Chemical and Volatile Compounds in Sweet Potato Brandy: Impact of Processing Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Cooking Methods
2.3. Preparation of Ipomoea batatas L. Wine
2.4. Determination of Basic Physicochemical Parameters
2.5. Sensory Evaluation
| Evaluation Indicators | Fraction | Evaluation Criteria |
|---|---|---|
| Color (20 points) | 16~20 | Clear, high gloss, transparent, golden yellow |
| 11~15 | Clear, slightly dull, golden yellow, no suspended matter | |
| 6~10 | Clear, glossless, light yellow, no obvious suspended matter | |
| 0~5 | Turbid, dull, dark yellow, with suspended matter | |
| Aroma (30 points) | 25~30 | Strong sweet potato aroma, prominent main aroma, rich aroma, long aftertaste |
| 20~25 | More obvious sweet potato aroma, natural and harmonious aroma | |
| 10~20 | Light sweet potato fragrance, no odor | |
| 0~10 | Lack of fragrance, peculiar smell | |
| Taste (40 points) | 30~40 | The brandy is full-bodied, mellow, and palatable, with harmonious flavor and lingering aftertaste |
| 20~30 | The taste is harmonious and pure, without any impurities | |
| 10~20 | The taste is light and not strong | |
| 0~10 | Light brandy, unbalanced taste | |
| Typicality (10 points) | 9~10 | Typical perfection, unique style |
| 6~8 | Typical and clear, good style | |
| 3~5 | Typical, general style | |
| 0~2 | The typicality of the brandy is not obvious |
2.6. Analytical Methods
2.7. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Main Components of Fermented Sweet Potato Wine and Distilled Brandy
3.1.1. Physicochemical Characteristics of Fermented Sweet Potato Wine
3.1.2. Physicochemical Characteristics of Distilled Sweet Potato Brandy
| Alcohol Content (%Vol, Brandy) | Soluble Solids Content (%, Wine) | Total Esters (mg/L, Brandy) | Reducing Sugars (g/L, Wine) | Total Acid (g/L, Wine) | pH Value (Wine) | |
|---|---|---|---|---|---|---|
| DB1 | 24.50 ± 0.15 a | 10.5 ± 0.10 a | 480 ± 5.00 a | 3.8 ± 0.05 b | 4.5 ± 0.05 a | 3.6 ± 0.05 a |
| DB2 | 22.80 ± 0.12 b | 9.8 ± 0.08 b | 450 ± 4.00 b | 4.2 ± 0.06 a | 4.0 ± 0.03 b | 3.7 ± 0.04 a |
| DB3 | 24.30 ± 0.13 a | 10.3 ± 0.12 a | 475 ± 5.00 a | 4.0 ± 0.04 a | 4.4 ± 0.04 a | 3.6 ± 0.05 a |
| DB4 | 22.50 ± 0.10 b | 9.5 ± 0.07 b | 440 ± 3.00 b | 4.1 ± 0.05 a | 4.0 ± 0.03 b | 3.8 ± 0.04 a |
| DB5 | 22.00 ± 0.12 c | 9.3 ± 0.09 b | 430 ± 3.50 c | 4.3 ± 0.06 a | 3.9 ± 0.03 b | 3.9 ± 0.05 a |
3.2. Sensory Evaluation

3.3. GC-IMS Analysis of Volatile Compounds in Fermented Sweet Potato (Ipomoea batatas L.) Brandy Following Diverse Processing Techniques
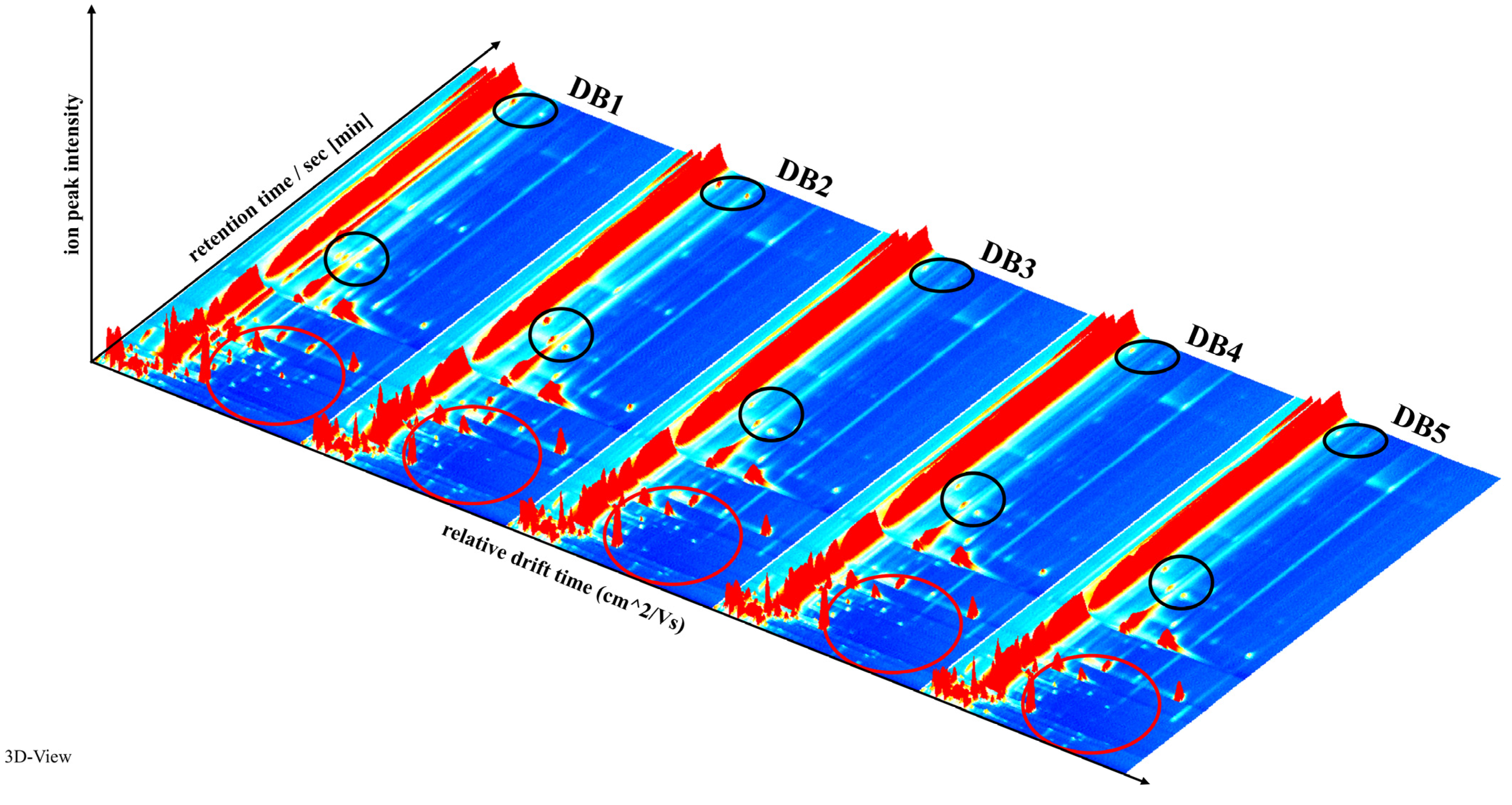

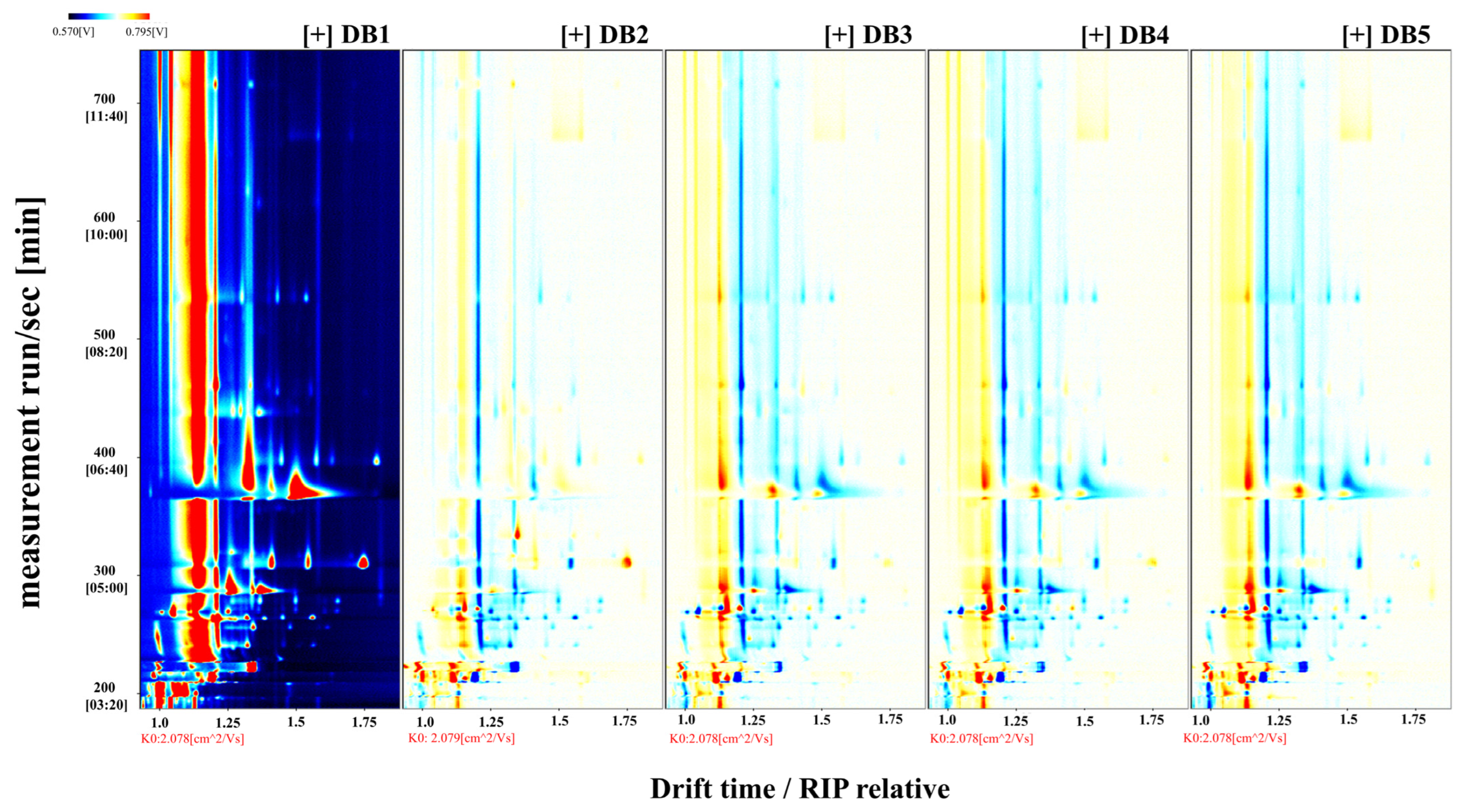
3.4. Characterization of Volatile Compounds in Sweet Potato Fermented Brandy Processed via Diverse Methodologies
| Count | Compound | CAS | Formula | a MW | b RI | c Rt [sec] | d Dt [a.u.] |
|---|---|---|---|---|---|---|---|
| 1 | 2-butoxyethanol | 111762 | C6H14O2 | 118.2 | 1453.6 | 715.276 | 1.20492 |
| 2 | Ethyl octanoate-D | 106321 | C10H20O2 | 172.3 | 1430.7 | 671.837 | 2.03135 |
| 3 | 1,3-diethylbenzene | 141935 | C10H14 | 134.2 | 1293.3 | 461.885 | 1.20966 |
| 4 | Ethyl hexanoate | 123660 | C8H16O2 | 144.2 | 1232.4 | 397.007 | 1.80067 |
| 5 | 3-Methyl-1-butanol | 123513 | C5H12O | 88.1 | 1204.8 | 370.638 | 1.48651 |
| 6 | Pentyl hexanoate | 540078 | C11H22O2 | 186.3 | 1347.5 | 535.298 | 1.54056 |
| 7 | Limonene | 138863 | C10H16 | 136.2 | 1232.4 | 396.955 | 1.5777 |
| 8 | 3-Methylbutyl 2-methylbutanoate | 27625350 | C10H20O2 | 172.3 | 1288.1 | 455.962 | 1.43329 |
| 9 | 3-Octanone | 106683 | C8H16O | 128.2 | 1272.6 | 438.713 | 1.30156 |
| 10 | 1-hydroxy-2-propanone | 116096 | C3H6O2 | 74.1 | 1273.1 | 439.206 | 1.23163 |
| 11 | ethyl pentanoate | 539822 | C7H14O2 | 130.2 | 1138.6 | 319.94 | 1.26416 |
| 12 | Isoamyl acetate-D | 123922 | C7H14O2 | 130.2 | 1124.9 | 310.576 | 1.75203 |
| 13 | 2-Methylpropanol | 78831 | C4H10O | 74.1 | 1086.6 | 287.413 | 1.36986 |
| 14 | Propyl propanoate | 106365 | C6H12O2 | 116.2 | 1036.6 | 264.63 | 1.32208 |
| 15 | 2-butanol | 78922 | C4H10O | 74.1 | 1016.3 | 255.901 | 1.34564 |
| 16 | isobutyl acetate | 110190 | C6H12O2 | 116.2 | 992.6 | 246.58 | 1.23794 |
| 17 | Ethyl propanoate | 105373 | C5H10O2 | 102.1 | 969.5 | 240.81 | 1.32208 |
| 18 | Ethyl Acetate | 141786 | C4H8O2 | 88.1 | 889.2 | 221.725 | 1.33779 |
| 19 | ethyl 3-methylbutanoate | 108645 | C7H14O2 | 130.2 | 1067.9 | 278.685 | 1.65689 |
| 20 | ethyl 2-methylbutanoate-M | 7452791 | C7H14O2 | 130.2 | 1048.7 | 269.956 | 1.25545 |
| 21 | Ethyl butanoate-D | 105544 | C6H12O2 | 116.2 | 1037 | 264.778 | 1.56217 |
| 22 | Methyl acetate | 79209 | C3H6O2 | 74.1 | 848.7 | 212.7 | 1.19344 |
| 23 | (E)-2-butenal | 123739 | C4H6O | 70.1 | 1053.8 | 272.274 | 1.2053 |
| 24 | Ethyl butanoate-M | 105544 | C6H12O2 | 116.2 | 1034 | 263.477 | 1.20435 |
| 25 | ethyl 2-methylbutanoate-D | 7452791 | C7H14O2 | 130.2 | 1053.6 | 272.138 | 1.65246 |
| 26 | pentyl formate | 638493 | C6H12O2 | 116.2 | 1017.3 | 256.304 | 1.61821 |
| 27 | Ethyl 2-methy lpropionate | 97621 | C6H12O2 | 116.2 | 970.9 | 241.147 | 1.56588 |
| 28 | 1 | unidentified | * | 0 | 1034.6 | 263.748 | 1.38702 |
| 29 | Acetone | 67641 | C3H6O | 58.1 | 844.5 | 211.805 | 1.11664 |
| 30 | Methanol | 67561 | CH4O | 32 | 891.9 | 222.355 | 0.98861 |
| 31 | Ethyl formate | 109944 | C3H6O2 | 74.1 | 828.6 | 208.358 | 1.06969 |
| 32 | 2 | unidentified | * | 0 | 792.1 | 200.692 | 1.13492 |
| 33 | Butanal | 123728 | C4H8O | 72.1 | 838.2 | 210.422 | 1.28146 |
| 34 | 2-Octanone | 111137 | C8H16O | 128.2 | 1287.2 | 454.973 | 1.75587 |
| 35 | Isoamyl acetate-M | 123922 | C7H14O2 | 130.2 | 1124.2 | 310.119 | 1.31645 |
| 36 | 1-pentanol | 71410 | C5H12O | 88.1 | 1273.4 | 439.568 | 1.27064 |
| 37 | 3-Hydroxy-2-butanone | 513860 | C4H8O2 | 88.1 | 1293.1 | 461.642 | 1.33433 |
| 38 | 3 | unidentified | * | 0 | 774.4 | 197.071 | 1.21516 |
| 39 | Ethyl octanoate-M | 106321 | C10H20O2 | 172.3 | 1431.9 | 674.043 | 1.49085 |
| 40 | Hexyl acetate | 142927 | C8H16O2 | 144.2 | 1272.1 | 438.223 | 1.36447 |

3.5. Comparative Spectroscopic Analysis of Fermented Brandy Derived from Sweet Potatoes Processed via Diverse Methods
| IMS Code | Compound | CAS# | Formula | a RI | b Rt [sec] | c Dt [a.u.] | # Odor Characteristics * | d MW |
|---|---|---|---|---|---|---|---|---|
| Esters | ||||||||
| 2 | Ethyl octanoate-D | 106321 | C10H20O2 | 1430.7 | 671.837 | 2.03135 | fruit, fat | 172.3 |
| 4 | Ethyl hexanoate | 123660 | C8H16O2 | 1232.4 | 397.007 | 1.80067 | apple peel, fruit | 144.2 |
| 6 | Pentyl hexanoate | 540078 | C11H22O2 | 1347.5 | 535.298 | 1.54056 | fruity | 186.3 |
| 8 | 3-Methylbutyl2-methylbutanoate | 27625350 | C10H20O2 | 1288.1 | 455.962 | 1.43329 | fruit, banana, grass | 172.3 |
| 11 | ethyl pentanoate | 539822 | C7H14O2 | 1138.6 | 319.94 | 1.26416 | yeast, fruit | 130.2 |
| 12 | Isoamyl acetate-D | 123922 | C7H14O2 | 1124.9 | 310.576 | 1.75203 | banana | 130.2 |
| 14 | Propyl propanoate | 106365 | C6H12O2 | 1036.6 | 264.63 | 1.32208 | pineapple | 116.2 |
| 16 | isobutyl acetate | 110190 | C6H12O2 | 992.6 | 246.58 | 1.23794 | fruit, apple, banana | 116.2 |
| 17 | Ethyl propanoate | 105373 | C5H10O2 | 969.5 | 240.81 | 1.32208 | fruity, sweet | 102.1 |
| 18 | Ethyl Acetate | 141786 | C4H8O2 | 889.2 | 221.725 | 1.33779 | pineapple | 88.1 |
| 19 | ethyl3-methylbutanoate | 108645 | C7H14O2 | 1067.9 | 278.685 | 1.65689 | fruit | 130.2 |
| 20 | Ethyl2-methylbutanoate-M | 7452791 | C7H14O2 | 1048.7 | 269.956 | 1.25545 | apple | 130.2 |
| 21 | Ethyl butanoate-D | 105544 | C6H12O2 | 1037 | 264.778 | 1.56217 | apple | 116.2 |
| 22 | Methyl acetate | 79209 | C3H6O2 | 848.7 | 212.7 | 1.19344 | —— | 74.1 |
| 24 | Ethyl butanoate-M | 105544 | C6H12O2 | 1034 | 263.477 | 1.20435 | apple | 116.2 |
| 25 | ethyl2-methylbutanoate-D | 7452791 | C7H14O2 | 1053.6 | 272.138 | 1.65246 | apple | 130.2 |
| 26 | pentyl formate | 638493 | C6H12O2 | 1017.3 | 256.304 | 1.61821 | —— | 116.2 |
| 27 | Ethyl 2-methy lpropionate | 97621 | C6H12O2 | 970.9 | 241.147 | 1.56588 | sweet, rubber | 116.2 |
| 31 | Ethyl formate | 109944 | C3H6O2 | 828.6 | 208.358 | 1.06969 | pungent | 74.1 |
| 35 | Isoamyl acetate-M | 123922 | C7H14O2 | 1124.2 | 310.119 | 1.31645 | banana | 130.2 |
| 39 | Ethyl octanoate-M | 106321 | C10H20O2 | 1431.9 | 674.043 | 1.49085 | fruit, fat | 172.3 |
| 40 | Hexyl acetate | 142927 | C8H16O2 | 1272.1 | 438.223 | 1.36447 | fruit, herb | 144.2 |
| Alcohols | ||||||||
| 1 | 2-butoxyethanol | 111762 | C6H14O2 | 1453.6 | 715.276 | 1.20492 | fruity, herbal flavor | 118.2 |
| 5 | 3-Methyl-1-butanol | 123513 | C5H12O | 1204.8 | 370.638 | 1.48651 | whiskey, malt, burnt | 88.1 |
| 10 | 1-hydroxy-2-propanone | 116096 | C3H6O2 | 1273.1 | 439.206 | 1.23163 | pungent, sweet, caramelly, ethereal | 74.1 |
| 13 | 2-Methylpropanol | 78831 | C4H10O | 1086.6 | 287.413 | 1.36986 | wine, solvent, bitter | 74.1 |
| 15 | 2-butanol | 78922 | C4H10O | 1016.3 | 255.901 | 1.34564 | wine | 74.1 |
| 30 | Methanol | 67561 | CH4O | 891.9 | 222.355 | 0.98861 | —— | 32 |
| 36 | 1-pentanol | 71410 | C5H12O | 1273.4 | 439.568 | 1.27064 | balsamic | 88.1 |
| Ketones | ||||||||
| 9 | 3-Octanone | 106683 | C8H16O | 1272.6 | 438.713 | 1.30156 | herb, butter, resin | 128.2 |
| 29 | Acetone | 67641 | C3H6O | 844.5 | 211.805 | 1.11664 | fruity | 58.1 |
| 34 | 2-Octanone | 111137 | C8H16O | 1287.2 | 454.973 | 1.75587 | soap, gasoline | 128.2 |
| 37 | 3-Hydroxy-2-butanone | 513860 | C4H8O2 | 1293.1 | 461.642 | 1.33433 | butter, cream | 88.1 |
| Aldehydes | ||||||||
| 23 | (E)-2-butenal | 123739 | C4H6O | 1053.8 | 272.274 | 1.2053 | flower | 70.1 |
| 33 | Butanal | 123728 | C4H8O | 838.2 | 210.422 | 1.28146 | pungent, green | 72.1 |
| Terpenoids | ||||||||
| 7 | Limonene | 138863 | C10H16 | 1232.4 | 396.955 | 1.5777 | lemon, orange | 136.2 |
| Others | ||||||||
| 3 | 1,3-diethylbenzene | 141935 | C10H14 | 1293.3 | 461.885 | 1.20966 | —— | 134.2 |

3.6. Hierarchical Cluster Analysis of Volatile Compounds in Sweet Potato Fermented Brandy Subjected to Diverse Processing Techniques
3.6.1. Dynamic PCA of Samples

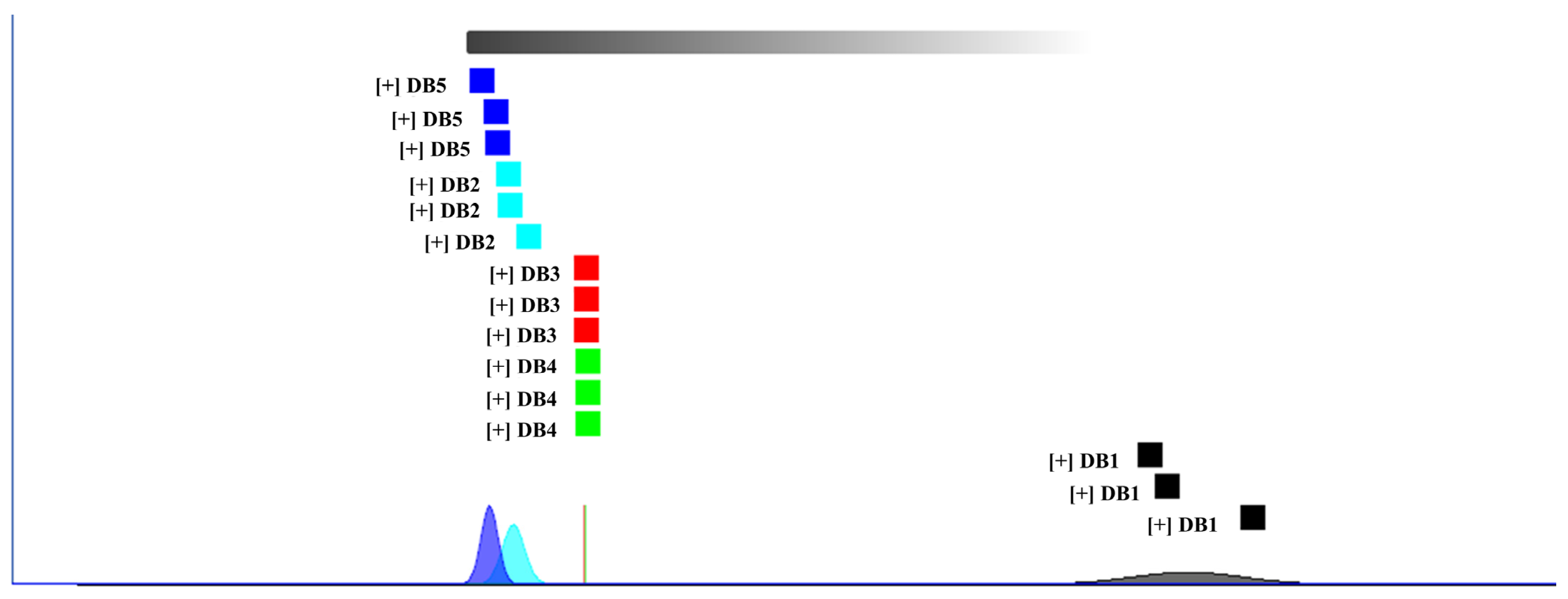
3.6.2. Fingerprint Similarity Analysis Using Euclidean Distance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, Y.; Zhang, R.; Jia, R.; Deng, Y.; Wang, Z. Impact of different cooking methods on the chemical profile of orange-fleshed sweet potato (Ipomoea batatas L.). LWT 2023, 173, 114288. [Google Scholar] [CrossRef]
- Badiora, O.A.; Morakinyo, T.A.; Taiwo, K.A. Some quality properties of yellow-fleshed sweet potato flour as affected by different drying methods. Food Prod. Process. Nutr. 2023, 5, 21. [Google Scholar] [CrossRef]
- Alam, M.K.; Sams, S.; Rana, Z.H.; Akhtaruzzaman, M.; Islam, S.N. Minerals, vitamin C, and effect of thermal processing on carotenoids composition in nine varieties orange-fleshed sweet potato (Ipomoea batatas L.). J. Food Compos. Anal. 2020, 92, 103582. [Google Scholar] [CrossRef]
- Kourouma, V.; Mu, T.-H.; Zhang, M.; Sun, H.-N. Effects of cooking process on carotenoids and antioxidant activity of orange-fleshed sweet potato. LWT 2019, 104, 134–141. [Google Scholar] [CrossRef]
- Alam, M.K. A comprehensive review of sweet potato (Ipomoea batatas [L.] Lam): Revisiting the associated health benefits. Trends Food Sci. Technol. 2021, 115, 512–529. [Google Scholar] [CrossRef]
- Chen, L.; Liu, B.; Li, D.; Wang, S.; Ma, X.; Zhang, Y. Effects of fermentation on flavor and antioxidant activity in ginkgo rice wine. Food Biosci. 2023, 53, 102652. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.; Ren, T.; Wang, J.; Niu, C.; Zheng, F.; Li, Q. Effect of Saccharomyces cerevisiae and non-Saccharomyces strains on alcoholic fermentation behavior and aroma profile of yellow-fleshed peach wine. LWT 2022, 155, 112993. [Google Scholar] [CrossRef]
- He, W.; Liu, S.; Heponiemi, P.; Heinonen, M.; Marsol-Vall, A.; Ma, X.; Yang, B.; Laaksonen, O. Effect of Saccharomyces cerevisiae and Schizosaccharomyces pombe strains on chemical composition and sensory quality of ciders made from Finnish apple cultivars. Food Chem. 2021, 345, 128833. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, B.; Fu, Y.; Shi, Y.-G.; Chen, F.-L.; Guan, H.-N.; Liu, L.-L.; Zhang, C.-Y.; Zhu, P.-Y.; Liu, Y.; et al. HS-GC-IMS with PCA to analyze volatile flavor compounds across different production stages of fermented soybean whey tofu. Food Chem. 2021, 346, 128880. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, J.; Dong, H.; Bai, W.; Yu, L.; Li, X. Variations of volatile flavour compounds in Cordyceps militaris chicken soup after enzymolysis pretreatment by SPME combined with GC-MS, GC × GC-TOF MS and GC-IMS. Int. J. Food Sci. Technol. 2020, 55, 509–516. [Google Scholar] [CrossRef]
- Martín-Gómez, A.; Arroyo-Manzanares, N.; Rodríguez-Estévez, V.; Arce, L. Use of a non-destructive sampling method for characterization of Iberian cured ham breed and feeding regime using GC-IMS. Meat Sci. 2019, 152, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Xiao, O.; Li, M.; Chen, J.; Li, R.; Quan, R.; Zhang, Z.; Kong, Z.; Dai, X. Influence of Triazole Pesticides on Wine Flavor and Quality Based on Multidimensional Analysis Technology. Molecules 2020, 25, 5596. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Meng, K.; Zheng, H.; Yu, H.; Zhang, Y.; Yang, X.; Lin, Z.; Xie, G. Metabolites comparison in post-fermentation stage of manual (mechanized) Chinese Huangjiu (yellow rice wine) based on GC–MS metabolomics. Food Chem. X 2022, 14, 100324. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, P.; Xiao, Z.; Zhu, J.; Sun, X.; Wang, R. Evaluation of the perceptual interaction among ester aroma compounds in cherry wines by GC–MS, GC–O, odor threshold and sensory analysis: An insight at the molecular level. Food Chemistry 2019, 275, 143–153. [Google Scholar] [CrossRef]
- Li, H.; Jiang, D.; Liu, W.; Yang, Y.; Zhang, Y.; Jin, C.; Sun, S. Comparison of fermentation behaviors and properties of raspberry wines by spontaneous and controlled alcoholic fermentations. Food Res. Int. 2020, 128, 108801. [Google Scholar] [CrossRef]
- Taiwo, K.A.; Baik, O.D. Effects of pre-treatments on the shrinkage and textural properties of fried sweet potatoes. LWT Food Sci. Technol. 2007, 40, 661–668. [Google Scholar] [CrossRef]
- Hao, Y.; Li, J.; Zhao, Z.; Xu, W.; Wang, L.; Lin, X.; Hu, X.; Li, C. Flavor characteristics of Shanlan rice wines fermented for different time based on HS-SPME-GC-MS-O, HS-GC-IMS, and electronic sensory analyses. Food Chem. 2024, 432, 137150. [Google Scholar] [CrossRef]
- Ye, M.; Yue, T.; Yuan, Y.; Li, Z. Application of FT-NIR Spectroscopy to Apple Wine for Rapid Simultaneous Determination of Soluble Solids Content, pH, Total Acidity, and Total Ester Content. Food Bioprocess Technol. 2014, 7, 3055–3062. [Google Scholar] [CrossRef]
- Jiang, J.; Yin, R.; Xie, Y.; Ma, X.; Cui, M.; Chen, Y.; Li, Y.; Hu, Y.; Niu, J.; Cheng, W.; et al. Effects of cofermentation of Saccharomyces cerevisiae and different lactic acid bacteria on the organic acid content, soluble sugar content, biogenic amines, phenol content, antioxidant activity and aroma of prune wine. Food Chem. X 2024, 22, 101502. [Google Scholar] [CrossRef]
- Shen, X.; Wang, H.; Yao, L.; Song, S.; Wang, H.; Sun, M.; Liu, Q.; Yu, C.; Feng, T. Volatile compounds analysis and sensory profiling of four colored roasted sweet potatoes via HS-SPME GC-O-MS and HS-SPME GC×GC-TOF MS. J. Food Compos. Anal. 2024, 131, 106256. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, J.; Yang, Y.; Deng, J.; Zhu, K.; Yi, Y.; Tang, J.; Jiang, X.; Zhu, C.; Laghi, L. Effects of S. cerevisiae strains on the sensory characteristics and flavor profile of kiwi wine based on E-tongue, GC-IMS and 1H-NMR. LWT 2023, 185, 115193. [Google Scholar] [CrossRef]
- Sarkar, S.; Basak, J.K.; Moon, B.E.; Kim, H.T. A Comparative Study of PLSR and SVM-R with Various Preprocessing Techniques for the Quantitative Determination of Soluble Solids Content of Hardy Kiwi Fruit by a Portable Vis/NIR Spectrometer. Foods 2020, 9, 1078. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, E.; Bautil, A.; Brijs, K.; Scheirlinck, I.; Van der Meulen, R.; Courtin, C.M. Sugar Levels Determine Fermentation Dynamics during Yeast Pastry Making and Its Impact on Dough and Product Characteristics. Foods 2022, 11, 1388. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.; Gómez-Mascaraque, L.G.; Le Feunteun, S.; Brodkorb, A. Boiling vs. baking: Cooking-induced structural transformations drive differences in the in vitro starch digestion profiles that are consistent with the in vivo glycemic indexes of white and sweet potatoes. Food Struct. 2023, 38, 100355. [Google Scholar] [CrossRef]
- Huang, J.; Li, H.; Wang, Y.; Wang, X.; Ren, Y.; Yue, T.; Gao, Z. Evaluation of the quality of fermented kiwi wines made from different kiwifruit cultivars. Food Biosci. 2021, 42, 101051. [Google Scholar] [CrossRef]
- Zhou, Y.; Fei, G.; Hasan, K.F.; Kang, Y.; Wu, Y.; Li, H.; Zhou, S. Cultivar difference characterization of kiwifruit wines on phenolic profiles, volatiles and antioxidant activity. Food Chem. X 2023, 18, 100691. [Google Scholar] [CrossRef]
- Sui, X.; Zhu, Z.; Cheng, F.; Sun, Y.; Jiang, X. Evaluation of physicochemical properties, antioxidant capacity, and volatile flavor compounds during the wine fermentation of Mei (Prunus mume) fruit. LWT 2024, 201, 116177. [Google Scholar] [CrossRef]
- Sioriki, E.; Lemarcq, V.; Alhakim, F.; Triharyogi, H.; Tuenter, E.; Cazin, C.S.; Nolan, S.P.; Pieters, L.; Van de Walle, D.; Dewettinck, K. Impact of alkalization conditions on the phytochemical content of cocoa powder and the aroma of cocoa drinks. LWT 2021, 145, 111181. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Z.; Chen, M.; Chen, G.; Lei, H.; Xu, H.; Yue, X. Effects of five different commercial strains of Saccharomyces cerevisiae on the physicochemical parameters, antioxidant activity, phenolic profiles and flavor components of jujube wine. LWT 2024, 198, 115989. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, S.K.C.; Stringer, S.J.; Zhang, Y. Characterization of titratable acids, phenolic compounds, and antioxidant activities of wines made from eight mississippi-grown muscadine varieties during fermentation. LWT 2017, 86, 302–311. [Google Scholar] [CrossRef]
- Yuan, L.; Li, G.; Yan, N.; Wu, J.; Due, J. Optimization of fermentation conditions for fermented green jujube wine and its quality analysis during winemaking. J. Food Sci. Technol. 2021, 59, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Mu, Y.; Xie, S.; Meng, D.; Zheng, Y.; Meng, X.; Lv, Z. Impact of UHT processing on volatile components and chemical composition of sea buckthorn (Hippophae rhamnoides) pulp: A prediction of the biochemical pathway underlying aroma compound formation. Food Chem. 2022, 390, 133142. [Google Scholar] [CrossRef] [PubMed]
- Ogliari, R.; Soares, J.M.; Teixeira, F.; Schwarz, K.; Da Silva, K.A.; Schiessel, D.L.; Novello, D. Chemical, Nutritional and Sensory Characterization of Sweet Potato Submitted To Different Cooking Methods. Int. J. Res. Granthaalayah 2020, 8, 147–156. [Google Scholar] [CrossRef]
- Tian, Z.; Dong, T.; Wang, S.; Sun, J.; Chen, H.; Zhang, N.; Wang, S. A comprehensive review on botany, chemical composition and the impacts of heat processing and dehydration on the aroma formation of fresh carrot. Food Chem. X 2024, 22, 101201. [Google Scholar] [CrossRef]
- Li, A.; Feng, X.; Yang, G.; Peng, X.; Du, M.; Song, J.; Kan, J. Impact of aroma-enhancing microorganisms on aroma attributes of industrial Douchi: An integrated analysis using E-nose, GC-IMS, GC–MS, and descriptive sensory evaluation. Food Res. Int. 2024, 182, 114181. [Google Scholar] [CrossRef]
- Wang, M.; Li, X.; Ding, H.; Chen, H.; Liu, Y.; Wang, F.; Chen, L. Comparison of the volatile organic compounds in Citrus reticulata ‘Chachi’ peel with different drying methods using E-nose, GC-IMS and HS-SPME-GC-MS. Front. Plant Sci. 2023, 14, 1169321. [Google Scholar] [CrossRef]
- Liu, B.; Yang, Y.; Ren, L.; Su, Z.; Bian, X.; Fan, J.; Wang, Y.; Han, B.; Zhang, N. HS-GC-IMS and PCA to Characterize the Volatile Flavor Compounds in Three Sweet Cherry Cultivars and Their Wines in China. Molecules 2022, 27, 9056. [Google Scholar] [CrossRef]
- Fan, X.; Jiao, X.; Liu, J.; Jia, M.; Blanchard, C.; Zhou, Z. Characterizing the volatile compounds of different sorghum cultivars by both GC-MS and HS-GC-IMS. Food Res. Int. 2021, 140, 109975. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Wu, J.; Wen, J.; Yu, Y.; An, K.; Zou, B. GC-IMS and olfactometry analysis on the tea aroma of Yingde black teas harvested in different seasons. Food Res. Int. 2021, 150, 110784. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, H.; Chen, Y.; Tang, C.; Jiang, B.; Wang, Z. Impact of different cooking methods on the flavor and chemical profile of yellow-fleshed table-stock sweetpotatoes (Ipomoea batatas L.). Food Chem. X 2023, 17, 100542. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Wang, Y.; Wang, W.; Shu, N.; Hou, Q.; Tang, F.; Shan, C.; Yang, X.; Guo, Z. Insights into the Aroma Profile of Sauce-Flavor Baijiu by GC-IMS Combined with Multivariate Statistical Analysis. J. Anal. Methods Chem. 2022, 2022, 4614330. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Maecker, R.; Vyhmeister, E.; Meisen, S.; Martinez, A.R.; Kuklya, A.; Telgheder, U. Identification of terpenes and essential oils by means of static headspace gas chromatography-ion mobility spectrometry. Anal. Bioanal. Chem. 2017, 409, 6595–6603. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Mesa, M.; Ropartz, D.; García-Campaña, A.M.; Rogniaux, H.; Dervilly-Pinel, G.; Le Bizec, B. Ion Mobility Spectrometry in Food Analysis: Principles, Current Applications and Future Trends. Molecules 2019, 24, 2706. [Google Scholar] [CrossRef]
- Ding, B.; Zhao, S.; Zhang, W.; Lin, Y.; Xiong, L. The Effect of Co-Culture with Different Pichia kluyveri and Saccharomyces cerevisiae on Volatile Compound and Characteristic Fingerprints of Mulberry Wine. Foods 2024, 13, 422. [Google Scholar] [CrossRef]
- Fan, C.; Shi, X.; Pan, C.; Zhang, F.; Zhou, Y.; Hou, X.; Hui, M. GC-IMS and GC/Q-TOFMS analysis of Maotai-flavor baijiu at different aging times. LWT 2024, 192, 115744. [Google Scholar] [CrossRef]
- Molteberg, E.L.; Magnus, E.M.; Bjørge, J.M.; Nilsson, A. Sensory and chemical studies of lipid oxidation in raw and heat-treated oat flours. Cereal Chem. 1996, 73, 579–587. [Google Scholar]
- Bi, J.; Yang, Z.; Li, Y.; Li, B.; Gao, Y.; Ping, C.; Chen, Z.; Li, C. Effects of different cooking methods on volatile flavor compounds in garlic. Int. J. Gastron. Food Sci. 2023, 31, 100642. [Google Scholar] [CrossRef]
- Bi, S.; Wang, A.; Wang, Y.; Xu, X.; Luo, D.; Shen, Q.; Wu, J. Effect of cooking on aroma profiles of Chinese foxtail millet (Setaria italica) and correlation with sensory quality. Food Chem. 2019, 289, 680–692. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, R.; Yao, Y.; Yang, Y.; Wang, B.; Wang, Z. Effect of cooking methods on metabolites of deep purple-fleshed sweetpotato. Food Chem. 2023, 429, 136931. [Google Scholar] [CrossRef]
- Ravi, R.; Nandwani, D.; Nwosisi, S. Volatile profiles of cooked organic sweetpotato by electronic nose. Org. Agric. 2021, 12, 17–32. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, L.; Zhang, C.; Feng, T.; Zhuang, H. Analysis of Volatile Flavor Compounds of Corn Under Different Treatments by GC-MS and GC-IMS. Front. Chem. 2022, 10, 725208. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-J.; Lin, L.-Y.; Yang, K.-M.; Chiang, Y.-C.; Chen, M.-H.; Chiang, P.-Y. Effects of Roasting Sweet Potato (Ipomoea batatas L. Lam.): Quality, Volatile Compound Composition, and Sensory Evaluation. Foods 2021, 10, 2602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, C.; Zhuang, H.; Liu, Y.; Feng, T.; Nie, B. Characterization of Volatile Component Changes in Peas under Different Treatments by GC-IMS and GC-MS. J. Food Qual. 2021, 2021, 6533083. [Google Scholar] [CrossRef]
- Mu, T.-H.; Sun, H.-N.; Ma, M.-M. Sweet potato snack foods. In Sweet Potato; Academic Press: Cambridge, MA, USA, 2019; pp. 303–324. [Google Scholar]
- Li, M.; Yang, R.; Zhang, H.; Wang, S.; Chen, D.; Lin, S. Development of a flavor fingerprint by HS-GC–IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef]
- Li, C.; Al-Dalali, S.; Wang, Z.; Xu, B.; Zhou, H. Investigation of volatile flavor compounds and characterization of aroma-active compounds of water-boiled salted duck using GC–MS–O, GC–IMS, and E-nose. Food Chem. 2022, 386, 132728. [Google Scholar] [CrossRef]
- Leng, P.; Hu, H.-W.; Cui, A.-H.; Tang, H.-J.; Liu, Y.-G. HS-GC-IMS with PCA to analyze volatile flavor compounds of honey peach packaged with different preservation methods during storage. LWT 2021, 149, 111963. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, C.; Jiang, B.; Mo, X.; Wang, Z. Optimization of HS-SPME for GC-MS Analysis and Its Application in Characterization of Volatile Compounds in Sweet Potato. Molecules 2021, 26, 5808. [Google Scholar] [CrossRef]
- Wang, F.; Gao, Y.; Wang, H.; Xi, B.; He, X.; Yang, X.; Li, W. Analysis of volatile compounds and flavor fingerprint in Jingyuan lamb of different ages using gas chromatography–ion mobility spectrometry (GC–IMS). Meat Sci. 2021, 175, 108449. [Google Scholar] [CrossRef]
- Xia, A.-N.; Tang, X.-J.; Dong, G.-Z.; Lei, S.-M.; Liu, Y.-G.; Tian, X.-M. Quality assessment of fermented rose jams based on physicochemical properties, HS-GC-MS and HS-GC-IMS. LWT 2021, 151, 112153. [Google Scholar] [CrossRef]
- Garrido-Delgado, R.; Dobao-Prieto, M.d.M.; Arce, L.; Valcárcel, M. Determination of volatile compounds by GC–IMS to assign the quality of virgin olive oil. Food Chemistry 2015, 187, 572–579. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, X.; Li, P.; Lv, Y.; Nan, H.; Wen, L.; Wang, Z. Research progress of wine aroma components: A critical review. Food Chem. 2023, 402, 134491. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, K.; Guo, H.; Geng, Y.; Lv, W.; Wang, S.; Guo, Y.; Qin, P.; Ren, G. Characterization of volatile compounds in differently coloured Chenopodium quinoa seeds before and after cooking by headspace-gas chromatography-ion mobility spectrometry. Food Chem. 2021, 348, 129086. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.-L.; Li, A.-H.; Su, J.; Wang, X.-C.; Chen, C.-Q.; Tao, Y.-S. Flavor modification of dry red wine from Chinese spine grape by mixed fermentation with Pichia fermentans and S. cerevisiae. LWT 2019, 109, 83–92. [Google Scholar] [CrossRef]
- Sánchez-Palomo, E.; Delgado, J.A.; Ferrer, M.A.; Viñas, M.A.G. The aroma of La Mancha Chelva wines: Chemical and sensory characterization. Food Res. Int. 2019, 119, 135–142. [Google Scholar] [CrossRef]
- Cao, W.; Shu, N.; Wen, J.; Yang, Y.; Jin, Y.; Lu, W. Characterization of the Key Aroma Volatile Compounds in Nine Different Grape Varieties Wine by Headspace Gas Chromatography–Ion Mobility Spectrometry (HS-GC-IMS), Odor Activity Values (OAV) and Sensory Analysis. Foods 2022, 11, 2767. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Y. Effect of ‘wheat Qu’ on the fermentation processes and volatile flavour-active compounds of Chinese rice wine (Huangjiu). J. Inst. Brew. 2013, 119, 71–77. [Google Scholar] [CrossRef]
- Zhou, S.-Q.; Feng, D.; Zhou, Y.-X.; Zhao, J.; Zhao, J.-Y.; Guo, Y.; Yan, W.-J. HS-GC-IMS detection of volatile organic compounds in cistanche powders under different treatment methods. LWT 2022, 165, 113730. [Google Scholar] [CrossRef]
- Guo, X.; Schwab, W.; Ho, C.-T.; Song, C.; Wan, X. Characterization of the aroma profiles of oolong tea made from three tea cultivars by both GC–MS and GC-IMS. Food Chem. 2022, 376, 131933. [Google Scholar] [CrossRef]
- Zhao, S.; Li, M.; Simal-Gandara, J.; Tian, J.; Chen, J.; Dai, X.; Kong, Z. Impact of chiral tebuconazole on the flavor components and color attributes of Merlot and Cabernet Sauvignon wines at the enantiomeric level. Food Chem. 2022, 373, 131577. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Zhu, T.; Wang, J.; Huang, M.; Wei, J.; Ye, H.; Wu, J.; Zhang, J.; Meng, N. Characterization of the key odorants and their content variation in Niulanshan Baijiu with different storage years using flavor sensory omics analysis. Food Chem. 2022, 376, 131851. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xia, Y.; Wang, G.; Tao, L.; Yu, J.; Ai, L. Effects of boiling, ultra-high temperature and high hydrostatic pressure on free amino acids, flavor characteristics and sensory profiles in Chinese rice wine. Food Chem. 2019, 275, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Huang, J.; Zhou, R.; Zhang, S.; Qin, H.; Tang, H.; Pan, Q.; Tang, H. Characterization of the differences in aroma-active compounds in strong-flavor Baijiu induced by bioaugmented Daqu using metabolomics and sensomics approaches. Food Chem. 2023, 424, 136429. [Google Scholar] [CrossRef]
- Xiao, Z.; Wu, Q.; Niu, Y.; Wu, M.; Zhu, J.; Zhou, X.; Chen, X.; Wang, H.; Li, J.; Kong, J. Characterization of the Key Aroma Compounds in Five Varieties of Mandarins by Gas Chromatography–Olfactometry, Odor Activity Values, Aroma Recombination, and Omission Analysis. J. Agric. Food Chem. 2017, 65, 8392–8401. [Google Scholar] [CrossRef]
- Zhang, Y.; Tong, X.; Chen, B.; Wu, S.; Wang, X.; Zheng, Q.; Jiang, F.; Qiao, Y. Novel application of HS-GC-IMS for characteristic fingerprints and flavor compound variations in citrus reticulatae pericarpium during storage with different Aspergillus niger fermentation. Food Chem. X 2023, 18, 100653. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, C.; Chu, B.; Gu, P.; Zhu, B.; Qian, W.; Chang, X.; Yu, M.; Zhang, Y.; Wang, X. Unraveling the relationship between key aroma components and sensory properties of fragrant peanut oils based on flavoromics and machine learning. Food Chem. X 2023, 20, 100880. [Google Scholar] [CrossRef]
- Xuan, X.; Sun, R.; Zhang, X.; Cui, Y.; Lin, X.; Sun, Y.; Deng, W.; Liao, X.; Ling, J. Novel application of HS-GC-IMS with PCA for characteristic fingerprints and flavor compound variations in NFC Chinese bayberry (Myrica rubra) juice during storage. LWT 2022, 167, 113882. [Google Scholar] [CrossRef]
- Li, X.; Dong, Y.; Jiang, P.; Qi, L.; Lin, S. Identification of changes in volatile compounds in sea cucumber Apostichopus japonicus during seasonings soaking using HS-GC-IMS. LWT 2022, 154, 112695. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, X.; Ma, Y.; Wang, Y.; Wang, D. Fingerprints and changes analysis of volatile compounds in fresh-cut yam during yellowing process by using HS-GC-IMS. Food Chem. 2022, 369, 130939. [Google Scholar] [CrossRef]
- Feng, T.; Sun, J.; Song, S.; Wang, H.; Yao, L.; Sun, M.; Wang, K.; Chen, D. Geographical differentiation of Molixiang table grapes grown in China based on volatile compounds analysis by HS-GC-IMS coupled with PCA and sensory evaluation of the grapes. Food Chem. X 2022, 15, 100423. [Google Scholar] [CrossRef]
- Huang, S.; Li, G.; Luo, Y.; Lan, H.; Li, X.; Kang, C.; Shuai, L.; Nie, H. Variations in volatile compounds in Chinese water chestnut (Eleocharis dulcis (Burm.f.) Trin. ex Hensch.) processed with different methods by HS-GC-IMS and HS-SPME-GC-MS. Food Chem. Adv. 2022, 1, 100072. [Google Scholar] [CrossRef]
- Duan, H.; Zhou, Y.; Wang, D.; Yan, W. Differences in Volatile Organic Compounds in Rhizoma gastrodiae (Tian Ma) of Different Origins Determined by HS-GC-IMS. Molecules 2023, 28, 4883. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, L.; Liu, Q.; Yin, Y.; Zhou, L.; Zhao, X.; Peng, X. Chemical and Volatile Compounds in Sweet Potato Brandy: Impact of Processing Methods. Foods 2025, 14, 1467. https://doi.org/10.3390/foods14091467
Li Y, Li L, Liu Q, Yin Y, Zhou L, Zhao X, Peng X. Chemical and Volatile Compounds in Sweet Potato Brandy: Impact of Processing Methods. Foods. 2025; 14(9):1467. https://doi.org/10.3390/foods14091467
Chicago/Turabian StyleLi, Yunying, Lin Li, Qian Liu, Yina Yin, Lin Zhou, Xinxin Zhao, and Xinyan Peng. 2025. "Chemical and Volatile Compounds in Sweet Potato Brandy: Impact of Processing Methods" Foods 14, no. 9: 1467. https://doi.org/10.3390/foods14091467
APA StyleLi, Y., Li, L., Liu, Q., Yin, Y., Zhou, L., Zhao, X., & Peng, X. (2025). Chemical and Volatile Compounds in Sweet Potato Brandy: Impact of Processing Methods. Foods, 14(9), 1467. https://doi.org/10.3390/foods14091467





