Use of Coffee Roasting By-Products (Coffee Silverskin) as Natural Preservative for Fresh-Cut Fennel Slices
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Bioactive Compounds from Coffee Silverskin
Chemical Characterization of CSE
2.2. Fresh-Cut Fennel Processing
2.3. Qualitative Analyses of Fresh-Cut Fennel
2.3.1. Sensory Analysis of Ready-to-Eat Fennel Slices
2.3.2. Color and Textural Analysis of Ready-to-Eat Fennel Slices
2.3.3. Total Soluble Solids, pH, Moisture, and Weight Loss
2.3.4. Quantification of Malic, Oxalic and Citric Acids
2.3.5. Quantification of Caffeic and Chlorogenic Acids
2.3.6. Microbiological Analysis
2.4. Statistical Analysis
3. Results
3.1. Chemical Characterization of Coffee Silverskin Extract
3.2. Quality Evaluations of Fresh-Cut Fennel Slices
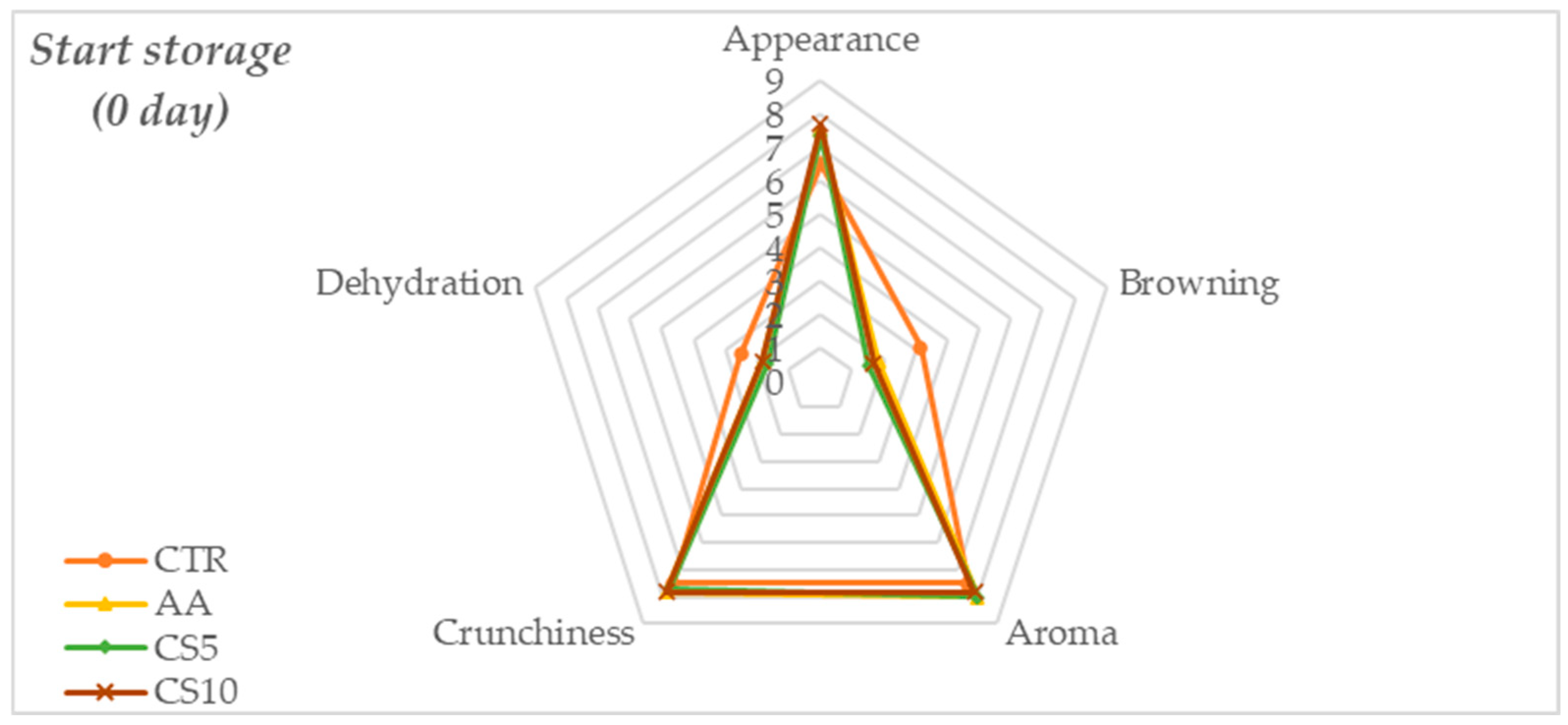
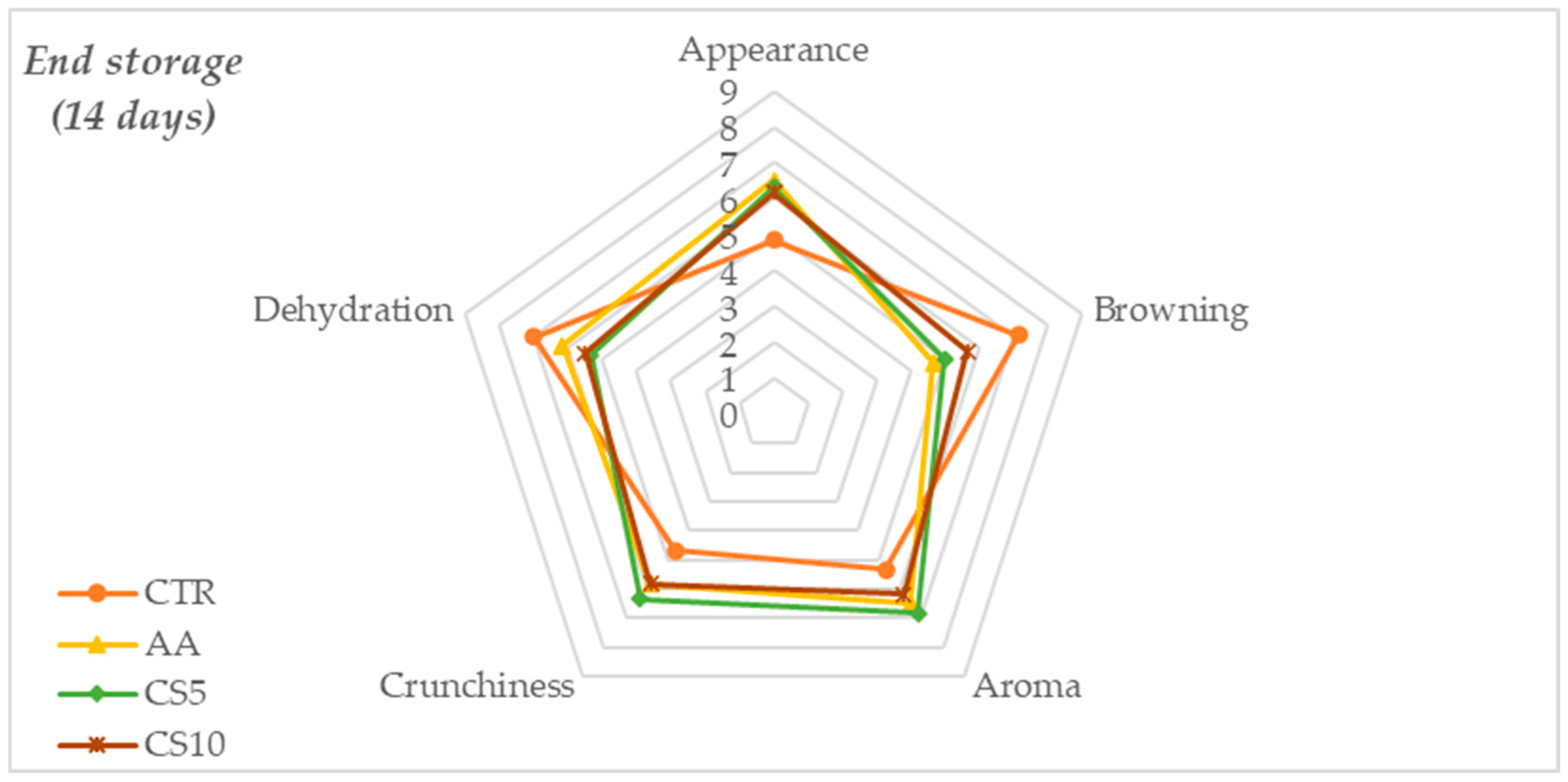
3.2.1. Sensory Analysis
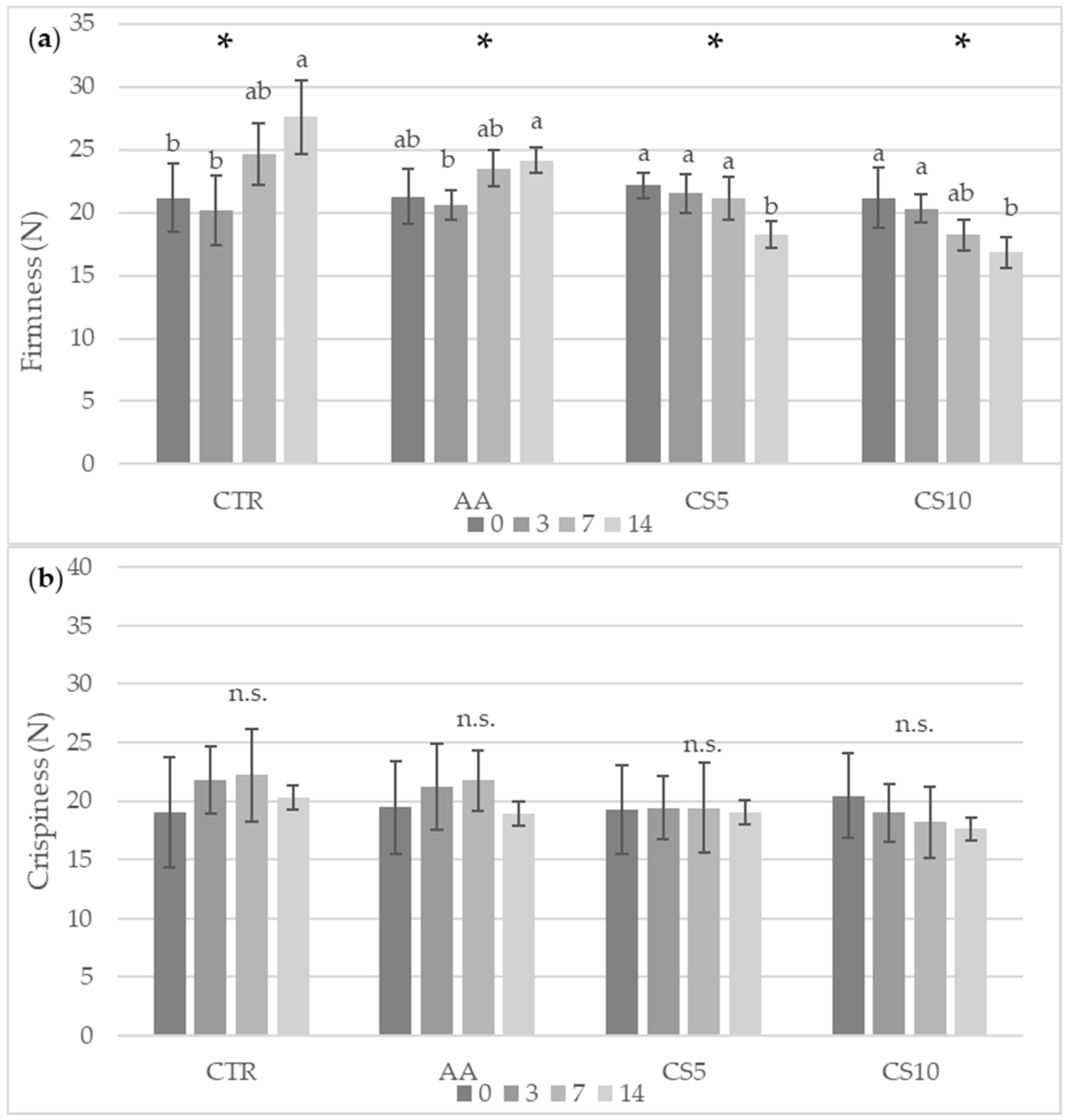
3.2.2. Color and Textural Analysis
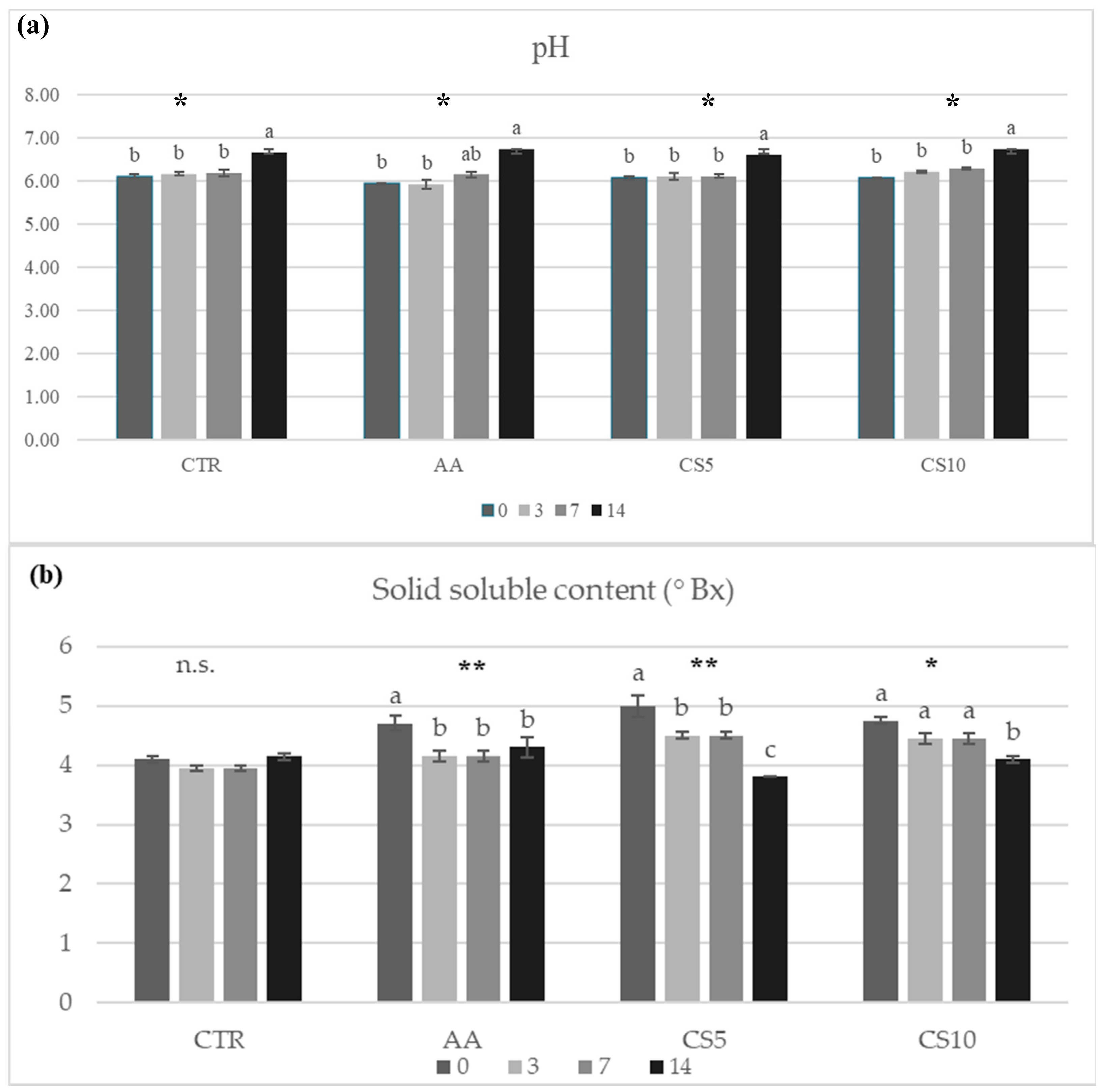
3.2.3. Total Soluble Solids, pH, Moisture and Weight Loss of Fresh-Cut Fennel Samples
3.2.4. Quantification of Malic, Oxalic, and Citric Acid
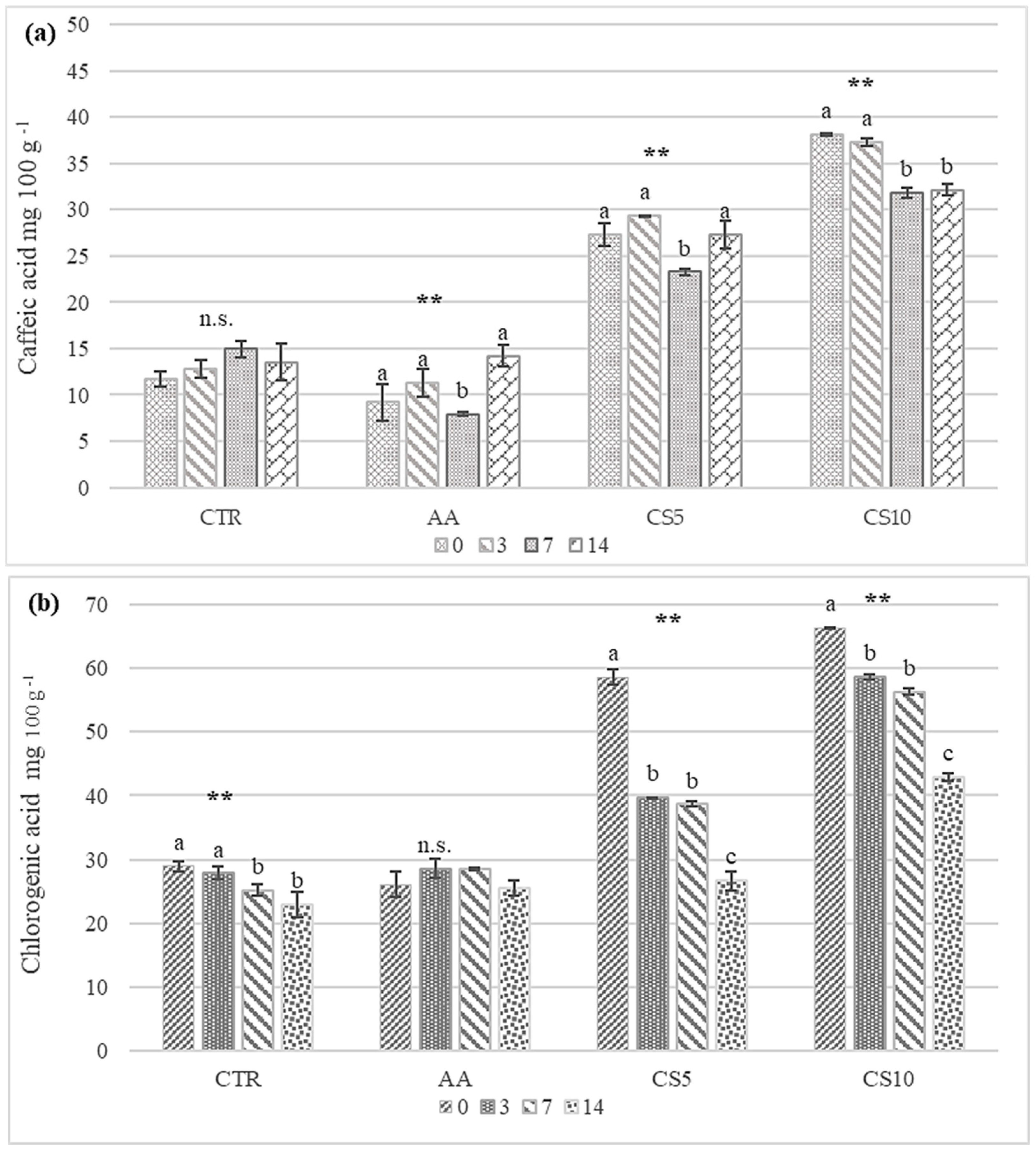
3.2.5. Quantification of Chlorogenic and Caffeic Acids
3.2.6. Microbiological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Ahmad, T.; Esposito, F.; Cirillo, T. Valorization of agro-food by-products: Advancing sustainability and Sustainable Development Goals 2030 through functional compound recovery. Food Biosci. 2024, 62, 105194. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, W.; Xu, Y.; Zhang, Y.; Pu, Y.; Cao, J.; Jiang, W. Applications of plant-derived food by-products to maintain quality of postharvest fruits and vegetables. Trends Food Sci. Technol. 2021, 116, 1105–1119. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Zhao, J.; Liu, L.; Zhao, L. Research progress on physical preservation technology of fresh-cut fruits and vegetables. Horticulturae 2024, 10, 1098. [Google Scholar] [CrossRef]
- Belgacem, I.; Schena, L.; Teixidó, N.; Romeo, F.V.; Ballistreri, G.; Abadias, M. Effectiveness of a pomegranate peel extract (PGE) in reducing Listeria monocytogenes in vitro and on fresh-cut pear, apple and melon. Eur. Food Res. Technol. 2020, 246, 1765–1772. [Google Scholar] [CrossRef]
- Lacivita, V.; Incoronato, A.L.; Conte, A.; Del Nobile, M.A. Pomegranate peel powder as a food preservative in fruit salad: A sustainable approach. Foods 2021, 10, 1359. [Google Scholar] [CrossRef]
- Akbari, E.; Gholami, M.; Ghobadi, C. Shelf-life and quality attributes in fresh-cut pear cv. Shahmive treated with different kinds of antioxidants. J. Food Sci. Technol. 2019, 56, 3998–4008. [Google Scholar] [CrossRef]
- Yousuf, B.; Srivastava, A.K.; Ahmad, S. Application of natural fruit extract and hydrocolloid-based coating to retain quality of fresh-cut melon. J. Food Sci. Technol. 2020, 57, 3647–3658. [Google Scholar] [CrossRef] [PubMed]
- Cefola, M.; Pace, B.; Imeneo, V.; Piscopo, A.; Martín-Belloso, O.; Soliva-Fortuny, R. Efficacy of Pectin-Based Coating Added with a Lemon Byproduct Extract on Quality Preservation of Fresh-Cut Carrots. Foods 2022, 11, 1314. [Google Scholar] [CrossRef]
- Jirasuteeruk, C.; Theerakulkait, C. Ultrasound-Assisted Extraction of Phenolic Compounds from Mango (Mangifera indica cv. Chok Anan) Peel and Its Inhibitory Effect on Enzymatic Browning of Potato Puree. Food Technol. Biotechnol. 2019, 57, 350–358. [Google Scholar] [CrossRef]
- Fang, X.; Han, J.; Lou, X.; Lv, Y.; Zhang, Y.; Xu, X.; Lv, Z.; Lu, G. Effect and Mechanism of Treatment with Different Concentrations of Citrus Peel Extract on the Browning of Fresh-Cut Sweet Potatoes. Foods 2023, 12, 3855. [Google Scholar] [CrossRef]
- Bishnoi, S.; Sharma, S.; Agrawal, H. Exploration of the Potential Application of Banana Peel for Its Effective Valorization: A Review. Indian J. Microbiol. 2023, 63, 398–409. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Kotsou, K.; Mantiniotou, M.; Bozinou, E.; Lalas, S.I. Recent Advances in the Antibacterial Activities of Citrullus lanatus (Watermelon) By-Products. Appl. Sci. 2023, 13, 11063. [Google Scholar] [CrossRef]
- Koirala, P.; Chunhavacharatorn, P.; Suttisansanee, U.; Benjakul, S.; Katewongsa, K.; Al-Asmari, F.; Nirmal, N. Antioxidant and Antimicrobial Activities of Mango Peel and Radish Peel—A Comparative Investigation. Front. Sustain. Food Syst. 2024, 8, 1354393. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Green Coffee Production in 2023, Crops/Regions/World List/Production Quantity/Year (Pick Lists); UN Food and Agriculture Organization, Corporate Statistical Database (FAOSTAT): Rome, Italy, 2025; Available online: https://www.fao.org/faostat/ (accessed on 1 April 2025).
- Boninsegna, M.A.; Cilea, I.; Piscopo, A.; De Bruno, A.; Poiana, M. Sustainable Use of Coffee Roasting By-Products: Development of High Value-Added Gummy Candies. J. Food Meas. Charact. 2024, 18, 9519–9531. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Borsetta, G.; Navarini, L.; Abouelenein, D.; Xiao, J.; Sagratini, G.; Vittori, S.; Caprioli, G.; Angeloni, S. Coffee Silverskin: Characterization of B-Vitamins, Macronutrients, Minerals and Phytosterols. Food Chem. 2022, 372, 131188. [Google Scholar] [CrossRef]
- Eckhardt, S.; Franke, H.; Schwarz, S.; Lachenmeier, D.W. Risk assessment of coffee cherry (cascara) fruit products for flour replacement and other alternative food uses. Molecules 2022, 27, 8435. [Google Scholar] [CrossRef] [PubMed]
- Nolasco, A.; Squillante, J.; Velotto, S.; D’Auria, G.; Ferranti, P.; Mamone, G.; Errico, M.E.; Avolio, R.; Castaldo, R.; Cirillo, T.; et al. Valorization of coffee industry wastes: Comprehensive physicochemical characterization of coffee silverskin and multipurpose recycling applications. J. Clean. Prod. 2022, 370, 133520. [Google Scholar] [CrossRef]
- Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Angeloni, S.; Mustafa, A.M.; Vittori, S.; Maggi, F.; Caprioli, G. Chemical composition, antioxidant, and enzyme inhibitory properties of different extracts obtained from spent coffee ground and coffee silverskin. Foods 2020, 9, 713. [Google Scholar] [CrossRef]
- Arya, S.S.; Venkatram, R.; More, P.R.; Vijayan, P. The wastes of coffee bean processing for utilization in food: A review. J. Food Technol. 2021, 59, 429–444. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Rai, D.; Sun, D.W.; Tiwari, B.K. Ultrasound-assisted extraction (UAE) of bioactive compounds from coffee silverskin: Impact on phenolic content, antioxidant activity, and morphological characteristics. J. Food Process Eng. 2019, 42, e13191. [Google Scholar] [CrossRef]
- Costa, A.S.G.; Alves, R.C.; Vinha, A.F.; Barreira, S.V.P.; Nunes, M.A.; Cunha, L.M.; Oliveira, M.B.P.P. Optimization of antioxidants extraction from coffee silverskin, a roasting by-product, for a sustainable process. Ind. Crops Prod. 2014, 53, 350–357. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Goulas, V.; Tsakona, S.; Manganaris, G.A.; Gekas, V. A knowledge base for the recovery of natural phenols with different solvents. Int. J. Food Prop. 2013, 16, 382–396. [Google Scholar] [CrossRef]
- Bertolino, M.; Barbosa-Pereira, L.; Ghirardello, D.; Botta, C.; Rolle, L.; Guglielmetti, A.; Borotto Dalla Vecchia, S.; Zeppa, G. Coffee silverskin as nutraceutical ingredient in yogurt: Its effect on functional properties and its bioaccessibility. J. Sci. Food Agric. 2019, 99, 4267–4275. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, W.C.; Da Silva Araújo, C.; Macedo, L.L.; Pimenta, C.J. Optimal extraction condition for the recovery of bioactive compounds and antioxidants from coffee silverskin. J. Food Process. Eng. 2022, 45, 14009. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; Ullate, M.; Martin-Cabrejas, M.A.; Martorell, P.; Genovés, S.; Ramon, D.; Del Castillo, M.D. A novel antioxidant beverage for body weight control based on coffee silverskin. Food Chem. 2014, 150, 227–234. [Google Scholar] [CrossRef]
- Ateş, G.; Elmacı, Y. Physical, chemical and sensory characteristics of fiber-enriched cakes prepared with coffee silverskin as wheat flour substitution. Food Meas. 2019, 13, 755–763. [Google Scholar] [CrossRef]
- Gocmen, D.; Sahan, Y.; Yildiz, E.; Coskun, M.; Aroufai, I.A. Use of coffee silverskin to improve the functional properties of cookies. J. Food Sci. Technol. 2019, 56, 2979–2988. [Google Scholar] [CrossRef]
- Thangavelu, K.P.; Tiwari, B.; Kerry, J.P.; Álvarez, C. A comparative study on the effect of ultrasound-treated apple pomace and coffee silverskin powders as phosphate replacers in Irish breakfast sausage formulations. Foods 2022, 11, 2763. [Google Scholar] [CrossRef]
- Capotorto, I.; Amodio, M.L.; Diaz, M.T.B.; De Chiara, M.L.V.; Colelli, G. Effect of anti-browning solutions on quality of fresh-cut fennel during storage. Postharvest Biol. Technol. 2018, 137, 21–30. [Google Scholar] [CrossRef]
- Escalona, V.H.; Aguayo, E.; Artès, F. Overall quality throughout shelf life of minimally fresh processed fennel. J. Food Sci. 2005, 70, 13–17. [Google Scholar] [CrossRef]
- Adams, J.B. Effect of enzymatic reactions on color of fruits and vegetables. In Enzymes in Fruit and Vegetable Processing; CRC Press–Taylor and Francis Group LLC: New York, NY, USA, 2010; pp. 19–44. [Google Scholar]
- Zhang, X.; Meng, W.; Chen, Y.; Peng, Y. Browning inhibition of plant extracts on fresh-cut fruits and vegetables—A review. J. Food Process. Preserv. 2022, 46, e16532. [Google Scholar] [CrossRef]
- Castro-Ibáñez, I.; Gil, M.I.; Allende, A. Ready-to-eat vegetables: Current problems and potential solutions to reduce microbial risk in the production chain. LWT-Food Sci. Technol. 2017, 85, 284–292. [Google Scholar] [CrossRef]
- Saini, R.K.; Ko, E.Y.; Keum, Y.S. Minimally processed ready-to-eat baby-leaf vegetables: Production, processing, storage, microbial safety, and nutritional potential. Food Rev. Int. 2017, 33, 644–663. [Google Scholar] [CrossRef]
- Wang, L.; Teplitski, M. Microbiological food safety considerations in shelf-life extension of fresh fruits and vegetables. Curr. Opin. Biotechnol. 2023, 80, 102895. [Google Scholar] [CrossRef]
- Suttirak, W.; Manurakchinakorn, S. Potential application of ascorbic acid, citric acid and oxalic acid for browning inhibition in fresh-cut fruits and vegetables. Walailak J. Sci. Tech. 2010, 7, 5–14. [Google Scholar]
- Valentino, M.; Volpe, S.; Cavella, S.; Masi, P.; Torrieri, E. Effect of biopolymer active coating on alteration kinetics of minimally processed fennel stored at different temperatures. Food Packag. Shelf Life 2023, 39, 101137. [Google Scholar] [CrossRef]
- Brzezińska, R.; Wirkowska-Wojdyła, M.; Piasecka, I.; Górska, A. Application of response surface methodology to optimize the extraction process of bioactive compounds from coffee silverskin. Appl. Sci. 2023, 13, 5388. [Google Scholar] [CrossRef]
- Guan, Y.; Lu, S.; Sun, Y.; Zheng, X.; Wang, R.; Lu, X.; Pang, L.; Cheng, J.; Wang, L. Tea Polyphenols Inhibit the Occurrence of Enzymatic Browning in Fresh-Cut Potatoes by Regulating Phenylpropanoid and ROS Metabolism. Plants 2024, 13, 125. [Google Scholar] [CrossRef]
- You, W.; Wang, C.; Zhang, J.; Ru, X.; Xu, F.; Wu, Z.; Jin, P.; Zheng, Y.; Cao, S. Exogenous Chlorogenic Acid Inhibits Quality Deterioration in Fresh-Cut Potato Slices. Food Chem. 2024, 446, 138866. [Google Scholar] [CrossRef]
- ISO 13299; Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile. International Standardisation Organisation: Geneva, Switzerland, 2016.
- ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. International Organization for Standardization: Geneva, Switzerland, 2007.
- Rizzo, V.; Muratore, G. Effects of Packaging on Shelf Life of Fresh Celery; Dipartimento di Orto Floro Arboricoltura e Tecnologie Agroalimentari (DOFATA), Sezione Tecnologie Agroalimentari, University of Catania: Catania, Italy, 2009. [Google Scholar]
- Varela, P.; Salvador, A.; Fiszman, S. Changes in apple tissue with storage time: Rheological, textural and microstructural analyses. J. Food Eng. 2007, 78, 622–629. [Google Scholar] [CrossRef]
- Alvarez, M.D.; Velarde, C.; Barrios, L.; Herranz, B. Understanding the crispy–crunchy texture of raw red pepper and its change with storage time. J. Texture Stud. 2020, 51, 120–133. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1994. [Google Scholar]
- AOAC. Official Methods of Analysis, 14th ed.; Association of Official Agricultural Chemists: Virginia, USA, 1984; pp. 414–420. [Google Scholar]
- Boninsegna, M.A.; De Bruno, A.; Piscopo, A. Improving the storage quality of ready-to-eat clementine fruits using lemon by-products. Agriculture 2024, 14, 1488. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Angeloni, S.; Navarini, L.; Angeloni, C.; Freschi, M.; Hrelia, S.; Vitali, L.A.; Sagratini, G.; Vittori, S.; Caprioli, G. Coffee silverskin extracts: Quantification of 30 bioactive compounds by a new HPLC-MS/MS method and evaluation of their antioxidant and antibacterial activities. Food Res. Int. 2020, 133, 109128. [Google Scholar] [CrossRef] [PubMed]
- More, P.R.; Jambrak, A.R.; Arya, S.S. Green, environment-friendly, and sustainable techniques for extraction of food bioactive compounds and waste valorization. Trends Food Sci. Technol. 2022, 128, 296–315. [Google Scholar] [CrossRef]
- Galani, J.H.Y.; Patel, J.S.; Patel, N.J.; Talati, J.G. Storage of fruits and vegetables in refrigerator increases their phenolic acids but decreases the total phenolics, anthocyanins and vitamin C with subsequent loss of their antioxidant capacity. Antioxidants 2017, 6, 59. [Google Scholar] [CrossRef]
- Erbaş, D. Effect of oxalic acid treatments and modified atmosphere packaging on the quality attributes of rocket leaves during different storage temperatures. Horticulturae 2023, 9, 718. [Google Scholar] [CrossRef]
- Rifna, E.J.; Misra, N.N.; Dwivedi, M. Recent advances in extraction technologies for recovery of bioactive compounds derived from fruit and vegetable waste peels: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 719–752. [Google Scholar] [CrossRef]
- Cheng, D.; Wang, G.; Tang, J.; Yao, C.; Li, P.; Song, Q.; Wang, C. Inhibitory effect of chlorogenic acid on polyphenol oxidase and browning of fresh-cut potatoes. Postharvest Biol. Technol. 2020, 168, 111282. [Google Scholar] [CrossRef]
- Mokrzycki, W.S.; Tatol, M. Colour difference ∆E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: Desirable levels, instrumental and sensory measurement, and the effects of processing. Crit. Rev. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef]
- Valentino, M.; Sequino, G.; De Filippis, F.; Di Monaco, R.; Cavella, S.; Torrieri, E. The effect of edible coating based on sodium caseinate and propyl gallate on the shelf life of minimally processed fennel during storage. Appl. Food Res. 2024, 4, 100462. [Google Scholar] [CrossRef]
- Artés, F.; Escalona, V.H.; Artés-Hdez, F. Modified atmosphere packaging of fennel. J. Food Sci. 2002, 67, 1550–1554. [Google Scholar] [CrossRef]
- Wen, Y.-T.; Liang, Y.-Q.; Chai, W.-M.; Wei, Q.-M.; Yu, Z.-Y.; Wang, L.-J. Effect of Ascorbic Acid on Tyrosinase and Its Anti-Browning Activity in Fresh-Cut Fuji Apple. J. Food Biochem. 2021, 45, e13995. [Google Scholar] [CrossRef]
- Landi, M.; Degl’Innocenti, E.; Guglielminetti, L.; Guidi, L. Role of Ascorbic Acid in the Inhibition of Polyphenol Oxidase and the Prevention of Browning in Different Browning-Sensitive Lactuca sativa var. capitata (L.) and Eruca sativa (Mill.) Stored as Fresh-Cut Produce. J. Sci. Food Agric. 2013, 93, 1814–1819. [Google Scholar] [PubMed]
- Zappia, A.; De Bruno, A.; Piscopo, A.; Poiana, M. Physico-chemical and microbiological quality of ready-to-eat rocket (Eruca vesicaria (L.) Cav.) treated with organic acids during storage in dark and light conditions. Food Sci. Biotechnol. 2019, 28, 965–973. [Google Scholar] [CrossRef]
- Lim, K.T.; Hu, C.; Kitts, D.D. Antioxidant activity of a Rhus verniciflua Stokes ethanol extract. Food Chem. Toxicol. 2001, 39, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Lorbeer, L.; Schwarz, S.; Franke, H.; Lachenmeier, D.W. Toxicological assessment of roasted coffee silver skin (Testa of Coffea sp.) as novel food ingredient. Molecules 2022, 27, 6839. [Google Scholar] [CrossRef] [PubMed]
- Fufa, D.D. Novel Approach to Enhance the Shelf Life of Fresh-Cut Fruits and Vegetables: A Review. J. Food Process. Technol. 2021, 12, 891. [Google Scholar]
- Umeohia, U.E.; Olapade, A.A. Physiological Processes Affecting Postharvest Quality of Fresh Fruits and Vegetables. Asian Food Sci. J. 2024, 23, 115915. [Google Scholar] [CrossRef]
- Guan, Y.; Hu, W.; Wang, L.; Yang, B. Different Cutting Methods Affect the Quality of Fresh-Cut Cucumbers by Regulating ROS Metabolism. Horticulturae 2023, 9, 514. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Domínguez, R.; Nieto, G.; Kumar, M.; Dhama, K.; Lorenzo, J.M. Bioactive Compounds from Fruits as Preservatives. Foods 2023, 12, 343. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent Developments in Shelf-Life Extension of Fresh-Cut Fruits and Vegetables by Application of Different Edible Coatings: A Review. LWT 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Degl’Innocenti, E.; Guidi, L.; Pardossi, A.; Tognoni, F. Biochemical study of leaf browning in minimally processed leaves of lettuce (Lactuca sativa L. var. Acephala). J. Agric. Food Chem. 2005, 53, 9980–9984. [Google Scholar] [CrossRef]
- Asrey, R.; Vinod, B.R.; Menaka, M.; Ahamed, S.; Kumar, A. Recent trends in postharvest treatments for fruits and vegetables. In Advances in Postharvest and Analytical Technology of Horticulture Crops; Springer Nature: Singapore, 2024. [Google Scholar]
- Rubio, A.; López-Orenes, A.; Ferrer, M.A.; Calderón, A.A. Influence of genotype on antioxidant activity and phenolic profile of fennel bulbs. Agronomy 2024, 14, 484. [Google Scholar] [CrossRef]
- Guan, Y.; Hu, W.; Jiang, A.; Xu, Y.; Sa, R.; Feng, K.; Zhao, M.; Yu, J.; Ji, Y.; Hou, M.; et al. Effect of Methyl Jasmonate on Phenolic Accumulation in Wounded Broccoli. Molecules 2019, 24, 3537. [Google Scholar] [CrossRef] [PubMed]
- Oliu, G.O.; Aguiló-Aguayo, I.; Martín-Belloso, O.; Soliva-Fortuny, R. Effects of pulsed light treatments on quality and antioxidant properties of fresh-cut mushrooms (Agaricus bisporus). Postharvest Biol. Technol. 2010, 56, 216–222. [Google Scholar] [CrossRef]
- Franca, A.S.; Basílio, E.P.; Resende, L.M.; Fante, C.A.; Oliveira, L.S. Coffee Silverskin as a Potential Ingredient for Functional Foods: Recent Advances and a Case Study with Chocolate Cake. Foods 2024, 13, 3935. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, S.T.; Nassur, R.D.C.M.R. Calcium Treatments. In Novel Postharvest Treatments of Fresh Produce; CRC Press: Boca Raton, FL, USA, 2017; pp. 51–78. [Google Scholar]
- Faulisi, M.V.; Palmeri, R.; Restuccia, C. Multifunctional Application of Food Grade Extracts from Fruit Processing Industry Wastes: A Sustainable Approach to Food and Health Preservation. Food Biosci. 2024, 62, 105204. [Google Scholar] [CrossRef]
- Gong, D.; Bi, Y.; Zong, Y.; Li, Y.; Sionov, E.; Prusky, D. Dynamic change of carbon and nitrogen sources in colonized apples by Penicillium expansum. Foods 2022, 11, 3367. [Google Scholar] [CrossRef]
- Lattanzio, V. Bioactive polyphenols: Their role in quality and storability of fruit and vegetables. J. Appl. Bot. 2003, 77, 128–146. [Google Scholar]
- Mesías, M.; Navarro, M.; Martínez-Saez, N.; Ullate, M.; Del Castillo, M.D.; Morales, F.J. Antiglycative and carbonyl trapping properties of the water-soluble fraction of coffee silverskin. Food Res. Int. 2014, 62, 1120–1126. [Google Scholar] [CrossRef]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Ritieni, A. In vitro bioaccessibility and antioxidant activity of coffee silverskin polyphenolic extract and characterization of bioactive compounds using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 2132. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gomez, B.; Lezama, A.; Amigo-Benavent, M.; Ullate, M.; Herrero, M.; Martín, M.Á.; Mesa, M.D.; Del Castillo, M.D. Insights on the health benefits of the bioactive compounds of coffee silverskin extract. J. Funct. Foods 2016, 25, 197–207. [Google Scholar] [CrossRef]
- Moreira, M.D.R.; Ponce, A.G.; del Valle, C.E.; Ansorena, R.; Roura, S.I. Effects of abusive temperatures on the postharvest quality of lettuce leaves: Ascorbic acid loss and microbial growth. J. Appl. Hortic. 2006, 8, 109–113. [Google Scholar] [CrossRef]
- Przekwas, J.; Wiktorczyk, N.; Budzyńska, A.; Wałecka-Zacharska, E.; Gospodarek-Komkowska, E. Ascorbic acid changes growth of food-borne pathogens in the early stage of biofilm formation. Microorganisms 2020, 8, 553. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Ożarowski, M. Plant organic acids as natural inhibitors of foodborne pathogens. Appl. Sci. 2024, 14, 6340. [Google Scholar] [CrossRef]
- Plaskova, A.; Mlcek, J. New insights on the application of water or ethanol-water plant extract rich in active compounds in food. Front. Nutr. 2023, 10, 1118761. [Google Scholar] [CrossRef]
- Inácio, H.P.; Santetti, G.S.; Dacoreggio, M.V.; da Silva Haas, I.C.; Baranzelli, J.; Emanuelli, T.; Hoff, R.B.; Kempka, A.P.; Fritzen Freire, C.B.; de Mello Castanho Amboni, R.D. Effects of different extraction methods on the phenolic profile, antioxidant and antimicrobial activity of the coffee grounds and coffee silverskin (Coffea arabica L.). JSFA Rep. 2023, 3, 354–363. [Google Scholar] [CrossRef]
- Chaves-Ulate, C.; Rodríguez-Sánchez, C.; Arias-Echandi, M.L.; Esquivel, P. Antimicrobial activities of phenolic extracts of coffee mucilage. JNFS 2023, 31, 50–56. [Google Scholar] [CrossRef]
- Ziemah, J.; Ullrich, M.S.; Kuhnert, N. Antibacterial activity potential of industrial food production waste extracts against pathogenic bacteria: Comparative analysis and characterization. Foods 2024, 13, 1902. [Google Scholar] [CrossRef]
- Castro-Díaz, R.; Silva-Beltrán, N.P.; Gámez-Meza, N.; Calderón, K. The antimicrobial effects of coffee and by-products and their potential applications in healthcare and agricultural sectors: A state-of-the-art review. Microorganisms 2025, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Kabir, F.; Katayama, S.; Tanji, N.; Nakamura, S. Antimicrobial effects of chlorogenic acid and related compounds. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 359–365. [Google Scholar]
- Feng, S.; Zeng, W.; Luo, F.; Zhao, J.; Yang, Z.; Sun, Q. Antibacterial activity of organic acids in aqueous extracts from pine needles (Pinus massoniana Lamb.). Food Sci. Biotechnol. 2010, 19, 35–41. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Mir, M.M.; Dar, B.N.; Greiner, R.; Roohinejad, S. Microbiological contamination of ready-to-eat vegetable salads in developing countries and potential solutions in the supply chain to control microbial pathogens. Food Control 2018, 85, 235–244. [Google Scholar] [CrossRef]



| Time (min) | Eluent A (%) | Eluent B (%) | Flow Rate (mL/min) |
|---|---|---|---|
| Initial | 98.00 | 2.00 | 0.40 |
| 3.00 | 80.00 | 20.00 | 0.40 |
| 9.00 | 50.00 | 50.00 | 0.40 |
| 14.00 | 50.00 | 50.00 | 0.40 |
| 16.00 | 80.00 | 20.00 | 0.40 |
| 18.00 | 95.00 | 5.00 | 0.40 |
| 20.00 | 95.00 | 5.00 | 0.40 |
| Attribute | Description Attribute | Description Score |
|---|---|---|
| Visual | ||
| Appearance | Evaluation related to the perception of fresh and freshly cut vegetables: Intensity of white and brightness, draining of surfaces, sheath and stem intact and free from defects. | 0 Total absence of required visual attributes (not acceptable); 5 slight perceptions of (acceptability limit); 9 Presence of visual attributes like as fresh vegetable perception (excellent acceptability). |
| Browning | Browning on cutting surface, sheath, and stem | 0 No browning (excellent acceptability); 5 Modest browning (acceptable limit); 9 Severe browning (not acceptable). |
| Olfactory | ||
| Aroma | The aroma of freshly cut fennel associated with fresh and herbaceous | 0 No presence (not acceptable); 5 Moderate Presence (limit acceptability); 9 Intense aromas (excellent acceptability) |
| Structural | ||
| Crunchiness | Sensory attributes associated with firmness, fractureability, and density | 0 No crunchy (not acceptable); 5 Moderate crunchy (limit acceptability); 9 Intense crunchy (excellent acceptability) |
| Dehydration | Dehydration of tissues | 0 No Dehydration (excellent acceptability); 5 Moderate Dehydration (limit acceptability); 9 Severe Dehydration (not acceptable) |
| Total Bioactive Compounds | |||
|---|---|---|---|
| TPC (mg GAE 100 mL−1) | TFC (mg ECE 100 mL −1) | TTC (mg TAE 100 mL−1) | |
| 234.74 | 18.07 | 2.94 | |
| Phenolic Acids (mg 100 mL−1) | |||
| Chlorogenic acid | Caffeic Acid | ||
| 33.99 | 15.21 | ||
| Antioxidant Assays (mmolTE 100 mL−1) | |||
| ABTS | DPPH | FRAP | |
| 33.42 | 21.99 | 5.75 | |
| Parameter | Sample | Time (Days) | Sign. | |||
|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 14 | |||
| L* | CTR | 70.27 ± 2.10 A | 68.90 ± 2.69 bAB | 69.65 ± 1.94 bA | 66.55 ± 1.41 bB | * |
| AA | 70.42 ± 1.18 B | 73.64 ± 2.25 aA | 73.08 ± 2.07 aA | 74.36 ± 2.49 aA | * | |
| CS5 | 73.84 ± 4.59 | 73.48 ± 2.65 a | 73.94 ± 2.74 a | 75.80 ± 3.94 a | n.s. | |
| CS10 | 70.15± 1.96 B | 74.85 ± 2.25 aA | 74.91 ± 2.10 aA | 75.27 ± 2.02 aA | * | |
| Sign. | n.s. | ** | ** | ** | ||
| a* | CTR | −1.1 ± 0.06 bC | −0.57 ± 0.04 abB | 0.29 ± 0.04 aA | 0.39 ± 0.02 aA | ** |
| AA | −1.06 ± 0.05 bB | −0.91 ± 0.05 aB | −0.16 ± 0.02 bA | −0.13 ± 0.07 bA | ** | |
| CS5 | −0.74 ± 0.04 a | −0.59 ± 0.09 ab | −0.66 ± 0.05 c | −0.71 ± 0.04 c | n.s. | |
| CS10 | −0.51 ± 0.04 a | −0.34 ± 0.02 b | −0.38 ± 0.09 ab | −0.59 ± 0.05 c | n.s. | |
| Sign. | * | * | ** | ** | ||
| b* | CTR | 11.82 ± 3.37 | 11.47 ± 2.90 | 10.93 ± 2.33 ab | 12.35 ± 2.64 | n.s. |
| AA | 11.43 ± 2.91 | 10.66 ± 2.95 | 10.99 ± 2.32 a | 12.32 ± 3.23 | n.s. | |
| CS5 | 11.79 ± 2.39 | 11.66 ± 2.83 | 10.84 ± 2.41ab | 10.59 ± 3.83 | n.s. | |
| CS10 | 11.61 ± 2.71 | 8.24 ± 4.44 | 9.00 ± 1.99 b | 11.62 ± 3.32 | n.s. | |
| Sign. | n.s. | n.s. | * | n.s. | ||
| Parameter | Sample | Time (Days) | Sign. | |||
|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 14 | |||
| Malic acid (mg 100 g−1) | CTR | 165.11 ± 0.91 bB | 174.88 ± 1.88 bA | 165.08 ± 0.94 bB | 152.34 ± 0.56 cC | ** |
| AA | 166.55 ± 1.37 bB | 182.76 ± 0.94 aA | 171.47 ± 1.88 bB | 171.02 ± 2.62 bB | ** | |
| CS5 | 199.39 ± 3.60 aA | 183.92 ± 1.30 aB | 182.84 ± 3.38 aB | 178.92 ± 1.00 aB | ** | |
| CS10 | 192.13 ± 3.14 a | 180.14 ± 0.21 a | 180.98 ± 1.64 a | 179.32 ± 0.94 a | ** | |
| Sign | ** | ** | ** | ** | ||
| Oxalic acid (mg 100 g−1) | CTR | 150.28 ± 1.82 abB | 140.51 ± 0.04 Cb | 169.3 ± 4.92 abA | 164.84 ± 9.14 Ba | * |
| AA | 156.13 ± 7.42 ab | 147.03 ± 5.36 c | 145.58 ± 7.17 c | 138.00 ± 3.84 c | n.s. | |
| CS5 | 160.15 ± 3.77 ab | 158.88 ± 2.02 b | 155.57 ± 3.76 bc | 161.22 ± 1.23 b | n.s. | |
| CS10 | 187.44 ± 13.62 a | 174.14 ± 0.93 a | 179.58 ± 0.45 a | 192.9 ± 0.38 a | n.s. | |
| Sign | * | ** | ** | ** | ||
| Citric acid (mg 100 g−1) | CTR | 165.34 ± 2.11 aB | 162.9 ± 3.28 Aa | 147.22 ± 0.16 bB | 117.59 ± 4.17 Ac | ** |
| AA | 146.6 ± 8.28 aB | 122.6 ± 6.16 abB | 101.85 ± 0.41 dc | 103.47 ± 7.84 aB | ** | |
| CS5 | 186.57 ± 1.95 aA | 181.97 ± 5.77 Aa | 164.27 ± 0.94 abB | 160.29 ± 0.24 bB | ** | |
| CS10 | 195.24 ± 5.63 Aa | 180.69 ± 5.28 aB | 187.81 ± 14.83 aB | 173.57 ± 3.87 bB | n.s. | |
| Sign | ** | ** | ** | ** | ||
| Parameter | Sample | Time of Storage | Sign. | |||
|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 14 | |||
| TBC | CTR | 3.11 ± 0.02 D | 4.83 ± 0.11 C | 5.98 ± 0.18 Ba | 7.01 ± 0.02 Aa | ** |
| AA | 2.99 ± 0.16 D | 4.79 ± 0.08 C | 5.28 ± 0.03 Ba | 6.49 ± 0.03 Aab | ** | |
| CS5 | 3.04 ± 0.12 D | 4.77 ± 0.21 C | 5.33 ± 0.09 Ba | 6.36 ± 0.08 Ab | ** | |
| CS10 | 2.95 ± 0.07 C | 5.04 ± 0.22 B | 4.84 ± 0.12 Bb | 6.37 ± 0.11 Ab | ** | |
| Sign. | n.s. | n.s. | ** | * | ||
| Yeast | CTR | 0.00 ± 0.00 C | 1.79 ± 0.17 B | 1.89 ± 0.17 B | 2.84 ± 0.34 A | ** |
| AA | 0.00 ± 0.00 C | 1.95 ± 0.24 B | 1.86 ± 0.28 B | 2.85 ± 0.21 A | ** | |
| CS5 | 0.00 ± 0.00 C | 1.70 ± 0.01 B | 1.96 ± 0.24 B | 2.20 ± 0.71 A | ** | |
| CS10 | 0.00 ± 0.00 B | 1.80 ± 0.17 A | 1.86 ± 0.28 A | 2.35 ± 0.92 A | ** | |
| Sign. | n.s. | n.s. | n.s. | n.s. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boninsegna, M.A.; De Bruno, A.; Giacondino, C.; Piscopo, A.; Crea, G.; Chinè, V.; Poiana, M. Use of Coffee Roasting By-Products (Coffee Silverskin) as Natural Preservative for Fresh-Cut Fennel Slices. Foods 2025, 14, 1493. https://doi.org/10.3390/foods14091493
Boninsegna MA, De Bruno A, Giacondino C, Piscopo A, Crea G, Chinè V, Poiana M. Use of Coffee Roasting By-Products (Coffee Silverskin) as Natural Preservative for Fresh-Cut Fennel Slices. Foods. 2025; 14(9):1493. https://doi.org/10.3390/foods14091493
Chicago/Turabian StyleBoninsegna, Miriam Arianna, Alessandra De Bruno, Corinne Giacondino, Amalia Piscopo, Giuseppe Crea, Valerio Chinè, and Marco Poiana. 2025. "Use of Coffee Roasting By-Products (Coffee Silverskin) as Natural Preservative for Fresh-Cut Fennel Slices" Foods 14, no. 9: 1493. https://doi.org/10.3390/foods14091493
APA StyleBoninsegna, M. A., De Bruno, A., Giacondino, C., Piscopo, A., Crea, G., Chinè, V., & Poiana, M. (2025). Use of Coffee Roasting By-Products (Coffee Silverskin) as Natural Preservative for Fresh-Cut Fennel Slices. Foods, 14(9), 1493. https://doi.org/10.3390/foods14091493










