Modulation of Antioxidant Capacity, Nutritional Composition, Probiotic Viability After Digestion and Sensory Attributes of Plant-Based Beverages Through Lactic Acid Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Beverage Preparation
2.3. Microbial Strains
2.4. Fermentation Process
2.5. Microbiological Analysis
2.6. Total Soluble Polyphenols and Total Antioxidant Capacity
2.7. Analysis of Phytochemicals Profileby UPLC-ESI-QTOF-MS/MS
2.8. Proximate Composition of the Beverages
2.9. Sensory Attributes
2.10. In Vitro Static Gastrointestinal Digestion
2.11. Survival of LAB Consortia
2.12. Statistical Analysis
3. Results and Discussion
3.1. Fermentation Process
3.2. Antioxidant Capacity Parameters
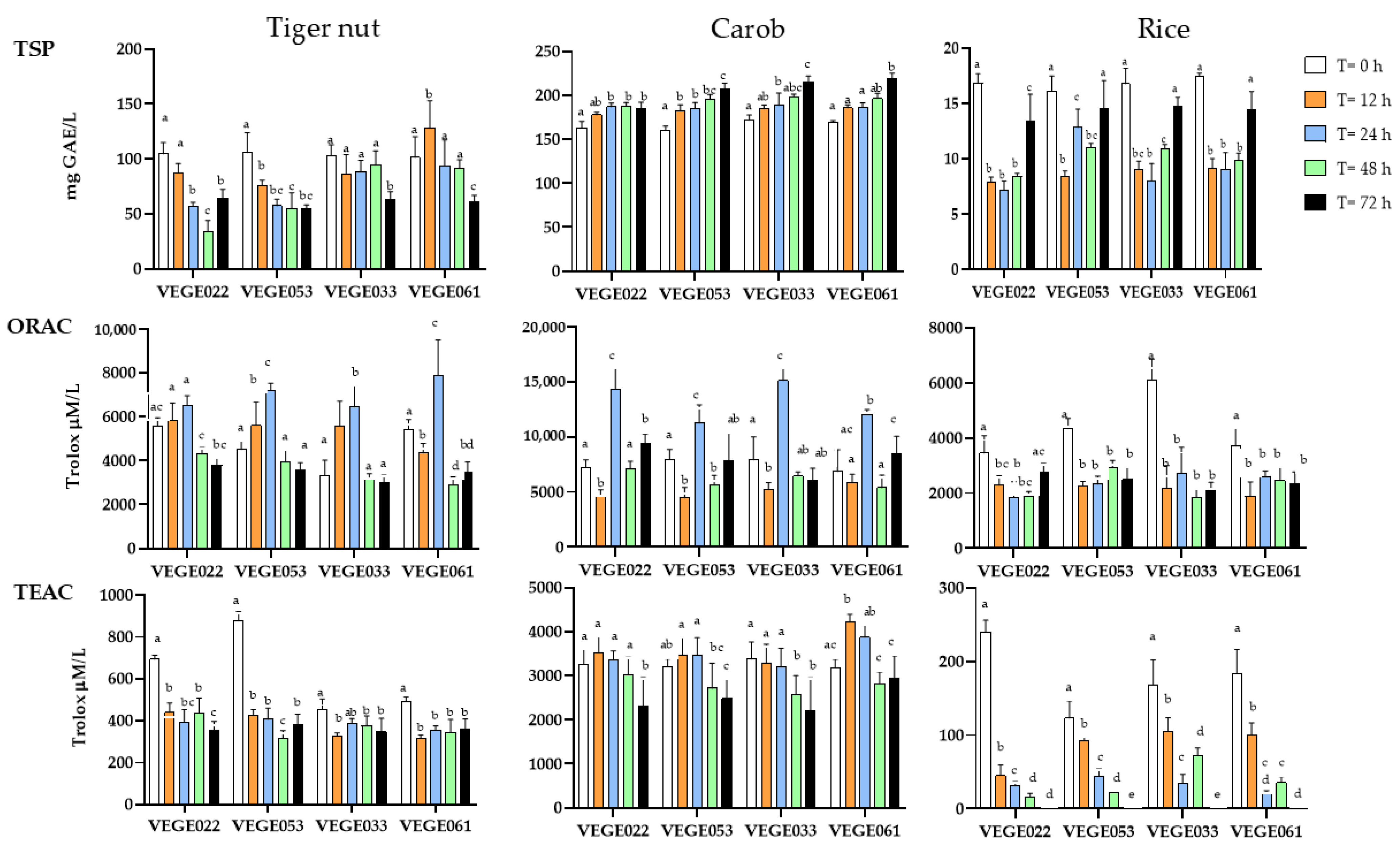
3.2.1. Total Soluble Polyphenols
3.2.2. Oxygen Radical Absorbance Capacity Assay (ORAC)
3.2.3. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
3.3. Phytochemical Compounds
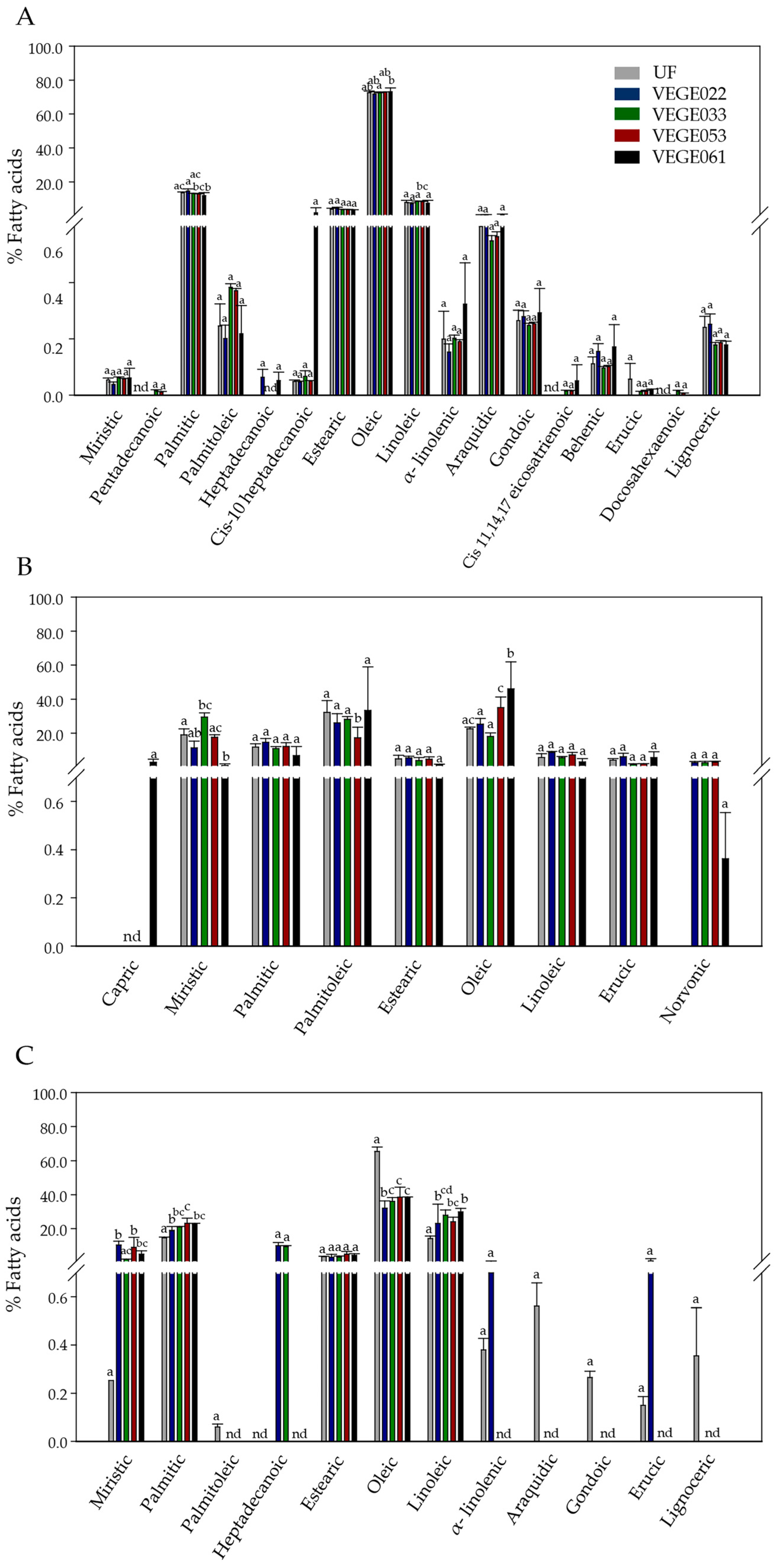
3.4. Proximate Chemical Composition
Fatty Acid Profile
3.5. Sensory Attributes
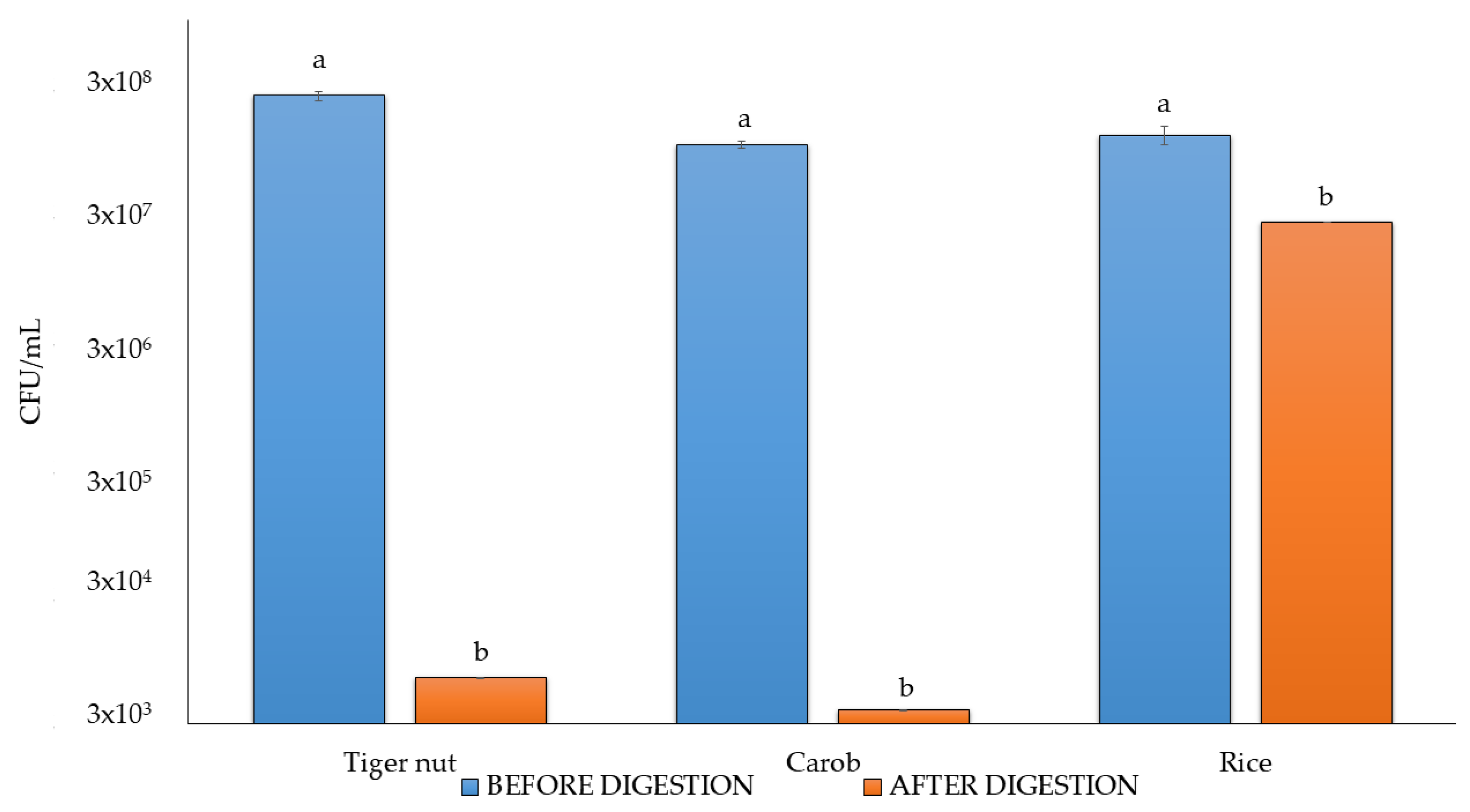
3.6. Viable Counts After Digestion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donat, M.G.; Lowry, A.L.; Alexander, L.V.; O’Gorman, P.A.; Maher, N. More Extreme Precipitation in the World’s Dry and Wet Regions. Nat. Clim. Change 2016, 6, 508–513. [Google Scholar] [CrossRef]
- Mirzabaev, A.; Bezner Kerr, R.; Hasegawa, T.; Pradhan, P.; Wreford, A.; Cristina Tirado von der Pahlen, M.; Gurney-Smith, H. Severe Climate Change Risks to Food Security and Nutrition. Clim. Risk Manag. 2023, 39, 100473. [Google Scholar] [CrossRef]
- Turkmen, N.; Akal, C.; Özer, B. Probiotic Dairy-Based Beverages: A Review. J. Funct. Foods 2019, 53, 62–75. [Google Scholar] [CrossRef]
- Vitali, M.; Gandía, M.; Garcia-Llatas, G.; Tamayo-Ramos, J.A.; Cilla, A.; Gamero, A. Exploring the Potential of Rice, Tiger Nut and Carob for the Development of Fermented Beverages in Spain: A Comprehensive Review on the Production Methodologies Worldwide. Beverages 2023, 9, 47. [Google Scholar] [CrossRef]
- Mukherjee, A.; Breselge, S.; Dimidi, E.; Marco, M.L.; Cotter, P.D. Fermented Foods and Gastrointestinal Health: Underlying Mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 248–266. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Hilbert, F.; Lindqvist, R.; et al. Update of the List of Qualified Presumption of Safety (QPS) Recommended Microbiological Agents Intentionally Added to Food or Feed as Notified to EFSA 19: Suitability of Taxonomic Units Notified to EFSA until September 2023. EFSA J. 2024, 22, e8517. [Google Scholar] [CrossRef]
- Grau-Fuentes, E.; Rodrigo, D.; Garzón, R.; Rosell, C.M. Understanding the Marketed Plant-Based Beverages: From Ingredients Technological Function to Their Nutritional Value. J. Funct. Foods 2023, 106, 105609. [Google Scholar] [CrossRef]
- Badejo, A.A.; Damilare, A.; Ojuade, T.D. Processing Effects on the Antioxidant Activities of Beverage Blends Developed from Cyperus esculentus, Hibiscus Sabdariffa, and Moringa Oleifera Extracts. Prev. Nutr. Food Sci. 2014, 19, 227–233. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Barba, F.J.; Putnik, P.; Bursać Kovačević, D.; Lorenzo, J.M.; Cantavella-Ferrero, Y. Enhancing Bioactive Antioxidants’ Extraction from “Horchata de Chufa” By-Products. Foods 2018, 7, 161. [Google Scholar] [CrossRef]
- THE 17 GOALS|Sustainable Development. Available online: https://sdgs.un.org/goals (accessed on 2 October 2024).
- Gan, R.-Y.; Shah, N.P.; Wang, M.-F.; Lui, W.-Y.; Corke, H. Fermentation Alters Antioxidant Capacity and Polyphenol Distribution in Selected Edible Legumes. Int. J. Food Sci. Technol. 2016, 51, 875–884. [Google Scholar] [CrossRef]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef]
- Cichońska, P.; Ziębicka, A.; Ziarno, M. Properties of Rice-Based Beverages Fermented with Lactic Acid Bacteria and Propionibacterium. Molecules 2022, 27, 2558. [Google Scholar] [CrossRef] [PubMed]
- Satir, G. The Effects of Fermentation with Water Kefir Grains on Two Varieties of Tigernut (Cyperus esculentus L). Milk. LWT 2022, 171, 114164. [Google Scholar] [CrossRef]
- Chait, Y.A.; Gunenc, A.G.; Bendali, F.B.; Hosseinian, F. Functional Fermented Carob Milk: Probiotic Variability and Polyphenolic Profile. J. Food Bioact. 2021, 14, 114–125. [Google Scholar] [CrossRef]
- Rodríguez, I.F.; Cattaneo, F.; Zech, X.V.; Svavh, E.; Pérez, M.J.; Zampini, I.C.; Isla, M.I. Aloja and Añapa, Two Traditional Beverages Obtained from Prosopis Alba Pods: Nutritional and Functional Characterization. Food Biosci. 2020, 35, 100546. [Google Scholar] [CrossRef]
- Fuloria, S.; Mehta, J.; Talukdar, M.P.; Sekar, M.; Gan, S.H.; Subramaniyan, V.; Rani, N.N.I.M.; Begum, M.Y.; Chidambaram, K.; Nordin, R.; et al. Synbiotic Effects of Fermented Rice on Human Health and Wellness: A Natural Beverage That Boosts Immunity. Front. Microbiol. 2022, 13, 950913. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Cilla, A.; Perales, S.; Lagarda, M.J.; Barberá, R.; Clemente, G.; Farré, R. Influence of Storage and in Vitro Gastrointestinal Digestion on Total Antioxidant Capacity of Fruit Beverages. J. Food Compos. Anal. 2011, 24, 87–94. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; García-Villalba, R.; Martínez-Díaz, F.; Ocaña-Castillo, B.; Monedero-Saiz, T.; Torrecillas-Sánchez, A.; Abellán, B.; González-Sarrías, A.; Espín, J.C. Metabolic Profiling of Dietary Polyphenols and Methylxanthines in Normal and Malignant Mammary Tissues from Breast Cancer Patients. Mol. Nutr. Food Res. 2019, 63, e1801239. [Google Scholar] [CrossRef]
- Cortés, C.; Esteve, M.J.; Frıgola, A.; Torregrosa, F. Quality Characteristics of Horchata (a Spanish Vegetable Beverage) Treated with Pulsed Electric Fields during Shelf-Life. Food Chem. 2005, 91, 319–325. [Google Scholar] [CrossRef]
- Thiex, N.; Novotny, L.; Crawford, A. Determination of Ash in Animal Feed: AOAC Official Method 942.05 Revisited. J. AOAC Int. 2012, 95, 1392–1397. [Google Scholar] [CrossRef]
- Tada, S.; Innami, S. A Simplified Modification of the AOAC Official Method for Determination of Total Dietary Fiber Using Newly Developed Enzymes. J. AOAC Int. 2007, 90, 217–224. [Google Scholar] [CrossRef]
- Boselli, E.; Velazco, V.; Fiorenza Caboni, M.; Lercker, G. Pressurized Liquid Extraction of Lipids for the Determination of Oxysterols in Egg-Containing Food. J. Chromatogr. A 2001, 917, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Kjeldahl, J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Z. Für Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Merrill, A.L.; Watt, B.K. Energy Value of Foods: Basis and Derivation; Human Nutrition Research Branch, Agricultural Research Service, U.S. Department of Agriculture: Washington, DC, USA, 1955; 105p. [Google Scholar]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Beaulieu, J.C.; Reed, S.S.; Obando-Ulloa, J.M.; McClung, A.M. Green Processing Protocol for Germinating and Wet Milling Brown Rice for Beverage Formulations: Sprouting, Milling and Gelatinization Effects. Food Sci. Nutr. 2020, 8, 2445–2457. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, X.; Zhang, T.; Zhao, C.; Guan, S.; Pu, Y.; Gao, F. Tiger Nut (Cyperus esculentus L.): Nutrition, Processing, Function and Applications. Foods 2022, 11, 601. [Google Scholar] [CrossRef]
- Deziderio, M.A.; de Souza, H.F.; Kamimura, E.S.; Petrus, R.R. Plant-Based Fermented Beverages: Development and Characterization. Foods 2023, 12, 4128. [Google Scholar] [CrossRef]
- Naissinger da Silva, M.; Tagliapietra, B.L.; Flores, V.d.A.; Pereira dos Santos Richards, N.S. In Vitro Test to Evaluate Survival in the Gastrointestinal Tract of Commercial Probiotics. Curr. Res. Food Sci. 2021, 4, 320–325. [Google Scholar] [CrossRef]
- Llorens, P.; Flavia Chiacchio, M.; Tagliamonte, S.; Juan-García, A.; Pallarés, N.; Carlos Moltó, J.; Vitaglione, P.; Juan, C. Potential Bioaccessibility and Bioavailability of Polyphenols and Functional Properties of Tiger Nut Beverage and Its By-Product during in Vitro Digestion. Food Funct. 2024, 15, 8143–8152. [Google Scholar] [CrossRef] [PubMed]
- Goulas, V.; Stylos, E.; Chatziathanasiadou, M.V.; Mavromoustakos, T.; Tzakos, A.G. Functional Components of Carob Fruit: Linking the Chemical and Biological Space. Int. J. Mol. Sci. 2016, 17, 1875. [Google Scholar] [CrossRef]
- Tounsi, L.; Kechaou, N. Influence of pectinase and cellulase extracts on carob juice yield and quality. Life Sci. J. 2021, 18, 10–20. [Google Scholar]
- Ma, Y.; Zhang, S.; Rong, L.; Wu, Z.; Sun, W. Polyphenol Composition and Antioxidant Activity of Japonica Rice Cultivars and Intake Status. Foods 2022, 11, 3788. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Elbrhami, A.A.; Popović, V.; Koutchma, T.; Warriner, K. Comparative Effects of Thermal, High Hydrostatic Pressure, and UV-C Processing on the Quality, Nutritional Attributes, and Inactivation of Escherichia Coli, Salmonella, and Listeria Introduced into Tiger Nut Milk. J. Food Prot. 2019, 82, 971–979. [Google Scholar] [CrossRef]
- Zou, J.; Hu, Y.; Li, K.; Liu, Y.; Li, M.; Pan, X.; Chang, X. Chestnuts in Fermented Rice Beverages Increase Metabolite Diversity and Antioxidant Activity While Reducing Cellular Oxidative Damage. Foods 2023, 12, 164. [Google Scholar] [CrossRef]
- Santos, M.V.; Banfi, S.; Santos, R.; Mota, M.; Raymundo, A.; Prista, C. Improving Chestnut Physicochemical Properties through Fermentation—Development of Chestnut Amazake. Food Chem. X 2023, 17, 100597. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Coelho, N.; Santos-Rufo, A.; Gonçalves, S.; Pérez-Santín, E.; Romano, A. The Influence of In Vitro Gastrointestinal Digestion on the Chemical Composition and Antioxidant and Enzyme Inhibitory Capacities of Carob Liqueurs Obtained with Different Elaboration Techniques. Antioxidants 2019, 8, 563. [Google Scholar] [CrossRef]
- Goufo, P.; Trindade, H. Rice Antioxidants: Phenolic Acids, Flavonoids, Anthocyanins, Proanthocyanidins, Tocopherols, Tocotrienols, γ-Oryzanol, and Phytic Acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef]
- Jung, T.-D.; Shin, G.-H.; Kim, J.-M.; Choi, S.-I.; Lee, J.-H.; Lee, S.J.; Park, S.J.; Woo, K.S.; Oh, S.K.; Lee, O.-H. Comparative Analysis of γ-Oryzanol, β-Glucan, Total Phenolic Content and Antioxidant Activity in Fermented Rice Bran of Different Varieties. Nutrients 2017, 9, 571. [Google Scholar] [CrossRef]
- Hernández-Olivas, E.; Asensio-Grau, A.; Calvo-Lerma, J.; García-Hernández, J.; Heredia, A.; Andrés, A. Content and Bioaccessibility of Bioactive Compounds with Potential Benefits for Macular Health in Tiger Nut Products. Food Biosci. 2022, 49, 101879. [Google Scholar] [CrossRef]
- Demarinis, C.; Montemurro, M.; Torreggiani, A.; Pontonio, E.; Verni, M.; Rizzello, C.G. Use of Selected Lactic Acid Bacteria and Carob Flour for the Production of a High-Fibre and “Clean Label” Plant-Based Yogurt-like Product. Microorganisms 2023, 11, 1607. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.R.; Velasco, J.I.; Fakhouri, F.M. Use of Rice on the Development of Plant-Based Milk with Antioxidant Properties: From Raw Material to Residue. LWT 2023, 173, 114271. [Google Scholar] [CrossRef]
- Shan, L.; Tyagi, A.; Ham, H.-J.; Oh, D.H. Uncovering the Antiinflammatory Potential of Lactiplantibacillus Plantarum Fermented Cannabis Sativa L Seeds. npj Sci. Food 2024, 8, 42. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, C.; Zhu, Y.; Zhou, C.; Xiong, Z.; Samy Eweys, A.; Zhou, H.; Dong, Y.; Xiao, X. Metabolomics Strategy for Revealing the Components in Fermented Barley Extracts with Lactobacillus Plantarum Dy-1. Food Res. Int. 2021, 139, 109808. [Google Scholar] [CrossRef]
- Pelegrín, C.J.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Chemical Composition and Bioactive Antioxidants Obtained by Microwave-Assisted Extraction of Cyperus esculentus L. By-Products: A Valorization Approach. Front. Nutr. 2022, 9, 944830. [Google Scholar] [CrossRef]
- Saeed, M.M.; Fernández-Ochoa, Á.; Saber, F.R.; Sayed, R.H.; Cádiz-Gurrea, M.d.l.L.; Elmotayam, A.K.; Leyva-Jiménez, F.J.; Segura-Carretero, A.; Nadeem, R.I. The Potential Neuroprotective Effect of Cyperus esculentus L. Extract in Scopolamine-Induced Cognitive Impairment in Rats: Extensive Biological and Metabolomics Approaches. Molecules 2022, 27, 7118. [Google Scholar] [CrossRef]
- Brejchova, K.; Balas, L.; Paluchova, V.; Brezinova, M.; Durand, T.; Kuda, O. Understanding FAHFAs: From Structure to Metabolic Regulation. Prog. Lipid Res. 2020, 79, 101053. [Google Scholar] [CrossRef]
- Yore, M.M.; Syed, I.; Moraes-Vieira, P.M.; Zhang, T.; Herman, M.A.; Homan, E.A.; Patel, R.T.; Lee, J.; Chen, S.; Peroni, O.D.; et al. Discovery of a Class of Endogenous Mammalian Lipids with Anti-Diabetic and Anti-Inflammatory Effects. Cell 2014, 159, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Kuda, O.; Brezinova, M.; Rombaldova, M.; Slavikova, B.; Posta, M.; Kopecky, J.; Kudova, E.; Rossmeisl, M.; Flachs, P. Distinct Biological Activities of Isomers from Several Families of Branched Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs). J. Lipid Res. 2021, 62, 100108. [Google Scholar] [CrossRef]
- Smit, B.A.; Engels, W.J.M.; Wouters, J.T.M.; Smit, G. Diversity of L-Leucine Catabolism in Various Microorganisms Involved in Dairy Fermentations, and Identification of the Rate-Controlling Step in the Formation of the Potent Flavour Component 3-Methylbutanal. Appl. Microbiol. Biotechnol. 2004, 64, 396–402. [Google Scholar] [CrossRef]
- Hidayat, S.; Yoshino, K.; Tokunaga, C.; Hara, K.; Matsuo, M.; Yonezawa, K. Inhibition of Amino Acid-mTOR Signaling by a Leucine Derivative Induces G1 Arrest in Jurkat Cells. Biochem. Biophys. Res. Commun. 2003, 301, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Sumi, K.; Sakuda, M.; Munakata, K.; Nakamura, K.; Ashida, K. α-Hydroxyisocaproic Acid Decreases Protein Synthesis but Attenuates TNFα/IFNγ Co-Exposure-Induced Protein Degradation and Myotube Atrophy via Suppression of iNOS and IL-6 in Murine C2C12 Myotube. Nutrients 2021, 13, 2391. [Google Scholar] [CrossRef]
- Razola-Díaz, M.d.C.; Gómez-Caravaca, A.M.; Guerra-Hernández, E.J.; Garcia-Villanova, B.; Verardo, V. New Advances in the Phenolic Composition of Tiger Nut (Cyperus esculentus L.) by-Products. Foods 2022, 11, 343. [Google Scholar] [CrossRef]
- Calderon-Montano, J.M.; Burgos-Moron, E.; Perez-Guerrero, C.; Lopez-Lazaro, M. A Review on the Dietary Flavonoid Kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. P-Coumaric Acid and Its Conjugates: Dietary Sources, Pharmacokinetic Properties and Biological Activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef] [PubMed]
- Elfazazi, K.; Harrak, H.; Achchoub, M.; Benbati, M. Physicochemical Criteria, Bioactive Compounds and Sensory Quality of Moroccan Traditional Carob Drink. Mater. Today Proc. 2020, 27, 3249–3253. [Google Scholar] [CrossRef]
- Santonocito, D.; Granata, G.; Geraci, C.; Panico, A.; Siciliano, E.A.; Raciti, G.; Puglia, C. Carob Seeds: Food Waste or Source of Bioactive Compounds? Pharmaceutics 2020, 12, 1090. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Romano, A.; Moreno-Rojas, J.M. Carob Pulp: A Nutritional and Functional By-Product Worldwide Spread in the Formulation of Different Food Products and Beverages. A Review. Processes 2021, 9, 1146. [Google Scholar] [CrossRef]
- Ozturk, T.; Ávila-Gálvez, M.Á.; Mercier, S.; Vallejo, F.; Bred, A.; Fraisse, D.; Morand, C.; Pelvan, E.; Monfoulet, L.E.; González-Sarrías, A. Impact of Lactic Acid Bacteria Fermentation on (Poly)Phenolic Profile and In Vitro Antioxidant and Anti-Inflammatory Properties of Herbal Infusions. Antioxidants 2024, 13, 562. [Google Scholar] [CrossRef]
- Custódio, L.; Escapa, A.L.; Fernandes, E.; Fajardo, A.; Aligué, R.; Alberício, F.; Neng, N.; Nogueira, J.M.F.; Romano, A. Phytochemical Profile, Antioxidant and Cytotoxic Activities of the Carob Tree (Ceratonia siliqua L.) Germ Flour Extracts. Plant Foods Hum. Nutr. 2011, 66, 78–84. [Google Scholar] [CrossRef]
- Braune, A.; Gütschow, M.; Engst, W.; Blaut, M. Degradation of Quercetin and Luteolin by Eubacterium Ramulus. Appl. Environ. Microbiol. 2001, 67, 5558–5567. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, H.; Wang, Y.; Nussio, L.G.; Yang, F.; Ni, K. Insights into Fermentation with Lactic Acid Bacteria on the Flavonoids Biotransformation of Alfalfa Silage. Chem. Biol. Technol. Agric. 2024, 11, 73. [Google Scholar] [CrossRef]
- Zannini, M.; Cattivelli, A.; Nissen, L.; Conte, A.; Gianotti, A.; Tagliazucchi, D. Identification, Bioaccessibility, and Antioxidant Properties of Phenolic Compounds in Carob Syrup. Foods 2024, 13, 2196. [Google Scholar] [CrossRef]
- Ortega, N.; Macià, A.; Romero, M.-P.; Reguant, J.; Motilva, M.-J. Matrix Composition Effect on the Digestibility of Carob Flour Phenols by an in-Vitro Digestion Model. Food Chem. 2011, 124, 65–71. [Google Scholar] [CrossRef]
- Chait, Y.A.; Gunenc, A.; Bendali, F.; Hosseinian, F. Simulated Gastrointestinal Digestion and in Vitro Colonic Fermentation of Carob Polyphenols: Bioaccessibility and Bioactivity. LWT 2020, 117, 108623. [Google Scholar] [CrossRef]
- Eicher, C.; Coulon, J.; Favier, M.; Alexandre, H.; Reguant, C.; Grandvalet, C. Citrate Metabolism in Lactic Acid Bacteria: Is There a Beneficial Effect for Oenococcus Oeni in Wine? Front. Microbiol 2024, 14, 1283220. [Google Scholar] [CrossRef]
- Zheng, Z.; Wei, L.; Zhu, M.; Qian, Z.; Liu, J.; Zhang, L.; Xu, Y. Effect of Lactic Acid Bacteria Co-Fermentation on Antioxidant Activity and Metabolomic Profiles of a Juice Made from Wolfberry and Longan. Food Res. Int. 2023, 174, 113547. [Google Scholar] [CrossRef]
- Duan, W.; Guan, Q.; Zhang, H.-L.; Wang, F.-Z.; Lu, R.; Li, D.-M.; Geng, Y.; Xu, Z.-H. Improving Flavor, Bioactivity, and Changing Metabolic Profiles of Goji Juice by Selected Lactic Acid Bacteria Fermentation. Food Chem. 2023, 408, 135155. [Google Scholar] [CrossRef] [PubMed]
- van Beek, S.; Priest, F.G. Decarboxylation of Substituted Cinnamic Acids by Lactic Acid Bacteria Isolated during Malt Whisky Fermentation. Appl. Environ. Microbiol. 2000, 66, 5322–5328. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, G.M.; Tlais, A.Z.A.; Losito, I.; Filannino, P.; Gobbetti, M.; Di Cagno, R. Triacylglycerols Hydrolysis and Hydroxy- and Epoxy-Fatty Acids Release during Lactic Fermentation of Plant Matrices: An Extensive Study Showing Inter- and Intra-Species Capabilities of Lactic Acid Bacteria. Food Chem. 2023, 412, 135552. [Google Scholar] [CrossRef] [PubMed]
- Ciulu, M.; Cádiz-Gurrea, M.d.l.L.; Segura-Carretero, A. Extraction and Analysis of Phenolic Compounds in Rice: A Review. Molecules 2018, 23, 2890. [Google Scholar] [CrossRef] [PubMed]
- Sęczyk, Ł.; Sugier, D.; Świeca, M.; Gawlik-Dziki, U. The Effect of in Vitro Digestion, Food Matrix, and Hydrothermal Treatment on the Potential Bioaccessibility of Selected Phenolic Compounds. Food Chem. 2021, 344, 128581. [Google Scholar] [CrossRef]
- Deng, Y.-J.; Wang, S.Y. Synergistic Growth in Bacteria Depends on Substrate Complexity. J. Microbiol. 2016, 54, 23–30. [Google Scholar] [CrossRef]
- Eke-Ejiofor, J.; Beleya, E.A. Chemical and Sensory Properties of Spiced Tigernut (Cyperus esculentus vassativa) Drink. Int. J. Biotechnol. Food Sci. 2018, 6, 52–58. [Google Scholar]
- Toufeili, I.; Itani, M.; Zeidan, M.; Al Yamani, O.; Kharroubi, S. Nutritional and Functional Potential of Carob Syrup Versus Date and Maple Syrups. Food Technol. Biotechnol. 2022, 60, 266–278. [Google Scholar] [CrossRef]
- Duman, E. Some Physico-Chemical Properties, Fatty Acid Compositions, Macro-Micro Minerals and Sterol Contents of Two Variety Tigernut Tubers and Oils Harvested from East Mediterranean Region. Food Sci. Technol. 2019, 39, 610–615. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Barba, F.J.; Lorenzo, J.M.; Dominguez, R.; Pateiro, M.; Mañes, J.; Moltó, J.C. Evaluating the Impact of Supercritical-CO2 Pressure on the Recovery and Quality of Oil from “Horchata” by-Products: Fatty Acid Profile, α-Tocopherol, Phenolic Compounds, and Lipid Oxidation Parameters. Food Res. Int. 2019, 120, 888–894. [Google Scholar] [CrossRef]
- Youssef, M.K.E.; El-Manfaloty, M.M.; Ali, H.M. Assessment of Proximate Chemical Composition, Nutritional Status, Fatty Acid Composition and Phenolic Compounds of Carob (Ceratonia siliqua L.). Food Public Health 2013, 3, 304–308. [Google Scholar]
- Beaulieu, J.C.; Moreau, R.A.; Powell, M.J.; Obando-Ulloa, J.M. Lipid Profiles in Preliminary Germinated Brown Rice Beverages Compared to Non-Germinated Brown and White Rice Beverages. Foods 2022, 11, 220. [Google Scholar] [CrossRef]
- Clemente-Villalba, J.; Cano-Lamadrid, M.; Issa-Issa, H.; Hurtado, P.; Hernández, F.; Carbonell-Barrachina, Á.A.; López-Lluch, D. Comparisonon Sensory Profile, Volatile Composition and Consumer’s Acceptancefor PDO or Non-PDO Tigernut (Cyperus esculentus L.) Milk. LWT 2021, 140, 110606. [Google Scholar] [CrossRef]
- Banwo, K.; Taiwo, O.T. Potential antioxidant activities and bioactive compounds in probiotic tiger nut date palm yogurt fermented with lactic acid bacteria. J. Food Sci. Technol. 2025, 62, 919–929. [Google Scholar] [CrossRef]
- Srour, N.; Daroub, H.; Toufeili, I.; Olabi, A. Developing a Carob-Based Milk Beverage Using Different Varieties of Carob Pods and Two Roasting Treatments and Assessing Their Effect on Quality Characteristics. J. Sci. Food Agric. 2016, 96, 3047–3057. [Google Scholar] [CrossRef]
- Allahdad, Z.; Manus, J.; Aguilar-Uscanga, B.R.; Salmieri, S.; Millette, M.; Lacroix, M. Physico-Chemical Properties and Sensorial Appreciation of a New Fermented Probiotic Beverage Enriched with Pea and Rice Proteins. Plant Foods Hum. Nutr. 2022, 77, 112–120. [Google Scholar] [CrossRef]
- Li, S.; Li, H.; Lu, L.; Shao, G.; Guo, Z.; He, Y.; Wang, Y.; Yang, X.; Chen, M.; Hu, X. Analysis of Rice Characteristic Volatiles and Their Influence on Rice Aroma. Curr. Res. Nutr. Food Sci. 2024, 9, 100794. [Google Scholar] [CrossRef]
- Faye, T.; Tamburello, A.; Vegarud, G.E.; Skeie, S. Survival of Lactic Acid Bacteria from Fermented Milks in an in Vitro Digestion Model Exploiting Sequential Incubation in Human Gastric and Duodenum Juice. J. Dairy Sci. 2012, 95, 558–566. [Google Scholar] [CrossRef]
- Treven, P.; Paveljšek, D.; Bogovič Matijašić, B.; Mohar Lorbeg, P. The Effect of Food Matrix Taken with Probiotics on the Survival of Commercial Probiotics in Simulation of Gastrointestinal Digestion. Foods 2024, 13, 3135. [Google Scholar] [CrossRef]
- Bourebaba, Y.; Marycz, K.; Mularczyk, M.; Bourebaba, L. Postbiotics as Potential New Therapeutic Agents for Metabolic Disorders Management. Biomed. Pharmacother. 2022, 153, 113138. [Google Scholar] [CrossRef]
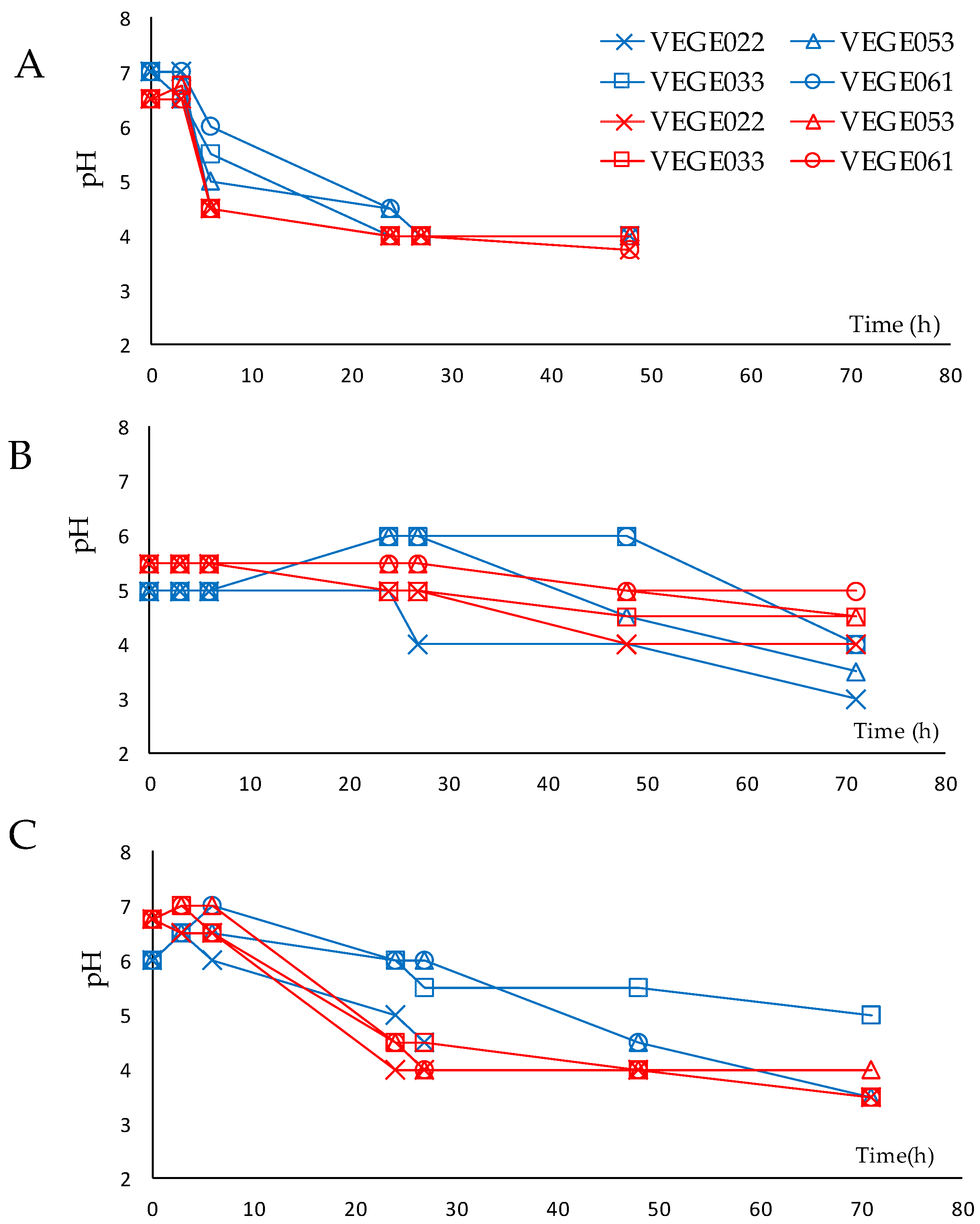
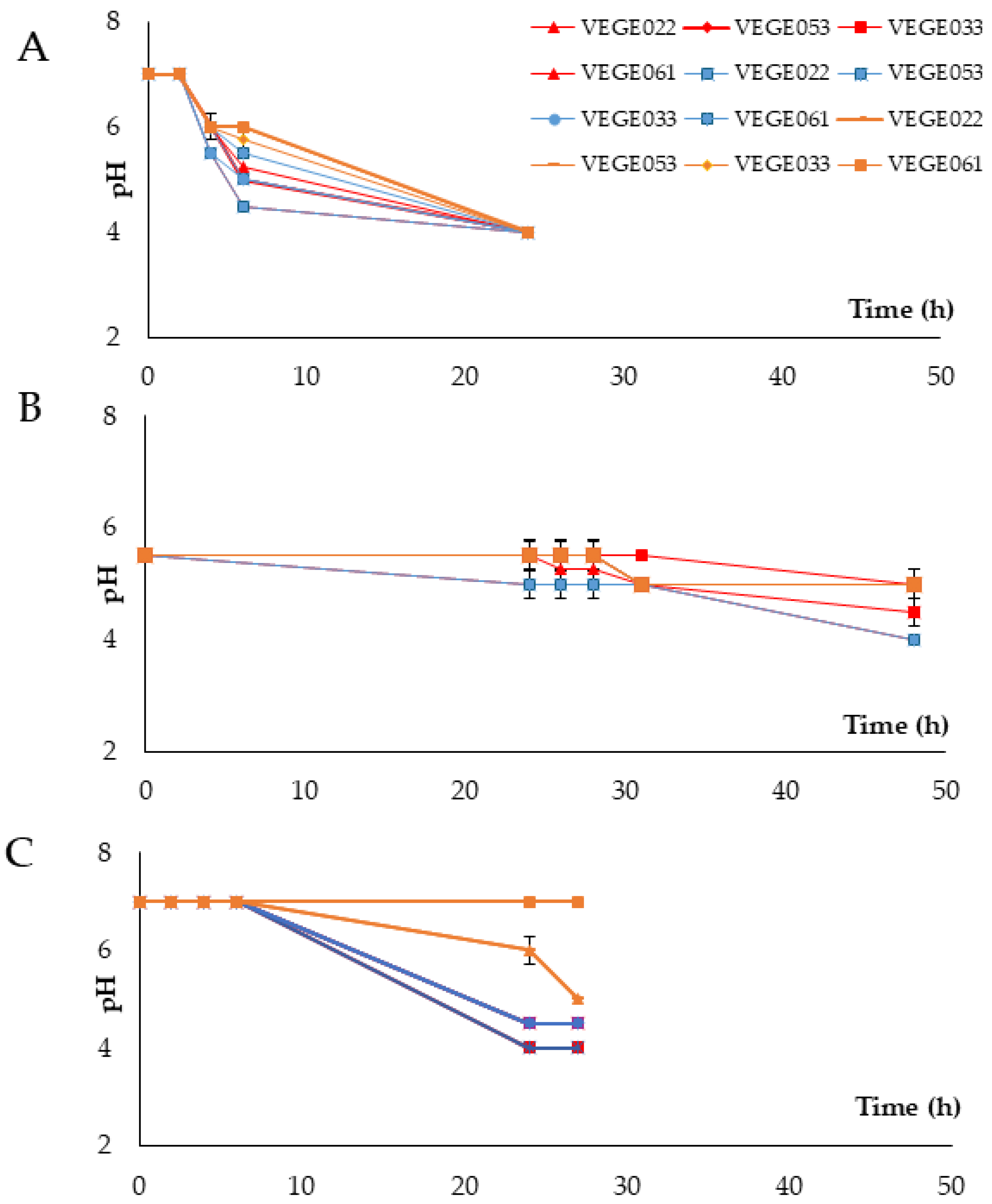
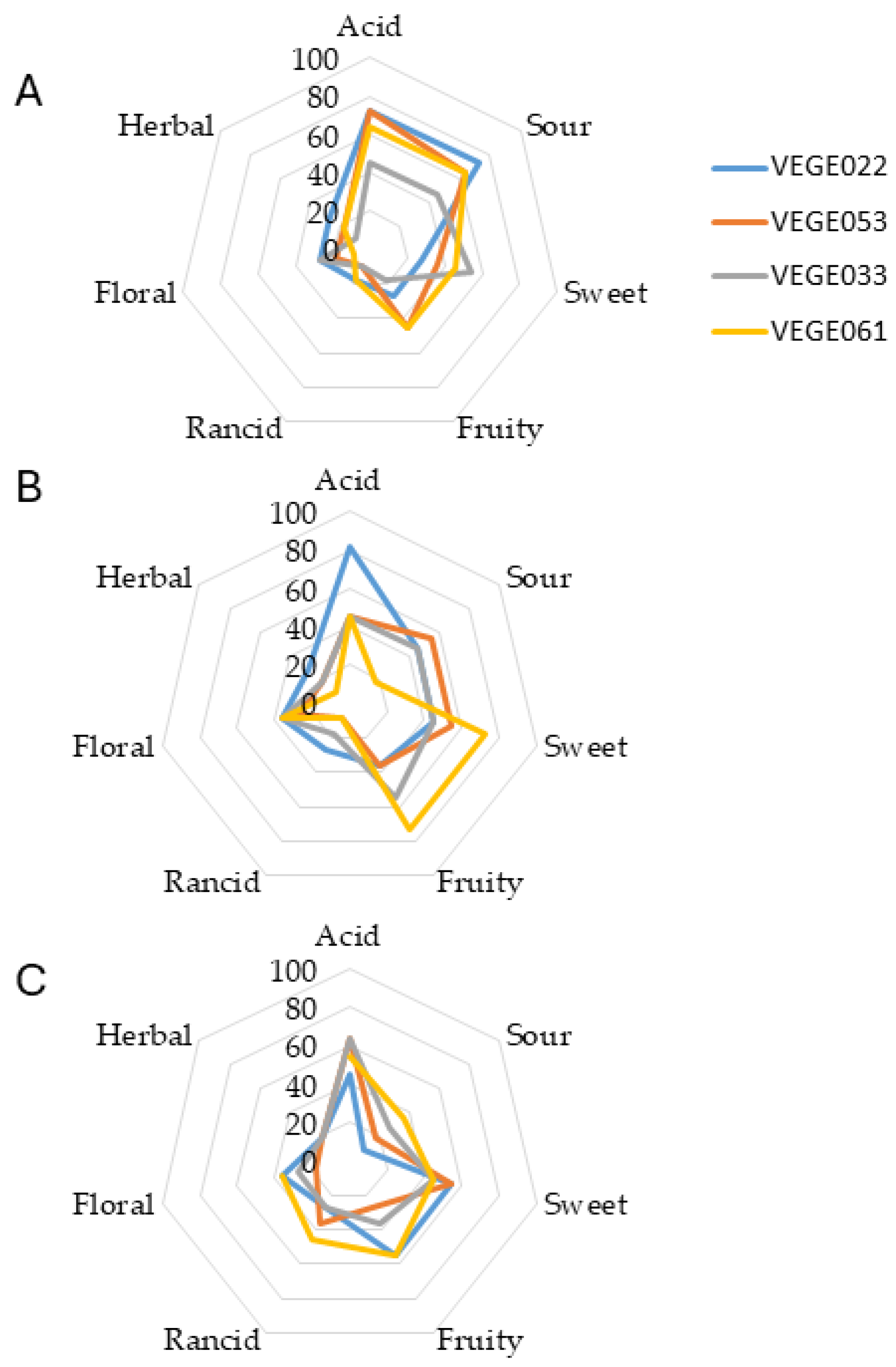
| Danisco® VEGE022 | Danisco® VEGE033 | Danisco® VEGE053 | Danisco® VEGE061 |
|---|---|---|---|
| Streptococcus thermophilus Lactobacillus delbrueckii subsp. bulgaricus Lactobacillus acidophilus NCFM® Bifidobacterium animalis subsp. lactis HN019 ® Lactobacillus plantarum | Streptococcus thermophilus Lactobacillus delbrueckii subsp. bulgaricus | Streptococcus thermophilus Lactobacillus delbrueckii subsp. bulgaricus Lactobacillus acidophilus NCFM® Bifidobacterium animalis subsp. lactis HN019 ® | Streptococcus thermophilus Lactobacillus delbrueckii subsp. bulgaricus Lactobacillus acidophilus NCFM® Bifidobacterium animalis subsp. lactis HN019 ® Lactobacillus paracasei |
| Sample | Starter | 30 °C | 37 °C | Level 1 * | Level 2 ** | Level 3 *** |
|---|---|---|---|---|---|---|
| Tiger nut | VEGE022 | 1.38 × 108 ± 1.17 × 108 | 3.00 × 108 ± 3.15 × 107 | 3.70 × 108 ± 1.00 × 107 | 4.25 × 108 ± 1.50 × 107 | 4.50 × 108 ± 3.00 × 107 |
| VEGE033 | 2.05 × 107 ± 1.20 × 106 | 1.02 × 108 ± 3.00 × 106 | 4.45 × 108 ± 3.50 × 107 | 4.50 × 108 ± 0 | 2.50 × 108 ± 1.50 × 106 | |
| VEGE053 | 1.77 × 105 ± 1.25 × 104 | 3.00 × 108 ± 0 | 2.06 × 108 ± 2.55 × 107 | 2.15 × 108 ± 1.65 × 107 | 2.35 × 108 ± 3.85 × 107 | |
| VEGE061 | 3.59 × 107 ± 3.51 × 107 | 1.04 × 108 ± 5.50 × 106 | 3.95 × 108 ± 1.65 × 108 | 3.15 × 108 ± 1.50 × 107 | 6.30 × 108 ± 1.10 × 108 | |
| VEGE022 | 1.26 × 106 ± 7.05 × 105 | 7.80 × 107 ± 1.70 × 107 | 8.35 × 107 ± 8.50 × 106 | 1.10 × 108 ± 5.00 × 105 | 9.35 × 107 ± 4.50 × 106 | |
| Carob | VEGE033 | 1.43 × 107 ± 5.35 × 106 | 1.08 × 107 ± 2.00 × 105 | 2.57 × 107 ± 3.00 × 105 | 8.00 × 107 ± 1.00 × 107 | 2.45 × 107 ± 3.50 × 106 |
| VEGE053 | 6.40 × 107 ± 3.10 × 107 | 5.60 × 105 ± 2.80 × 105 | 2.55 × 107 ± 8.00 × 105 | 3.70 × 107 ± 1.00 × 106 | 2.25 × 107 ± 5.50 × 106 | |
| VEGE061 | 6.80 × 107 ± 2.40 × 107 | 4.92 × 107 ± 2.88 × 107 | 1.45 × 107 ± 3.50 × 106 | 4.30 × 107 ± 1.60 × 107 | 1.85 × 107 ± 5.00 × 105 | |
| VEGE022 | 7.50 × 107 ± 4.00 × 106 | 7.00 × 107 ± 2.00 × 106 | 1.73 × 108 ± 1.85 × 107 | 1.45 × 108 ± 3.50 × 107 | 1.42 × 108 ± 1.40 × 107 | |
| Rice | VEGE033 | 4.09 × 107 ± 3.41 × 107 | 5.06 × 106 ± 4.54 × 106 | 4.70 × 106 ± 9.00 × 105 | 2.75 × 107 ± 6.50 × 106 | 3.60 × 106 ± 4.00 × 105 |
| VEGE053 | 4.50 × 107 ± 1.40 × 107 | 4.40 × 107 ± 2.00 × 107 | 5.85 × 106 ± 2.05 × 106 | 7.00 × 106 ± 2.00 × 106 | 1.15 × 106 ± 5.00 × 104 | |
| VEGE061 | 1.47 × 108 ± 1.15 × 108 | 6.55 × 107 ± 4.65 × 107 | 9.75 × 106 ± 5.00 × 104 | 1.70 × 108 ± 8.00 × 107 | n.d. |
| No. | Metabolites | Non-Fermented | VEGE022 | VEGE033 | VEGE053 | VEGE061 |
|---|---|---|---|---|---|---|
| 1 | Citric acid | 101.39 ± 4.00 a | 99.24 ± 2.52 a | 91.88 ± 7.32 a | 92.06 ± 10.13 a | 111.63 ± 19.11 a |
| 2 | Homovanillic acid * | 1.59 ± 0.97 a | 4.00 ± 0.36 b | 1.51 ± 0.49 a | 1.78 ± 1.17 a | 1.91 ± 0.70 a |
| 3 | L-leucic acid | 4.37 ± 0.20 a | 8.34 ± 0.29 b | 7.86 ± 1.92 b | 2.03 ± 0.69 a | 9.48 ± 1.77 b |
| 4 | S-leucic acid | 37.16 ± 1.60 a | 10.56 ± 0.20 b | 4.14 ± 1.59 c | 3.33 ± 1.57 c | 4.38 ± 1.10 c |
| 5 | Kaempferol 3′,7-diglucoside * | 1.46 ± 0.08 a (3.30 ± 0.25 µM) | 1.18 ± 0.02 bc (2.47 ± 0.07 µM) | 1.32 ± 0.12 ab (2.88 ± 0.36 µM) | 1.02 ± 0.03 c (1.97 ± 0.10 µM) | 1.06 ± 0.13 c (2.09 ± 0.41 µM) |
| 6 | 4-vinylphenol * | 2.81 ± 0.14 ab | 40.20 ± 0.04 b | 1.70 ± 0.47 ac | 2.38 ± 1.07 ac | 1.22 ± 0.23 c |
| 7 | Ethyl vanillin * | 19.20 ± 0.34 ab | 30.35 ± 0.22 b | 12.13 ± 4.43 a | 17.4 ± 8.55 a | 8.42 ± 2.02 a |
| 8 | Ferulic acid * | n.d. a | 0.34 ± 0.01 a | 2.88 ± 2.07 bc | 0.59 ± 0.14 ac | 3.62 ± 0.43 c |
| 9 | Sinapoyl alcohol | 0.91 ± 0.06 a | 1.21 ± 0.01 a | 1.11 ± 0.17 a | 0.89 ± 0.25 a | 1.0 ± 0.36 a |
| 10 | Dehydrodivanillin * | n.d. a | 0.89 ± 0.01 b | 0.92 ± 0.01 b | 0.58 ± 0.01 c | 0.60 ± 0.04 c |
| 11 | Trihydroxy octadecenoic acid isomer a | 28.89 ± 0.46 a | 28.35 ± 1.59 a | 30.27 ± 5.20 a | 17.70 ± 2.81 b | 13.01 ± 0.60 b |
| 12 | Trihydroxy octadecenoic acid isomer b | 22.69 ± 1.17 a | 20.62 ± 0.86 a | 22.69 ± 1.32 a | 12.20 ± 3.32 b | 6.20 ± 0.66 c |
| 13 | Dihydroxyoleic acid isomer a | 45.88 ± 0.88 a | 26.49 ± 1.61 b | 32.42 ± 5.75 b | 25.83 ± 1.54 b | 3.86 ± 3.60 c |
| 14 | Dihydroxyoleic acid isomer b | 43.76 ± 090 a | 26.96 ± 3.15 b | 27.09 ± 7.98 b | 19.90 ± 2.18 b | 3.02 ± 2.69 c |
| 15 | Dihydroxystearic acid isomer a | 18.43 ± 0.04 ab | 28.86 ± 17.43 b | 32.14 ± 0.12 b | 15.27 ± 1.60 ac | 1.95 ± 17.11 a |
| 16 | Dihydroxystearic acid isomer b | 71.66 ± 1.55 a | 84.16 ± 2.12 a | 81.49 ± 8.91 a | 71.82 ± 3.28 a | 14.28 ± 16.45 b |
| 17 | Hydroxylinoleic acid isomer a | 46.87 ± 0.46 a | 37.24 ± 5.45 ab | 27.20 ± 3.53 b | 40.02 ± 1.49 ab | 7.04 ± 9.47 c |
| 18 | Hydroxyoleic acid isomer a | 36.46 ± 0.80 a | 10.43 ± 0.99 b | 10.20 ± 3.28 bc | 15.75 ± 5.14 b | 2.30 ± 3.26 c |
| 19 | Hydroxyoleic acid isomer b | 3.91 ± 0.14 a | 1.46 ± 0.18 b | 2.92 ± 0.56 a | 3.24 ± 0.45 a | 0.36 ± 0.50 c |
| No | Metabolites | Non-Fermented | VEGE022 | VEGE033 | VEGE053 | VEGE061 |
|---|---|---|---|---|---|---|
| 1 | 2-Dehydro-D-xylonate | 19.27 × 106 ± 0.35 a | 36.15 ± 3.98 b | 11.76 ± 12.50 a | 22.35 ± 1.13 ab | 21.93 × 106 ± 0.96 ab |
| 2 | Citric acid | 19.27 × 106 ± 1.00 ab | 12.25 ± 2.63 b | 37.38 ± 35.25 abc | 54.17 ± 7.24 ac | 62.97 × 106 ± 0.70 c |
| 3 | 2-Deoxy-D-Ribose | 43.56 × 106 ± 1.00 a | 44.62 ± 2.82 a | 32.08 ±35.85 a | 45.55 ± 11.68 a | 52.22 × 106 ± 1.44 a |
| 4 | (R)-2-Methylmalate | 20.69 × 106 ± 0.77 a | 33.79 ± 2.86 a | 27.05 ± 12.88 a | 26.97 ± 10.41 a | 32.12 × 106 ± 5.02 a |
| 5 | 2-Galloylglucose * | 19.91 × 106 ± 0.64 a | 1.82 ± 0.10 b | 1.80 ± 0.15 b | 1.83 ± 0.12 b | 10.09 × 106 ± 11.92 ab |
| 6 | Succinic acid * | 16.13 × 106 ± 0.15 a | 15.68 ± 1.09 a | 13.17 ± 6.36 a | 14.19 ± 2.74 a | 15.36 × 106 ± 0.80 a |
| 7 | 2-Methylcitrate | 13.00 × 106 ± 0.03 a | 18.46 ± 1.61 a | 11.79 ± 15.23 a | 12.16 ± 10.83 a | 18.83 × 106 ± 1.29 a |
| 8 | Gallic acid * | 98.84 × 106 ± 1.97 a | 53.80 ± 61.87 ab | 98.48 ± 1.45 a | 12.42 ± 0.17 b | 12.57 × 106 ± 0.64 b |
| 9 | 2-O-Galloylsucrose * | 70.68 × 106 ± 0.78 a | 105.35 ± 2.67 a | 57.77 ± 72.28 a | 87.87 ± 26.28 a | 107.79 × 106 ± 0.47 a |
| 10 | Phloroglucinol * | n.d.a | 67.89 ± 4.31 b | n.d. a | n.d. a | n.d. a |
| 11 | Ethylmalonic acid | n.d. a | 19.79 ± 2.41 b | 12.10 ± 11.75 ab | 16.30 ± 5.94 b | 18.93 × 106 ± 1.01 b |
| 12 | b-D-Xylopyranosyl-(1-4)-a-L-rhamnopyranosyl-(1-2)-L-arabinose | 21.28 × 106 ± 1.64 a | 13.78 ± 0.34 a | 8.29 ± 9.94 a | 11.08 ± 5.58 a | 15.05 × 106 ± 0.98 a |
| 13 | a-L-Fucopyranosyl-(1-2)-b-D-galactopyranosyl-(1-2)-D-xylose | 28.67 × 106 ± 2.14 a | 49.37 ± 0.55 a | 25.20 ± 32.56 a | 55.94 ± 9.01 a | 63.31 × 106 ± 2.93 a |
| 14 | Cynaroside A * | n.d. a | 34.46 ± 0.51 b | 16.16 ± 22.57 ab | 24.35 ± 13.82 ab | 32.33 × 106 ± 1.00 b |
| 15 | 3′-Methoxyfukiic acid | n.d. a | 17.47 ± 0.49 b | 11.95 ± 7.17 b | 16.01 ± 0.05 b | 15.35 × 106 ± 0.16 b |
| 16 | Gallic acid 4-O-(6-galloylglucoside) * | n.d. a | 33.81 ± 0.34 b | 18.62 ± 21.92 ab | 25.14 ± 12.04 ab | 23.62 × 106 ± 0.68 ab |
| 17 | 3-propylmalic acid * | 38.58 × 106 ± 0.64 a | 48.32 ± 0.96 a | 34.11 ± 20.41 a | 30.26 ± 20.07 a | 40.31 × 106 ± 1.96 a |
| 18 | 3-O-Methylgallate * | 13.49 × 106 ± 0.75 a | 6.32 ± 0.11 a | 24.95 ± 18.09 a | 8.99 ± 6.81 a | 11.70 × 106 ± 0.75 a |
| 19 | Eriocitrin * | 22.60 × 106 ± 1.06 a | 14.30 ± 0.27 ab | 7.89 ± 10.99 b | 10.90 ± 3.51 ab | 14.75 × 106 ± 3.01 ab |
| 20 | Gallotannin * | n.d. a | 49.03 ± 1.10 b | 28.75 ± 36.05 ab | 45.65 ± 13.48 b | 50.31 × 106 ± 4.46 b |
| 21 | Gallotannin (isomer) * | n.d. a | 47.24 ± 0.47 b | 28.97 ± 34.57 ab | 39.88 ± 18.85 ab | 50.24 × 106 ± 4.29 b |
| 22 | Delphinidin 3-O-3″,6″-O-dimalonylglucoside * | n.d. a | 11.62 ± 0.08 bc | 8.84 ± 3.38 b | 10.44 ± 0.82 b | 10.29 × 106 ± 0.34 b |
| 23 | Ellagic acid+ * | 126.29 × 106 ± 15.64 a (126.05 ± 15.65 µM) | 71.10 ± 0.19 ab (70.86 ± 0.19 µM) | 40.72 ± 44.40 b (40.48 ± 44.40 µM) | 65.48 ± 10.63 b (65.24 ± 10.63 µM) | 72.20 ± 0.90 ab (71.96 ± 0.90 µM) |
| 24 | Myricitrin+ * | 25.83 × 106 ± 0.99 a (76.88 ± 2.98 µM) | 27.00 ± 0.64 a (80.38 ± 1.92 µM) | 13.20 ± 18.27 a (38.99 ± 54.81 µM) | 23.79 ± 4.68 a (70.75 ± 14.05 µM) | 28.90 ±2.84 a (86.09 ± 8.51 µM) |
| 25 | Quercetin 3-O-glucoside+ * | 15.38 × 106 ± 1.62 a (45.53 ± 4.87 µM) | 10.30 ± 0.66 ab (30.29 ± 1.98 µM) | 5.01 ± 6.91 b (14.42 ± 20.74 µM) | 9.79 ± 0.04 ab (28.74 ± 0.13 µM) | 9.44 ± 0.59 ab (27.70 ± 1.77 µM) |
| 26 | Benzoic acid * | n.d. a | 19.86 ± 0.12 a | 10.58 ± 14.22 a | 11.11 ± 9.65 a | 16.85 ± 2.37 a |
| 27 | Quercetin 3-arabinoside+ * | 27.09 × 106 ± 0.67 a (80.64 ± 2.01 µM) | 18.18 ± 0.23 a (53.93 ± 0.68 µM) | 11.52 ± 14.23 a (33.93 ± 42.70 µM) | 15.81 ± 7.13 a (46.82 ± 21.40 µM) | 22.08 ± 0.73 a (65.63 ± 2.19 µM) |
| 28 | Quercitrin+ * | 150.54 × 106 ± 4.97 a (451.00 ± 14.92 µM) | 108.17 ± 0.76 a (323.89 ± 2.28 µM) | 58.02 ± 79.22 a (173.45 ± 237.66 µM) | 97.13 ± 22.39 a (290.77 ± 67.17 µM) | 110.72 ± 5.87 a (331.54 ± 17.61 µM) |
| 29 | Isochinomin | 14.13 × 106 ± 0.86 a | 11.27 ± 0.40 a | 7.20 ± 10.03 a | 7.43 ± 5.60 a | 9.84 ± 0.22 a |
| 30 | Kaempferide 7-glucoside * | n.d. a | 10.83 ± 0.56 b | 5.86 ± 7.94 ab | 10.91 ± 5.60 b | 11.80 ± 1.08 b |
| 31 | Phloretin 2′-O-glucuronide+ * | 67.98 × 106 ± 4.01 a (203.32 ± 12.03 µM) | 55.37 ± 1.21 a (165.50 ± 3.64 µM) | 29.63 ± 41.40 a (88.28 ± 124.22 µM) | 53.95 ± 7.61 a (161.24 ± 22.84 µM) | 55.12 ± 0.20 a (164.76 ± 0.61 µM) |
| 32 | Apigenin 7-O-glucoside+ * | 19.16 × 106 ± 0.73 × 106 a (56.87 ± 2.19 μM) | 12.26 ± 1.41 ab (36.15 ± 4.24 µM) | 7.04 ± 9.31 b (20.50 ± 27.93 µM) | 11.47 ± 2.31 ab (33.79 ± 6.95 µM) | 11.85 ± 0.07 ab (34.92 ± 0.21 µM) |
| 33 | 6-Hydroxykaempferol+ * | 22.91 × 106 ± 1.96 a (68.12 ± 5.89 µM) | 26.75 ± 0.38 a (79.64 ± 1.14 µM) | 14.70 ± 20.54 a (43.49 ± 61.62 µM) | 23.43 ± 1.84 a (69.66 ± 5.52 µM) | 20.51 ± 0.73 a (60.90 ± 2.19 µM) |
| 34 | Eriodictyol * | n.d. a | 212.65 × 106 ± 0.56 a | 11.28 ± 15.74 a | 15.47 ± 9.00 a | 21.11 ± 5.84 a |
| 35 | Luteolin * | n.d. a | 99.00 ± 1.40 b | 50.266 ± 69.82 ab | 92.15 ± 9.25 b | 96.96 ± 0.70 b |
| 36 | Quercetin+ * | 28.86 × 106 ± 1.19 a (85.97 ± 3.57 µM) | 36.35 ± 6.77 a (108.44 ± 20.31 µM) | 15.64 ± 21.70 a (46.31 ± 65.10 µM) | 23.61 ± 11.89 a (70.20 ± 35.68 µM) | 28.27 ± 17.01 a (84.18 ± 51.05 µM) |
| 37 | Isorhamnetin+ * | n.d. a | 21.44 ± 0.65 b (63.71 ± 1.95 µM) | 9.18 ± 12.80 ab (26.93 ± 39.39 µM) | 15.64 ± 3.06 b (46.31 ± 9.19 µM) | 19.87 ± 3.26 b (58.99 ± 9.77 µM) |
| 38 | 9S,12S,13S-trihydroxy-10E-octadecenoic acid | 59.05 × 106 ± 1.31 a | 154.98 ± 2.15 a | 55.37 ± 77.59 a | 74.88 ± 86.75 a | 132.46 ± 3.12 a |
| 39 | 3′,5′-Dihydroxyflavanone * | n.d. a | 17.14 ±0.16 b | 11.61 ± 7.60 b | 16.40 ± 0.64 b | 16.56 ± 0.46 b |
| 40 | Octadecanedioic acid | 44.63 × 106 ± 0.85 a | 25.19 ± 1.17 ab | 11.64 ± 16.08 b | 15.81 ± 12.17 b | 24.06 ± 0.70 ab |
| 41 | 9,10-DiHOME | 57.42 × 106 ± 1.82 a | 36.72 ± 0.85 ab | 16.78 ± 21.98 b | 23.09 ± 11.12 b | 22.96 ± 7.78 b |
| 42 | L-Menthyl acetoacetate | n.d. a | 11.73 ± 1.24 a | 6.84 ± 9.43 a | 10.67 ± 4.77 a | 10.91 ± 0.17 a |
| 43 | 9,10-dihydroxy stearic acid | n.d. a | 44.72 ± 6.50 b | 26.97 ± 33.54 ab | 42.40 ± 10.13 b | 44.06 ± 6.36 b |
| 44 | Laserpitin | n.d. a | 15.29 ± 0.52 a | 12.32 ± 16.56 a | 18.01 ± 6.28 a | 14.11 ± 2.33 a |
| 45 | alpha, alpha’-Trehalose 6-palmitate | 22.41 × 106 ± 0.80 a | 6.23 ± 0.26 b | 5.31 ± 7.34 b | 9.42 ± 1.84 b | 10.02 ± 1.41 b |
| 46 | 12R-hydroxy-9Z-octadecenoic acid | 16.94 × 106 ± 0.69 a | 56.62 ± 0.71 a | 32.22 ± 42.29 a | 41.43 ± 30.28 a | 59.85 ± 2.83 a |
| 47 | 16-hydroxy hexadecanoic acid | 32.18 × 106 ± 2.91 ab | 3.03 ± 1.44 c | 6.73 ± 9.07 c | 13.10 ± 12.60 ac | 42.62 ± 12.02 b |
| No. | Metabolites | Non-Fermented | VEGE022 | VEGE033 | VEGE053 | VEGE061 |
|---|---|---|---|---|---|---|
| 1 | Citric acid | 7.36 ± 1.21 a | 2.29 ± 0.06 b | 2.01 ± 2.35 b | 0.93 ± 0.75 b | 2.84 ± 1.07 b |
| 2 | L-leucic acid | 0.30 ± 0.13 a | 0.33 ± 0.05 a | 1.34 ± 0.86 a | 1.19 ± 1.34 a | 2.14 ± 0.63 a |
| 3 | S-leucic acid | 0.32 ± 0.09 a | 3.29 ± 0.01 ab | 24.24 ± 8.47 bc | 18.24 ± 18.69 abc | 31.67 ±1.49 c |
| 4 | p-coumaric acid * | 1.91 ± 0.12 a | 0.20 ± 0.01 b | 0.87 ± 0.07 c | 0.86 ± 0.50 c | 0.86 ± 0.13 c |
| 5 | Ethyl vanillin * | n.d. a | 14.38 ± 0.62 c | 5.09 ± 2.58 ab | 6.43 ± 6.12 ab | 8.17 ± 1.10 bc |
| 6 | Sinapoyl alcohol | 4.78 ± 0.37 ab | 2.80 ± 0.11 a | 6.42 ± 0.39 b | 4.22 ± 2.97 ab | 6.73 ± 0.454 b |
| 7 | Trihydroxy octadecenoic acid isomer a | 79.88 ± 94.54 a | 112.14 ± 8.77 a | 120.86 ± 1.10 a | 104.55 ± 38.82 a | 142.30 ± 3.81 a |
| 8 | Trihydroxy octadecenoic acid isomer b | 108.79 ± 92.68 a | 51.52 ± 6.75 b | 72.97 ± 3.38 b | 53.91 ± 24.22 b | 77.29 ± 5.28 ab |
| 9 | Dihydroxyoleic acid isomer a | 26.19 ± 2.76 a | 55.09 ± 2.22 a | 31.16± 4.17 a | 36.50± 3.08 a | 23.29 ± 29.70 a |
| 10 | Dihydroxyoleic acid isomer b | 57.21 ± 4.37 a | 56.98 ± 4.79 a | 36.85 ± 1.19 b | 51.56 ± 2.43 a | 61.67 ± 10.41 a |
| 11 | Dihydroxystearic acid isomer a | 6.5 ± 0.64 a | 17.44 ± 3.36 a | 9.27 ± 1.41 a | 14.24 ± 8.96 a | 9.37 ± 0.35 a |
| 12 | Dihydroxystearic acid isomer b | 9.83 ± 1.15 a | 12.92 ± 0.60 b | 1.94 ± 0.30 c | 2.30 ± 0.88 c | 1.68 ± 0.28 c |
| 13 | Hydroxylinoleic acid isomer a | 89.01 ± 14.77 a | 28.38 ± 6.32 b | 1.63 ± 0.50 c | 2.11 ± 0.64 c | 0.78 ± 0.88 c |
| 14 | Hydroxyoleic acid isomer a | 18.13 ± 0.24 a | 3.62 ± 0.15 b | 3.98 ± 0.05 b | 4.47 ± 0.50 b | 4.84 ± 0.57 b |
| 15 | Hydroxyoleic acid isomer b | 14.49 ± 3.10 a | 4.65 ± 0.13 b | 5.99 ± 0.72 b | 4.88 ± 0.96 b | 6.40 ±0.20 b |
| Sample | Starter | Energetic Value (kcal) | Total Fat | Saturated Fat | Carbohydrates | Protein | Total Fiber | Moisture | Ash |
|---|---|---|---|---|---|---|---|---|---|
| Tiger nut | NF | 56.126 ± 0.332 a | 1.197 ± 0.045 a | 18.66 ± 0.45 a | 10.311 ± 0.218 a | 0.863 ± 0.0008 a | 0.031 ± 0.0063 a | 86.52 ± 0.0012 ab | 0.048 ± 0.0003 a |
| VEGE022 | 55.746 ± 1.648 a | 1.242 ± 0.112 a | 20.41 ± 0.10 a | 9.978 ± 0.227 a | 0.980 ± 0.100 a | 0.042 ± 0.0121 a | 86.66 ± 0.0033 ab | 0.047 ± 0.0004 a | |
| VEGE033 | 55.006 ± 0.938 a | 1.320 ± 0.485 a | 17.96 ± 0.06 a | 9.787 ± 0.926 a | 0.840 ± 0.07 a | 0.260 ± 0.0082 a | 86.96 ±0.0036 a | 0.051 ± 0.0002 a | |
| VEGE053 | 58.509 ± 0.884 a | 1.461 ± 0.014 a | 17.72 ± 0.21 a | 10.423 ± 0.225 a | 0.770 ± 0.07 a | 0.046 ± 0.0265 a | 86.17 ± 0.0026 b | 0.049 ± 0.0002 a | |
| VEGE061 | 56.083 a ± 4.695 a | 1.831 ± 0.160 a | 16.70 ± 0.53 a | 10.207 ± 1.062 a | 0.770 ± 0.07 a | 0.071 + 0.0629 a | 86.61 ± 0.0020 ab | 0.045 ± 0.0002 a | |
| Carob | NF | 10.210 ± 0.423 a | 0.064 ± 0.010 a | 35.60 ± 3.31 ac | 2.302 ± 0.121 a | 0.09 ± 0.00 | 0.231 ± 0.1898 a | 96.30 ± 0.0011 a | 0.028 ± 0.0002 a |
| VEGE022 | 61.703 ± 0.636 b | 0.071 ± 0.012 a | 39.98 ± 3.26 c | 15.129 ± 0.181 b | 0.12 ± 0.00 bc | 0.357 ± 0.2186 a | 83.45 ± 0.0016 b | 0.026 ± 0.000 a | |
| VEGE033 | 63.342 ± 3.373 c | 0.060 ± 0.014 a | 44.18 ± 1.32 a | 17.083 ± 0.813 c | 0.102 ± 0.00 a | 0.017 ± 0.0125 a | 81.88 ± 0.008 c | 0.024 ± 0.0007 a | |
| VEGE053 | 59.482 ± 0.153 b | 0.052 ± 0.004 a | 37.96 ± 2.06 ac | 14.671 ± 0.058 b | 0.072 ± 0.0073 a | 0.701 ± 0.1349 a | 83.63 ± 0.004 b | 0.027 ± 0.0002 a | |
| VEGE061 | 58.016 ± 0.378 b | 0.044 ± 0.007 a | 13.71 ± 8.12 b | 14.283 ± 0.100 b | 0.102 ± 0.0128 ac | n.d.a | 84.68 ± 0.009 d | 0.025 ± 0.0002 a | |
| Rice | NF | 3.170 ± 0.584 a | 0.186 ± 0.040 a | 19.52 ± 1.00 a | n.d.a | 0.256 ± 0.0466 ab | 0.01 ± 0.005 ab | 98.68 a ± 0.003 a | 0.016 ± 0.0007 a |
| VEGE022 | 34.572 ± 4.254 b | 0.257 ± 0.053 ab | 42.72 ± 11.8 b | 7.824 ± 1.163 c | 0.244 ± 0.0139 ab | n.d. a | 90.92 b ± 0.001 b | 0.023 ± 0.07 b | |
| VEGE033 | 41.859 ± 5.656 bc | 0.281 ± 0.099 ab | 36.07 ± 0.85 b | 9.567 ± 1.209 bc | 0.220 ± 0.0115 a | 0.02 ± 0.033 ab | 88.94 bc ± 0.001 a | 0.035 ± 0.0002 c | |
| VEGE053 | 45.991 ± 0.459 c | 0.214 ± 0.000 ab | 37.33 ± 4.27 b | 10.777 ± 0.189 c | 0.298 ± 0.0154 b | 0.05 ± 0.0352 b | 87.68 c ± 0.004 c | 0.032 ± 0.0001 c | |
| VEGE061 | 44.172 ± 0.937 c | 0.386 ± 0.0922 b | 32.03 ± 2.36 b | 9.735 ± 0.086 bc | 0.240 ± 0.0066 b | 0.03 ± 0.005 ab | 88.86 ± 0.002 bc | 0.035 ± 0.0007 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitali, M.; Gandía, M.; Garcia-Llatas, G.; González-Sarrías, A.; Vallejo, F.; Cilla, A.; Gamero, A. Modulation of Antioxidant Capacity, Nutritional Composition, Probiotic Viability After Digestion and Sensory Attributes of Plant-Based Beverages Through Lactic Acid Fermentation. Foods 2025, 14, 1447. https://doi.org/10.3390/foods14091447
Vitali M, Gandía M, Garcia-Llatas G, González-Sarrías A, Vallejo F, Cilla A, Gamero A. Modulation of Antioxidant Capacity, Nutritional Composition, Probiotic Viability After Digestion and Sensory Attributes of Plant-Based Beverages Through Lactic Acid Fermentation. Foods. 2025; 14(9):1447. https://doi.org/10.3390/foods14091447
Chicago/Turabian StyleVitali, Matteo, Mónica Gandía, Guadalupe Garcia-Llatas, Antonio González-Sarrías, Fernando Vallejo, Antonio Cilla, and Amparo Gamero. 2025. "Modulation of Antioxidant Capacity, Nutritional Composition, Probiotic Viability After Digestion and Sensory Attributes of Plant-Based Beverages Through Lactic Acid Fermentation" Foods 14, no. 9: 1447. https://doi.org/10.3390/foods14091447
APA StyleVitali, M., Gandía, M., Garcia-Llatas, G., González-Sarrías, A., Vallejo, F., Cilla, A., & Gamero, A. (2025). Modulation of Antioxidant Capacity, Nutritional Composition, Probiotic Viability After Digestion and Sensory Attributes of Plant-Based Beverages Through Lactic Acid Fermentation. Foods, 14(9), 1447. https://doi.org/10.3390/foods14091447














