Preparation and Encapsulation of DPP-IV Inhibitory Peptides: Challenges and Strategies for Functional Food Development
Abstract
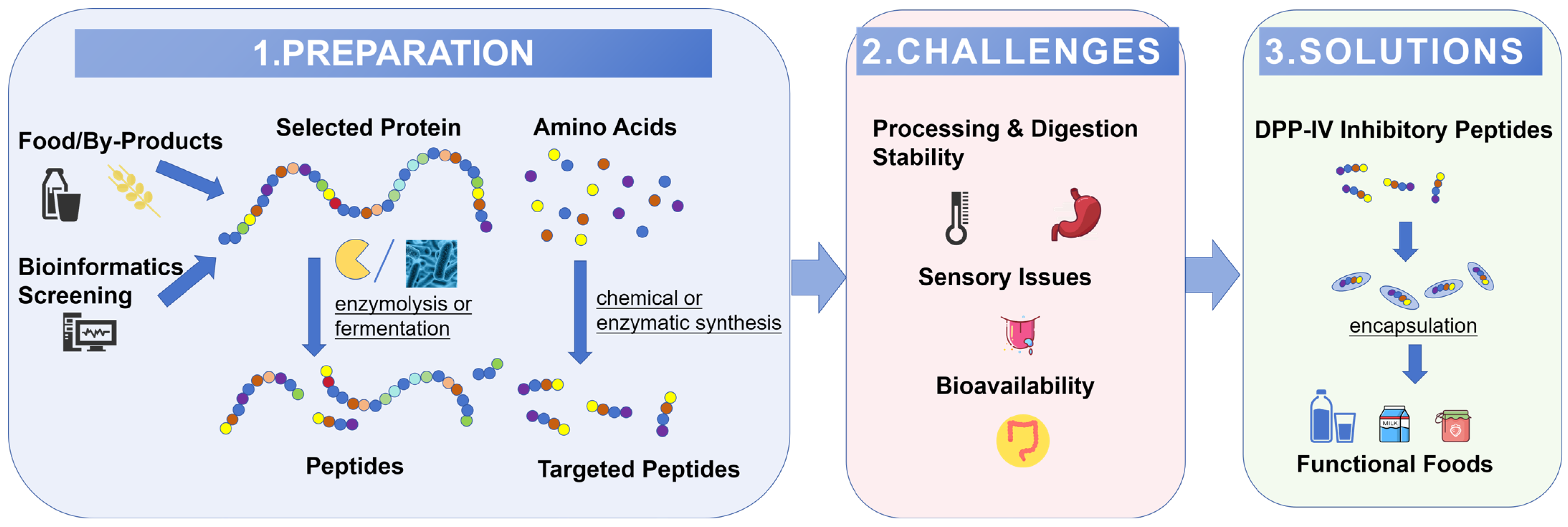
:1. Introduction
2. Strategies for Efficient Peptide Production
2.1. Selection of Protein Substrates for DPP-IV Inhibitory Peptides
2.1.1. Bioinformatics in DPP-IV Inhibitory Peptides Substrate Evaluation
2.1.2. Utilizing Protein-Rich Processing By-Products as Substrates
2.2. The Preparation of DPP-IV Inhibitory Peptides
2.2.1. Enzymatic Hydrolysis
2.2.2. Fermentation Technique
2.2.3. Peptide Synthesis: A Tool for Validation and Specialized Production
3. The Challenges of Application of DPP-IV Inhibitory Peptides in Food
3.1. The Impact on Food Sensory Perception
3.2. Processing Stability
3.3. Digestive Stability
3.4. Safety Considerations
4. The Delivery System of DPP-IV Inhibitory Peptides
4.1. The Significance and Ideal Characteristics of the Delivery System
4.2. Common Delivery System Types and Their Effects on DPP-IV Inhibitory Peptides
| Source of DPP-IV Inhibitory Peptides | Carrier Type | Encapsulation Materials | Preparation Approaches | Property Characterization | Stability and Efficacy | References |
|---|---|---|---|---|---|---|
| α-lactalbumin hydrolysates | W1/O/W2 double emulsion | Polyglycerol polyricinoleate in rice bran oil and pectin | Homogenization |
|
| [80] |
| Brewer’s spent grain hydrolysate | Microcapsules | Carrageenan, agar, maltodextrin | Spray drying |
|
| [87] |
| Lupin peptide and soybean peptide | Nanogels | An ionic self-assembling peptide RADA16 | Solvent-triggered co-assembly |
|
| [85] |
| Spirulina protein hydrolysates | Microcapsules | Alginate and chitosan | Extrusion |
|
| [88] |
| Tenebrio molitor hydrolysate | Nano-microcapsules | Arabic gum, pullulan, Tween 20 | Electrospraying, spray-drying |
|
| [89] |
| Hempseed protein hydrolysates | Hydrogel | RADA16 self-assembling peptide | Self-assembly |
|
| [86] |
| Collagen peptides | Liposomes | Lecithin from soybean, sodium alginate | Coacervation coating |
|
| [75] |
| Phaseolus lunatus seed protein hydrolysates | Microcapsules | Maltodextrin and gum arabic | Spray drying |
|
| [90] |
| Brewer’s spent grain-hydrolysate | Microcapsules | Agar and maltodextrin | Spray drying |
|
| [91] |
| Goat milk whey protein peptide (MW < 3 kDa) | Liposomes (GWP-LS) and niosomes (GWP-NS). | Lecithin, phytosterols (β-sitosterol, ergosterol, stigmasterol, mixed phytosterols) | Ethanol injection, stirring, and rotary evaporation |
|
| [92] |
| Gallic acid-Antarctic krill peptide copolymer | Nanocapsules | Polylactic acid-hydroxyacetic acid | Complex emulsion method, high-pressure microjet |
|
| [81] |
| Antarctic krill peptide | Composite nanoparticles | Bovine serum albumin and chitosan | Ionotropic gelation and coacervation |
|
| [83] |
| Rapeseed-derived cruciferin peptide (RCPP) and napin peptide (RNPP) | Nanoparticles | Chitosan and sodium alginate | Three-channel device |
|
| [84] |
| Low MW peptide fraction from a shrimp hydrolysate | Nanoliposomes | Partially purified soy phosphatidylcholine | Stirring and ultrasonication |
|
| [82] |
| Whey protein hydrolysate | Micro-hydrogels | Chitosan and gelatin | Spray drying | (Not explicitly mentioned in the document) |
| [93] |
| Rapeseed peptides | Nanogel | RADA16 | Self-assembly gelation |
|
| [94] |
4.3. Selection and Optimization of the Delivery System
5. Prospects
5.1. The Influence of Encapsulation on Food Sensory Perception
5.2. Safety
5.3. Co-Encapsulation
5.4. Targeted Delivery
5.5. Scalable Approaches
5.6. The Application Effect in Food
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DPP-IV | Dipeptidyl peptidase IV |
| GLP-1 | Glucagon-like peptide-1 |
| SGID | Simulated gastrointestinal digestion |
| LC | Loading capacity |
| EE | Encapsulation efficiency |
| MW | Molecular weight |
| PDI | Polydispersity index |
References
- Nong, N.T.P.; Hsu, J.-L. Characteristics of Food Protein-Derived Antidiabetic Bioactive Peptides: A Literature Update. Int. J. Mol. Sci. 2021, 22, 9508. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Cheng, J.; Wu, H. Discovery of Food-Derived Dipeptidyl Peptidase IV Inhibitory Peptides: A Review. Int. J. Mol. Sci. 2019, 20, 463. [Google Scholar] [CrossRef] [PubMed]
- Drummond, E.; Flynn, S.; Whelan, H.; Nongonierma, A.B.; Holton, T.A.; Robinson, A.; Egan, T.; Cagney, G.; Shields, D.C.; Gibney, E.R.; et al. Casein Hydrolysate with Glycemic Control Properties: Evidence from Cells, Animal Models, and Humans. J. Agric. Food Chem. 2018, 66, 4352–4363. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Wang, R.; Cheng, C.; Ma, Y.; Zhang, Y.; Lu, W. Preparation, Structural Properties, and in Vitro and in Vivo Activities of Peptides against Dipeptidyl Peptidase IV (DPP-IV) and α-Glucosidase: A General Review. Crit. Rev. Food Sci. Nutr. 2023, 64, 9844–9858. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kamata, A.; Nishimura, M.; Nishihira, J. Effect of One-Week Administration of Dipeptidyl Peptidase-IV Inhibitory Peptides from Chum Salmon Milt on Postprandial Blood Glucose Level: A Randomised, Placebo-Controlled, Double-Blind, Crossover, Pilot Clinical Trial. Food Funct. 2021, 12, 8544–8551. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Features of Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Peptides from Dietary Proteins. J. Food Biochem. 2019, 43, e12451. [Google Scholar] [CrossRef]
- Agyei, D.; Tsopmo, A.; Udenigwe, C.C. Bioinformatics and Peptidomics Approaches to the Discovery and Analysis of Food-Derived Bioactive Peptides. Anal. Bioanal. Chem. 2018, 410, 3463–3472. [Google Scholar] [CrossRef]
- Li, C.; Qi, R.; Yuan, J.; Han, L.; Wang, S.; Li, W.; Han, W. In Silico Study to Predict Potential Precursors of Human Dipeptidyl Peptidase-IV Inhibitors from Hazelnut. J. Biomol. Struct. Dyn. 2022, 40, 11664–11675. [Google Scholar] [CrossRef]
- Han, R. Potential Biological Properties of Peptides Derived from Oilseed Proteins. Ph.D. Thesis, University of Leeds, Leeds, UK, 2021. [Google Scholar]
- Du, Z.; Ding, X.; Xu, Y.; Li, Y. UniDL4BioPep: A Universal Deep Learning Architecture for Binary Classification in Peptide Bioactivity. Brief. Bioinform. 2023, 24, bbad135. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Zhu, R.; Zhang, H.; Li, D.; Li, H.; Tang, H.; Chen, L.; Peng, X.; Xu, X.; et al. Mechanistic Study of Novel Dipeptidyl Peptidase IV Inhibitory Peptides from Goat’s Milk Based on Peptidomics and In Silico Analysis. Foods 2024, 13, 1194. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, L.; Wu, G.; Liu, T.; Qi, X.; Zhang, H. Food-Derived Dipeptidyl Peptidase IV Inhibitory Peptides: Production, Identification, Structure-Activity Relationship, and Their Potential Role in Glycemic Regulation. Crit. Rev. Food Sci. Nutr. 2022, 64, 2053–2075. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Le Maux, S.; Hamayon, J.; FitzGerald, R.J. Strategies for the Release of Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Peptides in an Enzymatic Hydrolyzate of α-Lactalbumin. Food Funct. 2016, 7, 3437–3443. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Z.; Ma, M.; Huang, X.; Guyonnet, V.; Xiong, H. Exploration of Novel DPP-IV Inhibitory Peptides from Discarded Eggshell Membrane: An Integrated in Silico and in Vitro Study. Food Biosci. 2024, 59, 104036. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Santivarangkna, C.; Rajput, M.S.; Benjakul, S.; Maqsood, S. Valorization of Fish Byproducts: Sources to End-Product Applications of Bioactive Protein Hydrolysate. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1803–1842. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zheng, L.; Huang, M.; Zhao, M. Exploring Structural Features of Potent Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Peptides Derived from Tilapia (Oreochromis niloticus) Skin Gelatin by an Integrated Approach of Multivariate Analysis and Gly-Pro-Based Peptide Library. Food Chem. 2022, 397, 133821. [Google Scholar] [CrossRef]
- Harnedy-Rothwell, P.A.; McLaughlin, C.M.; O’Keeffe, M.B.; Le Gouic, A.V.; Allsopp, P.J.; McSorley, E.M.; Sharkey, S.; Whooley, J.; McGovern, B.; O’Harte, F.P.M.; et al. Identification and Characterisation of Peptides from a Boarfish (Capros aper) Protein Hydrolysate Displaying in Vitro Dipeptidyl Peptidase-IV (DPP-IV) Inhibitory and Insulinotropic Activity. Food Res. Int. 2020, 131, 108989. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Yang, W.; Li, X.; Qi, D.; Chen, H.; Liu, H.; Yu, S.; Pan, Y.; Liu, Y.; et al. Identification, Screening, and Comprehensive Evaluation of Novel DPP-IV Inhibitory Peptides from the Tilapia Skin Gelatin Hydrolysate Produced Using Ginger Protease. Biomolecules 2022, 12, 1866. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, Z.; Yokoyama, W.; Chiou, B.-S.; Chen, M.; Liu, F.; Zhong, F. Collagen Peptides with DPP-IV Inhibitory Activity from Sheep Skin and Their Stability to in Vitro Gastrointestinal Digestion. Food Biosci. 2021, 42, 101161. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Cadamuro, C.; Le Gouic, A.; Mudgil, P.; Maqsood, S.; FitzGerald, R.J. Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Properties of a Camel Whey Protein Enriched Hydrolysate Preparation. Food Chem. 2019, 279, 70–79. [Google Scholar] [CrossRef]
- Carrera-Alvarado, G.; Toldrá, F.; Mora, L. DPP-IV Inhibitory Peptides GPF, IGL, and GGGW Obtained from Chicken Blood Hydrolysates. Int. J. Mol. Sci. 2022, 23, 14140. [Google Scholar] [CrossRef]
- Chaipoot, S.; Punfa, W.; Ounjaijean, S.; Phongphisutthinant, R.; Kulprachakarn, K.; Parklak, W.; Phaworn, L.; Rotphet, P.; Boonyapranai, K. Antioxidant, Anti-Diabetic, Anti-Obesity, and Antihypertensive Properties of Protein Hydrolysate and Peptide Fractions from Black Sesame Cake. Molecules 2023, 28, 211. [Google Scholar] [CrossRef] [PubMed]
- Göksu, A.G.; Çakır, B.; Gülseren, İ. Sequence Alterations Affect the Antidiabetic Attributes of Hazelnut Peptide Fractions during the Industrial Manufacture and Simulated Digestion of Hazelnut Paste. J. Food Sci. Technol. 2023, 60, 171–180. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, L.; Song, W.; Zhang, C.; Hua, Y.; Chen, Y.; Li, X. Separation, Identification and Molecular Binding Mechanism of Dipeptidyl Peptidase IV Inhibitory Peptides Derived from Walnut (Juglans regia L.) Protein. Food Chem. 2021, 347, 129062. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gálvez, R.; Berraquero-García, C.; Ospina-Quiroga, J.L.; Espejo-Carpio, F.J.; Almécija, M.C.; Guadix, A.; García-Moreno, P.J.; Guadix, E. Influence of In Vitro Digestion on Dipeptidyl Peptidase-IV (DPP-IV) Inhibitory Activity of Plant-Protein Hydrolysates Obtained from Agro-Industrial By-Products. Foods 2024, 13, 2691. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Pradhan, R.; Manickavasagan, A.; Thimmanagari, M.; Dutta, A. Corn Distillers Solubles as a Novel Bioresource of Bioactive Peptides with ACE and DPP IV Inhibition Activity: Characterization, in Silico Evaluation, and Molecular Docking. Food Funct. 2022, 13, 8179–8203. [Google Scholar] [CrossRef]
- Pooja, K.; Rani, S.; Kanwate, B.; Pal, G.K. Physico-Chemical, Sensory and Toxicity Characteristics of Dipeptidyl Peptidase-IV Inhibitory Peptides from Rice Bran-Derived Globulin Using Computational Approaches. Int. J. Pept. Res. Ther. 2017, 23, 519–529. [Google Scholar] [CrossRef]
- Isidro-Llobet, A.; Kenworthy, M.N.; Mukherjee, S.; Kopach, M.E.; Wegner, K.; Gallou, F.; Smith, A.G.; Roschangar, F. Sustainability Challenges in Peptide Synthesis and Purification: From R&D to Production. J. Org. Chem. 2019, 84, 4615–4628. [Google Scholar] [CrossRef]
- Mu, X.; Wang, R.; Cheng, C.; Ma, Y.; Li, Q. Two Novel Peptides Derived from Oat with Inhibitory Activity against Dipeptidyl Peptidase-IV: The Related Mechanism Revealed by Molecular Docking and in Vitro and in Situ Effects. J. Food Meas. Charact. 2024, 18, 3087–3099. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Paolella, S.; Mudgil, P.; Maqsood, S.; FitzGerald, R.J. Identification of Novel Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Peptides in Camel Milk Protein Hydrolysates. Food Chem. 2018, 244, 340–348. [Google Scholar] [CrossRef]
- Zhou, L.; Xiao, C.; Gao, J.; Zhao, M.; Li, X.-G.; Mora, L.; Toldrá, F. Preparation and Identification of Novel DPP-IV Inhibitory Peptides from Musculus senhousei: Peptidomic Analysis, Molecular Simulation, and Validation. Food Biosci. 2024, 59, 103832. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Wang, S.; He, R.; Wang, Z.; Zhao, L.; Ge, W. Preparation, Characterization, and Mechanism of DPP-IV Inhibitory Peptides Derived from Bactrian Camel Milk. Int. J. Biol. Macromol. 2024, 277, 134232. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, X.; Wang, Y.; Luo, J.; Zhao, Y.; Han, G.; Han, L.; Yu, Q. Production and Identification of Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Peptides from Discarded Cowhide Collagen. Food Chem. 2023, 405, 134793. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Z.; Feng, Y.; Yu, S.; Li, Z.; Zhang, D.; Wang, C. DPP-IV Inhibitory Peptides from Coix Seed Prolamins: Release, Identification, and Analysis of the Interaction between Key Residues and Enzyme Domains. J. Agric. Food Chem. 2023, 71, 14575–14592. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Jiang, C.; Wang, S.; Jing, H.; Mo, L.; Ma, C.; Wang, H. Preparation, Identification, and Inhibitory Mechanism of Dipeptidyl Peptidase IV Inhibitory Peptides from Goat Milk Whey Protein. J. Food Sci. 2023, 88, 3577–3593. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, C.; Li, L.; Yang, X.; Chen, S.; Wu, Y.; Feng, Y. Novel Insight into the Formation and Inhibition Mechanism of Dipeptidyl Peptidase-IV Inhibitory Peptides from Fermented Mandarin Fish (Chouguiyu). Food Sci. Hum. Wellness 2023, 12, 2408–2416. [Google Scholar] [CrossRef]
- Mudgil, P.; Gan, C.-Y.; Yap, P.-G.; Redha, A.A.; Alsaadi, R.H.S.; Mohteshamuddin, K.; Aguilar-Toalá, J.E.; Vidal-Limon, A.M.; Liceaga, A.M.; Maqsood, S. Exploring the Dipeptidyl Peptidase-IV Inhibitory Potential of Probiotic-Fermented Milk: An in Vitro and in Silico Comprehensive Investigation into Peptides from Milk of Different Farm Animals. J. Dairy Sci. 2024, 107, 10153–10173. [Google Scholar] [CrossRef]
- Helal, A.; Pierri, S.; Tagliazucchi, D.; Solieri, L. Effect of Fermentation with Streptococcus Thermophilus Strains on In Vitro Gastro-Intestinal Digestion of Whey Protein Concentrates. Microorganisms 2023, 11, 1742. [Google Scholar] [CrossRef]
- Simsek, S. Angiotensin I-Converting Enzyme, Dipeptidyl Peptidase-IV, and α-Glucosidase Inhibitory Potential of Hazelnut Meal Protein Hydrolysates. Food Meas. 2021, 15, 4490–4496. [Google Scholar] [CrossRef]
- Pacheco, A.F.C.; Pacheco, F.C.; Cunha, J.S.; Nalon, G.A.; Gusmão, J.V.F.; dos Santos, F.R.; Andressa, I.; Paiva, P.H.C.; Tribst, A.A.L.; Leite Junior, B.R.d.C. Physicochemical Properties and In Vitro Antioxidant Activity Characterization of Protein Hydrolysates Obtained from Pumpkin Seeds Using Conventional and Ultrasound-Assisted Enzymatic Hydrolysis. Foods 2025, 14, 782. [Google Scholar] [CrossRef]
- Boukil, A.; Perreault, V.; Chamberland, J.; Mezdour, S.; Pouliot, Y.; Doyen, A. High Hydrostatic Pressure-Assisted Enzymatic Hydrolysis Affect Mealworm Allergenic Proteins. Molecules 2020, 25, 2685. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, K.; Liu, X.; Zhang, X. Isolation and identification of dipeptidyl peptidase-IV inhibitory peptides from Sacha inchi meal. J. Sci. Food Agric. 2023, 103, 2926–2938. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Toldrá, F. Advanced Enzymatic Hydrolysis of Food Proteins for the Production of Bioactive Peptides. Curr. Opin. Food Sci. 2023, 49, 100973. [Google Scholar] [CrossRef]
- Hall, F.; Liceaga, A. Effect of Microwave-Assisted Enzymatic Hydrolysis of Cricket (Gryllodes sigillatus) Protein on ACE and DPP-IV Inhibition and Tropomyosin-IgG Binding. J. Funct. Foods 2020, 64, 103634. [Google Scholar] [CrossRef]
- Zhao, R.; Lu, S.; Li, S.; Shen, H.; Wang, Y.; Gao, Y.; Shen, X.; Wang, F.; Wu, J.; Liu, W.; et al. Enzymatic Preparation and Processing Properties of DPP-IV Inhibitory Peptides Derived from Wheat Gluten: Effects of Pretreatment Methods and Protease Types. Foods 2024, 13, 216. [Google Scholar] [CrossRef]
- Guan, C.; Iwatani, S.; Xing, X.; Yamamoto, N. Strategic Preparations of DPP-IV Inhibitory Peptides from Val-Pro-Xaa and Ile-Pro-Xaa Peptide Mixtures. Int. J. Pept. Res. Ther. 2021, 27, 735–743. [Google Scholar] [CrossRef]
- Taga, Y.; Hayashida, O.; Kusubata, M.; Ogawa-Goto, K.; Hattori, S. Production of a Novel Wheat Gluten Hydrolysate Containing Dipeptidyl Peptidase-IV Inhibitory Tripeptides Using Ginger Protease. Biosci. Biotechnol. Biochem. 2017, 81, 1823–1828. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, L.; Udenigwe, C.C.; Lin, L.; Zhao, M. Enzymatic Characterization and Synergistic Effects of Protease Combinations on DPP-IV Inhibitory Peptide Release from Bovine Casein. Food Biosci. 2024, 60, 104276. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Maux, S.L.; Esteveny, C.; FitzGerald, R.J. Response Surface Methodology Applied to the Generation of Casein Hydrolysates with Antioxidant and Dipeptidyl Peptidase IV Inhibitory Properties. J. Sci. Food Agric. 2017, 97, 1093–1101. [Google Scholar] [CrossRef]
- Sóti, V.; Lenaerts, S.; Cornet, I. Of Enzyme Use in Cost-Effective High Solid Simultaneous Saccharification and Fermentation Processes. J. Biotechnol. 2018, 270, 70–76. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Enhancing Bioactive Peptide Release and Identification Using Targeted Enzymatic Hydrolysis of Milk Proteins. Anal. Bioanal. Chem. 2018, 410, 3407–3423. [Google Scholar] [CrossRef]
- Olvera-Rosales, L.B.; Cruz-Guerrero, A.E.; Jaimez-Ordaz, J.; Pérez-Escalante, E.; Quintero-Lira, A.; Ramírez-Moreno, E.; Contreras-López, E.; González-Olivares, L.G. Differences in the Proteolytic System of Lactic Acid Bacteria Affect the Release of DPP-IV Inhibitory Peptides from Whey Proteins. Dairy 2023, 4, 515–526. [Google Scholar] [CrossRef]
- Yang, D.; Li, C.; Li, L.; Wang, Y.; Chen, S.; Zhao, Y.; Hu, X.; Rong, H. Discovery and Functional Mechanism of Novel Dipeptidyl Peptidase IV Inhibitory Peptides from Chinese Traditional Fermented Fish (Chouguiyu). Curr. Res. Food Sci. 2022, 5, 1676–1684. [Google Scholar] [CrossRef]
- Shen, H.; Li, Y.; Song, H.; Bai, J.; Peng, N.; Ge, X.; Zhao, S. Quality Improvement of Soybean Meal by Simultaneous Microbial Fermentation and Enzymolysis and Untargeted Metabolomic Analysis of Its Metabolites. Food Biosci. 2024, 59, 104090. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, K.; Zhang, Z.; Zhang, C.; Sun, Y.; Feng, Z. Fermented Soybean Foods: A Review of Their Functional Components, Mechanism of Action and Factors Influencing Their Health Benefits. Food Res. Int. 2022, 158, 111575. [Google Scholar] [CrossRef]
- Sato, K.; Miyasaka, S.; Tsuji, A.; Tachi, H. Isolation and Characterization of Peptides with Dipeptidyl Peptidase IV (DPPIV) Inhibitory Activity from Natto Using DPPIV from Aspergillus oryzae. Food Chem. 2018, 261, 51–56. [Google Scholar] [CrossRef]
- Elisha, C.; Bhagwat, P.; Pillai, S. Emerging Production Techniques and Potential Health Promoting Properties of Plant and Animal Protein-Derived Bioactive Peptides. Crit. Rev. Food Sci. Nutr. 2024, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.; Brijesha, N.; Aparna, H.S. Discovery, Synthesis, and in Vitro Evaluation of a Novel Bioactive Peptide for ACE and DPP-IV Inhibitory Activity. Eur. J. Med. Chem. 2019, 180, 99–110. [Google Scholar] [CrossRef]
- Yang, J.; Sun-Waterhouse, D.; Cui, C.; Dong, K.; Zhao, M. γ-Glu-Met Synthesised Using a Bacterial Glutaminase as a Potential Inhibitor of Dipeptidyl Peptidase IV. Int. J. Food Sci. Technol. 2018, 53, 1166–1175. [Google Scholar] [CrossRef]
- Alyas, J.; Rafiq, A.; Amir, H.; Khan, S.U.; Sultana, T.; Ali, A.; Hameed, A.; Ahmad, I.; Kazmi, A.; Sajid, T.; et al. Human Insulin: History, Recent Advances, and Expression Systems for Mass Production. Biomed. Res. Ther. 2021, 8, 4540–4561. [Google Scholar] [CrossRef]
- De Oliveira, K.B.S.; Leite, M.L.; Rodrigues, G.R.; Duque, H.M.; da Costa, R.A.; Cunha, V.A.; Costa, L.S.d.L.; da Cunha, N.B.; Franco, O.L.; Dias, S.C. Strategies for Recombinant Production of Antimicrobial Peptides with Pharmacological Potential. Expert Rev. Clin. Pharmacol. 2020, 13, 367–390. [Google Scholar] [CrossRef]
- Ding, Y.; Ting, J.P.; Liu, J.; Al-Azzam, S.; Pandya, P.; Afshar, S. Impact of Non-Proteinogenic Amino Acids in the Discovery and Development of Peptide Therapeutics. Amino Acids 2020, 52, 1207–1226. [Google Scholar] [CrossRef]
- He, B.; Lian, Y.; Xue, H.; Zhou, Y.; Wei, Y.; Ma, J.; Tan, Y.; Wu, Y. DPP-IV Inhibitory Peptide against In Vitro Gastrointestinal Digestion Derived from Goat’s Milk Protein and Its Activity Enhancement via Amino Acid Substitution. Foods 2024, 13, 2721. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Pino, F. Bioactive Food-Derived Peptides for Functional Nutrition: Effect of Fortification, Processing and Storage on Peptide Stability and Bioactivity within Food Matrices. Food Chem. 2023, 406, 135046. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Sun-Waterhouse, D.; Chen, J.; Cui, C.; Wang, W. Bitter-Tasting Hydrophobic Peptides Prepared from Soy Sauce Using Aqueous Ethanol Solutions Influence Taste Sensation. Int. J. Food Sci. Technol. 2020, 55, 146–156. [Google Scholar] [CrossRef]
- Li-Chan, E.C. Bioactive Peptides and Protein Hydrolysates: Research Trends and Challenges for Application as Nutraceuticals and Functional Food Ingredients. Curr. Opin. Food Sci. 2015, 1, 28–37. [Google Scholar] [CrossRef]
- Cermeño, M.; O’Brien, N.; FitzGerald, R.J. Angiotensin Converting Enzyme and Dipeptidyl Peptidase-IV Inhibitory Activities of Transglutaminase Treated Sodium Caseinate Hydrolysates. Int. Dairy J. 2018, 78, 85–91. [Google Scholar] [CrossRef]
- Pei, J.; Gao, X.; Pan, D.; Hua, Y.; He, J.; Liu, Z.; Dang, Y. Advances in the Stability Challenges of Bioactive Peptides and Improvement Strategies. Curr. Res. Food Sci. 2022, 5, 2162–2170. [Google Scholar] [CrossRef]
- Harnedy-Rothwell, P.A.; McLaughlin, C.M.; Crowe, W.; Allsopp, P.J.; McSorley, E.M.; Devaney, M.; Whooley, J.; McGovern, B.; Parthsarathy, V.; O’Hart, F.P.M.; et al. Stability to Thermal Treatment of Dipeptidyl peptidase-IV Inhibitory Activity of a Boarfish (Capros aper) Protein Hydrolysate When Incorporated into Tomato-based Products. Int. J. Food Sci. Technol. 2021, 56, 158–165. [Google Scholar] [CrossRef]
- Kamdem, J.P.; Tsopmo, A. Reactivity of Peptides within the Food Matrix. J. Food Biochem. 2017, 43, e12489. [Google Scholar] [CrossRef]
- Sun, X.; Udenigwe, C.C. Chemistry and Biofunctional Significance of Bioactive Peptide Interactions with Food and Gut Components. J. Agric. Food Chem. 2020, 68, 12972–12977. [Google Scholar] [CrossRef]
- López-Sánchez, J.; Ponce-Alquicira, E.; Pedroza-Islas, R.; de la Peña-Díaz, A.; Soriano-Santos, J. Effects of Heat and pH Treatments and in Vitro Digestion on the Biological Activity of Protein Hydrolysates of Amaranthus hypochondriacus L. Grain. J. Food Sci. Technol. 2016, 53, 4298–4307. [Google Scholar] [CrossRef]
- Stewart, A.L.; Lorts, A.R.; Seal, E.L.; Takas, N.J.; Fortenberry, R.C. The Role of Sodium Ions in the Solubility of Peptides. Struct. Chem. 2016, 27, 1855–1862. [Google Scholar] [CrossRef]
- Fleury, L.; Deracinois, B.; Dugardin, C.; Nongonierma, A.B.; FitzGerald, R.J.; Flahaut, C.; Cudennec, B.; Ravallec, R. In Vivo and In Vitro Comparison of the DPP-IV Inhibitory Potential of Food Proteins from Different Origins after Gastrointestinal Digestion. Int. J. Mol. Sci. 2022, 23, 8365. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Yuan, Y.; Chen, L.; Chen, M.; Chiou, B.-S.; Liu, F.; Zhong, F. Effects of Gastrointestinal Digestion on the Cell Bioavailability of Sodium Alginate Coated Liposomes Containing DPP-IV Inhibition Active Collagen Peptides. Food Biosci. 2023, 56, 103426. [Google Scholar] [CrossRef]
- Ferrazzano, L.; Catani, M.; Cavazzini, A.; Martelli, G.; Corbisiero, D.; Cantelmi, P.; Fantoni, T.; Mattellone, A.; Luca, C.D.; Felletti, S.; et al. Sustainability in Peptide Chemistry: Current Synthesis and Purification Technologies and Future Challenges. Green. Chem. 2022, 24, 975–1020. [Google Scholar] [CrossRef]
- Ahmed, T.; Sun, X.; Udenigwe, C.C. Role of Structural Properties of Bioactive Peptides in Their Stability during Simulated Gastrointestinal Digestion: A Systematic Review. Trends Food Sci. Technol. 2022, 120, 265–273. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, Q.; Lin, L.; Zeng, X.-A.; Sun, B.; Zhao, M. In Vitro Metabolic Stability of a Casein-Derived Dipeptidyl Peptidase-IV (DPP-IV) Inhibitory Peptide VPYPQ and Its Controlled Release from Casein by Enzymatic Hydrolysis. J. Agric. Food Chem. 2019, 67, 10604–10613. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Zheng, J.; Bu, T.; He, G.; Wu, J. Safety Considerations on Food Protein-Derived Bioactive Peptides. Trends Food Sci. Technol. 2020, 96, 199–207. [Google Scholar] [CrossRef]
- Puri, B.; Meena, S.; Kumar, M.H.S.; Shelke, P.A.; Sabikhi, L. Ashutosh Encapsulation and Assessment of Antidiabetic Potential of α-Lactalbumin-Derived Hydrolysates. J. Agric. Food Chem. 2023, 71, 5547–5553. [Google Scholar] [CrossRef]
- Li, M.; Chen, P.; Lin, Y.; Miao, S.; Bao, H. Preparation and Characterization of a Hypoglycemic Complex of Gallic Acid–Antarctic Krill Polypeptide Based on Polylactic Acid–Hydroxyacetic Acid (PLGA) and High-Pressure Microjet Microencapsulation. Foods 2024, 13, 1177. [Google Scholar] [CrossRef]
- Montero, P.; Mosquera, M.; Marín-Peñalver, D.; Alemán, A.; Martínez-Álvarez, Ó.; Gómez-Guillén, M.C. Changes in Structural Integrity of Sodium Caseinate Films by the Addition of Nanoliposomes Encapsulating an Active Shrimp Peptide Fraction. J. Food Eng. 2019, 244, 47–54. [Google Scholar] [CrossRef]
- Gao, Y.; Luo, D.; Li, X.; Xue, B.; Xie, J.; Sun, T. Preparation and Characterization of Bovine Serum Albumin/Chitosan Composite Nanoparticles for Delivery of Antarctic Krill Peptide. J. Sci. Food Agric. 2024, 105, 162–170. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, X.; Castañeda-Reyes, E.D.; Wu, Y.; Zhang, S.; Wu, Z.; Wang, Z.; Dai, L.; Xu, B.; Xu, F. Chitosan and Sodium Alginate Nanocarrier System: Controlling the Release of Rapeseed-Derived Peptides and Improving Their Therapeutic Efficiency of Anti-Diabetes. Int. J. Biol. Macromol. 2024, 265, 130713. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, R.; Bollati, C.; Gelain, F.; Arnoldi, A.; Lammi, C. A Supramolecular Approach to Develop New Soybean and Lupin Peptide Nanogels with Enhanced Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Activity. J. Agric. Food Chem. 2019, 67, 3615–3623. [Google Scholar] [CrossRef] [PubMed]
- Lammi, C.; Bollati, C.; Gelain, F.; Arnoldi, A.; Pugliese, R. Enhancement of the Stability and Anti-DPPIV Activity of Hempseed Hydrolysates Through Self-Assembling Peptide-Based Hydrogels. Front. Chem. 2019, 6, 670. [Google Scholar] [CrossRef]
- Garzón, A.G.; Cian, R.E.; Drago, S.R. Effects of Agar-Carrageenan Wall Materials and Core-to-Wall Material Ratio on Physicochemical Properties and in Vitro Bioaccessibility of Microencapsulated Bioactive Peptides. Food Hydrocoll. 2023, 139, 108570. [Google Scholar] [CrossRef]
- Thongcumsuk, B.; Woraprayote, W.; Janyaphisan, T.; Cheunkar, S.; Oaew, S. Microencapsulation and Peptide Identification of Purified Bioactive Fraction from Spirulina Protein Hydrolysates with Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Activity. Food Biosci. 2023, 56, 103438. [Google Scholar] [CrossRef]
- Berraquero-García, C.; Martínez-Sánchez, L.; Guadix, E.M.; García-Moreno, P.J. Encapsulation of Tenebrio Molitor Hydrolysate with DPP-IV Inhibitory Activity by Electrospraying and Spray-Drying. Nanomaterials 2024, 14, 840. [Google Scholar] [CrossRef]
- Cian, R.E.; Campos-Soldini, A.; Chel-Guerrero, L.; Drago, S.R.; Betancur-Ancona, D. Bioactive Phaseolus Lunatus Peptides Release from Maltodextrin/Gum Arabic Microcapsules Obtained by Spray Drying after Simulated Gastrointestinal Digestion. Int. J. Food Sci. Technol. 2019, 54, 2002–2009. [Google Scholar] [CrossRef]
- Garzón, A.G.; Ferreira, M.d.R.; Cian, R.E.; Oliva, M.E.; D’Alessandro, M.E.; Drago, S.R. Microencapsulated Bioactive Peptides from Brewer’s Spent Grain Promotes Antihypertensive and Antidiabetogenic Effects on a Hypertensive and Insulin-Resistant Rat Model. J. Food Biochem. 2022, 46, e14283. [Google Scholar] [CrossRef]
- Du, X.; Huang, X.; Wang, L.; Mo, L.; Jing, H.; Bai, X.; Wang, H. Nanosized Niosomes as Effective Delivery Device to Improve the Stability and Bioaccessibility of Goat Milk Whey Protein Peptide. Food Res. Int. 2022, 161, 111729. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Miralles, B.; Recio, I.; López-Rubio, A. Microencapsulation of a Whey Protein Hydrolysate within Micro-Hydrogels: Impact on Gastrointestinal Stability and Potential for Functional Yoghurt Development. J. Funct. Foods 2016, 26, 290–300. [Google Scholar] [CrossRef]
- Xu, F.; Chen, H.; Ju, X.; de Mejia, E.G. Enhancement of DPP-IV Inhibitory Activity and GLP-1 Release Through RADA16-Assisted Molecular Designed Rapeseed Peptide Nanogels. Curr. Dev. Nutr. 2021, 5, 614. [Google Scholar] [CrossRef]
- McClements, D.J. Recent Developments in Encapsulation and Release of Functional Food Ingredients: Delivery by Design. Curr. Opin. Food Sci. 2018, 23, 80–84. [Google Scholar] [CrossRef]
- Cortés-Morales, E.A.; Mendez-Montealvo, G.; Velazquez, G. Interactions of the Molecular Assembly of Polysaccharide-Protein Systems as Encapsulation Materials. A Review. Adv. Colloid. Interface Sci. 2021, 295, 102398. [Google Scholar] [CrossRef]
- Li, M.; Guo, Q.; Lin, Y.; Bao, H.; Miao, S. Recent Progress in Microencapsulation of Active Peptides—Wall Material, Preparation, and Application: A Review. Foods 2023, 12, 896. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Cui, Z.; Guo, S.; Zhang, X.; Huo, Y.; Mao, S. Mucoadhesive versus Mucopenetrating Nanoparticles for Oral Delivery of Insulin. Acta Biomater. 2021, 135, 506–519. [Google Scholar] [CrossRef]
- Paul, P.; Nandi, G.; Abosheasha, M.A.; Bera, H. Alginate-Based Systems for Protein and Peptide Delivery. In Tailor-Made and Functionalized Biopolymer Systems; Bera, H., Layek, B., Singh, J., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Thorston, UK, 2021; pp. 85–113. ISBN 978-0-12-821437-4. [Google Scholar]
- Wang, S.; Gao, Z.; Liu, L.; Li, M.; Zuo, A.; Guo, J. Preparation, in Vitro and in Vivo Evaluation of Chitosan-Sodium Alginate-Ethyl Cellulose Polyelectrolyte Film as a Novel Buccal Mucosal Delivery Vehicle. Eur. J. Pharm. Sci. 2022, 168, 106085. [Google Scholar] [CrossRef]
- Wu, P.; Chen, L.; Chen, M.; Chiou, B.-S.; Xu, F.; Liu, F.; Zhong, F. Use of Sodium Alginate Coatings to Improve Bioavailability of Liposomes Containing DPP-IV Inhibitory Collagen Peptides. Food Chem. 2023, 414, 135685. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, Protection, and Delivery of Bioactive Proteins and Peptides Using Nanoparticle and Microparticle Systems: A Review. Adv. Colloid. Interface Sci. 2018, 253, 1–22. [Google Scholar] [CrossRef]
- Koshani, R.; Jafari, S.M. Ultrasound-Assisted Preparation of Different Nanocarriers Loaded with Food Bioactive Ingredients. Adv. Colloid. Interface Sci. 2019, 270, 123–146. [Google Scholar] [CrossRef] [PubMed]
- Lopez, F.L.; Bowles, A.; Gul, M.O.; Clapham, D.; Ernest, T.B.; Tuleu, C. Effect of Formulation Variables on Oral Grittiness and Preferences of Multiparticulate Formulations in Adult Volunteers. Eur. J. Pharm. Sci. 2016, 92, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Nejatian, M.; Abbasi, S. Chapter 19—Application of Bio-Based Emulsifiers in the Formulation of Food-Grade Nanoemulsions. In Bio-Based Nanoemulsions for Agri-Food Applications; Abd-Elsalam, K.A., Murugan, K., Eds.; Nanobiotechnology for Plant Protection; Elsevier: Amsterdam, The Netherlands, 2022; pp. 311–327. ISBN 978-0-323-89846-1. [Google Scholar]
- Fatani, S.H.; Babakr, A.T.; NourEldin, E.M.; Almarzouki, A.A. Lipid Peroxidation Is Associated with Poor Control of Type-2 Diabetes Mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, S64–S67. [Google Scholar] [CrossRef] [PubMed]
- Bamidele, O.P.; Emmambux, M.N. Encapsulation of Bioactive Compounds by “Extrusion” Technologies: A Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3100–3118. [Google Scholar] [CrossRef]
| Method | Advantages | Disadvantages | Food Applicability |
|---|---|---|---|
| Enzymatic Hydrolysis | Mild conditions Safe and environmentally friendly Scalable | Limited specificity Potential bitterness High cost of proteases Incomplete hydrolysis | High (aligned with clean-label trends) |
| Fermentation | Natural Rich flavor Cost-effective | Time-consuming Low yield Poor specificity Complex composition | Moderate (depends on strain safety) |
| Chemical Synthesis | High purity Sequence control | High cost Synthetic origin | Low (limited to research validation) |
| Enzymatic Synthesis | Mild conditions Food-grade materials Scalable | Enzyme selection Optimization complex High cost and liability of enzymes | Moderate (suitable for specific peptides) |
| Source | Preparation Method | IC50 of Hydrolysate (mg/mL) | Peptide Sequence | Size (AA) | IC50 of Peptides (µM) | Reference |
|---|---|---|---|---|---|---|
| ENZYMOLYSIS | ||||||
| Oat protein isolate | Alcalase | 0.41 | SPVAEVPFLR | 10 | 167.8 | [29] |
| LDATDMVALVG | 11 | 269.1 | ||||
| Camel milk proteins | Trypsin | 0.52 | LPVP | 4 | 87 | [30] |
| MPVQA | 5 | 93.3 | ||||
| YPVEPF | 6 | 138 | ||||
| LLQLEAIR | 8 | 177.8 | ||||
| SPVVPF | 6 | 214.1 | ||||
| ILDKVGINY | 9 | 321.5 | ||||
| ILDKEGIDY | 9 | 347.8 | ||||
| ILELA | 5 | 721.1 | ||||
| Chicken blood | Alcalase and Protana Prime | GPF | 3 | 940 | [21] | |
| IGL | 3 | 2220 | ||||
| GGGW | 4 | 2730 | ||||
| Musculus senhousei | Neutrase | DPF | 3 | 1399.73 | [31] | |
| LTWR | 4 | 1788.67 | ||||
| Bactrian camel milk | α-Chymotrypsin and Proteinase K | QPY | 3 | 655.60 | [32] | |
| FPH | 3 | 1039.29 | ||||
| LPAAP | 5 | 199.66 | ||||
| WPEYL | 5 | 380.16 | ||||
| YPPQVM | 6 | 1067.49 | ||||
| IPAPSFPRL | 9 | 425.01 | ||||
| Discarded cowhide collagen | Compound protease and Papain | 3.04 | GPVG | 4 | 386.77 | [33] |
| FGPGP | 5 | 3309.21 | ||||
| APGGAP | 6 | 382.07 | ||||
| GPPGPT | 6 | 1197.14 | ||||
| GPVGPPG | 7 | 196.67 | ||||
| Coix seed prolamins | Papain and Alcalase | LPFYPN | 6 | 70.24 | [34] | |
| TFFPQ | 5 | 176.87 | ||||
| ATFFPQ | 6 | 268.31 | ||||
| Tilapia skin gelatin | Ginger protease | GPXGPPGPGP | 9 | 1012.3 | [18] | |
| Boarfish (Capros aper) | Alcalase 2.4L and Flavourzyme 500L | IPV | 3 | 5.61 | [17] | |
| APIT | 4 | 34.73 | ||||
| VPTP | 4 | 38.93 | ||||
| GPIN | 4 | 48.96 | ||||
| IPGA | 4 | 66.37 | ||||
| GPSL | 4 | 68.13 | ||||
| GPSI | 4 | 72.85 | ||||
| APVP | 4 | 73.15 | ||||
| APLT | 4 | 91.1 | ||||
| MPAVP | 4 | 115.27 | ||||
| GPGI | 4 | 116.27 | ||||
| GPLN | 4 | 116.37 | ||||
| PAVP | 4 | 126.51 | ||||
| GPGL | 4 | 131.9 | ||||
| LPGA | 4 | 154.12 | ||||
| AALP | 4 | 164.37 | ||||
| IPVDM | 5 | 21.72 | ||||
| LPVYD | 5 | 51.36 | ||||
| LPVDM | 5 | 53.5 | ||||
| APLER | 5 | 63.67 | ||||
| VPDPR | 5 | 79.1 | ||||
| APLDK | 5 | 90.37 | ||||
| goat milk whey protein | Papain | 0.34 | FNPTY | 5 | 62.32 | [35] |
| LDADGSY | 7 | 52.16 | ||||
| SPPEFLR | 7 | 56.22 | ||||
| YPVEPFT | 7 | 175.7 | ||||
| FERMENTATION | ||||||
| fermented Mandarin fish (Chouguiyu) | Lysobacter, Lactococcus | GEKVDFDDIQK | 11 | - | [36] | |
| Lysobacter, Lactococcus | VVDADEMYLKGK | 12 | - | |||
| Lactococcus, Peptostreptococcus | GQKDSYVGDEAQ | 12 | - | |||
| Bacillus, Kocuria | KAGARALTDAETAT | 14 | - | |||
| cow milk | Limosilactobacillus fermentum | 0.18 | [37] | |||
| camel milk | Limosilactobacillus fermentum | 0.37 | ||||
| goat milk | Limosilactobacillus fermentum | 0.36 | ||||
| sheep milk | Limosilactobacillus fermentum | 0.26 | ||||
| whey protein concentrate | Streptococcus thermophilus | IPA | 3 | 49 | [38] | |
| IPP | 3 | 169 | ||||
| LPVP | 4 | 87 | ||||
| VLGP | 4 | 580 | ||||
| VPYPQ | 5 | 41 | ||||
| LPVPQ | 5 | 44 | ||||
| APFPE | 5 | 49 | ||||
| YPFPGP | 6 | 749 | ||||
| PQNIPPL | 7 | 1500 | ||||
| TPEVDDEALEK | 11 | 320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Zhou, Y.; Shen, H.; Guan, L.; Wang, Y.; Shen, X.; Wang, F.; Yao, X. Preparation and Encapsulation of DPP-IV Inhibitory Peptides: Challenges and Strategies for Functional Food Development. Foods 2025, 14, 1479. https://doi.org/10.3390/foods14091479
Zhao R, Zhou Y, Shen H, Guan L, Wang Y, Shen X, Wang F, Yao X. Preparation and Encapsulation of DPP-IV Inhibitory Peptides: Challenges and Strategies for Functional Food Development. Foods. 2025; 14(9):1479. https://doi.org/10.3390/foods14091479
Chicago/Turabian StyleZhao, Rui, Ye Zhou, Huifang Shen, Lijun Guan, Yao Wang, Xinting Shen, Fei Wang, and Xinmiao Yao. 2025. "Preparation and Encapsulation of DPP-IV Inhibitory Peptides: Challenges and Strategies for Functional Food Development" Foods 14, no. 9: 1479. https://doi.org/10.3390/foods14091479
APA StyleZhao, R., Zhou, Y., Shen, H., Guan, L., Wang, Y., Shen, X., Wang, F., & Yao, X. (2025). Preparation and Encapsulation of DPP-IV Inhibitory Peptides: Challenges and Strategies for Functional Food Development. Foods, 14(9), 1479. https://doi.org/10.3390/foods14091479





