Comprehensive Characterization and Comparison of Aroma Profiles of Tricholoma matsutake Soup During the Cooking Process by HS-GC-IMS and HS-SPME-GC-MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of T. matsutake Soup
2.2. E-Nose Analysis
2.3. HS-GC-IMS Analysis
2.4. HS-SPME-GC-MS Analysis
2.5. ROAV Analysis
2.6. Data Analysis
3. Results and Discussion
3.1. Analysis of E-Nose Results
3.2. Difference Analysis of Volatile Components in T. matsutake Soup Cooking Process Based on HS-GC-IMS
3.3. Difference Analysis of Volatile Components in T. matsutake Soup Cooking Process Based on HS-SPME-GC-MS
3.4. ROAV Analysis of Volatile Components of T. matsutake Soup Based on HS-SPME-GC-MS
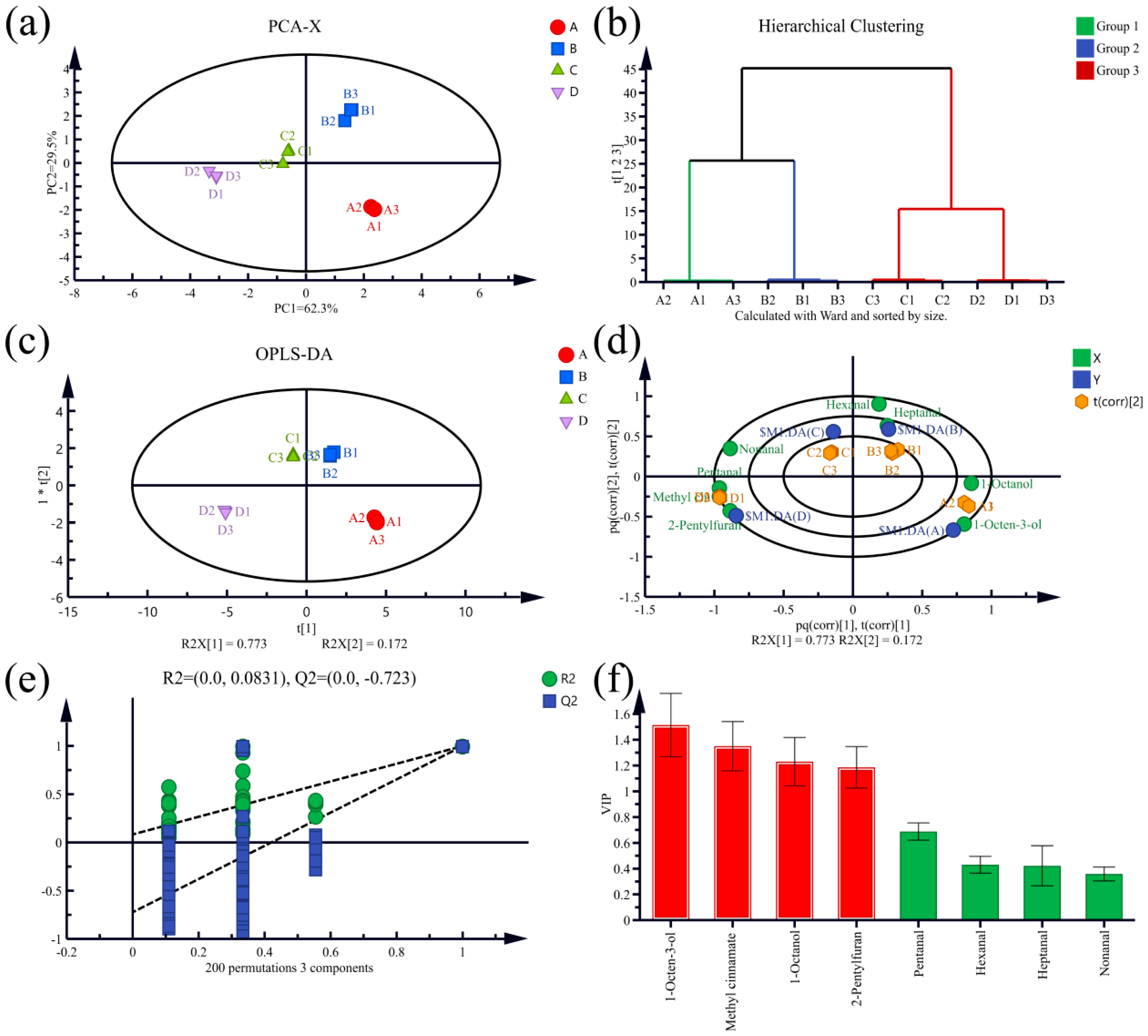
3.5. Multivariate Statistical Analysis of Volatile Components of T. matsutake Soup Based on HS-SPME-GC-MS
3.6. Combined Analysis by HS-GC-IMS and HS-SPME-GC-MS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Wang, Y.; Chen, Y.; Teng, M.; He, J.; Wang, X.; Kong, F.; Teng, L.; Wang, D. Investigation of the immunomodulatory activity of Tricholoma matsutake mycelium in cyclophosphamide-induced immunosuppressed mice. Mol. Med. Rep. 2017, 16, 4320–4326. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, T.; Huang, C.; Liu, L.; Kwame, A.W.; Zhu, Y.; Ren, J. Preventive intervention with Agaricus blazei murill polysaccharide exerts anti-tumor immune effect on intraperitoneal metastasis colorectal cancer. Int. J. Biol. Macromol. 2024, 282 Pt 3, 136810. [Google Scholar] [CrossRef]
- Ding, X.; Tang, J.; Cao, M.; Guo, C.; Zhang, X.; Zhong, J.; Zhang, J.; Sun, Q.; Feng, S.; Yang, Z.; et al. Structure elucidation and antioxidant activity of a novel polysaccharide isolated from Tricholoma matsutake. Int. J. Biol. Macromol. 2010, 47, 271–275. [Google Scholar] [CrossRef]
- Ma, N.; Tao, H.; Du, H.; Zhao, L.; Hu, Q.; Xiao, H. Antifatigue effect of functional cookies fortified with mushroom powder (Tricholoma Matsutake) in mice. J. Food Sci. 2020, 85, 4389–4395. [Google Scholar] [CrossRef] [PubMed]
- Sissons, J.; Davila, M.; Du, X. Sautéing and roasting effect on free amino acid profiles in portobello and shiitake mushrooms, and the effect of mushroom- and cooking-related volatile aroma compounds on meaty flavor enhancement. Int. J. Gastron. Food Sci. 2022, 28, 100550. [Google Scholar] [CrossRef]
- Xie, L.; Guo, S.; Rao, H.; Lan, B.; Zheng, B.; Zhang, N. Characterization of volatile flavor compounds and aroma active components in button mushroom (Agaricus bisporus) across various cooking methods. Foods 2024, 13, 685. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, H.H.; Claver, I.P.; Zhu, K.X.; Peng, W.; Zhou, H.M. Effect of different cooking methods on the flavour constituents of mushroom (Agaricus bisporus (Lange) Sing) soup. Int. J. Food Sci. Technol. 2011, 46, 1100–1108. [Google Scholar] [CrossRef]
- Yi, L.; Dong, N.; Yun, Y.; Deng, B.; Ren, D.; Liu, S.; Liang, Y. Chemometric methods in data processing of mass spectrometry-based metabolomics: A review. Anal. Chim. Acta 2016, 914, 17–34. [Google Scholar] [CrossRef]
- Xu, L.; Wang, S.; Tian, A.; Liu, T.; Benjakul, S.; Xiao, G.; Ying, X.; Zhang, Y.; Ma, L. Characteristic volatile compounds, fatty acids and minor bioactive components in oils from green plum seed by HS-GC-IMS, GC-MS and HPLC. Food Chem. X 2023, 17, 100530. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, F.; Wu, W.; Wang, P.; Ye, N. Comparison of volatiles in different jasmine tea grade samples using electronic nose and automatic thermal desorption-gas chromatography-mass spectrometry followed by multivariate statistical analysis. Molecules 2020, 25, 380. [Google Scholar] [CrossRef]
- Sun, X.; Liu, X.; Yang, W.; Feng, A.; Sun, T.; Jiang, Q.; Xie, W. Establishment of intermittent ultrasound-complex enzyme deodorization technology for tilapia based on GC-IMS and HS-SPME-GC/MS analysis. Food Biosci. 2024, 61, 104806. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Liu, Y.; Wang, D. Analyzing volatile compounds of young and mature Docynia delavayi fruit by HS-SPME-GC-MS and rOAV. Foods 2022, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Kh, A.A.; Mi, S.; Tian, H.; Xu, X.; Abdo, A.A.A.; Aleryani, H.; Wang, X. Evaluation of flavor characteristics in Chinese wheat flour paste using electronic-nose, electronic-tongue, and headspace-gas chromatography-ion mobility spectrometry at different fermentation stages. J. Sci. Food Agric. 2024, 105, 2454–2465. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Li, Y.; Zhu, H.; Liu, Y.; Quan, K. Effect of Lactobacillus plantarum fermentation on the volatile flavors of mung bean. LWT 2021, 146, 111434. [Google Scholar] [CrossRef]
- Brunton, N.P.; Cronin, D.A.; Monahan, F.J.; Durcan, R. A comparison of solid-phase microextraction (SPME) fibres for measurement of hexanal and pentanal in cooked turkey. Food Chem. 2020, 68, 339–345. [Google Scholar] [CrossRef]
- Xie, Q.; Xu, B.; Xu, Y.; Yao, Z.; Zhu, B.; Li, X.; Sun, Y. Effects of different thermal treatment temperatures on volatile flavour compounds of water-boiled salted duck after packaging. LWT 2022, 154, 112625. [Google Scholar] [CrossRef]
- Li, C.; Zou, Y.; Liao, G.; Zheng, Z.; Chen, G.; Zhong, Y.; Wang, G. Identification of characteristic flavor compounds and small molecule metabolites during the ripening process of Nuodeng ham by GC-IMS, GC-MS combined with metabolomics. Food Chem. 2024, 440, 138188. [Google Scholar] [CrossRef]
- Li, M.; Yang, R.; Zhang, H.; Wang, S.; Chen, D.; Lin, S. Development of a flavor fingerprint by HS-GC-IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef]
- Cho, I.H.; Kim, S.Y.; Choi, H.K.; Kim, Y.S. Characterization of aroma-active compounds in raw and cooked pine-mushrooms (Tricholoma matsutake Sing.). J. Agric. Food Chem. 2006, 54, 6332–6335. [Google Scholar] [CrossRef]
- Pei, F.; Yang, W.; Ma, N.; Fang, Y.; Zhao, L.; An, X.; Xin, Z.; Hu, Q. Effect of the two drying approaches on the volatile profiles of button mushroom (Agaricus bisporus) by headspace GC-MS and electronic nose. LWT-Food Sci. Technol. 2016, 72, 343–350. [Google Scholar] [CrossRef]
- Jaffe, T.R.; Wang, H.; Iv, E.C. Determination of a lexicon for the sensory flavor attributes of smoked food products. J. Sens. Stud. 2017, 32, e12262. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, J.; Wei, Q.; Wu, L.; Li, T.; Zhang, W. Comprehensive characterization and comparison of aroma profiles of rambutan seed oils using GC-MS and GC-IMS combined with chemometrics. Front. Nutr. 2024, 11, 1486368. [Google Scholar] [CrossRef]
- Sun, X.; Yu, Y.; Saleh, A.S.M.; Yang, X.; Ma, J.; Gao, Z.; Zhang, D.; Li, W.; Wang, Z. Characterization of aroma profiles of Chinese four most famous traditional red-cooked chickens using GC-MS, GC-IMS, and E-nose. Food Res. Int. 2023, 173, 113335. [Google Scholar] [CrossRef]
- Wei, G.; Dan, M.; Zhao, G.; Wang, D. Recent advances in chromatography-mass spectrometry and electronic nose technology in food flavor analysis and detection. Food Chem. 2022, 405, 134814. [Google Scholar] [CrossRef]
- Wang, H.; Chambers, E. Sensory characteristics of various concentrations of phenolic compounds potentially associated with smoked aroma in foods. Molecules 2018, 23, 780. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; de Freitas, V. Tannins in food: Insights into the molecular perception of astringency and bitter taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; He, J.J.; Zhou, Y.Z.; Li, Y.L.; Zhou, H.J. Aroma effects of key volatile compounds in Keemun black tea at different grades: HS-SPME-GC-MS, sensory evaluation, and chemometrics. Food Chem. 2022, 373, 131587. [Google Scholar] [CrossRef]
- Xu, M.; Wang, J.; Zhu, L. Tea quality evaluation by applying E-nose combined with chemometrics methods. J. Food Sci. Technol. 2021, 58, 1549–1561. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Yan, F.; Tang, Y.; Yu, B.; Chen, B.; Lu, L.; Yuan, L.; Wu, Z.; Chen, H. Monitoring changes in the volatile compounds of tea made from summer tea leaves by GC-IMS and HS-SPME-GC-MS. Foods 2022, 12, 146. [Google Scholar] [CrossRef]
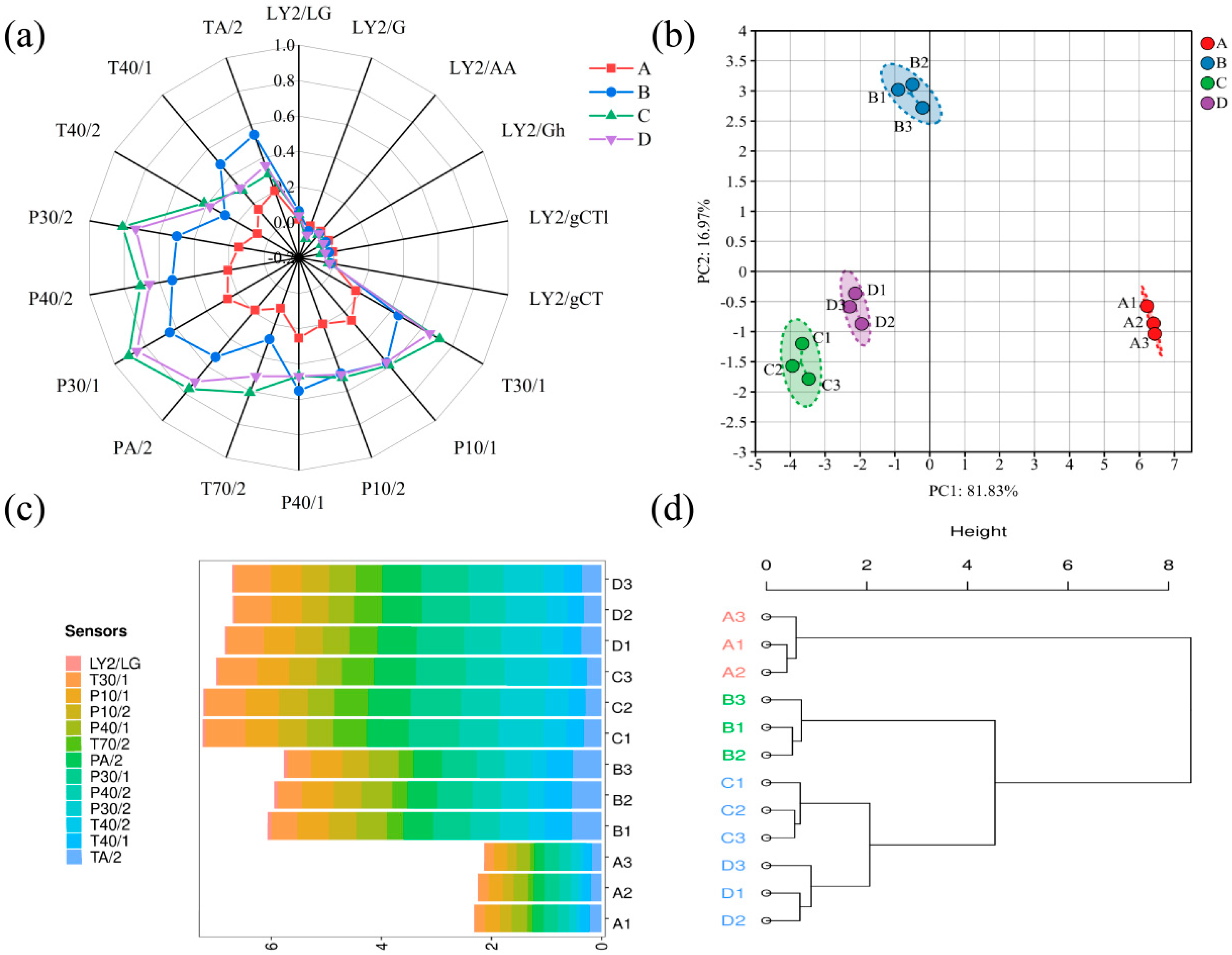
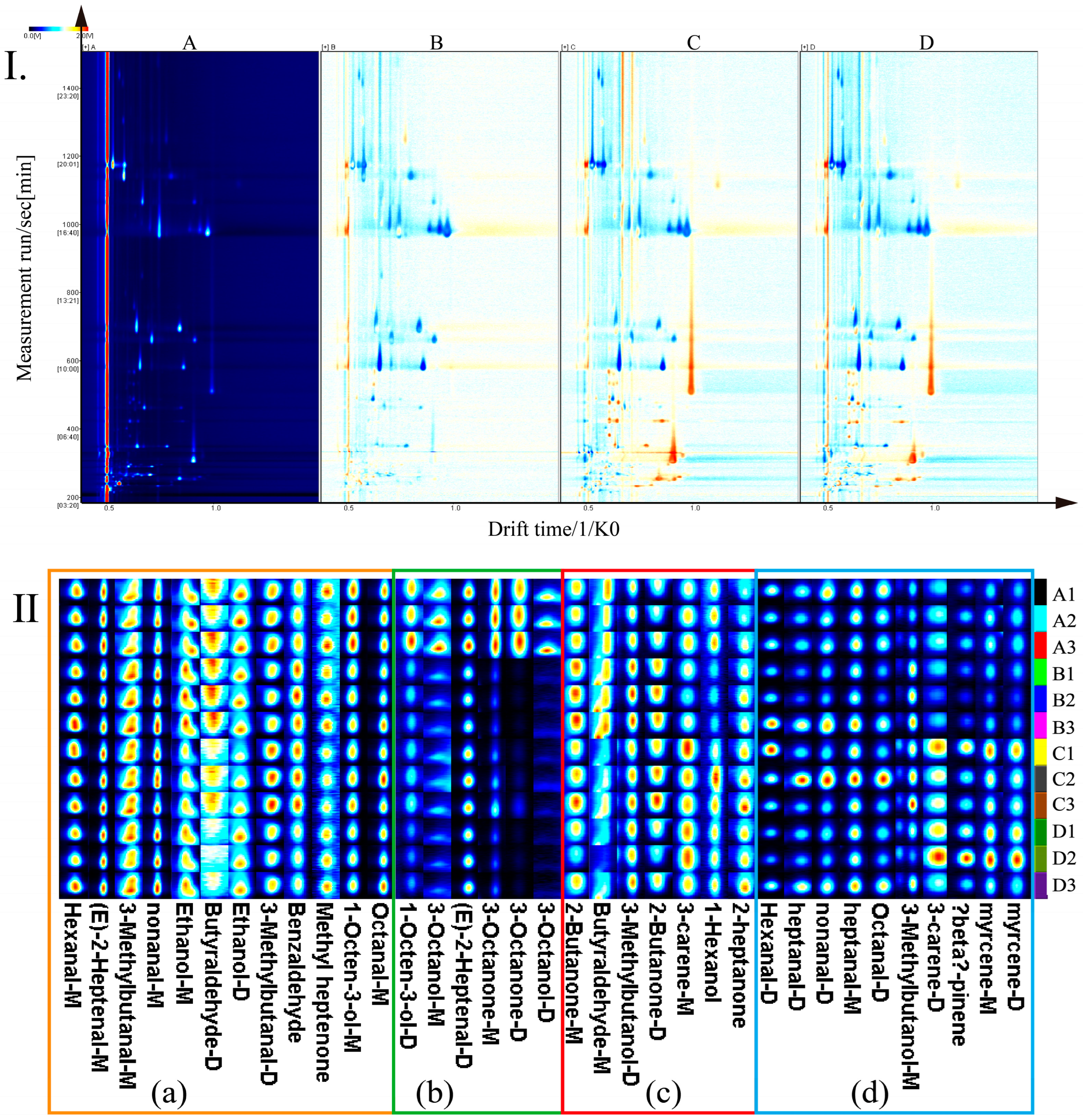

| Levels | Sensors Name | Response Characteristic | Reference Materials |
|---|---|---|---|
| 1 | LY2/LG | Sensitive to gases with strong oxidation capacity | Nitrogen oxides, sulfides, chlorine, fluorine |
| 2 | LY2/G | Sensitive to poisonous gases | Carbohydrates, amines, ammonia |
| 3 | LY2/AA | Sensitive to organic compounds | Ammonia, acetone, ethanol |
| 4 | LY2/GH | Sensitive to toxic gases | Amines, ammonia |
| 5 | LY2/gCTl | Sensitive to toxic gases | Sulphide |
| 6 | LY2/gCT | Sensitive to flammable gases | Butane, propane |
| 7 | T30/1 | Sensitive to polar compounds | Propane, hydrogen chloride |
| 8 | P10/1 | Sensitive to non-polar compounds | Carbohydrates, ammonia |
| 9 | P10/2 | Sensitive to non-polar compounds | Methane, octane |
| 10 | P40/1 | Sensitive to strong oxidizing gas | Chlorine and fluorine |
| 11 | T70/2 | Sensitive to aromatic compounds | Toluene |
| 12 | PA/2 | Sensitive to organic compounds | Ethanol, amines |
| 13 | P30/1 | Sensitive to combustible gases and organic compounds | Hydrocarbons, ethanol, ammonia |
| 14 | P40/2 | Sensitive to gases with strong oxidizing power | Sulfide, chlorine, fluoride |
| 15 | P30/2 | Sensitive to organic compounds | Hydrogen sulfide, ketones |
| 16 | T40/2 | Sensitive to gases with strong oxidizing capacity | Chlorine and fluorine |
| 17 | T40/1 | Sensitive to gases with strong oxidizing capacity | Fluorine |
| 18 | TA/2 | Sensitive to organic compounds | Ethanol |
| Compounds | Relative Content (%) | Odor Description * | ||||
|---|---|---|---|---|---|---|
| RI | A | B | D | E | ||
| (E)-2-Heptenal-D | 1291.6 | 8.54 ± 0.53 a | 6.80 ± 0.91 b | 7.69 ± 0.81 ab | 7.38 ± 0.5 ab | Almond, fat |
| (E)-2-Heptenal-M | 1293.5 | 9.87 ± 0.41 a | 11.14 ± 1.42 a | 10.32 ± 1.24 a | 11.03 ± 0.51 a | Almond, fat |
| 3-Methylbutanal-D | 881.9 | 2.91 ± 0.07 a | 3.20 ± 0.36 a | 3.51 ± 0.40 a | 3.22 ± 0.04 a | Almond, malt |
| 3-Methylbutanal-M | 880.6 | 1.57 ± 0.07 b | 2.15 ± 0.24 ab | 1.87 ± 0.22 a | 2.05 ± 0.09 a | Almond, malt |
| Benzaldehyde | 1511.5 | 0.95 ± 0.03 c | 1.41 ± 0.22 a | 1.21 ± 0.13 ab | 1.10 ± 0.09 bc | Bitter almond |
| Butyraldehyde-D | 845.6 | 0.07 ± 0.01 b | 0.09 ± 0.01 a | 0.06 ± 0.01 b | 0.06 ± 0.01 b | Banana |
| Butyraldehyde-M | 845.1 | 0.51 ± 0.02 b | 0.81 ± 0.06 a | 0.48 ± 0.12 b | 0.41 ± 0.15 b | Banana |
| Heptanal-D | 1166.2 | 0.54 ± 0.05 a | 0.62 ± 0.22 a | 0.73 ± 0.26 a | 0.71 ± 0.13 a | Citrus, green |
| Heptanal-M | 1165.7 | 2.30 ± 0.10 b | 2.76 ± 0.25 a | 2.90 ± 0.31 a | 3.02 ± 0.20 a | Citrus, green |
| Hexanal-D | 1055.2 | 1.48 ± 0.12 a | 1.98 ± 0.58 a | 2.08 ± 0.98 a | 1.40 ± 0.30 a | Apple, fresh, green |
| Hexanal-M | 1055.8 | 3.61 ± 0.01 b | 4.81 ± 0.22 a | 4.31 ± 0.56 a | 4.24 ± 0.23 a | Apple, fresh, green |
| Nonanal-D | 1393.6 | 8.08 ± 1.63 a | 8.32 ± 3.58 a | 8.68 ± 4.05 a | 7.75 ± 1.37 a | Green, lemon |
| Nonanal-M | 1393.6 | 16.39 ± 0.67 c | 19.96 ± 0.79 a | 17.86 ± 0.91 a | 19.19 ± 0.30 b | Green, lemon |
| Octanal-D | 1275.2 | 2.24 ± 0.24 a | 1.85 ± 0.51 a | 2.42 ± 1.01 a | 2.31 ± 0.61 a | Citrus, fat |
| Octanal-M | 1274.9 | 6.69 ± 0.30 a | 7.22 ± 0.32 a | 7.37 ± 0.56 a | 7.69 ± 0.62 a | Citrus, fat |
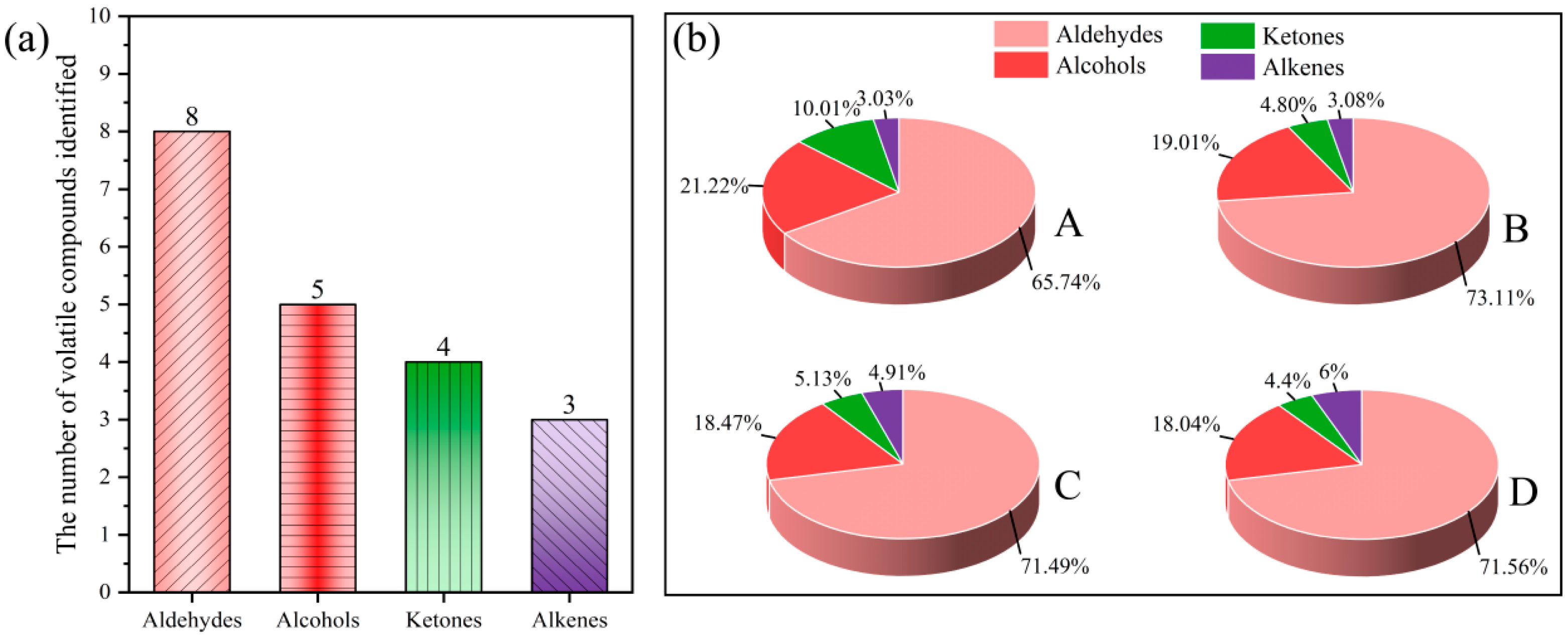
| Aldehydes | 65.73 ± 2.10 b | 73.11 ± 2.86 a | 71.49 ± 3.30 a | 71.57 ± 2.71 a | - | |
| 1-Hexanol | 1348.9 | 0.73 ± 0.06 bc | 0.61 ± 0.10 c | 0.90 ± 0.08 a | 0.87 ± 0.08 ab | Banana, grass |
| 1-Octen-3-ol-D | 1452.7 | 2.17 ± 0.19 a | 1.49 ± 0.24 b | 1.46 ± 0.16 b | 1.20 ± 0.07 b | Earth, mushroom |
| 1-Octen-3-ol-M | 1453 | 9.56 ± 0.42 a | 9.21 ± 1.16 a | 8.85 ± 1.09 a | 8.46 ± 0.48 a | Earth, mushroom |
| 3-Methylbutanol-M | 1210.5 | 0.62 ± 0.05 b | 1.04 ± 0.08 a | 1.12 ± 0.18 a | 1.04 ± 0.09 a | Cocoa, malt |
| 3-Methylbutanol-D | 1228.1 | 0.83 ± 0.05 b | 1.30 ± 0.11 a | 1.17 ± 0.14 a | 1.30 ± 0.08 a | Cocoa, malt |
| 3-Octanol-D | 1398.9 | 2.35 ± 0.19 a | 0.95 ± 0.09 b | 0.92 ± 0.11 b | 0.84 ± 0.05 b | Mushroom, oil |
| 3-Octanol-M | 1401.4 | 3.25 ± 0.28 a | 2.10 ± 0.18 b | 2.33 ± 0.18 b | 2.05 ± 0.16 b | Mushroom, oil |
| Ethanol-D | 895.7 | 1.07 ± 0.07 b | 1.36 ± 0.16 b | 1.06 ± 0.14 a | 1.40 ± 0.11 a | Pungent |
| Ethanol-M | 897.5 | 0.65 ± 0.04 b | 0.95 ± 0.12 b | 0.66 ± 0.08 a | 0.88 ± 0.04 a | Pungent |
| Alcohols | 21.22±1.18 a | 19.01 ± 2.06 a | 18.47 ± 1.73 a | 18.04 ± 0.94 a | - | |
| 2-Butanone-D | 867.2 | 0.55 ± 0.03 b | 0.98 ± 0.12 a | 0.89 ± 0.16 a | 0.79 ± 0.08 a | Pleasant fruit |
| 2-Butanone-M | 865.5 | 0.49 ± 0.01 b | 0.79 ± 0.07 a | 0.61 ± 0.11 b | 0.58 ± 0.10 b | Pleasant fruit |
| 2-Heptanone | 1162.7 | 0.41 ± 0.03 b | 0.63 ± 0.06 a | 0.63 ± 0.05 a | 0.69 ± 0.01 a | Blue cheese, fruit, green |
| 3-Octanone-D | 1235 | 2.62 ± 0.20 a | 0.18 ± 0.03 b | 0.37 ± 0.21 b | 0.19 ± 0.02 b | Butter, herb |
| 3-Octanone-M | 1236 | 5.58 ± 0.26 a | 1.74 ± 0.29 b | 2.25 ± 0.69 b | 1.64 ± 0.02 b | Butter, herb |
| Methyl heptenone | 1331.8 | 0.37 ± 0.02 b | 0.48 ± 0.06 a | 0.37 ± 0.02 b | 0.49 ± 0.02 a | Citrus, Mushroom |
| Ketones | 10.01 ± 0.52 a | 4.80 ± 0.61 b | 5.13 ± 1.22 b | 4.40 ± 0.11 b | - | |
| Beta-pinene | 1098.9 | 0.33 ± 0.06 b | 0.33 ± 0.05 b | 0.60 ± 0.19 ab | 0.81 ± 0.34 a | Pine |
| 3-Carene-D | 1134.5 | 0.49 ± 0.09 b | 0.48 ± 0.09 b | 0.87 ± 0.21 ab | 1.06 ± 0.37 a | Lemon |
| 3-Carene-M | 1134 | 1.02 ± 0.12 b | 1.11 ± 0.16 b | 1.55 ± 0.35 ab | 1.76 ± 0.40 a | Lemon |
| Myrcene-D | 1179.1 | 0.54 ± 0.08 b | 0.55 ± 0.10 b | 0.89 ± 0.27 ab | 1.17 ± 0.46 a | Fruits, herbs |
| Myrcene-M | 1178.5 | 0.66 ± 0.08 b | 0.61 ± 0.09 b | 1.00 ± 0.25 ab | 1.20 ± 0.38 a | Fruits, herbs |
| Alkenes | 3.03 ± 0.43 b | 3.08 ± 0.49 b | 4.91 ± 1.27 ab | 6.00 ± 1.94 a | - | |
| Compounds | RI | Relative Content (%) | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| 2-(Methylamino)ethanol | 8.32 | 0.05 ± 0.01 a | ND | 0.15 ± 0.01 a | ND |
| 1-Octanol | 17.136 | 4.88 ± 0.04 ab | 4.63 ± 0.05 c | 0.45 ± 0.01 d | 0.50 ± 0.01 a |
| Cubenol | 23.025 | 1.30 ± 0.02 | ND | ND | ND |
| 1-Octen-3-ol | 15.643 | 14.64 ± 0.11 a | 4.73 ± 0.02 b | 3.38 ± 0.07 c | 3.17 ± 0.01 d |
| 3-Octanol | 14.917 | 0.30 ± 0.02 a | 0.24 ± 0.02 b | 0.24 ± 0.01 b | 0.17 ± 0.01 c |
| 4-Sec-Butylcyclohexanol | 12.961 | 0.45 ± 0.01 | ND | ND | ND |
| Cedrol | 23.636 | 19.8 ± 0.02 c | 22.26 ± 0.03 a | 20.89 ± 0.01 b | 16.45 ± 0.03 d |
| 2-Isopropyl-5-methyl-1-hexanol | 17.872 | ND | 1.82 ± 0.06 b | 3.24 ± 0.11 a | ND |
| (Z)-2-Decen-1-ol | 9.769 | 0.03 ± 0.01 c | 0.07 ± 0.01 b | 0.10 ± 0.01 a | 0.03 ± 0.01 c |
| Ledol | 25.834 | ND | 0.06 ± 0.01 | ND | ND |
| 1,3-Dioxolane-4-methanol | 3.58 | ND | ND | 0.13 ± 0.01 | ND |
| widdrol | 23.928 | ND | ND | 6.17 ± 0.02 a | 5.31 ± 0.01 b |
| 2-Propyl-1-heptanol | 18.459 | 2.40 ± 0.02 d | 15.13 ± 0.01 b | 23.59 ± 0.02 a | 2.81 ± 0.02 c |
| (S)-2-Amino-1-propanol | 4.035 | ND | ND | ND | 0.18 ± 0.01 |
| 4-(Butylnitrosamino)-1-butanol | 15.01 | ND | ND | ND | 0.69 ± 0.01 |
| Z,Z-2,5-Pentadecadien-1-ol | 16.08 | ND | ND | ND | 0.31 ± 0.02 |
| Sclareol | 23.626 | ND | ND | ND | 1.53 ± 0.02 |
| 1,3-Octanediol | 7.195 | ND | 0.01 ± 0.01 bc | 0.02 ± 0.01 ab | 0.03 ± 0.01 a |
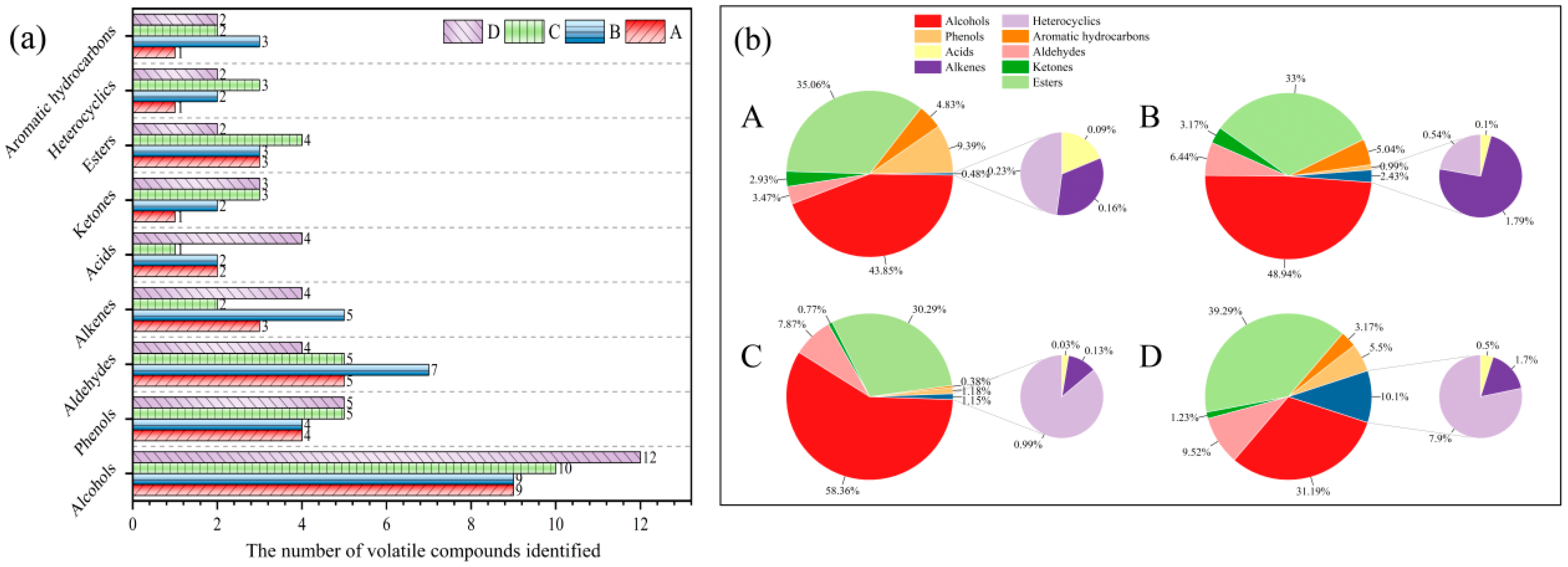
| Alcohols | 43.85 ± 0.15 c | 48.94 ± 0.04 b | 58.36 ± 0.08 a | 31.19 ± 0.05 d | |
| Pentanal | 5 | 0.08 ± 0.01 cd | 0.21 ± 0.02 c | 1.71 ± 0.01 b | 2.91 ± 0.01 a |
| Heptanal | 10.605 | 0.01 ± 0.00 c | 0.39 ± 0.01 a | 0.04 ± 0.01 b | 0.01 ± 0.01 c |
| Succindialdehyde | 12.835 | 0.14 ± 0.01 a | 0.01 ± 0.01 b | ND | ND |
| Nonanal | 15.769 | 0.27 ± 0.03 c | 0.80 ± 0.02 b | 0.80 ± 0.01 b | 0.97 ± 0.03 a |
| 5-Methylhexanal | 10.863 | ND | 0.08 ± 0.02 | ND | ND |
| Hexanal | 8.643 | ND | 0.54 ± 0.02 a | 0.35 ± 0.01 b | ND |
| Benzaldehyde | 17.24 | 2.97 ± 0.04 d | 4.41 ± 0.02 c | 4.98 ± 0.02 b | 5.62 ± 0.02 a |
| Aldehydes | 3.47 ± 0.05 d | 6.44 ± 0.03 c | 7.87 ± 0.02 b | 9.51 ± 0.01 a | |
| 8-Paradol | 22.346 | ND | 2.76 ± 0.11 | ND | ND |
| 3,3-Dimethyl-4-methylamino-butan-2-one | 4.53 | ND | ND | 0.31 ± 0.01 | ND |
| 2-Isopropyl-1-oxaspiro[4.4]nonan-4-one | 14.96 | ND | ND | ND | 0.33 ± 0.01 |
| 3-Octanone | 3.555 | 2.93 ± 0.06 a | 0.41 ± 0.01 d | 0.44 ± 0.01 c | 0.64 ± 0.01 b |
| 2-Heptanone | 5.374 | ND | ND | 0.03 ± 0.01 | 0.26 ± 0.01 |
| Ketones | 2.93 ± 0.06 b | 3.17 ± 0.11 a | 0.78 ± 0.01 d | 1.23 ± 0.00 c | |
| Methyl cinnamate | 21.856 | 14.29 ± 0.02 d | 16.39 ± 0.02 c | 18.50 ± 0.01 b | 26.90 ± 0.02 a |
| (E)-9-Octadecenoic acid methyl ester | 20.664 | 13.62 ± 0.02 | ND | ND | ND |
| 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | 20.91 | 7.15 ± 0.05 c | 11.04 ± 0.04 b | 6.68 ± 0.02 d | 12.38 ± 0.07 a |
| Phenyl cyanate | 22.201 | ND | 5.57 ± 0.02 | ND | ND |
| 3-hydroxy-2,2,4-trimethylpentyl isobutyrate | 20.822 | ND | ND | 4.39 ± 0.01 | ND |
| 1,2-Benzenediol,4-(1,1-dimethylethyl)-1,2-diacetate | 24.094 | ND | ND | 0.72 ± 0.02 | ND |
| Esters | 35.06 ± 0.07 b | 33.00 ± 0.04 c | 30.29 ± 0.02 d | 39.29 ± 0.07 a | |
| 2,4-Dimethylhexanedioic acid | 27.195 | 0.08 ± 0.01 | ND | ND | ND |
| 12-Methylaminolauric acid | 3.7 | 0.01 ± 0.00 | ND | ND | 0.02 ± 0.01 |
| 2-Octynoic acid | 13.631 | ND | 0.02 ± 0.01 b | 0.03 ± 0.01 b | 0.08 ± 0.02 a |
| Tridecanoic acid | 28.874 | ND | 0.08 ± 0.02 b | ND | 0.29 ± 0.01 a |
| Dihydroxyfumaric acid | 2.1 | ND | ND | ND | 0.12 ± 0.01 |
| Acids | 0.09 ± 0.01 b | 0.10 ± 0.02 b | 0.03 ± 0.01 c | 0.51 ± 0.01 a | |
| 1-Methyldecylamine | 3.293 | 0.07 ± 0.02 c | 0.49 ± 0.01 a | 0.11 ± 0.01 b | 0.12 ± 0.01 b |
| 1-Methyldodecylamine | 3.973 | 0.02 ± 0.01 | ND | ND | ND |
| 2-Pentanamine | 2.533 | 0.07 ± 0.02 | ND | ND | ND |
| 2-Aminononadecane | 3.795 | ND | 0.45 ± 0.01 a | ND | 0.02 ± 0.01 b |
| Pentadecane | 10.77 | ND | 0.08 ± 0.01 | ND | ND |
| 1-Undecene | 11.779 | ND | 0.23 ± 0.01 a | 0.02 ± 0.01 b | ND |
| 1,3,5,7-Cyclooctatetraene | 12.283 | ND | 0.54 ± 0.01 | ND | ND |
| 5-methyl-1-Heptene | 14.81 | ND | ND | ND | 1.45 ± 0.03 |
| CIS-3-Dodecene | 15.33 | ND | ND | ND | 0.12 ± 0.01 |
| Alkenes | 0.16 ± 0.02 c | 1.79 ± 0.01 a | 0.13 ± 0.01 d | 1.70 ± 0.01 b | |
| Heptylbenzene | 18.147 | 4.83 ± 0.03 a | 4.65 ± 0.05 b | 0.38 ± 0.02 c | 0.07 ± 0.01 d |
| Butylbenzene | 11.131 | ND | 0.16 ± 0.03 | ND | ND |
| n-Butylbenzene | 13.323 | ND | 0.23 ± 0.03 | ND | ND |
| 1-Phenyl-decan | 22.044 | ND | ND | ND | 3.10 ± 0.01 |
| Aromatic hydrocarbons | 4.83 ± 0.03 b | 5.04 ± 0.01 a | 0.38 ± 0.02 c | 3.17 ± 0.02 d | |
| 2-Pentylfuran | 11.629 | 0.23 ± 0.02 d | 0.53 ± 0.01 c | 0.87 ± 0.03 b | 7.55 ± 0.01 a |
| Indole | 27.35 | ND | ND | 0.10 ± 0.01 | 0.35 ± 0.01 |
| 3-Piperidinol | 11.445 | ND | 0.01 ± 0.00 | 0.02 ± 0.01 | ND |
| Heterocyclics | 0.23 ± 0.02 d | 0.54 ± 0.05 c | 0.99 ± 0.01 b | 7.90 ± 0.02 a | |
| Phenol | 22.33 | 0.63 ± 0.03 b | ND | ND | 4.13 ± 0.03 a |
| 2,4-Di-tert-butylphenol | 25.174 | 8.76 ± 0.03 a | 0.99 ± 0.01 d | 1.18 ± 0.02 c | 1.37 ± 0.03 b |
| Phenols | 9.39 ± 0.02 a | 0.99 ± 0.01 d | 1.18 ± 0.02 c | 5.50 ± 0.05 b | |
| Compounds | Threshold # (mg/kg) | ROAV | Odor Description * | |||
|---|---|---|---|---|---|---|
| A | B | C | D | |||
| 1-Octanol | 0.054 | 4.32 | 5.76 | 0.49 | 0.38 | Mushroom, oil |
| 1-Octen-3-ol | 0.007 | 100.00 | 45.31 | 28.70 | 18.53 | Earth, mushroom |
| 3-Octanol | 0.1 | 0.14 | 0.16 | 0.14 | 0.07 | Mushroom, oil |
| Pentanal | 0.008 | 0.49 | 1.74 | 12.71 | 14.87 | Almond, bitter, oil |
| Heptanal | 0.01 | 0.06 | 2.61 | 0.22 | 0.06 | Citrus, green |
| Nonanal | 0.015 | 0.87 | 3.59 | 3.17 | 2.65 | Green, lemon |
| Hexanal | 0.0075 | ND | 4.79 | 2.75 | ND | Apple, fresh, green |
| Benzaldehyde | 0.3 | 0.47 | 0.99 | 0.99 | 0.77 | Bitter almond, burnt sugar, hyacinth-like |
| 3-Octanone | 1 | 0.14 | 0.03 | 0.03 | 0.03 | Butter, herb |
| 2-Heptanone | 0.2 | ND | ND | 0.01 | 0.05 | Blue cheese, fruit, green |
| Methyl cinnamate | 0.011 | 62.11 | 100.00 | 100.00 | 100.00 | Balsamic, strawberry |
| 2-Pentanamine | 170 | <0.01 | ND | ND | ND | - |
| 2-Pentylfuran | 0.0048 | 2.31 | 7.35 | 10.77 | 64.27 | Butter, floral, fruit |
| Indole | 0.5 | ND | ND | 0.01 | 0.03 | Burnt, mothball |
| 3-Piperidinol | 10,000 | ND | <0.01 | <0.01 | ND | - |
| Phenol | 5.5 | 0.01 | ND | ND | 0.03 | - |
| Levels | Compounds | VIP |
|---|---|---|
| 1 | 1-Octen-3-ol | 1.51387 |
| 2 | Methyl cinnamate | 1.35069 |
| 3 | 1-Octanol | 1.23024 |
| 4 | 2-Pentylfuran | 1.18552 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, N.-N.; Xu, C.-J.; Xiong, S.-L.; Li, X.; Gu, S.-Y.; Zhu, Z.-Y.; Liu, Y.; Zhu, N.; Zhou, Y.; Xiao, H. Comprehensive Characterization and Comparison of Aroma Profiles of Tricholoma matsutake Soup During the Cooking Process by HS-GC-IMS and HS-SPME-GC-MS. Foods 2025, 14, 1478. https://doi.org/10.3390/foods14091478
Yan N-N, Xu C-J, Xiong S-L, Li X, Gu S-Y, Zhu Z-Y, Liu Y, Zhu N, Zhou Y, Xiao H. Comprehensive Characterization and Comparison of Aroma Profiles of Tricholoma matsutake Soup During the Cooking Process by HS-GC-IMS and HS-SPME-GC-MS. Foods. 2025; 14(9):1478. https://doi.org/10.3390/foods14091478
Chicago/Turabian StyleYan, Ni-Na, Cheng-Jian Xu, Shuang-Li Xiong, Xiang Li, Si-Yu Gu, Zhong-Yan Zhu, Yang Liu, Nan Zhu, Yu Zhou, and Hang Xiao. 2025. "Comprehensive Characterization and Comparison of Aroma Profiles of Tricholoma matsutake Soup During the Cooking Process by HS-GC-IMS and HS-SPME-GC-MS" Foods 14, no. 9: 1478. https://doi.org/10.3390/foods14091478
APA StyleYan, N.-N., Xu, C.-J., Xiong, S.-L., Li, X., Gu, S.-Y., Zhu, Z.-Y., Liu, Y., Zhu, N., Zhou, Y., & Xiao, H. (2025). Comprehensive Characterization and Comparison of Aroma Profiles of Tricholoma matsutake Soup During the Cooking Process by HS-GC-IMS and HS-SPME-GC-MS. Foods, 14(9), 1478. https://doi.org/10.3390/foods14091478







