Ilex latifolia Improves the Anti-Tumor Effectiveness of Rapamycin Against Breast Cancer In Vitro and In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of I. latifolia Extracts
2.2. Characterization of I. latifolia Extracts
2.3. Cell Culture
2.4. Cell Treatment
2.5. Cell Counting Assay
2.6. CCK-8 Cell Cytotoxicity Assay
2.7. Cell Cycle Analysis
2.8. Western Blot
2.9. Quantitative PCR Analysis
2.10. Experimental Animals and Treatment
2.11. Immunohistochemistry of Tumor Tissue
2.12. Data Analysis
3. Results
3.1. The Main Components of Ilex latifolia Extract Are Triterpene Saponins and Phenolic Acid
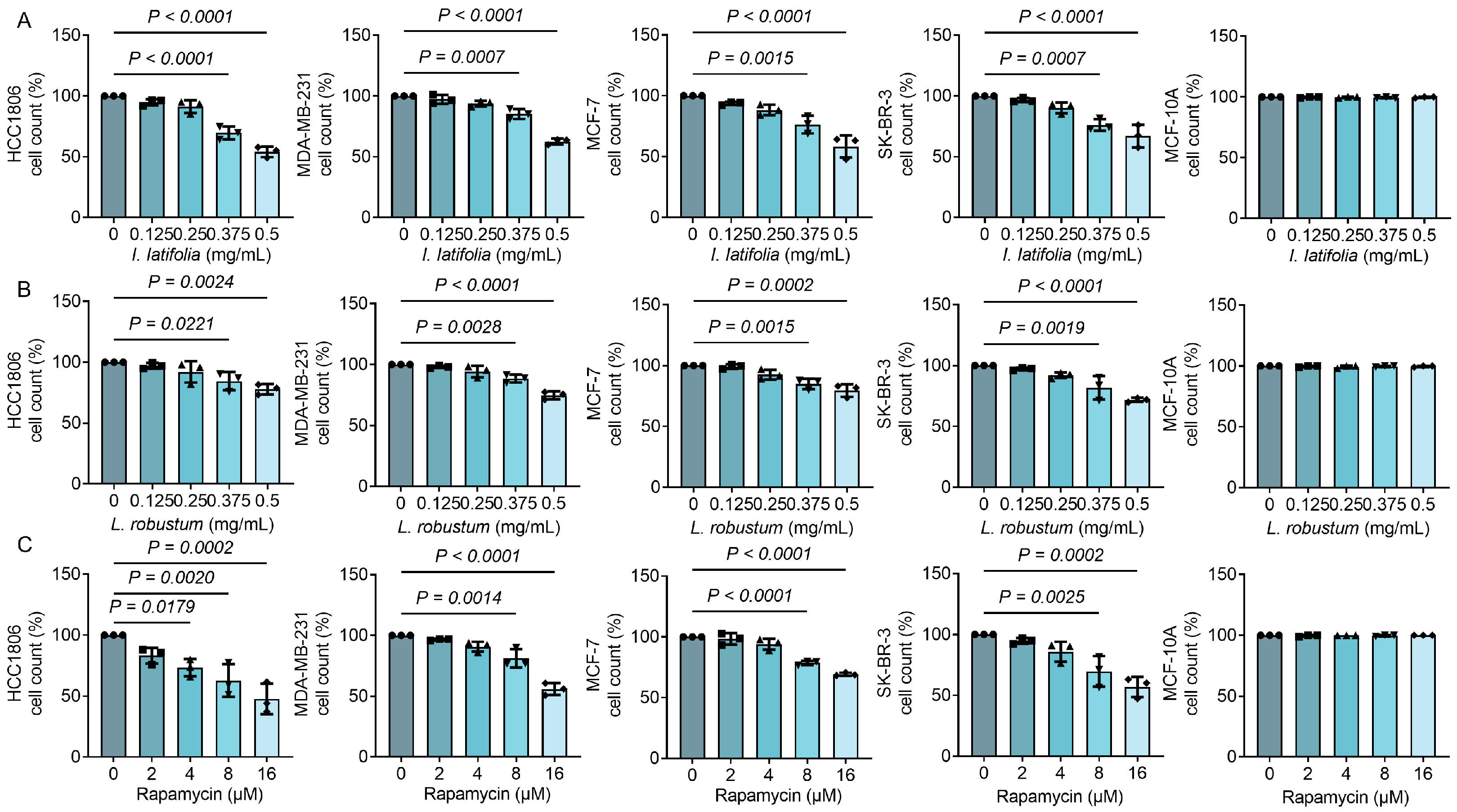
3.2. Ilex latifolia, Ligustrum robustum, and Rapamycin Inhibit Breast Cancer Cell Proliferation in a Way Dependent on Dosage
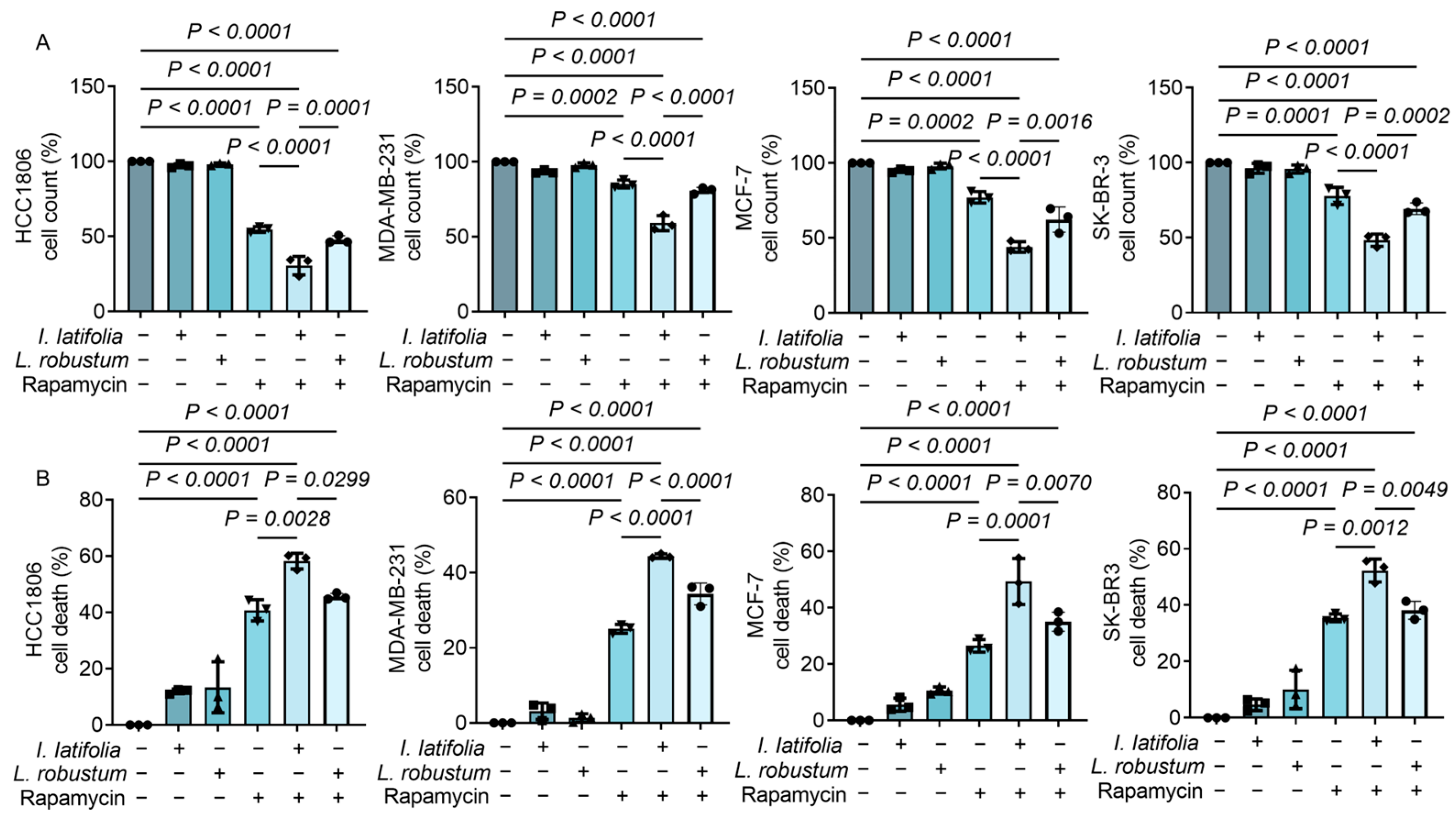
3.3. Ilex latifolia Amplifies the Antiproliferative Effect of Rapamycin on Breast Cancer Cells
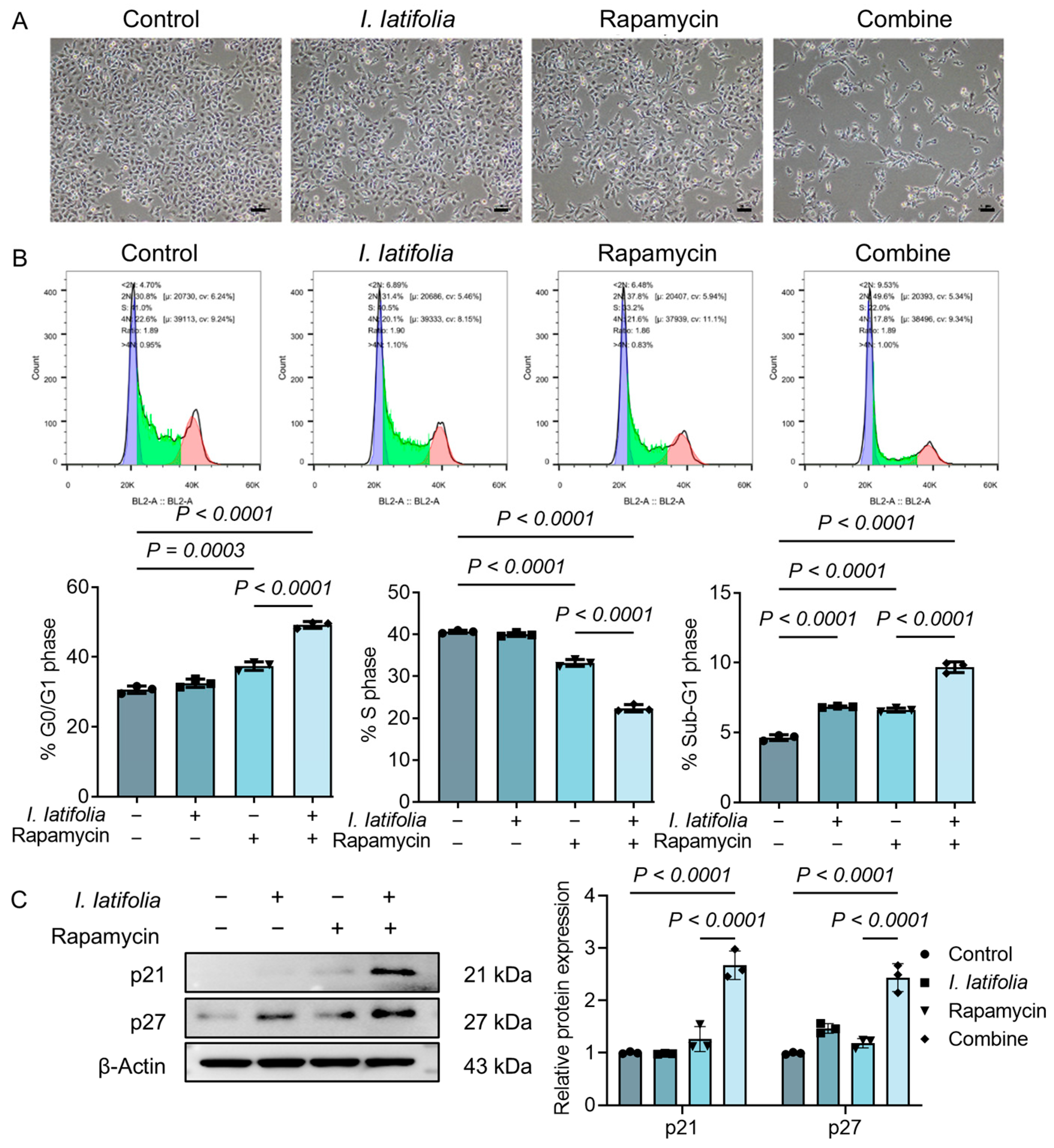
3.4. Ilex latifolia Enhances Rapamycin-Induced Tumor Cell Cycle Arrest
3.5. Ilex latifolia Augments Rapamycin-Induced Modulation of Apoptosis and Inflammation in Breast Cancer Cells
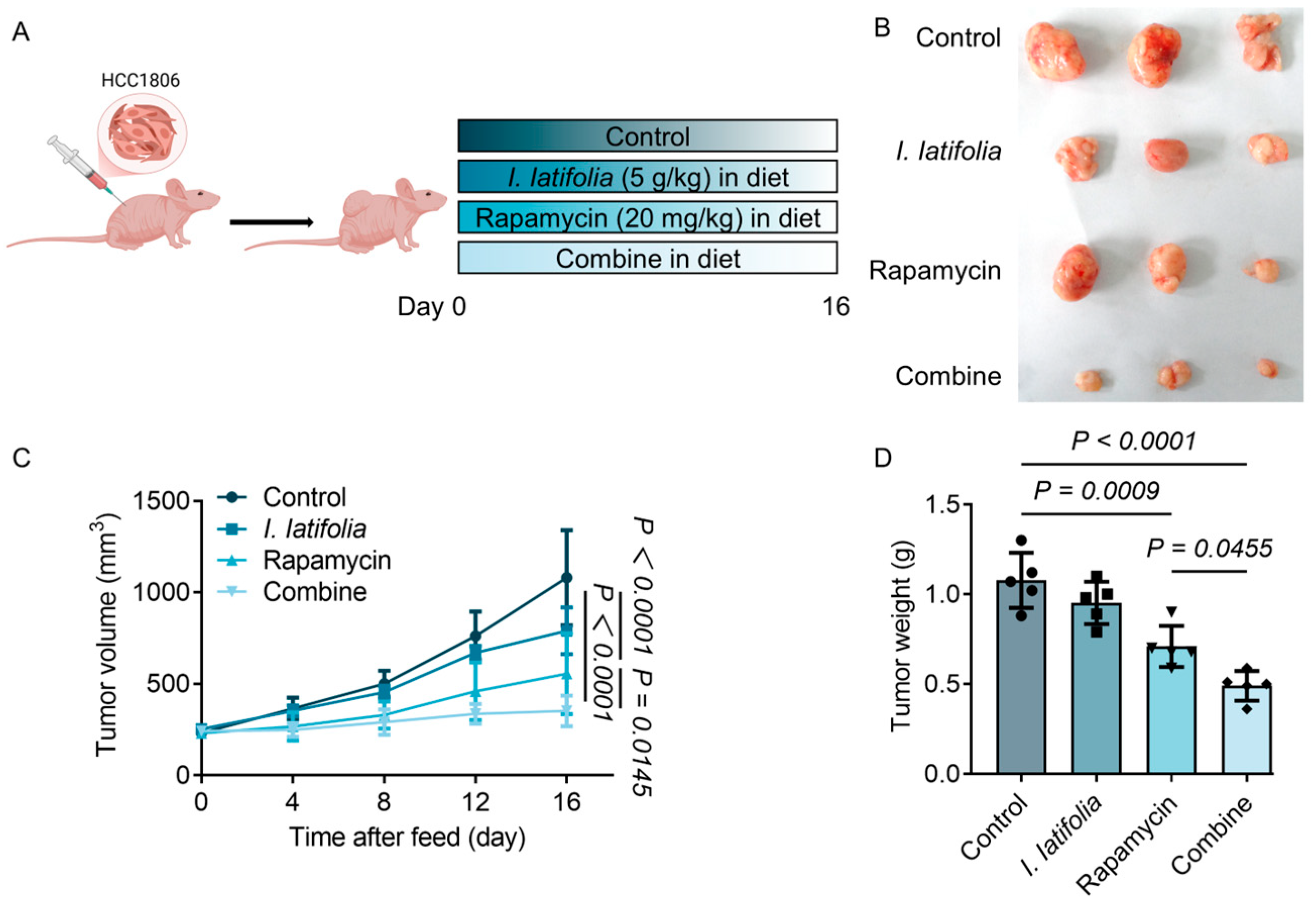
3.6. Combined Treatment of Ilex latifolia and Rapamycin Inhibits Breast Cancer Cell Growth in Mice
3.7. Ilex latifolia Enhances the Anti-Tumor Effect of Rapamycin in Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | One-way analysis of variance |
| ER | Estrogen receptor |
| HDL-C | High-density lipoprotein cholesterol |
| HER2 | Human epidermal growth factor receptor 2 |
| I. latifolia | Ilex latifolia |
| LDL-C | Low-density lipoprotein cholesterol |
| L. robustum | Ligustrum robustum |
| mTOR | Mammalian target of rapamycin |
| PR | Progesterone receptor |
| SD | Standard deviation |
| TC | Total cholesterol |
| TG | Triglycerides |
| TNBC | Triple-negative breast cancer |
References
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Zagami, P.; Carey, L.A. Triple negative breast cancer: Pitfalls and progress. npj Breast Cancer 2022, 8, 95. [Google Scholar] [CrossRef]
- Zardavas, D.; Irrthum, A.; Swanton, C.; Piccart, M. Clinical management of breast cancer heterogeneity. Nat. Rev. Clin. Oncol. 2015, 12, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Dash, R. Natural Products for the Management and Prevention of Breast Cancer. Evid. Based Complement. Altern. Med. 2018, 2018, 8324696. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Meng, X.; Zhang, J.-J.; Li, H.-B. Dietary Natural Products for Prevention and Treatment of Breast Cancer. Nutrients 2017, 9, 728. [Google Scholar] [CrossRef]
- Seto, B. Rapamycin and mTOR: A serendipitous discovery and implications for breast cancer. Clin. Transl. Med. 2012, 1, 29. [Google Scholar] [CrossRef]
- Li, J.; Kim, S.G.; Blenis, J. Rapamycin: One drug, many effects. Cell Metab. 2014, 19, 373–379. [Google Scholar] [CrossRef]
- Yu, F.Y.; Zheng, K.; Wu, Y.F.; Gao, S.-W.; Weng, Q.-Y.; Zhu, C.; Wu, Y.-P.; Li, M.; Qin, Z.-N.; Lou, J.-F.; et al. Rapamycin Exacerbates Staphylococcus aureus Pneumonia by Inhibiting mTOR-RPS6 in Macrophages. J. Inflamm. Res. 2023, 16, 5715–5728. [Google Scholar] [CrossRef]
- Sun, C.Y.; Li, Y.Z.; Cao, D.; Zhou, Y.-F.; Zhang, M.-Y.; Wang, H.-Y. Rapamycin and trametinib: A rational combination for treatment of NSCLC. Int. J. Biol. Sci. 2021, 17, 3211–3223. [Google Scholar] [CrossRef]
- Jobu, Y.; Nishigawa, M.; Furihata, K.; Uchida, K.; Taniuchi, K. Inhibitory effects of the combination of rapamycin with gemcitabine plus paclitaxel on the growth of pancreatic cancer tumors. Hum. Cell 2025, 38, 44. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gong, W.; Hua, Z.; Chen, B.; Zhao, G.; Liu, Z.; Thiele, C.J.; Li, Z. Combination of Rapamycin and MK-2206 Induced Cell Death via Autophagy and Necroptosis in MYCN-Amplified Neuroblastoma Cell Lines. Front. Pharmacol. 2020, 11, 31. [Google Scholar] [CrossRef]
- Li, L.; Xu, L.J.; Ma, G.Z.; Dong, Y.M.; Peng, Y.; Xiao, P.G. The large-leaved Kudingcha (Ilex latifolia Thunb and Ilex kudingcha C.J. Tseng): A traditional Chinese tea with plentiful secondary metabolites and potential biological activities. J. Nat. Med. 2013, 67, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, R.; Chen, Y.; Wang, L.; Zhou, S.; Wang, H. Discovery of an EGFR tyrosine kinase inhibitor from Ilex latifolia in breast cancer therapy. Bioorg. Med. Chem. Lett. 2019, 29, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, Y.-L.; Yu, Y.; Zang, J.; Wu, Y.; He, Z. Ilex latifolia Thunb protects mice from HFD-induced body weight gain. Sci. Rep. 2017, 7, 14660. [Google Scholar] [CrossRef]
- Feng, R.-B.; Fan, C.-L.; Liu, Q.; Liu, Z.; Zhang, W.; Li, Y.-L.; Tang, W.; Wang, Y.; Li, M.-M.; Ye, W.-C. Crude triterpenoid saponins from Ilex latifolia (Da Ye Dong Qing) ameliorate lipid accumulation by inhibiting SREBP expression via activation of AMPK in a non-alcoholic fatty liver disease model. Chin. Med. 2015, 10, 23. [Google Scholar] [CrossRef]
- Lau, K.-M.; He, Z.-D.; Dong, H.; Fung, K.-P.; But, P.P.-H. Anti-oxidative, anti-inflammatory and hepato-protective effects of Ligustrum robustum. J. Ethnopharmacol. 2002, 83, 63–71. [Google Scholar] [CrossRef]
- Yi, F.; Zhao, X.-L.; Peng, Y.; Xiao, P.G. Genus llex L.: Phytochemistry, Ethnopharmacology, and Pharmacology. Chin. Herb. Med. 2016, 8, 209–230. [Google Scholar] [CrossRef]
- Hu, T.; He, X.-W.; Jiang, J.-G. Functional Analyses on Antioxidant, Anti-inflammatory, and Antiproliferative Effects of Extracts and Compounds from Ilex latifolia Thunb., a Chinese Bitter Tea. J. Agric. Food Chem. 2014, 62, 8608–8615. [Google Scholar] [CrossRef]
- Chen, J.; Du, Y.; Long, Y.; Tao, D.; Hu, M.; Jiang, Y.; Wan, Y.; Yang, D. Polyphenols in Ilex latifolia Thunb. inhibit human lung cancer cell line A549 by regulation of the PI3K-Akt signaling pathway. BMC Complement. Med. Ther. 2022, 22, 85. [Google Scholar] [CrossRef]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010, 32, 35–48. [Google Scholar] [CrossRef]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef]
- Yellen, P.; Saqcena, M.; Salloum, D.; Feng, J.; Preda, A.; Xu, L.; Rodrik-Outmezguine, V.; Foster, D.A. High-dose rapamycin induces apoptosis in human cancer cells by dissociating mTOR complex 1 and suppressing phosphorylation of 4E-BP1. Cell Cycle 2011, 10, 3948–3956. [Google Scholar] [CrossRef]
- Percie Du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Chang, S.B.; Miron, P.; Miron, A.; Iglehart, J.D. Rapamycin Inhibits Proliferation of Estrogen-Receptor-Positive Breast Cancer Cells. J. Surg. Res. 2007, 138, 37–44. [Google Scholar] [CrossRef]
- Steelman, L.S.; Martelli, A.M.; Cocco, L.; Libra, M.; Nicoletti, F.; Abrams, S.L.; McCubrey, J.A. The therapeutic potential of mTOR inhibitors in breast cancer. Br. J. Clin. Pharmacol. 2016, 82, 1189–1212. [Google Scholar] [CrossRef] [PubMed]
- Al-Astani Tengku Din, T.A.; Shamsuddin, S.H.; Idris, F.M.; Mansor, W.N.A.W.; Jalal, M.I.A.; Jaafar, H. Rapamycin and PF4 induce apoptosis by upregulating Bax and down-regulating survivin in MNU-induced breast cancer. Asian Pac. J. Cancer Prev. 2014, 15, 3939–3944. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Liang, Y.; Mira, R.R.; Lu, Y.; Gu, J.; Zheng, Q. Mammalian target of rapamycin (mTOR) inhibitors and combined chemotherapy in breast cancer: A meta-analysis of randomized controlled trials. Int. J. Clin. Exp. Med. 2014, 7, 3333–3343. [Google Scholar]
- Alayev, A.; Berger, S.M.; Kramer, M.Y.; Schwartz, N.S.; Holz, M.K. The combination of rapamycin and resveratrol blocks autophagy and induces apoptosis in breast cancer cells. J. Cell. Biochem. 2015, 116, 450–457. [Google Scholar] [CrossRef]
- Guo, N.; Yan, A.; Gao, X.; Chen, Y.; He, X.; Hu, Z.; Mi, M.; Tang, X.; Gou, X. Berberine sensitizes rapamycin-mediated human hepatoma cell death in vitro. Mol Med Rep. 2014, 10, 3132–3138. [Google Scholar] [CrossRef]
- Wüpper, S.; Lüersen, K.; Rimbach, G. Chemical Composition, Bioactivity and Safety Aspects of Kuding Tea-From Beverage to Herbal Extract. Nutrients 2020, 12, 2796. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Bailly, C.; Vergoten, G. Esculentosides: Insights into the potential health benefits, mechanisms of action and molecular targets. Phytomedicine 2020, 79, 153343. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Anthraquinone Production from Cell and Organ Cultures of Rubia Species: An Overview. Metabolites 2022, 13, 39. [Google Scholar] [CrossRef]
- Shin, S.; Fu, J.; Shin, W.K.; Huang, D.; Min, S.; Kang, D. Association of food groups and dietary pattern with breast cancer risk: A systematic review and meta-analysis. Clin. Nutr. 2023, 42, 282–297. [Google Scholar] [CrossRef]
- De Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Nutrition and Breast Cancer: A Literature Review on Prevention, Treatment and Recurrence. Nutrients 2019, 11, 1514. [Google Scholar] [CrossRef]
- Martin, L.J.; Li, Q.; Melnichouk, O.; Greenberg, C.; Minkin, S.; Hislop, G.; Boyd, N.F. A Randomized Trial of Dietary Intervention for Breast Cancer Prevention. Cancer Res. 2011, 71, 123–133. [Google Scholar] [CrossRef]
- Aune, D.; Chan, D.S.; Vieira, A.R.; Rosenblatt, D.A.N.; Vieira, R.; Greenwood, D.C.; Norat, T. Fruits, vegetables and breast cancer risk: A systematic review and meta-analysis of prospective studies. Breast Cancer Res. Treat. 2012, 134, 479–493. [Google Scholar] [CrossRef]
- Xiao, Y.; Ke, Y.; Wu, S.; Huang, S.; Li, S.; Lv, Z.; Yeoh, E.-K.; Lao, X.; Wong, S.; Kim, J.H.; et al. Association between whole grain intake and breast cancer risk: A systematic review and meta-analysis of observational studies. Nutr. J. 2018, 17, 87. [Google Scholar] [CrossRef] [PubMed]
- Losada-Echeberría, M.; Herranz-López, M.; Micol, V.; Barrajón-Catalán, E. Polyphenols as Promising Drugs against Main Breast Cancer Signatures. Antioxidants 2017, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Kim, Y.; Ha, S.E.; Kim, H.H.; Bhosale, P.B.; Abusaliya, A.; Jeong, S.H.; Kim, G.S. Function and Application of Flavonoids in the Breast Cancer. Int. J. Mol. Sci. 2022, 23, 7732. [Google Scholar] [CrossRef] [PubMed]
- Fabian, C.J.; Kimler, B.F.; Hursting, S.D. Omega-3 fatty acids for breast cancer prevention and survivorship. Breast Cancer Res. 2015, 17, 62. [Google Scholar] [CrossRef]


| Gene | Species | Forward | Reverse |
|---|---|---|---|
| BAX | Human | 5′-CCCGAGAGGTCTTTTTCCGAG-3′ | 5′-CCAGCCCATGATGGTTCTGAT-3′ |
| BCL2 | Human | 5′-GGTGGGGTCATGTGTGTGG-3′ | 5′-CGGTTCAGGTACTCAGTCATCC-3′ |
| P53 | Human | 5′-CAGCACATGACGGAGGTTGT-3′ | 5′-TCATCCAAATACTCCACACGC-3′ |
| IL6 | Human | 5′-ACTCACCTCTTCAGAACGAATTG-3′ | 5′-CCATCTTTGGAAGGTTCAGGTTG-3′ |
| IL1B | Human | 5′-ATGATGGCTTATTACAGTGGCAA-3′ | 5′-GTCGGAGATTCGTAGCTGGA-3′ |
| NFKB1 | Human | 5′-AACAGAGAGGATTTCGTTTCCG-3′ | 5′-TTTGACCTGAGGGTAAGACTTCT-3′ |
| ACTB | Human | 5′-CATGTACGTTGCTATCCAGGC-3′ | 5′-CTCCTTAATGTCACGCACGAT-3′ |
| Bax | Mouse | 5′-TGAAGACAGGGGCCTTTTTG-3′ | 5′-AATTCGCCGGAGACACTCG-3′ |
| Bcl2 | Mouse | 5′-GTCGCTACCGTCGTGACTTC-3′ | 5′-CAGACATGCACCTACCCAGC-3′ |
| Il6 | Mouse | 5′-TAGTCCTTCCTACCCCAATTTCC-3′ | 5′-TTGGTCCTTAGCCACTCCTTC-3′ |
| Il1b | Mouse | 5′-GCAACTGTTCCTGAACTCAACT-3′ | 5′-ATCTTTTGGGGTCCGTCAACT-3′ |
| Actb | Mouse | 5′-AATCCCATCACCATCTTCCA-3′ | 5′-TGGACTCCACGACGTACTCA-3′ |
| Constituents Class | Representative Subclass | Relative Content (%) |
|---|---|---|
| Saponins | Triterpenoid saponins | 46.89349 |
| Polyphenols | Phenolic acids | 45.96911 |
| Alkaloids | Pyrrolizidine alkaloids | 2.914044 |
| Terpenoids | Sesquiterpenes | 1.920293 |
| Organic acids | Citric acid derivatives | 1.270804 |
| Lipids | Glyceride | 0.630561 |
| Aromatic compounds | Benzene compounds | 0.170388 |
| Carbohydrates | Polysaccharides | 0.117496 |
| Steroids | Phytosterols | 0.04531 |
| Glycosides | Cyanogenic glycosides | 0.03653 |
| Amino acids | Essential amino acids | 0.016108 |
| Ketones | Quinones | 0.009137 |
| Alcohols | Aliphatic Alcohols | 0.006725 |
| No | Component Name | tR (min) | Formula | Molecular Ion (m/z) | Peak Area | Ion Mode | Relative Content (%) |
|---|---|---|---|---|---|---|---|
| 1 | 1,4-dicaffeoylquinic acid | 7.41 | C25H24O12 | 515.1198 | 13,226,556 | [M−H]− | 17.17072603 |
| 2 | 1-caffeoylquinic acid | 5.08 | C16H18O9 | 353.0884 | 8,470,651 | [M−H]− | 10.99660619 |
| 3 | esculentoside L | 11.70 | C48H76O20 | 971.4829 | 6,761,731 | [M−H]− | 8.778084822 |
| 4 | ruberythric acid | 6.08 | C25H26O13 | 533.1303 | 4,749,673 | [M−H]− | 6.166029449 |
| 5 | cynanoside O | 12.82 | C48H74O19 | 953.4716 | 4,566,480 | [M−H]− | 5.928208144 |
| 6 | marsdenoside H | 13.73 | C48H76O19 | 955.4881 | 3,383,373 | [M−H]− | 4.39229765 |
| 7 | clinodiside A | 12.04 | C48H78O19 | 957.5033 | 2,835,076 | [M−H]− | 3.680498027 |
| 8 | clinodiside B | 13.41 | C54H88O23 | 1103.5600 | 2,428,726 | [M−H]− | 3.152974118 |
| 9 | nelumboroside A | 6.83 | C27H30O16 | 609.1455 | 1,692,296 | [M−H]− | 2.196940078 |
| 10 | soyasaponin I | 14.33 | C48H78O18 | 941.5083 | 1,652,682 | [M−H]− | 2.14551315 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.; Wu, Y.; Guo, X.; Tian, H.; Ou, H.; Xiong, Z.; Xiao, Y.; Xiao, L.; Li, J.; Wu, H.; et al. Ilex latifolia Improves the Anti-Tumor Effectiveness of Rapamycin Against Breast Cancer In Vitro and In Vivo. Foods 2025, 14, 1477. https://doi.org/10.3390/foods14091477
Ren Z, Wu Y, Guo X, Tian H, Ou H, Xiong Z, Xiao Y, Xiao L, Li J, Wu H, et al. Ilex latifolia Improves the Anti-Tumor Effectiveness of Rapamycin Against Breast Cancer In Vitro and In Vivo. Foods. 2025; 14(9):1477. https://doi.org/10.3390/foods14091477
Chicago/Turabian StyleRen, Zhengnan, Yikuan Wu, Xiaoying Guo, Haizhi Tian, Hongjing Ou, Zihan Xiong, Yu Xiao, Longquan Xiao, Jing Li, Haibo Wu, and et al. 2025. "Ilex latifolia Improves the Anti-Tumor Effectiveness of Rapamycin Against Breast Cancer In Vitro and In Vivo" Foods 14, no. 9: 1477. https://doi.org/10.3390/foods14091477
APA StyleRen, Z., Wu, Y., Guo, X., Tian, H., Ou, H., Xiong, Z., Xiao, Y., Xiao, L., Li, J., Wu, H., & Wang, X. (2025). Ilex latifolia Improves the Anti-Tumor Effectiveness of Rapamycin Against Breast Cancer In Vitro and In Vivo. Foods, 14(9), 1477. https://doi.org/10.3390/foods14091477







