In Vitro Digestion Patterns of Advanced Glycation End Products and α-Dicarbonyls in Biscuits and the Modulatory Effects of Ferulic Acid and Epicatechin
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Materials
2.2. In Vitro Digestion of Food Systems
2.3. AGEs Determination by LC-ESI-MS/MS
2.4. α-DCs Determination by LC-ESI-MS/MS
2.5. Establishment of Glucose–Lysine Simulation System
2.6. Detection of Adducts Formed During Digestion In Vitro
2.7. Statistical Analysis
3. Results and Discussion
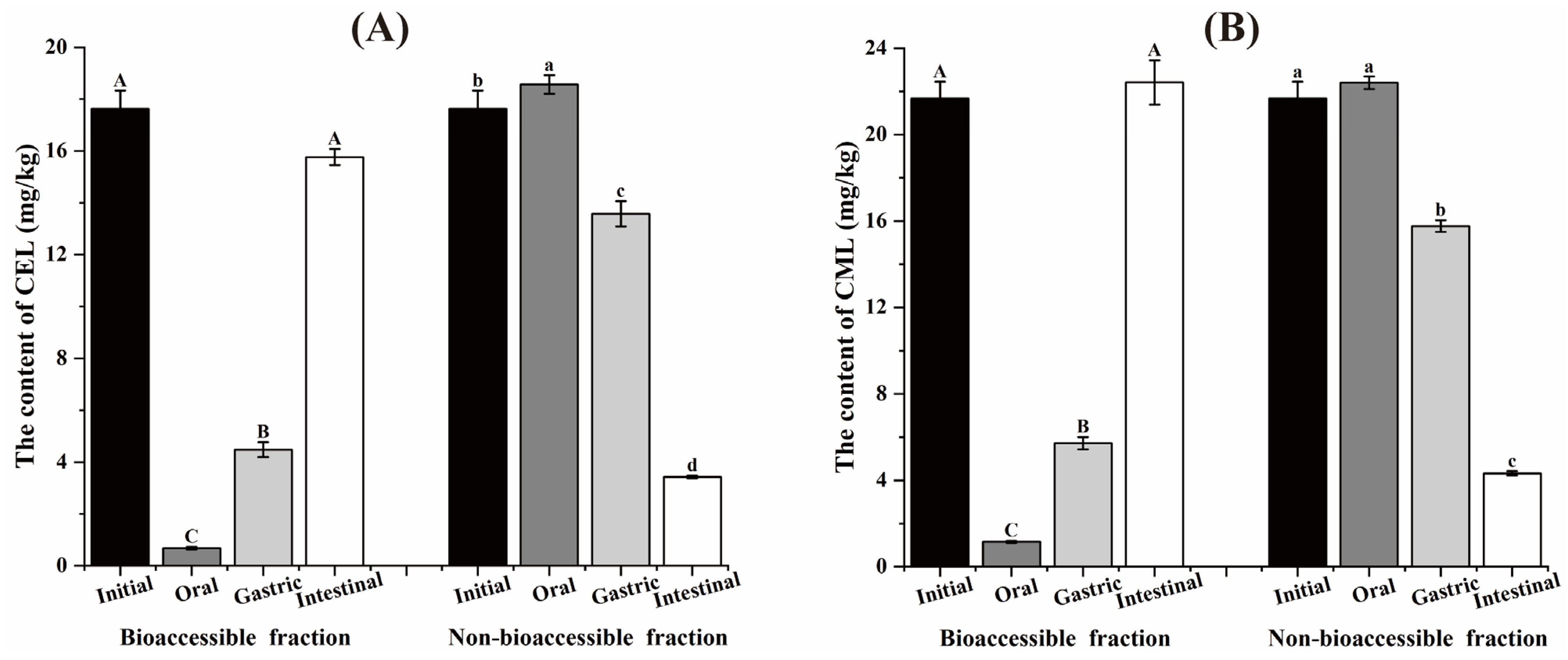
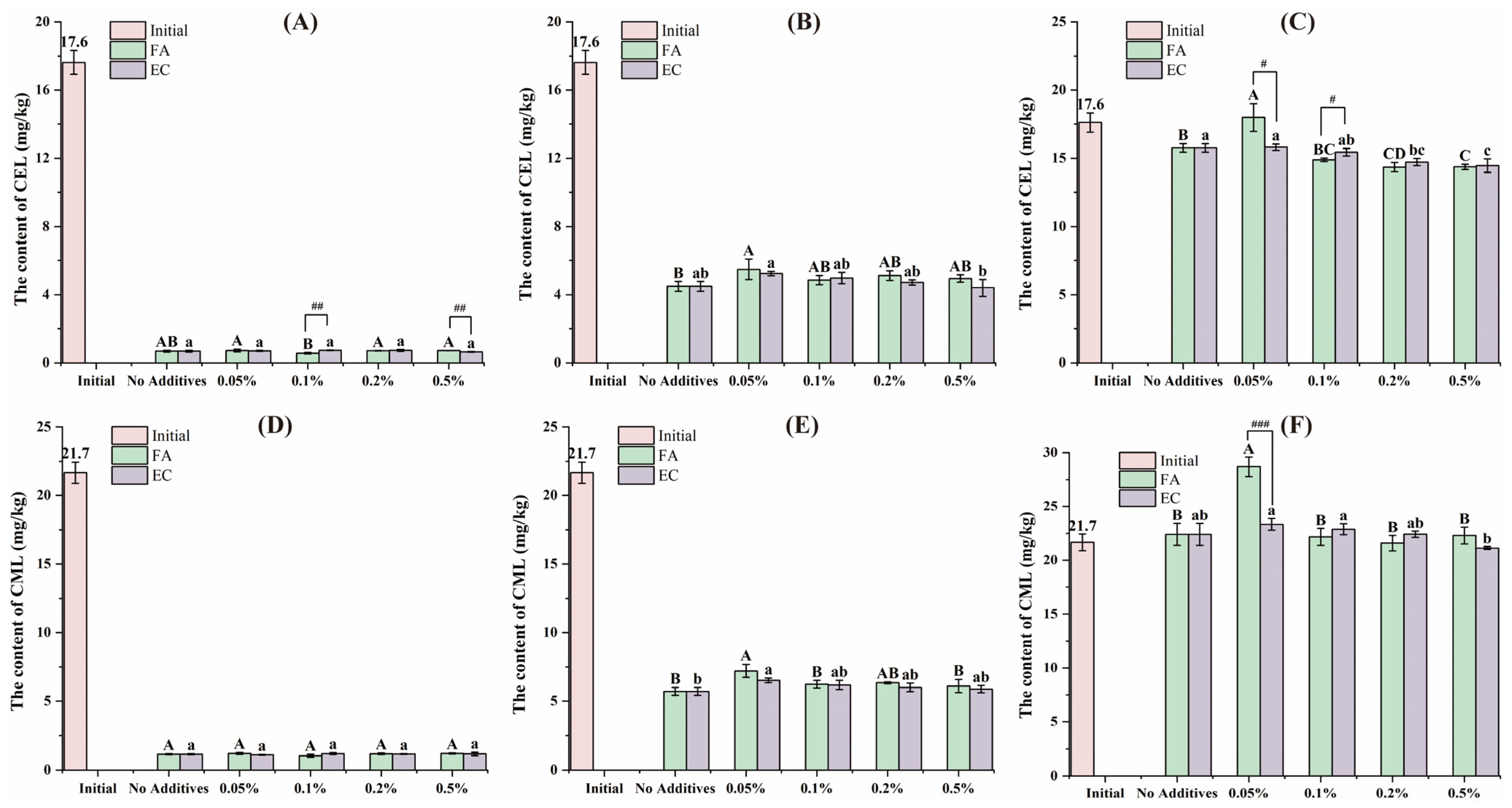
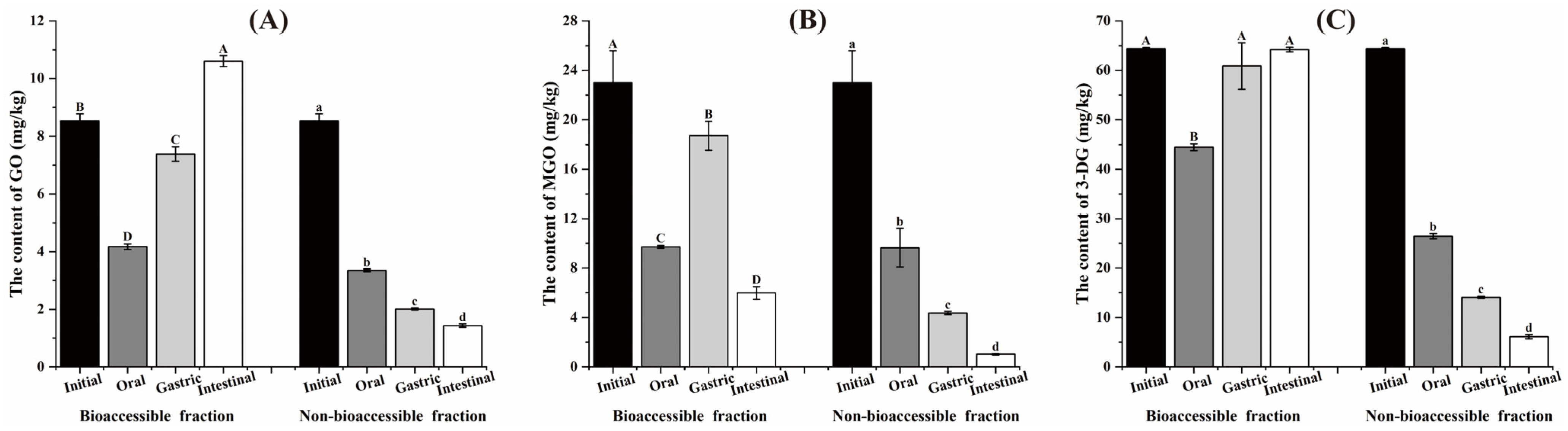
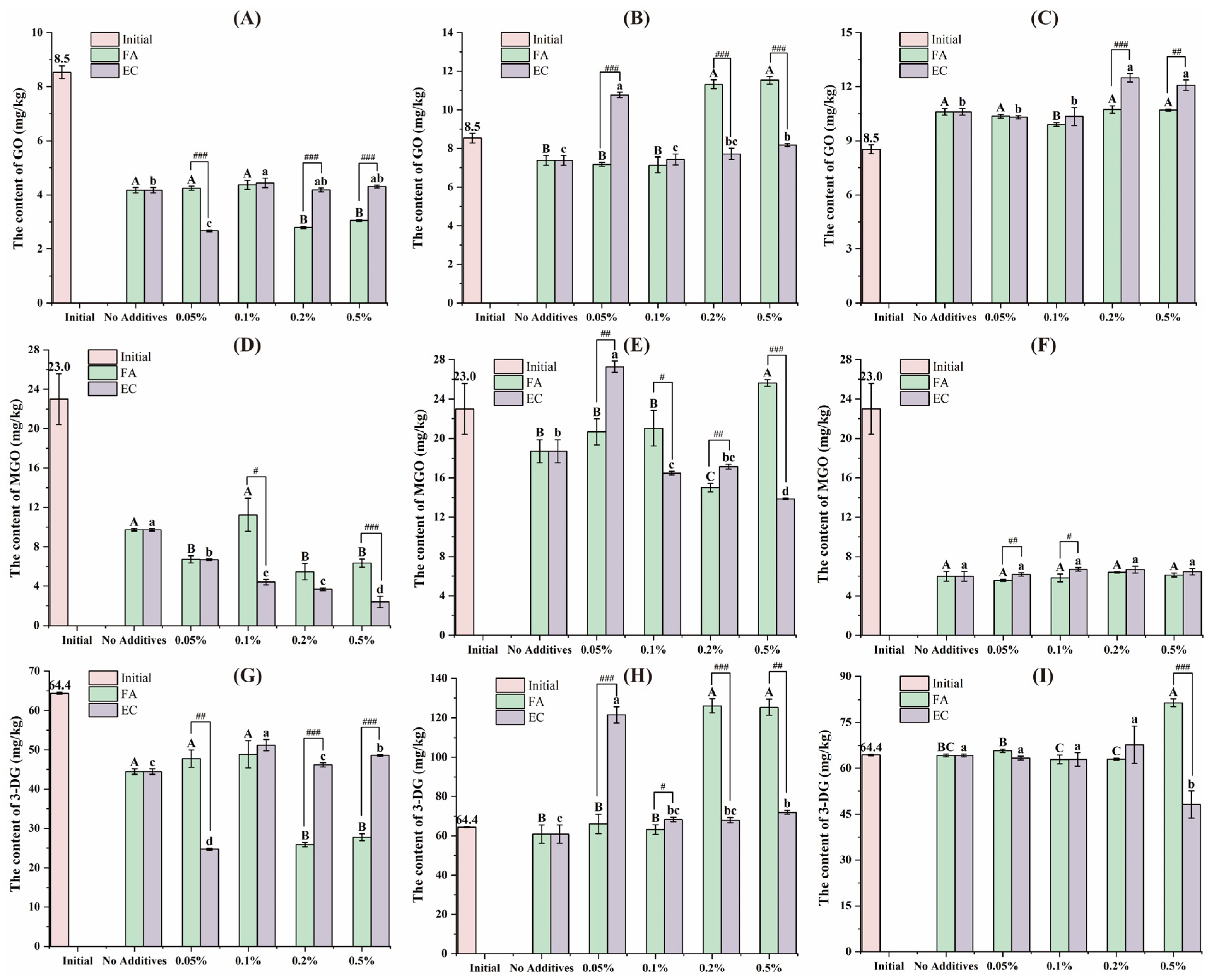
3.1. Variation of the AGEs Content During the In Vitro Digestion Process
3.2. Variation of the α-DCs Content During the In Vitro Digestion Process
3.3. Bioaccessibility of Contaminants After Digestion In Vitro
3.4. The Content of Contaminants in the Insoluble Fraction After Digestion In Vitro
3.5. Structural Identification of Adducts Formed During Digestion In Vitro
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poutanen, K.S.; Kårlund, A.O.; Gómez-Gallego, C.; Johansson, D.P.; Scheers, N.M.; Marklinder, I.M.; Eriksen, A.K.; Silventoinen, P.C.; Nordlund, E.; Sozer, N.; et al. Grains—A major source of sustainable protein for health. Nutr. Rev. 2022, 80, 1648–1663. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Niu, L.; Zhu, L.; Yuan, H.; Kilmartin, P.A.; Jiang, Y. Contents of α-dicarbonyl compounds in commercial black tea and affected by the processing. Food Res. Int. 2024, 178, 113876. [Google Scholar] [CrossRef]
- Yan, S.; Wu, L.; Xue, X. α-Dicarbonyl compounds in food products: Comprehensively understanding their occurrence, analysis, and control. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1387–1417. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, K.; Chen, Y.; Ouyang, Y.; Feng, Y.; Li, S.; Zhang, L.; Feng, N. Effect of lotus seedpod oligomeric procyanidins on AGEs formation in simulated gastrointestinal tract and cytotoxicity in Caco-2 cells. Food Funct. 2021, 12, 3527–3538. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Huang, M.; Cheng, Y.; Khan, I.A.; Huang, J. A comprehensive review of Nε-carboxymethyllysine and Nε-carboxyethyllysine in thermal processed meat products. Trends Food Sci. Technol. 2020, 98, 30–40. [Google Scholar] [CrossRef]
- Yu, L.; Li, Y.; Gao, C.; Yang, Y.; Zeng, M.; Chen, J. Nε-carboxymethyl-lysine and Nε-carboxyethyl-lysine contents in commercial meat products. Food Res. Int. 2022, 155, 111048. [Google Scholar] [CrossRef]
- Cincotta, F.; Brighina, S.; Condurso, C.; Arena, E.; Verzera, A.; Fallico, B. Sugars Replacement as a Strategy to Control the Formation of α-Dicarbonyl and Furanic Compounds during Cookie Processing. Foods 2021, 10, 2101. [Google Scholar] [CrossRef]
- Hamzalıoğlu, A.; Gökmen, V. Potential reactions of thermal process contaminants during digestion. Trends Food Sci. Technol. 2020, 106, 198–208. [Google Scholar] [CrossRef]
- Shang, F.; Zhu, R.; Li, H.; Zhen, T.; Li, T.; Song, L.; Pan, Z.; Zhang, Q.; Lan, H.; Duan, Z. Galactooligosaccharides in infant formulas: Maillard reaction characteristics and influence on formation of advanced glycation end products. Food Funct. 2024, 15, 2197–2207. [Google Scholar] [CrossRef]
- Hamzalıoğlu, A.; Gökmen, V. Investigations on the reactions of α-dicarbonyl compounds with amino acids and proteins during in vitro digestion of biscuits. Food Funct. 2016, 7, 2544–2550. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Yang, X.; Zhu, L.; Wu, G.; Qi, X.; Zhang, H. Trapping of reactive carbonyl species by fiber-bound polyphenols from whole grains under simulated physiological conditions. Food Res. Int. 2022, 156, 111142. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J.; Huang, Y.; Wang, W.; Xie, J.; Xie, M.; Shen, M. Quantitative assessment of furosine, furfurals, and advanced glycation end products in different types of commercially available cheeses. Food Control 2022, 136, 108866. [Google Scholar] [CrossRef]
- Wang, W.; Kou, Y.; Du, Y.; Li, M.; Zhang, J.; Yan, A.; Xie, J.; Shen, M. Investigation on the Contents of Nε-carboxymethyllysine, Nε-carboxyethyllysine, and N-nitrosamines in Commercial Sausages on the Chinese Market. Foods 2023, 12, 724. [Google Scholar] [CrossRef]
- Kocadağlı, T.; Gökmen, V. Investigation of α-Dicarbonyl Compounds in Baby Foods by High-Performance Liquid Chromatography Coupled with Electrospray Ionization Mass Spectrometry. J. Agric. Food Chem. 2014, 62, 7714–7720. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Saez, N.; Fernandez-Gomez, B.; Cai, W.; Uribarri, J.; del Castillo, M.D. In vitro formation of Maillard reaction products during simulated digestion of meal-resembling systems. Food Res. Int. 2019, 118, 72–80. [Google Scholar] [CrossRef]
- Feng, N.; Feng, Y.; Zhang, F.; Yan, J.; Niu, M.; Shi, L.; Xiong, H.; Zhou, M.; Wu, Q. Inhibition mechanism of mango vinegar on protein-AGEs digestion in vitro gastrointestinal environment. LWT 2023, 186, 115246. [Google Scholar] [CrossRef]
- Arribas-Lorenzo, G.; Morales, F.J. Analysis, distribution, and dietary exposure of glyoxal and methylglyoxal in cookies and their relationship with other heat-induced contaminants. J. Agric. Food Chem. 2010, 58, 2966–2972. [Google Scholar] [CrossRef]
- Degen, J.; Hellwig, M.; Henle, T. 1,2-dicarbonyl compounds in commonly consumed foods. J. Agric. Food Chem. 2012, 60, 7071–7079. [Google Scholar] [CrossRef]
- Papetti, A.; Mascherpa, D.; Marrubini, G.; Gazzani, G. Effect of In Vitro Digestion on Free α-Dicarbonyl Compounds in Balsamic Vinegars. J. Food Sci. 2013, 78, C514–C519. [Google Scholar] [CrossRef] [PubMed]
- Yaman, M.; Ede-Cintesun, E.; ÖZgÖLet, M.; Serdar, E.; Mizrak, Ö.F.; Yildirim-Servi, E. Bioaccessibility Of α-Dicarbonyl Compounds in Breads and Other Bakery Products Using an in Vitro Stimulated Gastrointestinal Digestive Systems. Lat. Am. Appl. Res.—Int. J. 2023, 53, 111–117. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, C.; Ou, J.; Liu, F.; Ou, S.; Zheng, J. Glyoxal in Foods: Formation, Metabolism, Health Hazards, and Its Control Strategies. J. Agric. Food Chem. 2024, 72, 2434–2450. [Google Scholar] [CrossRef]
- Zheng, J.; Guo, H.; Ou, J.; Liu, P.; Huang, C.; Wang, M.; Simal-Gandara, J.; Battino, M.; Jafari, S.M.; Zou, L.; et al. Benefits, deleterious effects and mitigation of methylglyoxal in foods: A critical review. Trends Food Sci. Technol. 2021, 107, 201–212. [Google Scholar] [CrossRef]
- Yuan, X.; Feng, S.; Li, J.; Guo, R.; Nie, C.; Zhai, R.; Tu, A.; Cao, X.; Zhang, M.; Li, J. Generation of advanced glycation end products from glycated protein or fructose/glyoxal-protein adducts under in vitro simulated gastrointestinal digestion. Food Chem. 2025, 463, 141175. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, H.; Zhu, L.; Wu, G.; Zhang, H. Influence of in vitro gastrointestinal digestion and colonic fermentation on carbonyl scavenging capacity of fiber-bound polyphenols from quinoa. Food Funct. 2023, 14, 10581–10590. [Google Scholar] [CrossRef]
- Zhu, H.; Poojary, M.M.; Andersen, M.L.; Lund, M.N. Trapping of Carbonyl Compounds by Epicatechin: Reaction Kinetics and Identification of Epicatechin Adducts in Stored UHT Milk. J. Agric. Food Chem. 2020, 68, 7718–7726. [Google Scholar] [CrossRef]
- Jiang, K.; Huang, C.; Jiao, R.; Bai, W.; Zheng, J.; Ou, S. Adducts formed during protein digestion decreased the toxicity of five carbonyl compounds against Caco-2 cells. J. Hazard. Mater. 2019, 363, 26–33. [Google Scholar] [CrossRef]
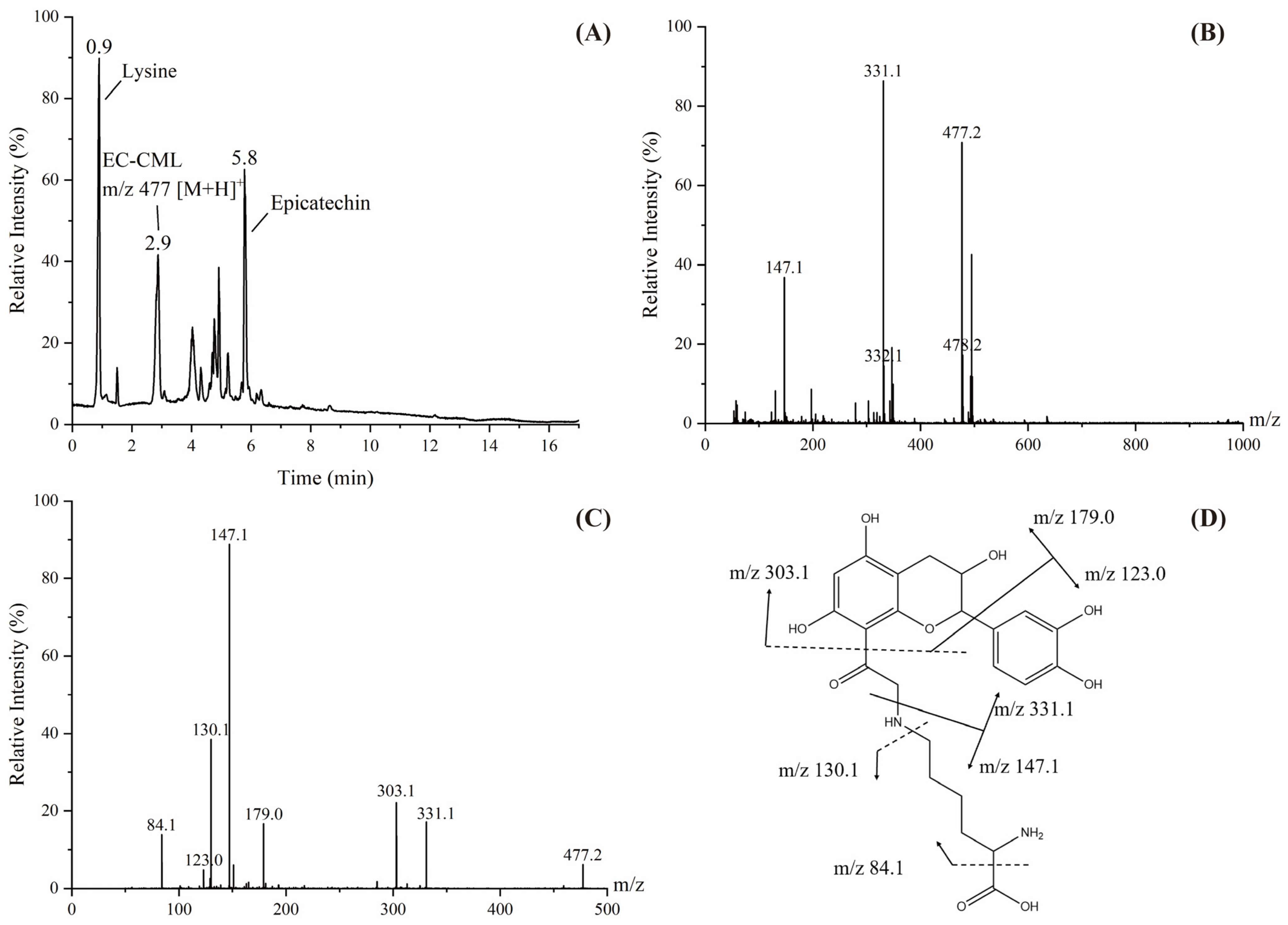
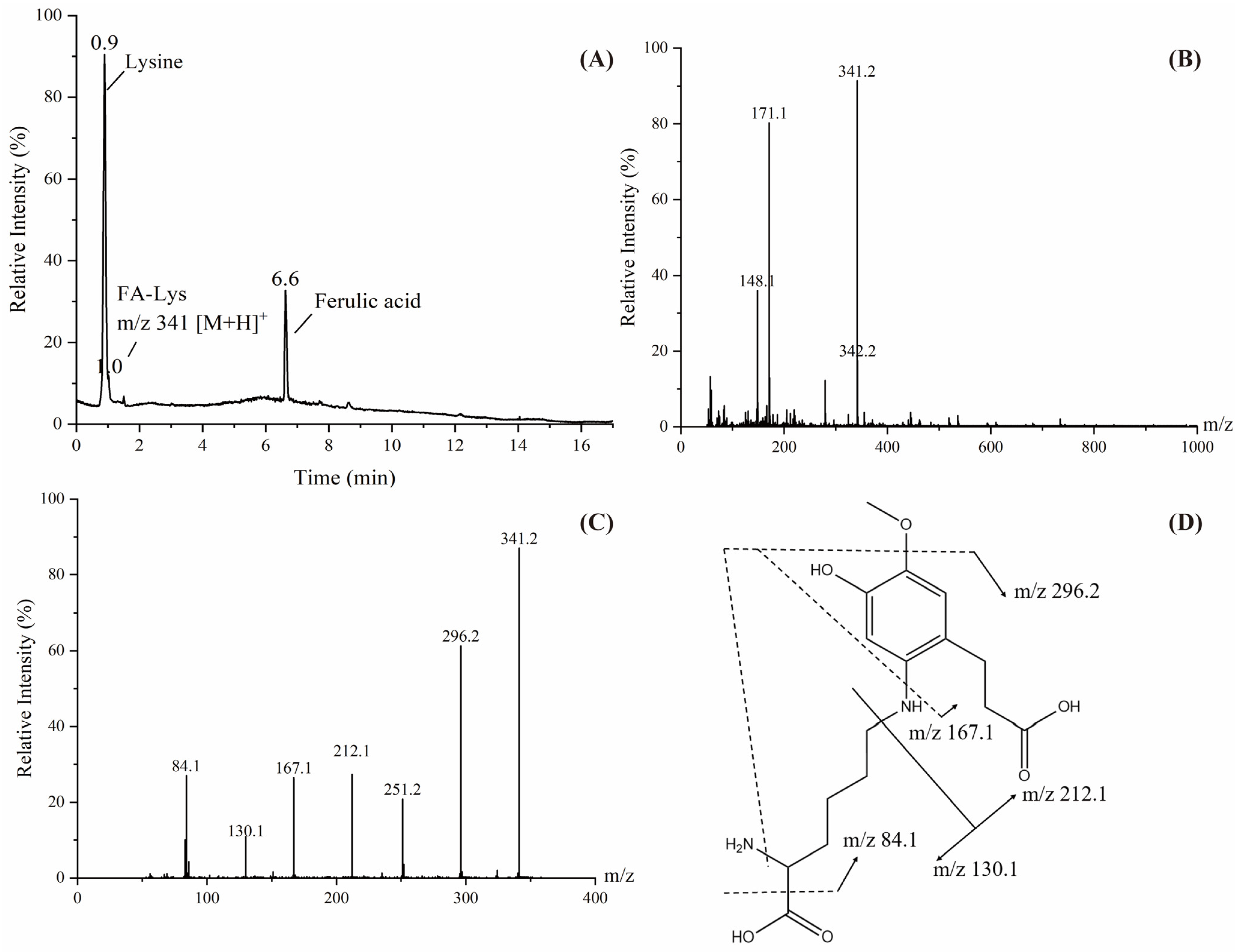
| CEL (%) | CML (%) | GO (%) | MGO (%) | 3-DG (%) | ||
|---|---|---|---|---|---|---|
| No Additives | 89.5 | 103.4 | 124.3 | 26.0 | 99.7 | |
| FA | 0.05% | 102.1 | 132.5 | 121.4 | 24.2 | 102.1 |
| 0.1% | 84.5 | 102.3 | 116.0 | 25.3 | 97.7 | |
| 0.2% | 81.4 | 99.7 | 125.8 | 27.8 | 97.9 | |
| 0.5% | 81.5 | 103.0 | 125.4 | 26.6 | 126.6 | |
| EC | 0.05% | 89.7 | 107.7 | 120.7 | 26.9 | 98.4 |
| 0.1% | 87.7 | 105.7 | 121.2 | 29.1 | 97.8 | |
| 0.2% | 83.5 | 103.5 | 146.4 | 29.0 | 105.2 | |
| 0.5% | 82.1 | 97.6 | 141.5 | 28.1 | 74.8 | |
| CEL (mg/kg) | CML (mg/kg) | GO (mg/kg) | MGO (mg/kg) | 3-DG (mg/kg) | ||
|---|---|---|---|---|---|---|
| Initial | 17.6 | 21.7 | 8.5 | 23.0 | 64.4 | |
| No Additives | 3.4 | 4.3 | 1.4 | 1.0 | 6.1 | |
| FA | 0.05% | 3.0 | 3.9 | 1.5 | 1.1 | 5.8 |
| 0.1% | 3.0 | 4.3 | 1.4 | 1.1 | 5.1 | |
| 0.2% | 3.5 | 4.6 | 1.6 | 1.1 | 6.1 | |
| 0.5% | 3.0 | 4.2 | 1.4 | 1.2 | 4.9 | |
| EC | 0.05% | 3.0 | 3.7 | 1.4 | 1.1 | 6.0 |
| 0.1% | 2.9 | 3.5 | 1.3 | 0.9 | 6.2 | |
| 0.2% | 3.5 | 4.1 | 1.7 | 1.2 | 3.3 | |
| 0.5% | 3.4 | 4.2 | 1.3 | 1.0 | 1.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.; Hu, H.; Liu, Y.; Li, J.; Huang, Y.; Wang, H.; Wang, Z.; Wang, Y. In Vitro Digestion Patterns of Advanced Glycation End Products and α-Dicarbonyls in Biscuits and the Modulatory Effects of Ferulic Acid and Epicatechin. Foods 2025, 14, 1429. https://doi.org/10.3390/foods14081429
Peng X, Hu H, Liu Y, Li J, Huang Y, Wang H, Wang Z, Wang Y. In Vitro Digestion Patterns of Advanced Glycation End Products and α-Dicarbonyls in Biscuits and the Modulatory Effects of Ferulic Acid and Epicatechin. Foods. 2025; 14(8):1429. https://doi.org/10.3390/foods14081429
Chicago/Turabian StylePeng, Xiaoxiang, Huiyu Hu, Yuwei Liu, Jia Li, Yilun Huang, Haiwa Wang, Ziyi Wang, and Yuting Wang. 2025. "In Vitro Digestion Patterns of Advanced Glycation End Products and α-Dicarbonyls in Biscuits and the Modulatory Effects of Ferulic Acid and Epicatechin" Foods 14, no. 8: 1429. https://doi.org/10.3390/foods14081429
APA StylePeng, X., Hu, H., Liu, Y., Li, J., Huang, Y., Wang, H., Wang, Z., & Wang, Y. (2025). In Vitro Digestion Patterns of Advanced Glycation End Products and α-Dicarbonyls in Biscuits and the Modulatory Effects of Ferulic Acid and Epicatechin. Foods, 14(8), 1429. https://doi.org/10.3390/foods14081429





