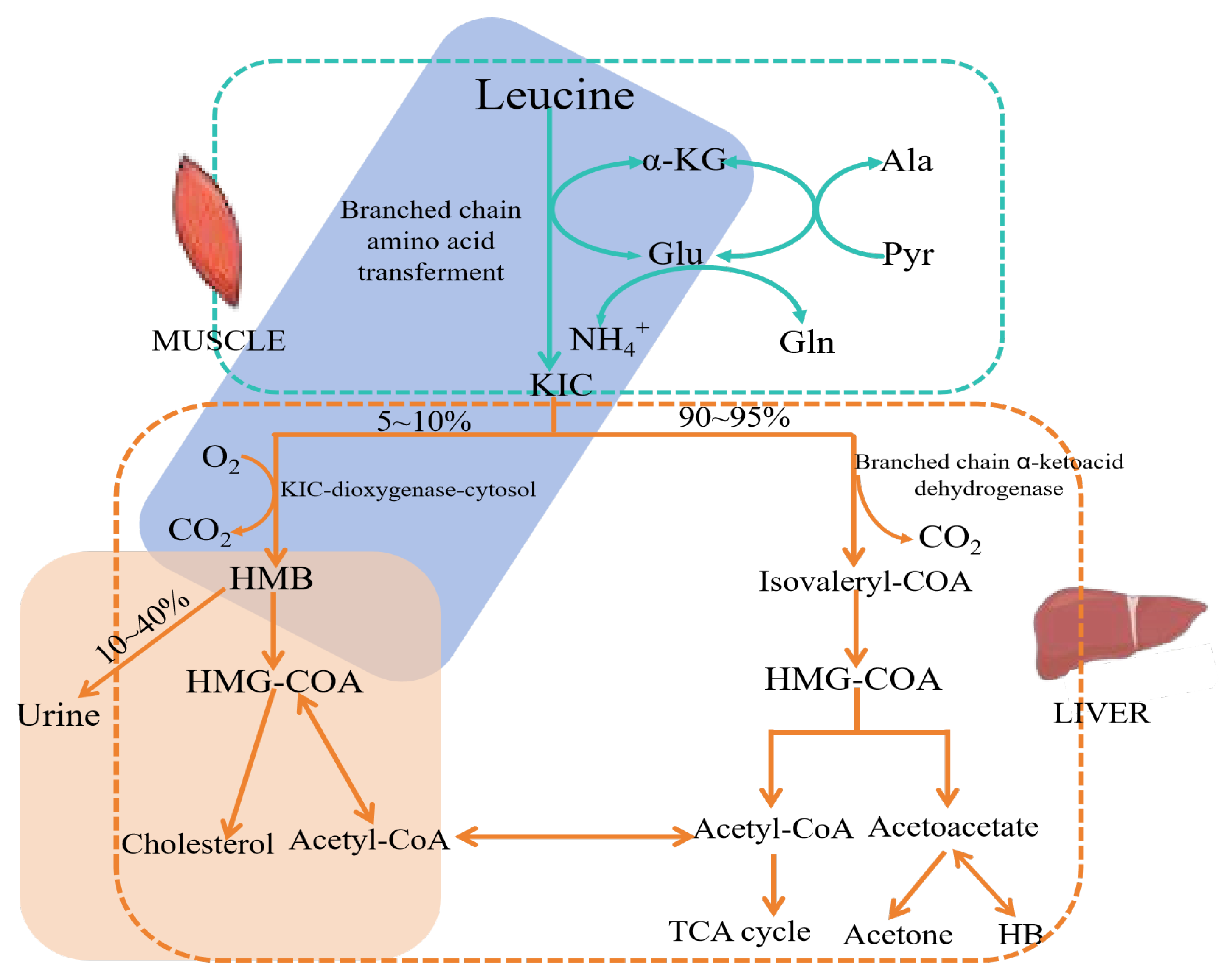
3. The Metabolism of HMB
4. Advances in HMB Synthesis
4.1. Chemical Synthesis of HMB
4.2. Biotransformation of HMB
5. The Determination Methods of HMB
5.1. High-Performance Liquid Chromatography (HPLC)
5.2. Gas Chromatography (GC)
5.3. HPLC-MS/MS and GC-MS/MS
5.4. Infrared Absorption Spectroscopy
6. The Physiological Functions and Applications of HMB
6.1. Muscle Preservation and Disease Treatment
6.2. Impact on Body Composition and Physical Performance
6.3. Ergogenic Use in Young Trained/Untrained Subjects
6.4. Applications in Animal Nutrition
| Study Design | Results | Author (Year) |
|---|---|---|
| 68 HIV infected patients, HMB 3 g/d + 14 g/d Arg + 14 g/d Gln, 8 weeks | Increased CD3 and CD8 cells, decreased HIV viral, gained BW and LBM, and altered the course of lean tissue loss in patients with AIDS associated wasting | Clark et al., 2000 [39] |
| 32 patients with solid tumors, 3 g/d HMB + 14 g/d Arg + 14 g/d Gln, 24 weeks | Increased the FFM of advanced (stage IV) cancer | May et al., 2002 [49] |
| 11 diabetic dialysis patients, 2.6 g/d HMB + 14.8 g/d Gln + 14.8 g/d Arg, 4 weeks | Had a positive contribution to the wound healing | Sipahi et al., 2013 [50] |
| Malnourished and sarcopenic men and women, 3.0 g/d HMB + 40 g/d protein + 998 IU vitamin D3, 4 weeks | Improved leg muscle strength and quality in not severe sarcopenia | Cramer et al., 2016 [51] |
| patients with I–IIIstage colon cancer, 3 g/d HMB + nutritional supplement, 5 weeks | Improved PG-SGA and MNA scores, greater hemoglobin and albumin | Yang et al., 2022 [52] |
| 24 healthy older adults (60–79 years old) during 10 days of bed rest, 3 g/d, 8 weeks | Prevented the decline in LBM | Deutz et al., 2013 [54] |
| 39 women and 38 men, 2 g/d HMB + 5 g/d Arg + 1.5 g/d Lys, 1 year | Increased lean tissue; increased body cell mass and lean mass; increased protein turnover | Baier et al., 2008 [55] |
| healthy older women, 1.5 g/d HMB, 8 weeks | Improved some muscle strength and physical functioning parameters | Tian et al., 2021 [15] |
| 20–40 years old women and men, 3.0 g/d + 3 times per week RT, 4 weeks | Increased body strength, fat-free weight; decreased fat percentage | Panton et al., 2000 [59] |
| 19–29 years men, 0, 1.5 or 3.0 g/d + normal, 117 g/d and 175 g/d Protein, 7 weeks | Either 1.5 or 3 g HMB/day could partly prevent exercise-induced proteolysis and/or muscle damage and improved in muscle function associated with resistance training | Nissen et al., 1996 [6] |
| 37 untrained college-aged men, 0, 3 or 6 g HMB + 3 d·wk−1 RT, 8 weeks | Increased peak isometric, isokinetic torque values and FFM; decreased plasma CPK activity | Gallagher et al., 2000 [23] |
| 20 RT males, 3 g/d HMB-FA | Blunt increases in muscle damage and prevent declines in perceived readiness | Wilson et al., 2013 [16] |
| 16 healthy men, 3 g/d HMB + RT, 6 weeks | Increased 1RM leg press; decreased CORT and ACTH; improved GH and IGF-1 | Asadi et al., 2017 [60] |
| 20 sows, 4 g/d HMB, from the 35th day of gestation to parturition | Increased birth weight; decreased the rate of stillborn piglets and the feed intake | Wan et al., 2016 [73] |
| 24 Alpine goats, 50 mg/kg HMB, 60 days | Higher body weight, more favorable musculature development | Zabek et al., 2016 [74] |
| 48 Bovans Brown, 0.02% HMB in diet | Increased mean daily, total egg weight, trabecular bone structure; decreased cholesterol content and alterations in the fatty acid profile | Tomaszewska et al., 2024 [75] |
7. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Challenges and Opportunities of Population Ageing in the Least Developed Countries; World Population Ageing 2023; United Nations: San Francisco, CA, USA, 2023.
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing J. Br. Geriatr. Soc. Br. Soc. Res. Ageing 2019, 48, 16–31. [Google Scholar]
- Ikeda, K.; Takahashi, M.; Aburaya, S.; Harada, D.; Ikeda, M.; Kitagawa, Y.; Soma, Y.; Izumi, Y.; Bamba, T.; Furuse, M. Produced β-hydroxybutyrate after β-hydroxy-β-methylbutyrate (HMB) administration may contribute HMB function in mice. Biochem. Biophys. Rep. 2021, 27, 101097. [Google Scholar]
- He, X.; Duan, Y.H.; Yao, K.; Li, F.N.; Hou, Y.Q.; Wu, G.Y.; Yin, Y.L. β-Hydroxy-β-methylbutyrate, mitochondrial biogenesis, and skeletal muscle health. Amino Acids 2016, 48, 653–664. [Google Scholar] [PubMed]
- Pinheiro, C.H.d.J.; Gerlinger-Romero, F.; Guimaraes-Ferreira, L.; de Souza, A.L., Jr.; Vitzel, K.F.; Nachbar, R.T.; Nunes, M.T.; Curi, R. Metabolic and functional effects of beta-hydroxy-beta-methylbutyrate (HMB) supplementation in skeletal muscle. Eur. J. Appl. Physiol. 2012, 112, 2531–2537. [Google Scholar] [PubMed]
- Nissen, S.; Sharp, R.; Ray, M.; Rathmacher, J.A.; Rice, D.; Fuller, J.C.; Connelly, A.S.; Abumrad, N. Effect of leucine metabolite β-hydroxy-β-methylbutyrate on muscle metabolism during resistance-exercise training. J. Appl. Physiol. 1996, 81, 2095–2104. [Google Scholar]
- Nissen, S.L.; Abumrad, N.N. Nutritional role of the leucine metabolite β-hydroxy β-methylbutyrate (HMB). J. Nutr. Biochem. 1997, 8, 300–311. [Google Scholar]
- Nissen, S.; Sharp, R.L.; Panton, L.; Vukovich, M.; Trappe, S.; Fuller, J.C. β-Hydroxy-β-Methylbutyrate (HMB) Supplementation in Humans Is Safe and May Decrease Cardiovascular Risk Factors. J. Nutr. 2000, 130, 1937–1945. [Google Scholar]
- Nissen, S.L.; Sharp, R.L. Effect of dietary supplements on lean mass and strength gains with resistance exercise: A meta-analysis. Scand. J. Med. Sci. Sports 2003, 13, 651–659. [Google Scholar]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar]
- Smith, H.J.; Mukerji, P.; Tisdale, M.J. Attenuation of Proteasome-Induced Proteolysis in Skeletal Muscle by β-Hydroxy-β-Methylbutyrate in Cancer-Induced Muscle Loss. Cancer Res. 2005, 65, 277–283. [Google Scholar]
- Holecek, M.; Muthny, T.; Kovarik, M.; Sispera, L. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on protein metabolism in whole body and in selected tissues. Food Chem. Toxicol. 2009, 47, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Arazi, H.; Taati, B.; Suzuki, K. A review of the effects of leucine metabolite (β-hydroxy-β-methylbutyrate) supplementation and resistance training on inflammatory markers: A new approach to oxidative stress and cardiovascular risk factors. Antioxidants 2018, 7, 148. [Google Scholar] [CrossRef]
- Baxter, J.H.; Carlos, J.L.; Thurmond, J.; Rehani, R.N.; Bultman, J.; Frost, D. Dietary toxicity of calcium β-hydroxy-β-methylbutyrate (CaHMB). Food Chem. Toxicol. 2005, 43, 1731–1741. [Google Scholar] [PubMed]
- Tian, L.; Chen, J.Y. Research progress on the safety and effectiveness of calcium β-hydroxy-β-methyl butyrate. J. Food Saf. Qual. 2021, 12, 6565–6572. [Google Scholar]
- Wilson, J.M.; Lowery, R.P.; Joy, J.M.; Walters, J.A.; Baier, S.M.; Fuller, J.C.; Stout, J.R.; Norton, L.E.; Sikorski, E.M.; Wilson, S.M.C.; et al. β-Hydroxy-β-methylbutyrate free acid reduces markers of exercise-induced muscle damage and improves recovery in resistance-trained men. Br. J. Nutr. 2013, 110, 538–544. [Google Scholar]
- Fuller, J.C.; Sharp, R.L.; Angus, H.F.; Baier, S.M.; Rathmacher, J.A. Free acid gel form of β-hydroxy-β-methylbutyrate (HMB) improves HMB clearance from plasma in human subjects compared with the calcium HMB salt. Br. J. Nutr. 2010, 105, 367–372. [Google Scholar] [PubMed]
- Rathmacher, J.; Nissen, S.; Panton, L.; Clark, R.; May, P. Supplementation with a combination of beta-hydroxy-beta-methylbutyrate (HMB), arginine, and glutamine is safe and could improve hematological parameters. J. Parenter. Enter. Nutr. 2004, 28, 65–75. [Google Scholar]
- Cruz-Jentoft, A.J. Beta-Hydroxy-Beta-Methyl Butyrate (HMB): From Experimental Data to Clinical Evidence in Sarcopenia. Curr. Protein Pept. Sci. 2018, 19, 668–672. [Google Scholar]
- Wilson, J.M.; Fitschen, P.J.; Campbell, B.; Wilson, G.J.; Zanchi, N.; Taylor, L.; Wilborn, C.; Kalman, D.S.; Stout, J.R.; Hoffman, J.R.; et al. International Society of Sports Nutrition Position Stand: Beta-hydroxy-beta-methylbutyrate (HMB). J. Int. Soc. Sports Nutr. 2013, 10, 6. [Google Scholar]
- Pitchford, L.M.; Fuller, J.C., Jr.; Rathmacher, J.A. Genotoxicity assessment of calcium β-hydroxy-β-methylbutyrate—ScienceDirect. Regul. Toxicol. Pharmacol. 2018, 100, 68–71. [Google Scholar]
- Kreider, R.; Ferreira, M.P.; Almada, A. Effects of calcium beta-hydroxy-beta-methylbutyrate (HMB) supplementation during resistance-training on markers of catabolism, body composition and strength. Int. J. Sports Med. 1999, 20, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, P.M.; Carrithers, J.A.; Godard, M.P.; Schulze, K.E.; Trappe, A.S.W. Beta-hydroxy-beta-methylbutyrate ingestion, Part I: Effects on strength and fat free mass. Med. Sci. Sports Exerc. 2000, 32, 2109–2115. [Google Scholar] [CrossRef]
- Rathmacher, J.; Fuller, J.; Baier, S.; Nissen, S.; Abumrad, N. Method of Administering β-hydroxy-β-methylbutyrate (HMB). U.S. Patent 2012053240A1, 1 March 2012. [Google Scholar]
- Fuller, J.C.; Arp, L.H.; Diehl, L.M.; Landin, K.L.; Baier, S.M.; Rathmacher, J.A. Subchronic toxicity study of β-hydroxy-β-methylbutyric free acid in Sprague–Dawley rats. Food Chem. Toxicol. 2014, 67, 145–153. [Google Scholar] [CrossRef]
- Wilson, G.J.; Wilson, J.M.; Manninen, A.H. Effects of beta-hydroxy-beta-methylbutyrate (HMB) on exercise performance and body composition across varying levels of age, sex, and training experience: A review. Nutr. Metab. 2008, 5, 1. [Google Scholar]
- Chinese Institute of Food Science and Technology. Scientific Consensus on Application of Calcium β-hydroxy-β-methylbutyrate (CaHMB). J. Chin. Inst. Food Sci. Technol. 2024, 1–9. [Google Scholar]
- Zanchi, N.E.; Gerlinger-Romero, F.; Guimarães-Ferreira, L.; Filho, M.r.A.d.S.; Felitti, V.; Lira, F.S.; Seelaender, M.; Lancha, A.H., Jr. HMB supplementation: Clinical and athletic performance-related effects and mechanisms of action. Amino Acids 2011, 40, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Koevering, M.T.V.; Nissen, S. Oxidation of leucine and α-ketoisocaproate to β-hydroxy-β-methylbutyrate in vivo. Am. J. Physiol. 1992, 262, E27–E31. [Google Scholar]
- Tan, M.; Zhang, X.L.; Zhang, Y.Q. Application of β-Hydroxy-β-Methylbutyric Acid in Food Industry. Food Ind. 2020, 41, 277–280. [Google Scholar]
- Meng, J.; Kong, X.H.; Tan, X.Z.; Li, X.H. Improvement on the Production Technology of Calcium β-Hydroxy-β-Methyl Butyrate. Shangdong Chem. Ind. 2000, 29, 7–9. [Google Scholar]
- Tang, S.K. Preparation Method of Calcium Beta-Hydroxy-Beta-Methylbutyrate. China Patent CN201711166893.7, 24 April 2018. [Google Scholar]
- Lee, I.Y.; Nissen, S.L.; Rosazza, J.P.N. Conversion of beta-methylbutyric acid to beta-hydroxy-beta-methylbutyric acid by Galactomyces reessii. Appl. Environ. Microbiol. 1997, 63, 4191–4195. [Google Scholar]
- Dhar, A.; Dhar, K.; Rosazza, J. Purification and characterization of a Galactomyces reessii hydratase that converts 3-methylcrotonic acid to 3-hydroxy-3-methylbutyric acid. J. Ind. Microbiol. Biotechnol. 2002, 28, 81–87. [Google Scholar] [PubMed]
- Fei, H.M.; Ye, Q. Production of β-Hydroxy-β-methylbutyric Acid from β-Methylbutyric Acid with Galactomyces reessii. J. East China Univ. Sci. Technol. 2002, 601–605. [Google Scholar]
- Gao, R.C.; Li, Z.M. Biosynthesis of 3-Hydroxy-3-Methylbutyrate from l-Leucine by Whole-Cell Catalysis. J. Agric. Food Chem. 2021, 69, 3712–3719. [Google Scholar] [PubMed]
- Huang, S.J.; Lai, M.J.; Chen, A.Y.; Lan, E.I. De novo biosynthesis of 3-hydroxy-3-methylbutyrate as anti-catabolic supplement by metabolically engineered Escherichia coli. Metab. Eng. 2024, 84, 48–58. [Google Scholar]
- Zhang, C.Y.; Jin, J.Y.; Qiu, Y.J.; Fan, L.Q.; Zhao, L.M. Collision Between Tradition and Future: Technology and Application Progressin Food Fermentation Engineering. Curr. Biotechnol. 2021, 11, 418–429. [Google Scholar]
- Yang, X.L.; Nai, S.J.; Wei, J.L.; Zheng, C.Z.; Cai, Z.Y.; Lin, Z.M. Determination of β-Hydroxy-β-Methylbutyrate Calcium and Adulteration Identification. Guangzhou Chem. 2018, 43, 30–34, 40. [Google Scholar]
- Zhu, G.F.; Yu, Q.; Lu, X.; Zhou, X.; Yang, Z.D.; Wei, L.H.; Sun, Y. Determination of Calcium β-Hydroxy-β-Methylbutyrate Content in Sports Nutrition Food. Food Sci. Technol. 2023, 48, 248–253. [Google Scholar]
- Baxter, J.H.; Phillips, R.R.; Dowlati, L.; Goehring, K.C.; Johns, P.W. Direct Determination of β-Hydroxy-β-Methylbutyrate (HMB) in Liquid Nutritional Products. Food Anal. Methods 2010, 4, 341–346. [Google Scholar]
- Huang, Y.Q.; Pang, B.N.; Lin, Y.K. Reserarch Progress on the Application and Detection of β-Hydroxy-β-Methylbutyric Acid and Its Calcium Salt in Food. Food Ind. 2024, 45, 217–220. [Google Scholar]
- Deshpande, P.; Jie, Z.; Subbarayan, R.; Mamidi, V.K.; Chunduri, R.H.B.; Das, T.; Shreeram, S. Development and validation of LC-MS/MS method for the estimation of β-hydroxy-β-methylbutyrate in rat plasma and its application to pharmacokinetic studies. Biomed. Chromatogr. BMC 2013, 27, 142–147. [Google Scholar]
- Wang, M.; Zhu, X.; Li, Q.; Di, D. Determination of β-Hydroxy-β-Methylbutyric Acid and Its Calcium Salt in Health Drinks by GC-MS/MS. China Food Saf. Mag. 2023, 17, 106–109. [Google Scholar]
- Jówko, E.; Ostaszewski, P.; Jank, M.; Sacharuk, J.; Nissen, S. Creatine and β-hydroxy-β-methylbutyrate (HMB) additively increase lean body mass and muscle strength during a weight-training program. Nutrition 2001, 17, 558–566. [Google Scholar] [PubMed]
- Molfino, A.; Gioia, G.; Fanelli, F.R.; Muscaritoli, M. Beta-hydroxy-beta-methylbutyrate supplementation in health and disease: A systematic review of randomized trials. Amino Acids 2013, 45, 1273–1292. [Google Scholar]
- Wu, H.M.; Xia, Y.; Jiang, J.; Du, H.M.; Guo, X.Y.; Liu, X.; Li, C.L.; Huang, G.W.; Niu, K.J. Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2015, 61, 168–175. [Google Scholar] [PubMed]
- Clark, R.H.; Feleke, G.; Din, M.; Yasmin, T.; Singh, G.; Khan, F.A.; Rathmacher, J.A. Nutritional treatment for acquired immunodeficiency virus-associated wasting using beta-hydroxy beta-methylbutyrate, glutamine, and arginine: A randomized, double-blind, placebo-controlled study. JPEN J. Parenter. Enter. Nutr. 2000, 24, 133–139. [Google Scholar]
- May, P.E.; Barber, A.; D’Olimpio, J.T.; Hourihane, A.; Abumrad, N.N. Reversal of cancer-related wasting using oral supplementation with a combination of β-hydroxy-β-methylbutyrate, arginine, and glutamine. Am. J. Surg. 2002, 183, 471–479. [Google Scholar] [CrossRef]
- Sipahi, S.; Gungor, O.; Gunduz, M.; Cilci, M.; Demirci, M.C.; Tamer, A. The effect of oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine and glutamine on wound healing: A retrospective analysis of diabetic haemodialysis patients. BMC Nephrol. 2013, 14, 8. [Google Scholar]
- Cramer, J.T.; Cruz-Jentoft, A.J.; Landi, F.; Hickson, M.; Zamboni, M.; Pereira, S.L.; Hustead, D.S.; Mustad, V.A. Impacts of High-Protein Oral Nutritional Supplements Among Malnourished Men and Women with Sarcopenia: A Multicenter, Randomized, Double-Blinded, Controlled Trial. J. Am. Med. Dir. Assoc. 2016, 17, 1044–1055. [Google Scholar] [CrossRef]
- Yang, L.Q.; Wang, L.; Tian, H.M.; Wang, K.H.; Liu, M.; Yu, Z.; Shi, H.P. Effect of HMB on cancer-related fatigue and quality of life in elderly surgical patients with stageI-III colon cancer. Electron. J. Metab. Nutr. Cancer 2022, 9, 229–233. [Google Scholar]
- Zanker, O.; Duque, V. The Effect of β-hydroxy-β-methylbutyrate (HMB) on Sarcopenia and Functional Frailty in Older Persons: A Systematic Review. J. Nutr. Health Aging 2019, 23, 145–150. [Google Scholar]
- Deutz, N.E.; Pereira, S.L.; Hays, N.P.; Oliver, J.S.; Edens, N.K.; Evans, C.M.; Wolfe, R.R. Effect of β-hydroxy-β-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin. Nutr. 2013, 32, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Baier, S.; Johannsen, D.; Abumrad, N.; Rathmacher, J.A.; Nissen, S.; Flakoll, P. Year-long Changes in Protein Metabolism in Elderly Men and Women Supplemented with a Nutrition Cocktail of β-Hydroxy-β-methylbutyrate (HMB), L-Arginine, and L-Lysine. J. Parenter. Enter. Nutr. 2008, 33, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.Q.; Wang, Q.; Wan, H.; Lu, X.; Gao, W. Leucine metabolite β-hydroxy-β-methyl butyrate (HMB) supplementation on muscle mass during resistance training in older subjects: Meta-analysis. Aging Pathobiol. Ther. 2022, 4, 4–13. [Google Scholar] [CrossRef]
- Thomson, J.S.; Watson, P.E.; Rowlands, D.S. Effects of nine weeks of β-Hydroxy-β-Methylbutyrate supplementation on strength and body composition in resistance trained men. J. Strength Cond. Res. 2009, 23, 827–835. [Google Scholar] [CrossRef]
- Palisin, T.; Stacy, J.J. β-Hydroxy-β-methylbutyrate and its use in athletics. Curr. Sports Med. Rep. 2005, 4, 220–223. [Google Scholar] [CrossRef]
- Panton, L.B.; Rathmacher, J.A.; Baier, S.; Steven Nissen, D. Nutritional supplementation of the leucine metabolite β-hydroxy-β-methylbutyrate (hmb) during resistance training. Nutrition 2000, 16, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Arazi, H.; Suzuki, K. Effects of Hydroxy-methylbutyrate-free Acid Supplementation on Strength, Power and Hormonal Adaptations Following Resistance Training. Nutrients 2017, 9, 1316. [Google Scholar] [CrossRef]
- Arazi, H.; Rohani, H.; Ghiasi, A.; Davaran, M. The effect of HMB supplementation on cardiovascular risk factors after four weeks of resistance training in amateur athletes. Int. Cardiovasc. Res. J. 2015, 9, 89–93. [Google Scholar]
- Shirato, M.; Tsuchiya, Y.; Sato, T.; Hamano, S.; Gushiken, T.; Kimura, N.; Ochi, E. Effects of combined β-hydroxy-β-methylbutyrate (HMB) and whey protein ingestion on symptoms of eccentric exercise-induced muscle damage. J. Int. Soc. Sports Nutr. 2022, 13, 7. [Google Scholar] [CrossRef]
- Hojjat, S.; Alikhani, E.; Havasian, M.R.; Mahboubi, N. Investigating the Interactive Effect of HMB-FA and Extrovert Physical Exercise on LDH Muscular Damage in Mature Male Rats. J. Res. Med. Dent. Sci. 2017, 5, 79–83. [Google Scholar]
- Routhier, D.D.; Stacy, J.J. HMB use and its relationship to exercise-induced muscle damage and performance during exercise. Int. Sport. J. 2007, 8, 68–77. [Google Scholar]
- Slater, G.; Jenkins, D.; Logan, P.; Lee, H.; Vukovich, M.; Rathmacher, J.A.; Hahn, A.G. β-hydroxy-β-methylbutyrate (HMB) supplementation does not affect changes in strength or body composition during resistance training in trained men. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 384–396. [Google Scholar]
- Nissen, S.; Fuller, J.C., Jr.; Sell, J.; Ferket, P.R.; RIVES, D.V. The effect of β-hydroxy-β-methylbutyrate on growth, mortality, and carcass qualities of broiler chickens. Poult. Sci. 1994, 73, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Zhang, H.; Wu, S.; Yue, H.; Zuo, J.; Feng, D.; Qi, G. Effect of β-hydroxy-β-methylbutyrate calcium on growth, blood parameters, and carcass qualities of broiler chickens. Poult. Sci. 2013, 92, 753–759. [Google Scholar]
- Tous, N.; Guiomar, R.L.; Vilà, B.; Martinell, M.G.; Furnols, M.F.; Esteve-Garcia, E. Addition of arginine and leucine to low or normal protein diets: Performance, carcass characteristics and intramuscular fat of finishing pigs. Span. J. Agric. Res. 2016, 14, 9. [Google Scholar]
- Gerlinger-Romero, F.; Guimarães-Ferreira, L.; Giannocco, G.; Nunes, M.T. Chronic supplementation of beta-hydroxy-beta methylbutyrate (HMB) increases the activity of the GH/IGF-I axis and induces hyperinsulinemia in rats. Growth Horm. IGF Res. 2011, 21, 57–62. [Google Scholar] [PubMed]
- Kornasio, R.; Riederer, I.; Butler-Browne, G.; Mouly, V.; Uni, Z.; Halevy, O. β-hydroxy-β-methylbutyrate (HMB) stimulates myogenic cell proliferation, differentiation and survival via the MAPK/ERK and PI3K/Akt pathways. Biochim. Biophys. Acta BBA Mol. Cell Res. 2009, 1793, 755–763. [Google Scholar] [CrossRef]
- Smith, H.J.; Wyke, S.M.; Tisdale, M.J. Mechanism of the attenuation of proteolysis-inducing factor stimulated protein degradation in muscle by β-hydroxy-β-methylbutyrate. Cancer Res. 2004, 64, 8731–8735. [Google Scholar]
- Tatara, M.R.; Sliwa, E.; Krupski, W. Prenatal programming of skeletal development in the offspring: Effects of maternal treatment with β-hydroxy-β-methylbutyrate (HMB) on femur properties in pigs at slaughter age. Bone 2007, 40, 1615–1622. [Google Scholar]
- Wan, H.; Zhu, J.; Xiang, S.; Yin, H. Effects of Dietary Supplementation of β-hydroxy-β-methylbutyrate on Sow Performance and mRNA Expression of Myogenic Markers in Skeletal Muscle of Neonatal Piglets. Reprod. Domest. Anim. 2016, 51, 135–142. [Google Scholar]
- Zabek, K.; Wojcik, R.; Milewski, S.; Malaczewska, J.; Siwicki, A.K. Effect of β-hydroxy-β-methylbutyrate acid on meat performance traits and selected indicators of humoral immunity in goats. Jpn. J. Vet. Res. 2016, 64, 247–256. [Google Scholar] [PubMed]
- Tomaszewska, E.; Witkiewicz, S.; Arczewska-Wosek, A.; Wojtysiak, D.; Dobrowolski, P.; Domaradzki, P.; Puzio, I.; Rudyk, H.; Brezvyn, O.; Muszyński, S. β-Hydroxy-β-methylbutyrate: A feed supplement influencing performance, bone metabolism, intestinal morphology, and muscle quality of laying hens: A preliminary one-point study. Poult. Sci. 2024, 103, 103597. [Google Scholar] [PubMed]

| Countries | Year of Approval | Forms of HMB’s Utilization | Range of Application and Dosage |
|---|---|---|---|
| China | 2011, 2017, 2022 | CaHMB | As a new resource food, used in foods for sports nutrition and foods for special medical purposes, ≤3 g/d (2011). As a new food raw material, used in beverages, milk and dairy products, cocoa products, chocolate and chocolate products, confectionery, and baked goods, ≤6 g/d (2017), ≤6 g/d (2022) |
| USA | 2005, 2009 | CaHMB | As medical and general foodstuffs, 3 g/d (2005), ≤6 g/d (2009) |
| EU | 1997 | CaHMB | As general food ingredients used in special dietary foods such as whole nutrition food, and nutritional supplements. ≤1.5 g/serving and ≤6 g/d |
| Canada | 2014 | CaHMB | As a new resource food and as an ingredient in natural health products, ≤1.5 g/serving and ≤6 g/d |
| Japan | 2009 | HMB | As functional food, 1.6~4.8 g/d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Liu, G.; Wang, Z.; Lei, Z.; Chen, W.; Wang, C. Physiological Benefits, Applications, and Future Directions of β-Hydroxy-β-Methylbutyrate (HMB) in Food and Health Industries. Foods 2025, 14, 1294. https://doi.org/10.3390/foods14081294
Zhou S, Liu G, Wang Z, Lei Z, Chen W, Wang C. Physiological Benefits, Applications, and Future Directions of β-Hydroxy-β-Methylbutyrate (HMB) in Food and Health Industries. Foods. 2025; 14(8):1294. https://doi.org/10.3390/foods14081294
Chicago/Turabian StyleZhou, Sijing, Guijun Liu, Zhong Wang, Ziteng Lei, Wei Chen, and Chengtao Wang. 2025. "Physiological Benefits, Applications, and Future Directions of β-Hydroxy-β-Methylbutyrate (HMB) in Food and Health Industries" Foods 14, no. 8: 1294. https://doi.org/10.3390/foods14081294
APA StyleZhou, S., Liu, G., Wang, Z., Lei, Z., Chen, W., & Wang, C. (2025). Physiological Benefits, Applications, and Future Directions of β-Hydroxy-β-Methylbutyrate (HMB) in Food and Health Industries. Foods, 14(8), 1294. https://doi.org/10.3390/foods14081294







