Effect of Ultrasound Combined with Plasma-Activated Water on Lethal and Sublethal Injury Against Escherichia coli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains Culture
2.2. Preparation of PAW
2.3. Samples Treatment
2.4. Inhibition Efficiency
2.5. Inactivation Kinetics Model
2.5.1. Linear Model
2.5.2. Weibull Model
2.6. Scanning Electron Microscopy (SEM)
2.7. Cell Membrane Structure Observation
2.7.1. Determination of Cell Membrane Integrity
2.7.2. Determination of Cell Membrane Permeability
2.7.3. Determination of Cell Membrane Potential
2.7.4. Analysis of Membrane Fatty Acid Composition
2.7.5. Membrane Protein Structure
2.8. Measurement of Oxidative Damage Indexes of E. coli
2.8.1. Intracellular ROS Content
2.8.2. Determination of Oxidative Damage in E. coli
2.8.3. Determination of DNA Oxidative Damage in E. coli
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of US-PAW Treatments on Lethal and Sublethal Injury on E. coli
3.2. Synergistic Inhibition of Combined Treatment
3.3. Kinetic Model Fit Analysis
3.4. SEM Observations
3.5. Effect of US-PAW Treatment on Cell Membrane Structure
3.5.1. Determination of Cell Membrane Integrity
3.5.2. Determination of Cell Membrane Permeability
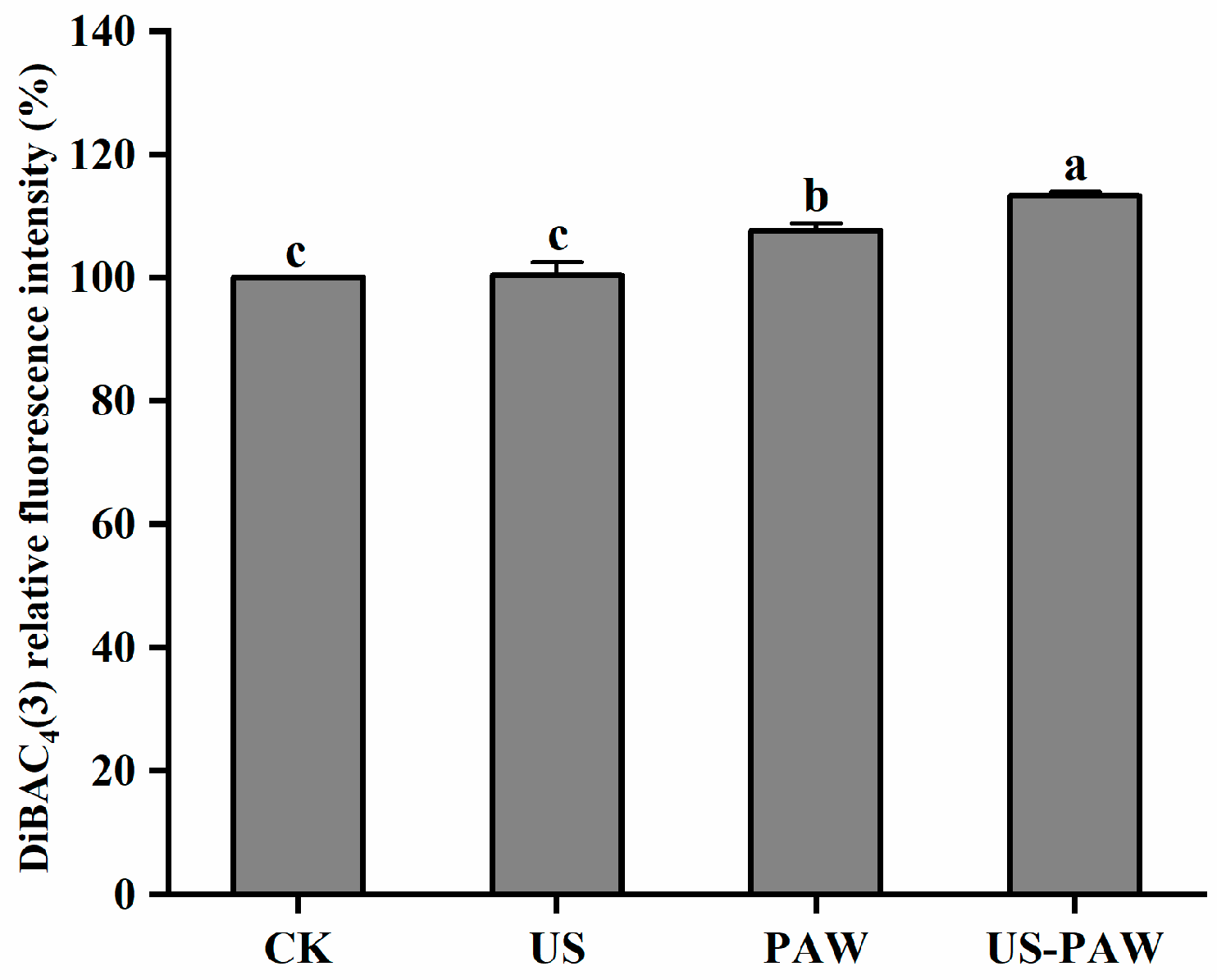
3.5.3. Determination of Cell Membrane Potential
3.5.4. Analysis of Membrane Fatty Acid Composition
3.5.5. Membrane Protein Structure
3.6. Effect of US-PAW Treatment on Oxidative Damage of E. coli
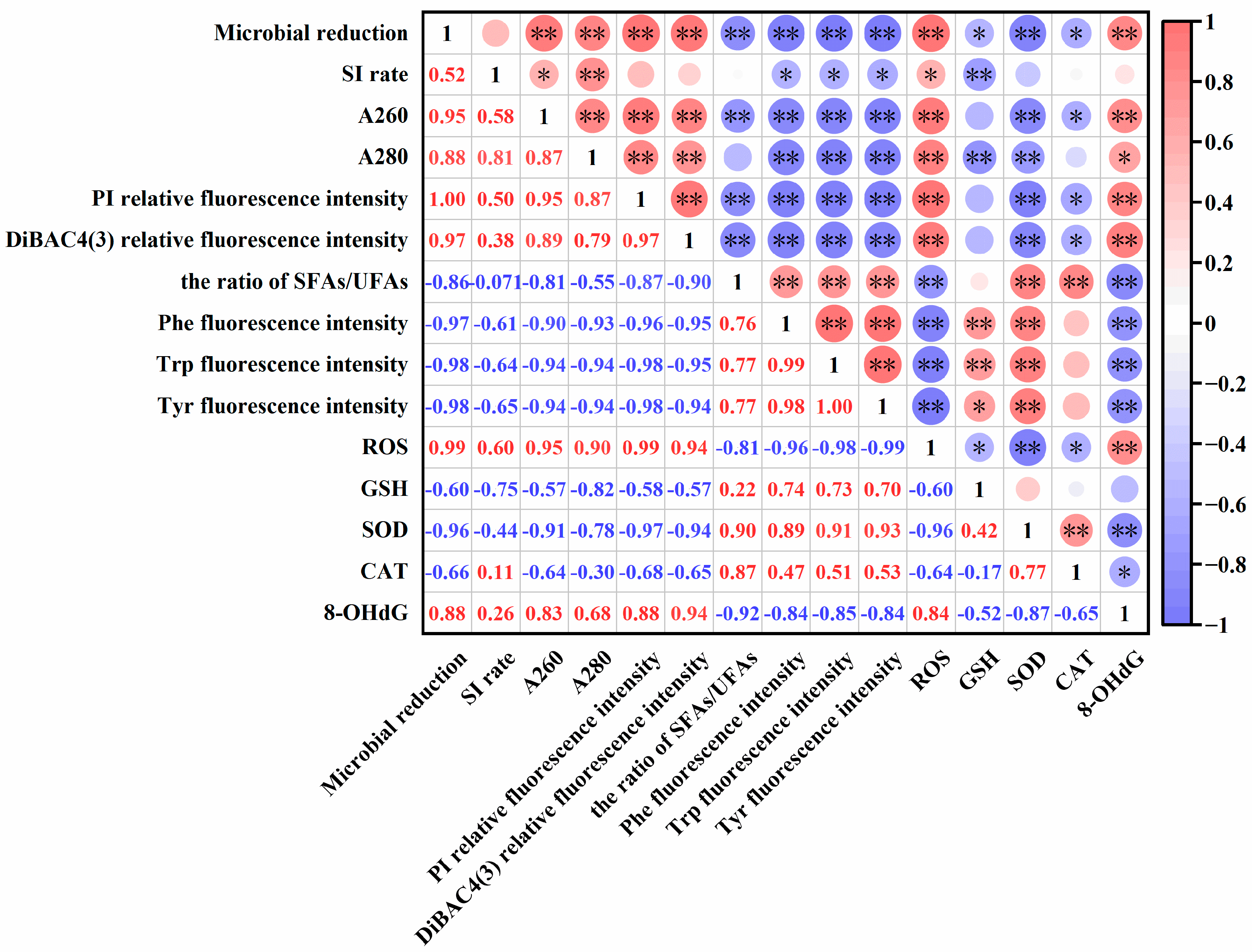
3.7. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PAW | plasma-activated water |
| US-PAW | ultrasound Combined with plasma-activated water |
| US | ultrasound |
| E. coli | Escherichia coli |
| SI | sublethal injury |
References
- Abebe, E.; Gugsa, G.; Ahmed, M. Review on major food-borne zoonotic bacterial pathogens. J. Trop. Med. 2020, 2020, 4674235. [Google Scholar] [CrossRef] [PubMed]
- Farid, N. Synthetic and natural antimicrobials as a control against food borne pathogens: A review. Heliyon 2023, 9, e17021. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization. Diarrhoeal Disease. Available online: https://www.who.int/health-topics/foodborne-diseases#tab=tab_1 (accessed on 7 March 2024).
- Khouryieh, H.A. Novel and emerging technologies used by the U.S. food processing industry. Innov. Food Sci. Emerg. Technol. 2021, 67, 102559. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Ojha, S.; Burgess, C.M.; Sun, D.-W.; Tiwari, B.K. Inactivation efficacy and mechanisms of plasma activated water on bacteria in planktonic state. J. Appl. Microbiol. 2020, 129, 1248–1260. [Google Scholar] [CrossRef]
- Qian, J.; Yan, W.; Zhang, W.; Zhang, J.; Wang, J.; Raghavan, V. Plasma-activated water: Perspective of the theoretical model, safety assessment and application in animal-derived products. Trends Food Sci. Technol. 2024, 143, 104282. [Google Scholar] [CrossRef]
- Pan, Y.; Cheng, J.H.; Lv, X.; Sun, D.-W. Assessing the inactivation efficiency of Ar/O2 plasma treatment against Listeria monocytogenes cells: Sublethal injury and inactivation kinetics. LWT-Food Sci. Technol. 2019, 111, 318–327. [Google Scholar] [CrossRef]
- Hartsell, S.E. The longevity and behavior of pathogenic bacteria in frozen foods: The Influence of Plating Media. Am. J. Public Health 1951, 41, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Forghani, F.; Daliri, E.B.M.; Li, J.; Liao, X.; Liu, D.; Ye, X.; Chen, S.; Ding, T. Microbial response to some nonthermal physical technologies. Trends Food Sci. Technol. 2020, 95, 107–117. [Google Scholar] [CrossRef]
- Shao, L.; Zhao, Y.; Zou, B.; Li, X.; Dai, R. Recovery and virulence factors of sublethally injured Staphylococcus aureus after ohmic heating. Food Microbiol. 2022, 102, 103899. [Google Scholar] [CrossRef]
- Shao, L.; Sun, Y.; Zou, B.; Zhao, Y.; Li, X.; Dai, R. Sublethally injured microorganisms in food processing and preservation: Quantification, formation, detection, resuscitation and adaption. Food Res. Int. 2023, 165, 112536. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, W.; Li, L. Proteomics-based mechanistic study of sub-lethally injured Saccharomyces cerevisiae by pulsed electric fields. Food Biosci. 2022, 50, 101989. [Google Scholar] [CrossRef]
- Zhang, R.; Lan, L.; Shi, H. Sublethal injury and recovery of Escherichia coli O157:H7 after freezing and thawing. Food Control 2021, 120, 107488. [Google Scholar] [CrossRef]
- Nasiłowska, J.; Kocot, A.; Osuchowska, P.N.; Sokołowska, B. High-ressure-induced sublethal injuries of food pathogens—Microscopic assessment. Foods 2021, 10, 2940. [Google Scholar] [CrossRef]
- Lan, L.; Zhang, R.; Zhang, X.; Shi, H. Sublethal injury and recovery of Listeria monocytogenes and Escherichia coli O157:H7 after exposure to slightly acidic electrolyzed water. Food Control 2019, 106, 106746. [Google Scholar] [CrossRef]
- Royintarat, T.; Choi, E.H.; Boonyawan, D.; Seesuriyachan, P.; Wattanutchariya, W. Chemical-free and synergistic interaction of ultrasound combined with plasma-activated water (PAW) to enhance microbial inactivation in chicken meat and skin. Sci. Rep. 2020, 10, 1559. [Google Scholar] [CrossRef] [PubMed]
- Okon, E.J.; Hu, J.C.; Wen, D.S. Novel technique for treating grass carp (Ctenopharyngodon idella) by combining plasma functionalized liquids and ultrasound: Effects on bacterial inactivation and quality attributes. Ultrason. Sonochem. 2021, 76, 105660. [Google Scholar]
- Sun, R.; Xu, W.; Xiong, L.; Jiang, N.; Xia, J.; Zhu, Y.; Wang, C.; Liu, Q.; Ma, Y.; Luo, H. The combined effects of ultrasound and plasma-activated water on microbial inactivation and quality attributes of crayfish during refrigerated storage. Ultrason. Sonochem. 2023, 98, 106517. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ahn, J.; Liu, D.; Chen, S.; Ye, X.; Ding, T. Evaluation of ultrasound-induced damage to Escherichia coli and Staphylococcus aureus by flow cytometry and transmission electron microscopy. Appl. Environ. Microbiol. 2016, 82, 1828–1837. [Google Scholar] [CrossRef]
- Li, J.; Ding, T.; Liao, X.; Chen, S.; Ye, X.; Ding, T. Synergetic effects of ultrasound and slightly acidic electrolyzed water against Staphylococcus aureus evaluated by flow cytometry and electron microscopy. Ultrason. Sonochem. 2017, 38, 711–719. [Google Scholar] [CrossRef]
- Li, J.; Suo, Y.; Liao, X.; Ahn, J.; Liu, D.; Chen, S.; Ye, X.; Ding, T. Analysis of Staphylococcus aureus cell viability, sublethal injury and death induced by synergistic combination of ultrasound and mild heat. Ultrason. Sonochem. 2017, 39, 101–110. [Google Scholar] [CrossRef]
- Akkermans, S.; Verheyen, D.; Smet, C.; Van Impe, J.F.M. A population balance model to describe the evolution of sublethal injury. Foods 2021, 10, 1674. [Google Scholar] [CrossRef]
- Peleg, M.; Cole, M.B. Reinterpretation of microbial survival curves. Crit. Rev. Food Sci. Nutr. 1998, 38, 353–380. [Google Scholar] [CrossRef]
- Wang, L.H.; Wang, M.S.; Zeng, X.A.; Liu, Z.W. Temperature-mediated variations in cellular membrane fatty acid composition of Staphylococcus aureus in resistance to pulsed electric fields. Biochim. Biophys. Acta Biomembr. 2016, 1858, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, H.; Zhang, C.; Sun, L.; Liu, S.; Yue, R. Excellent antimicrobial properties of silver-loaded mesoporous silica SBA-15. J. Appl. Microbiol. 2014, 116, 1106–1118. [Google Scholar] [CrossRef]
- Xiang, Q.; Kang, C.; Niu, L.; Zhao, D.; Li, K.; Bai, Y. Antibacterial activity and a membrane damage mechanism of plasma-activated water against Pseudomonas deceptionensis CM2. LWT-Food Sci. Technol. 2018, 96, 395–401. [Google Scholar] [CrossRef]
- Xing, K.; Xing, Y.; Liu, Y.; Zhang, Y.; Shen, X.; Li, X.; Miao, X.; Feng, Z.; Peng, X.; Qin, S. Fungicidal effect of chitosan via inducing membrane disturbance against Ceratocystis fimbriata. Carbohydr. Polym. 2018, 192, 95–103. [Google Scholar] [CrossRef]
- Liu, Z.; Zeng, X.; Ngadi, M.; Han, Z. Effect of cell membrane fatty acid composition of Escherichia coli on the resistance to pulsed electric field (PEF) treatment. LWT-Food Sci. Technol. 2017, 76, 18–25. [Google Scholar] [CrossRef]
- Wu, Y.; Bai, J.; Zhong, K.; Huang, Y.; Gao, H. A dual antibacterial mechanism involved in membrane disruption and DNA binding of 2R,3R-dihydromyricetin from pine needles of Cedrus deodara against Staphylococcus aureus. Food Chem. 2017, 218, 463–470. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, R.; Tian, Y.; Su, B.; Wang, K.; Yu, S.; Zhang, J.; Fang, J. Sterilization efficiency of a novel electrochemical disinfectant against Staphylococcus aureus. Environ. Sci. Technol. 2016, 50, 3184–3192. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Liu, D.; Zhu, M.; Chen, J.; Zhang, J.; Zhang, F.; Jiang, J.; Guo, L.; Wang, X.; et al. Combination of NOx mode and O3 mode air discharges for water activation to produce a potent disinfectant. Plasma Sources Sci. Technol. 2022, 31, 05LT01. [Google Scholar] [CrossRef]
- Rathore, V.; Patel, D.; Butani, S.; Nema, S.K. Investigation of physicochemical properties of plasma activated water and its bactericidal efficacy. Plasma Chem. Plasma Process. 2021, 41, 871–902. [Google Scholar] [CrossRef]
- Xu, W.; Sun, R.; Jiang, N.; Wang, Q.; Wang, C.; Liu, Q.; Luo, H. Synergistic effects of ultrasound and plasma-activated water against Listeria innocua in crayfish disinfection by metabolomics analysis. Food Biosci. 2024, 61, 104597. [Google Scholar] [CrossRef]
- Kharel, K.; Yemmireddy, K.V.; Graham, J.C.; Prinyawiwatkul, W.; Adhikari, A. Hot water treatment as a kill-step to inactivate Escherichia coli O157:H7, Salmonella enterica, Listeria monocytogenes and Enterococcus faecium on in-shell pecans. LWT-Food Sci. Technol. 2018, 97, 555–560. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Wang, S.; Huangfu, L.; Zhang, M.; Xiang, Q. Synergistic antimicrobial activity of plasma-activated water and propylparaben: Mechanism and applications for fresh produce sanitation. LWT-Food Sci. Technol. 2021, 146, 111447. [Google Scholar] [CrossRef]
- Chen, R.; Zhu, Q.; Fang, Z.; Huang, Z.; Sun, J.; Peng, M.; Shi, P. Aluminum induces oxidative damage in Saccharomyces cerevisiae. Can. J. Microbiol. 2020, 66, 713–722. [Google Scholar] [CrossRef]
- Ni, J.B.; Luo, S.Y.; Bi, Y.X.; Zielinska, S.; Ding, C.-J.; Tao, J.-L.; Ning, Z.; Tian, W.-L.; Peng, W.-J.; Fang, X.-M. The combined effects of ultrasound and plasma-activated water on silkworm pupae: Physicochemical properties, microbiological diversity and ultrastructure. Ultrason. Sonochem. 2024, 107, 106927. [Google Scholar] [CrossRef]
- Froehling, A.; Baier, M.; Ehlbeck, J.; Knorr, D.; Schlüter, O. Atmospheric pressure plasma treatment of Listeria innocua and Escherichia coli at polysaccharide surfaces: Inactivation kinetics and flow cytometric characterization. Innov. Food Sci. Emerg. Technol. 2012, 13, 142–150. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Zhou, R.; Zhang, T.; Ostrikov, K.K.; Mugunthan, S.; Rice, S.A.; Cullen, P.J. Interactions of plasma-activated water with biofilms: Inactivation, dispersal effects and mechanisms of action. NPJ Biofilms Microbiomes 2021, 7, 11. [Google Scholar] [CrossRef]
- Mitchell, P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol. Rev. 1966, 41, 445–501. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Zhang, Y.; Jie, M.; Cheng, Y.; Xiang, Q.; Zhang, Z.; Bai, Y. Synergetic effect of petit-high pressure carbon dioxide combined with cinnamon (Cinnamomum cassia) essential oil against Salmonella typhimurium. Int. J. Food Sci. Technol. 2022, 57, 2954–2967. [Google Scholar] [CrossRef]
- Bai, F.; Guo, D.; Wang, Y.; Zhang, S.; Li, J.; Zhi, K.; Shi, C.; Xia, X. The combined bactericidal effect of nisin and thymoquinone against Listeria monocytogenes in Tryptone Soy Broth and sterilized milk. Food Control 2022, 135, 108771. [Google Scholar] [CrossRef]
- Beales, N. Adaptation of microorganisms to cold temperatures, weak acid preservatives, low pH, and osmotic stress: A review. Compr. Rev. Food Sci. Food Saf. 2004, 3, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, M.R.; Bakshi, K.; Volkin, D.B.; Russell Middaugh, C. Fluorescence spectroscopy of peptides. Methods Mol. Biol. 2014, 1088, 237–246. [Google Scholar] [PubMed]
- Ye, X.; Li, X.; Yuan, L.; Ge, L.; Zhang, B.; Zhou, S. Interaction of houttuyfonate homologues with the cell membrane of gram-positive and gram-negative bacteria. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 412–418. [Google Scholar] [CrossRef]
- Facey, S.J.; Kuhn, A. Biogenesis of bacterial inner-membrane proteins. Cell. Mol. Life Sci. 2010, 67, 2343–2362. [Google Scholar] [CrossRef]
- Guo, L.; Sun, Y.; Zhu, Y.; Wang, B.; Xu, L.; Huang, M.; Li, Y.; Sun, J. The antibacterial mechanism of ultrasound in combination with sodium hypochlorite in the control of Escherichia coli. Food Res. Int. 2020, 129, 108887. [Google Scholar] [CrossRef]
- Duan, L.; Jiang, T.; Zhou, Y.; Bai, X.; Wang, Y.; Lü, X.; Xia, X.; Lin, L.; Shi, C. The inactivation of Shigella flexneri by synergistic effect of ultrasound combined with basil essential oil nanoemulsion and application in cabbage cleaning. Food Control 2024, 156, 110142. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, M.; Xu, B. Application of ultrasonic technology in postharvested fruits and vegetables storage: A review. Ultrason. Sonochem. 2020, 69, 105261. [Google Scholar]
- Jiang, Y.; Leung, A.W.; Hua, H.; Rao, X.; Xu, C. Photodynamic action of LED-activated curcumin against Staphylococcus aureus involving intracellular ROS increase and membrane damage. Int. J. Photoenergy 2014, 2014, 637601. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The applications and mechanisms of superoxide dismutase in medicine, Food, and Cosmetics. Antioxidants 2023, 12, 1675. [Google Scholar] [CrossRef]
- Ezraty, B.; Gennaris, A.; Barras, F.; Collet, J.-F. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017, 15, 385–396. [Google Scholar] [PubMed]
- Ki, S.H.; Noh, H.; Ahn, G.R.; Kim, S.H.; Kaushik, N.K.; Choi, E.H.; Lee, G.J. Influence of nonthermal atmospheric plasma-activated water on the structural, optical, and biological properties of Aspergillus brasiliensis Spores. Appl. Sci. 2020, 10, 6378. [Google Scholar] [CrossRef]
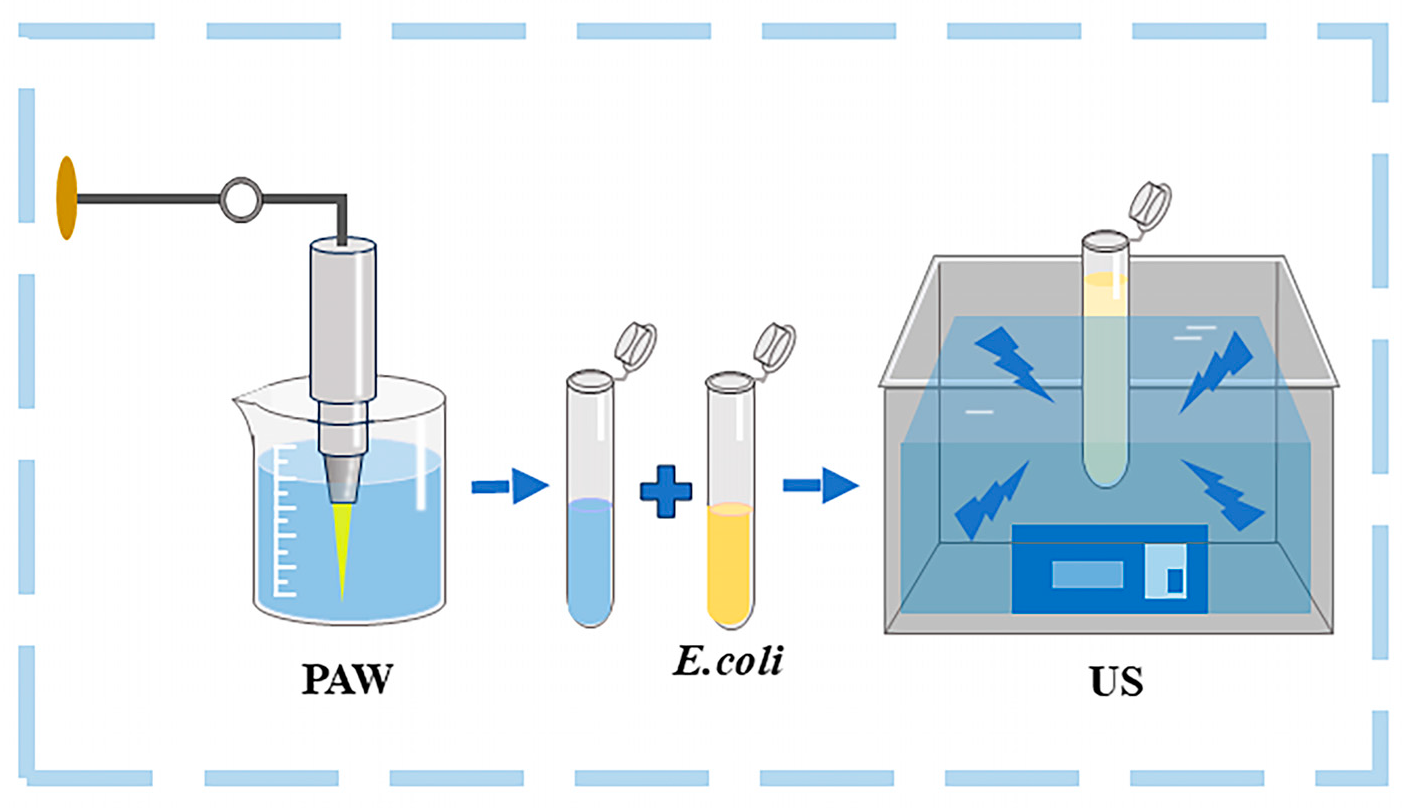
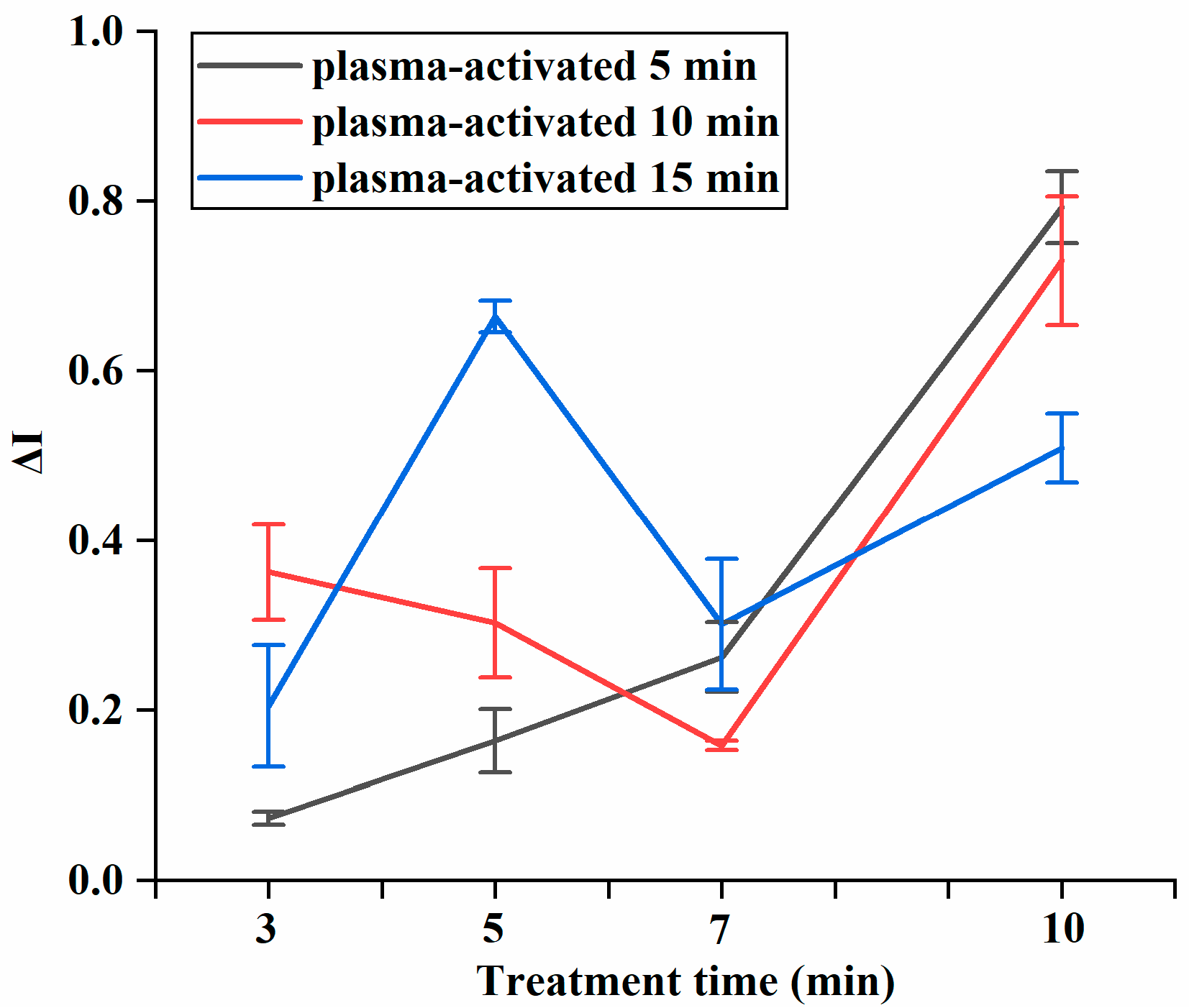


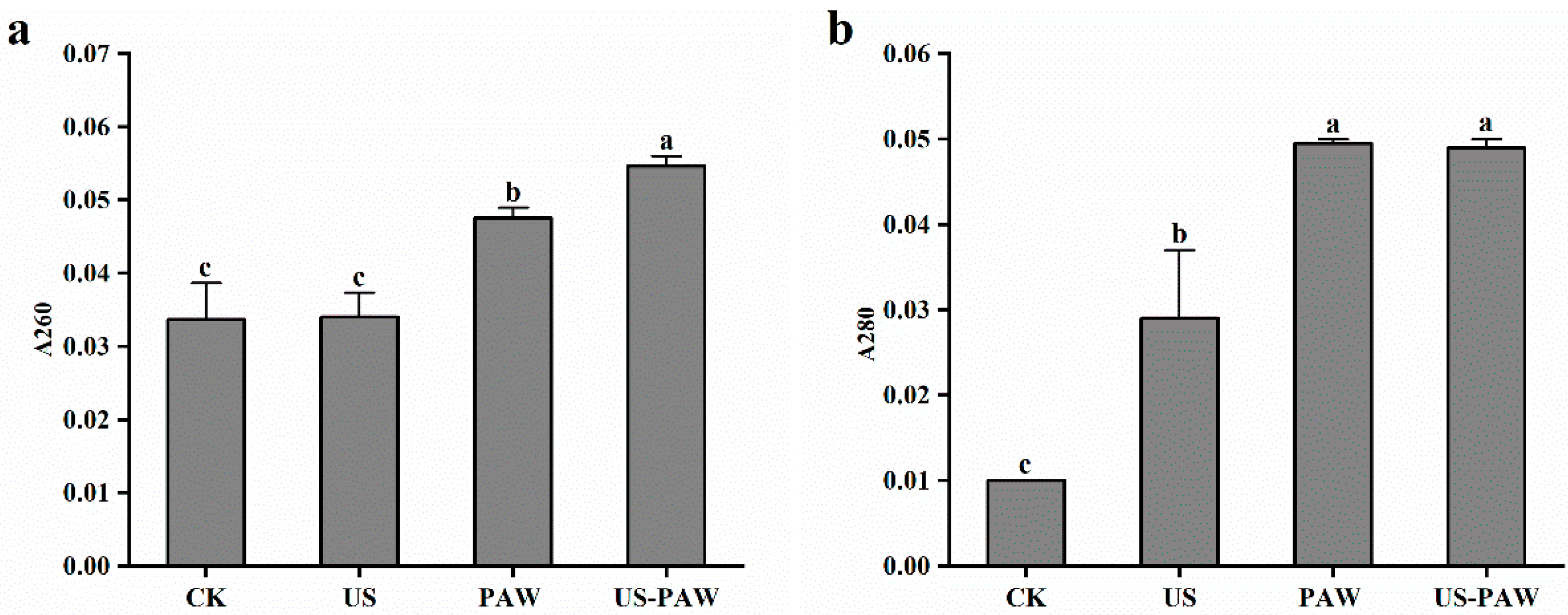
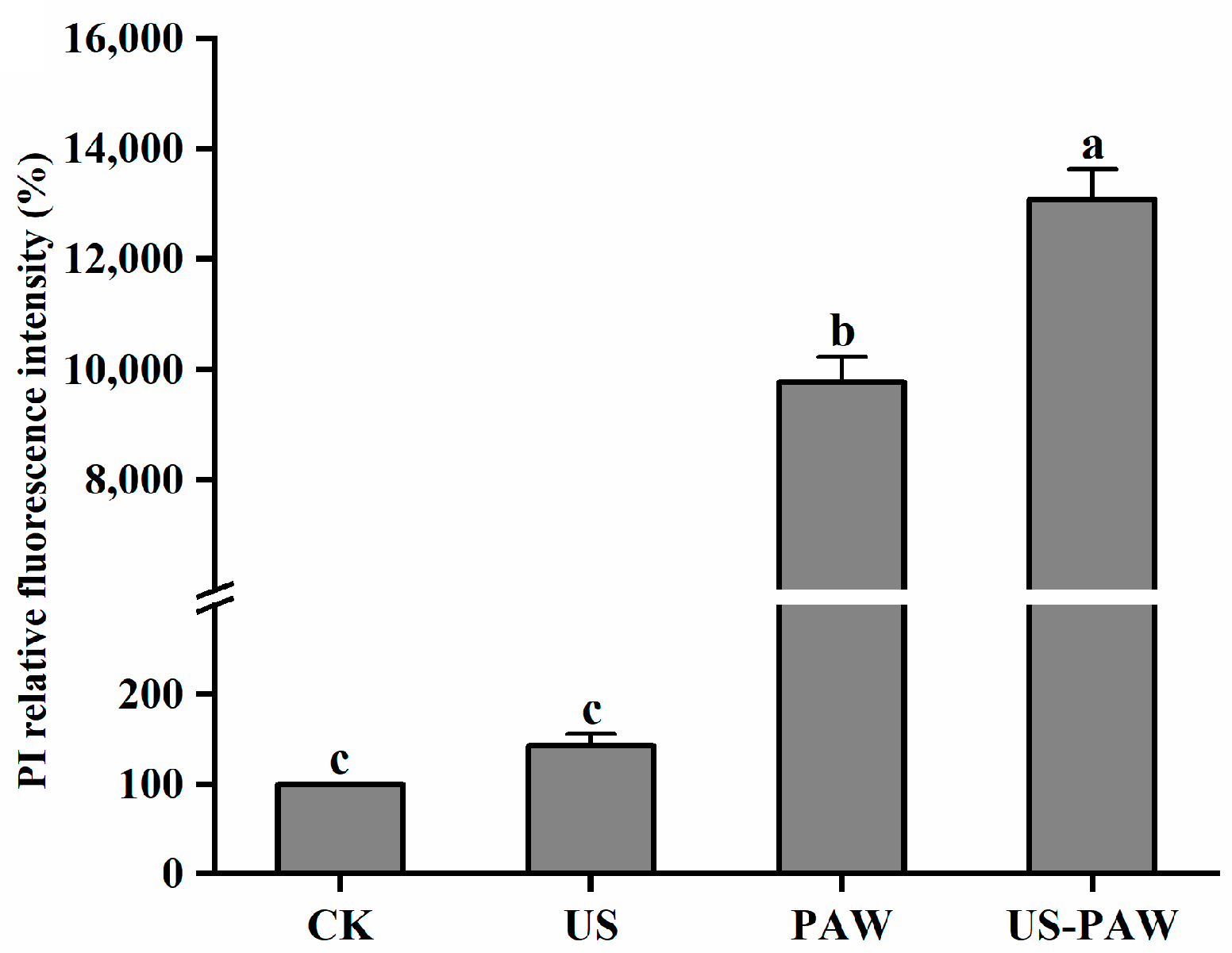



| Treatments | Microbial Log Reduction (log CFU/mL) | |||
|---|---|---|---|---|
| 3 min | 5 min | 7 min | 10 min | |
| US | 0.01 ± 0.01 Cd | 0.03 ± 0.01 Ce | 0.13 ± 0.03 Bf | 0.19 ± 0.04 Ag |
| PAW5 | 0.26 ± 0.04 Cc | 0.30 ± 0.01 Cde | 0.48 ± 0.07 Be | 0.86 ± 0.13 Af |
| PAW10 | 0.75 ± 0.12 Db | 1.66 ± 0.06 Cc | 2.49 ± 0.12 Bc | 3.59 ± 0.08 Ad |
| PAW15 | 1.03 ± 0.15 Da | 1.74 ± 0.02 Cbc | 3.32 ± 0.22 Bb | 4.83 ± 0.07 Ac |
| US-PAW5 | 0.31 ± 0.02 Cc | 0.49 ± 0.12 Cd | 0.87 ± 0.07 Bd | 2.12 ± 0.19 Ae |
| US-PAW10 | 1.12 ± 0.15 Da | 1.99 ± 0.28 Cb | 2.71 ± 0.19 Bc | 4.89 ± 0.07 Ab |
| US-PAW15 | 1.25 ± 0.14 Da | 2.43 ± 0.17 Ca | 3.75 ± 0.06 Ba | 5.24 ± 0.40 Aa |
| Treatments | SI Rate (%) | |||
|---|---|---|---|---|
| 3 min | 5 min | 7 min | 10 min | |
| US | 23.80 ± 2.10 Bd | 22.28 ± 1.45 BCc | 51.18 ± 5.21 Aa | 16.13 ± 3.25 Cc |
| PAW5 | 42.15 ± 2.11 Ab | 38.15 ± 1.40 ABb | 35.64 ± 4.01 BCb | 31.01 ± 1.59 Cb |
| PAW10 | 45.21 ± 3.12 Ab | 37.31 ± 2.24 Bb | 34.25 ± 1.45 Bb | 28.14 ± 1.45 Cb |
| PAW15 | 77.09 ± 2.95 Aa | 65.51 ± 5.20 Ba | 54.52 ± 5.01 Ca | 40.48 ± 3.25 Da |
| US-PAW5 | 41.14 ± 4.21 Ab | 41.41 ± 2.64 Ab | 32.15 ± 1.48 Bb | 31.02 ± 4.15 Bb |
| US-PAW10 | 45.52 ± 3.41 Ab | 35.91 ± 1.43 Bb | 35.04 ± 6.05 Bd | 13.30 ± 2.15 Cc |
| US-PAW15 | 32.09 ± 2.63 Cc | 62.21 ± 5.41 Aa | 50.56 ± 3.41 Ba | 29.30 ± 4.17 Cb |
| Treatments | Linear Model | Weibull Model | |||
|---|---|---|---|---|---|
| D | R2 | σ | ρ | R2 | |
| US | 114.9186 | 0.4726 | 19.3897 | 2.3447 | 0.8921 |
| PAW5 | 15.8633 | 0.7481 | 12.1841 | 1.3172 | 0.8127 |
| PAW10 | 8.5232 | 0.7950 | 7.0133 | 1.4392 | 0.9272 |
| PAW15 | 2.8332 | 0.9861 | 3.2383 | 1.1375 | 0.9965 |
| US-PAW5 | 2.6605 | 0.9988 | 2.5542 | 0.9692 | 0.9992 |
| US-PAW10 | 2.7765 | 0.8856 | 3.2828 | 1.3296 | 0.9827 |
| US-PAW15 | 1.5255 | 0.8826 | 2.1950 | 1.3236 | 0.9195 |
| Fatty Acids (%) | CK | US | PAW | US-PAW |
|---|---|---|---|---|
| C4:0 | 18.83 ± 1.95 a | 13.12 ± 1.36 ab | 7.75 ± 5.15 b | 8.04 ± 0.01 b |
| C12:0 | 0.53 ± 0.09 c | 1.17 ± 0.01 c | 2.87 ± 0.64 b | 4.17 ± 0.45 a |
| C14:0 | 3.15 ± 0.84 a | 3.26 ± 0.51 a | 3.27 ± 0.14 a | 3.21 ± 0.39 a |
| C15:0 | 3.42 ± 0.96 a | 3.54 ± 0.59 a | 4.17 ± 0.29 a | 4.47 ± 0.56 a |
| C16:0 | 46.71 ± 0.37 b | 53.09 ± 1.76 a | 49.46 ± 2.86 b | 38.26 ± 0.02 c |
| C17:0 | 1.91 ± 0.50 a | 1.95 ± 0.34 a | 2.20 ± 0.17 a | 2.14 ± 0.23 a |
| C18:0 | 3.19 ± 0.09 b | 3.14 ± 0.41 b | 4.71 ± 0.02 a | 5.31 ± 0.43 a |
| C16:1 | 13.56 ± 0.65 a | 9.03 ± 0.73 b | 12.69 ± 0.79 a | 14.07 ± 1.13 a |
| C18:1 | 1.02 ± 0.31 b | 1.14 ± 0.05 ab | 0.85 ± 0.27 b | 1.69 ± 0.31 a |
| C18:2 | 3.56 ± 0.44 b | 4.21 ± 0.36 ab | 3.74 ± 0.22 ab | 4.46 ± 0.21 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, X.; Nie, M.; Zhang, Z.; Xiong, L.; Feng, J.; Zhang, Z.; Li, D.; Bao, Y.; Wu, H. Effect of Ultrasound Combined with Plasma-Activated Water on Lethal and Sublethal Injury Against Escherichia coli. Foods 2025, 14, 1457. https://doi.org/10.3390/foods14091457
Wen X, Nie M, Zhang Z, Xiong L, Feng J, Zhang Z, Li D, Bao Y, Wu H. Effect of Ultrasound Combined with Plasma-Activated Water on Lethal and Sublethal Injury Against Escherichia coli. Foods. 2025; 14(9):1457. https://doi.org/10.3390/foods14091457
Chicago/Turabian StyleWen, Xin, Meimei Nie, Zhongyuan Zhang, Lingming Xiong, Jialin Feng, Zhi Zhang, Dajing Li, Yihong Bao, and Haihong Wu. 2025. "Effect of Ultrasound Combined with Plasma-Activated Water on Lethal and Sublethal Injury Against Escherichia coli" Foods 14, no. 9: 1457. https://doi.org/10.3390/foods14091457
APA StyleWen, X., Nie, M., Zhang, Z., Xiong, L., Feng, J., Zhang, Z., Li, D., Bao, Y., & Wu, H. (2025). Effect of Ultrasound Combined with Plasma-Activated Water on Lethal and Sublethal Injury Against Escherichia coli. Foods, 14(9), 1457. https://doi.org/10.3390/foods14091457







