A Comprehensive Study on the Development of an Isotonic Sports Drink Preserved by UV-C Light Assisted by Mild Heat and Loaded with Yerba Mate (Ilex paraguariensis St. Hill.) Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Isotonic Sports Drink Design
Osmolality Study
2.3. Isotonic Sports Drink Processing
Validation Study
2.4. Yerba Mate Extract Development
2.5. Yerba Mate Extract Addition to the Isotonic Sports Drink
2.6. Sensory Studies
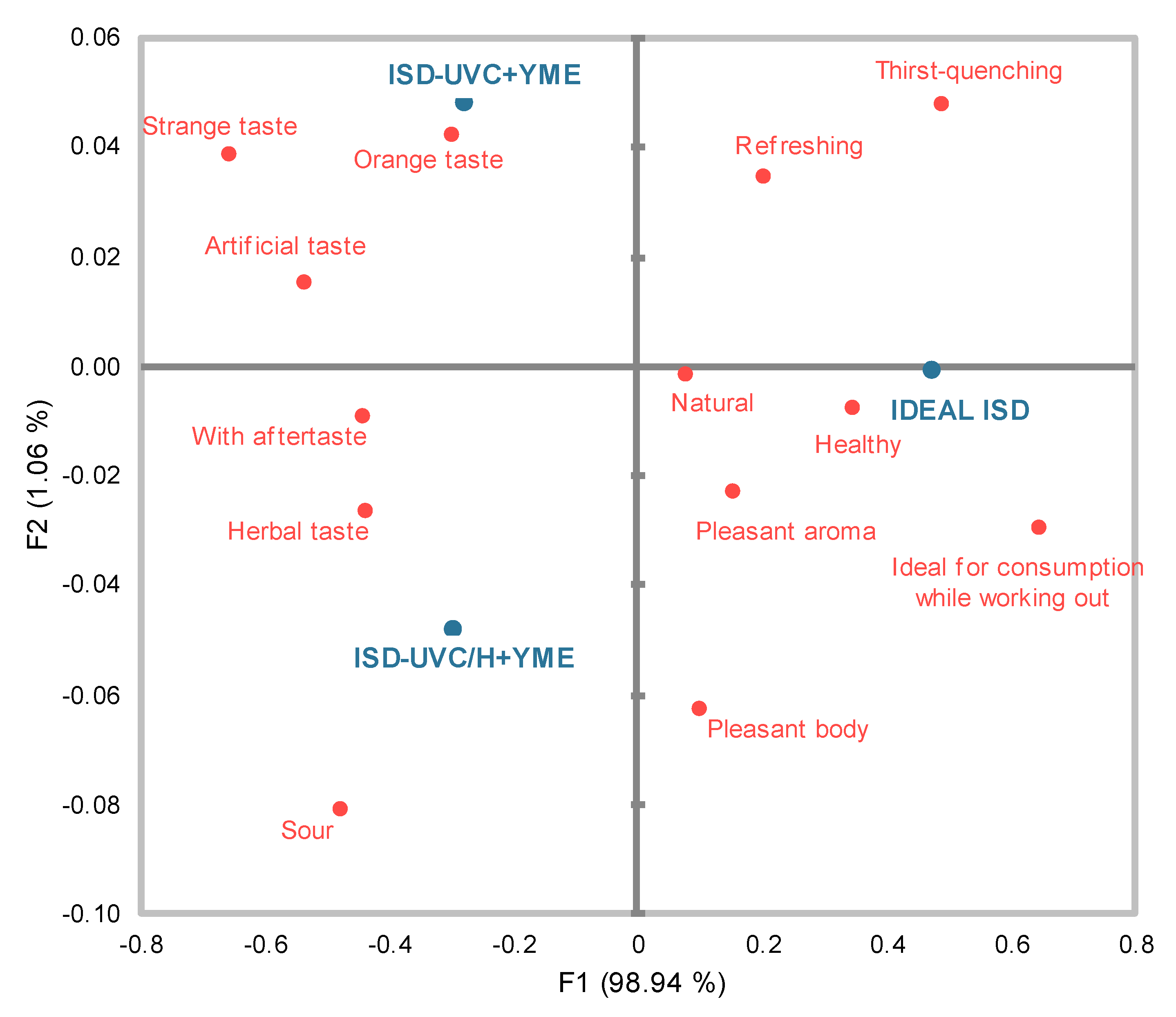
2.6.1. Consumer Field Test
2.6.2. Check-All-That-Apply Test
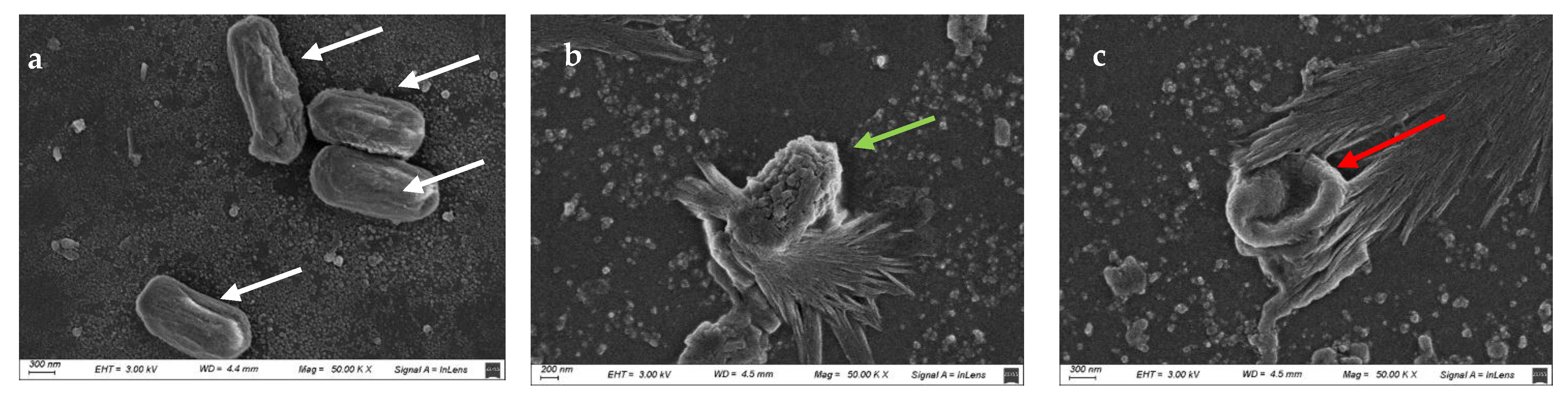
2.7. Study of the Morphological Damage Induced by the UV-C/H Treatment
2.8. ISD-UVC/H+YME Storage Studies
2.8.1. Native Microbiota Study
2.8.2. Physicochemical Characterization
2.8.3. Turbiscan Stability Index
2.9. Statistical Analysis
3. Results and Discussion
3.1. Isotonic Sports Drink Design
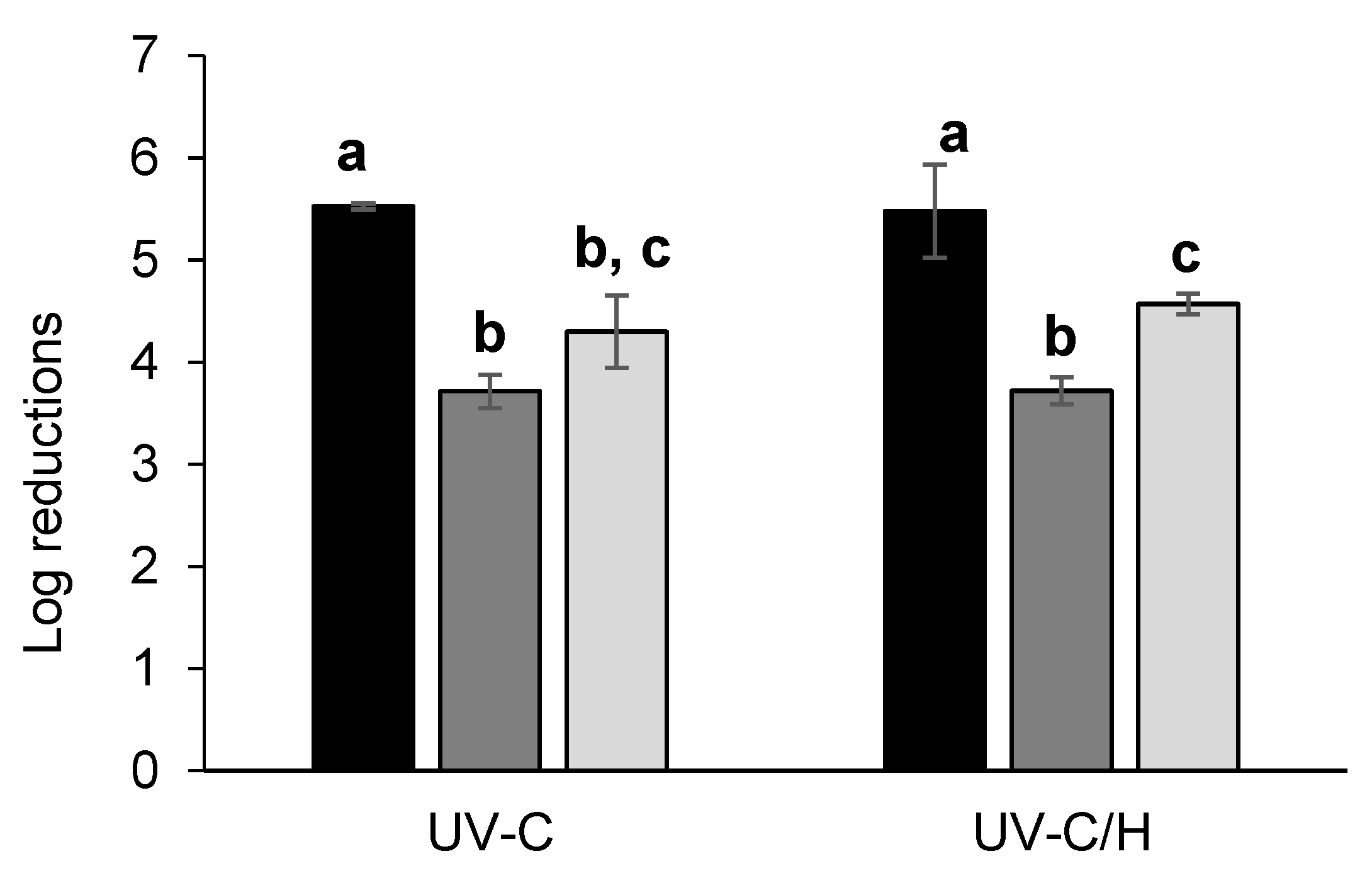
3.2. Selection of the UV-C Treatment
3.2.1. Validation Study
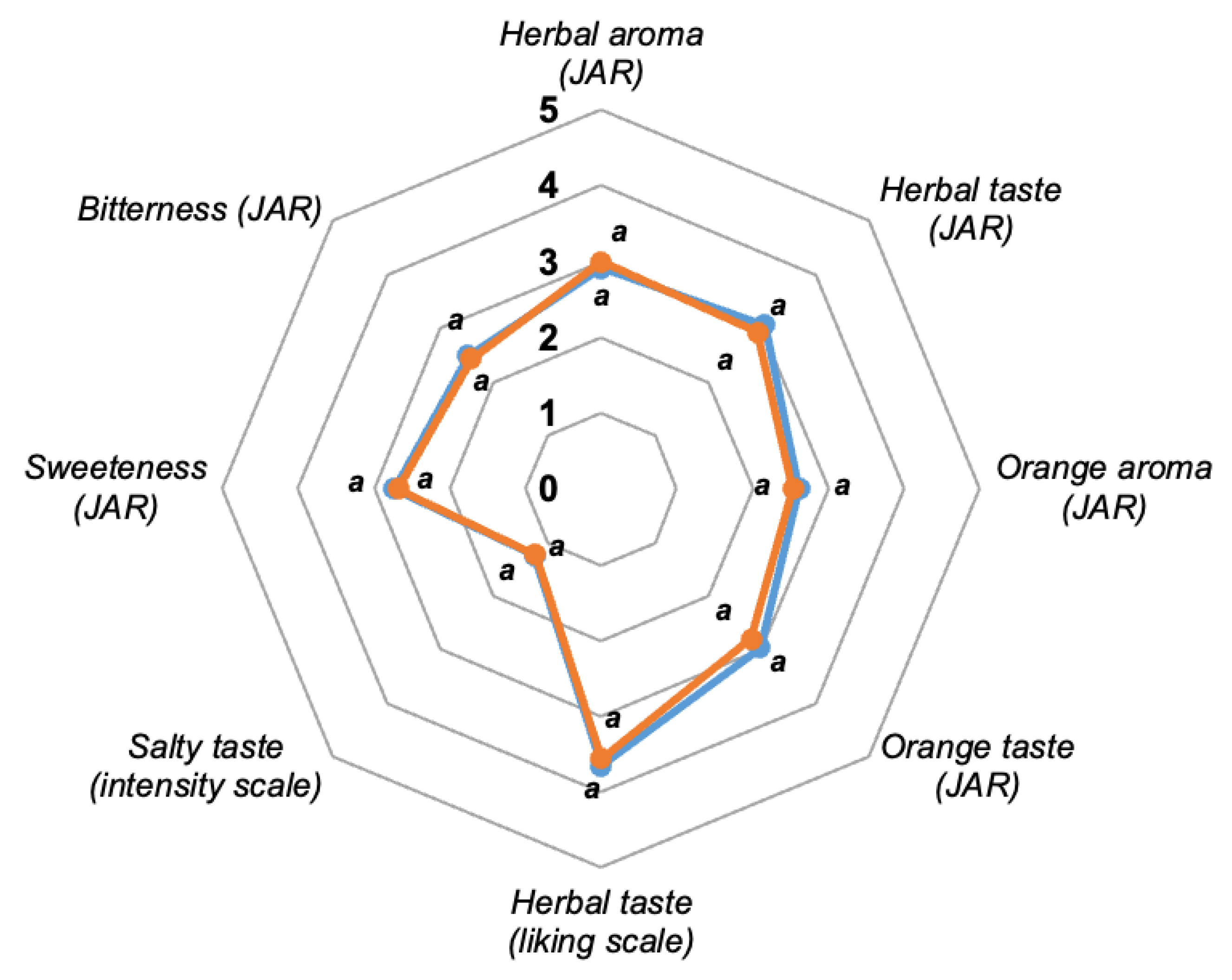
3.2.2. Sensory Studies
Consumer Field Test
Check-All-That-Apply Test
3.3. UV-C/H Induced Damage
3.4. Storage Stability Studies
3.4.1. Native Microbiota Evolution
3.4.2. Physicochemical Parameters
3.4.3. Particle Sedimentation Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beverage Trends: Global Market Overview. Available online: https://www.innovamarketinsights.com/trends/beverage-trends/?_gl=1*oatgiu*_up*MQ..*_ga*MTg5MjA3NzgzMi4xNzE4MDQ0NzQ4*_ga_N8YDYBR8H0*MTcxODA0NDc0Ny4xLjEuMTcxODA0NDgzNS4wLjAuMA (accessed on 18 June 2024).
- Sadowska, A.; Świderski, F.; Laskowski, W. Osmolality of components and their Application in the design of functional recovery drinks. Appl. Sci. 2020, 10, 7663. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, Y.; Qu, X.; Zhou, L.; Wang, Z.; Gao, Z.; Yuan, Y. Development of a colourimetric and fluorescence dual-mode immunoassay for the precise identification of Alicyclobacillus acidoterrestris in apple juice. Food Control 2021, 124, 107898. [Google Scholar] [CrossRef]
- Aneja, K.R.; Dhiman, R.; Aggarwal, N.K.; Aneja, A. Emerging preservation techniques for controlling spoilage and pathogenic microorganisms in fruit juices. Int. J. Microbiol. 2014, 758942. [Google Scholar] [CrossRef]
- Sauer, A.; Moraru, C.I. Inactivation of Escherichia coli ATCC 25922 and Escherichia coli O157:H7 in apple juice and apple cider, using pulsed light treatment. J. Food Protect. 2009, 72, 937–944. [Google Scholar] [CrossRef]
- Guerrero, S.; Ferrario, M.; Schenk, M.; Fenoglio, D.; Andreone, A. Use of short-wave ultraviolet light in a hurdle approach for food preservation design. In Electromagnetic Technologies in Food Science, 1st ed.; López, V.G., Bhat, R., Eds.; John Wiley & Sons Inc.: London, UK, 2021; pp. 128–180. ISBN 978-1119759515. [Google Scholar]
- Koutchma, T. Principles and Applications of UV Light Technology. In Ultraviolet Light in Food Technology: Principles and Applications, 2nd ed.; Koutchma, T., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–48. [Google Scholar] [CrossRef]
- Gök, S.B. UV-C treatment of apple and grape juices by modified UV-C reactor based on dean vortex technology: Microbial, physicochemical and sensorial parameters evaluation. Food Bioproc. Tech. 2021, 14, 1055–1066. [Google Scholar] [CrossRef]
- Fenoglio, D.; Ferrario, M.; Schenk, M.; Guerrero, S. UV-C light inactivation of single and composite microbial populations in a tangerine-orange juice blend. Evaluation of some physicochemical parameters. Food Bioprod. Process. 2019, 117, 149–159. [Google Scholar] [CrossRef]
- Ferrario, M.; Schenk, M.; García Carrillo, M.; Guerrero, S. Development and quality assessment of a turbid carrot-orange juice blend processed by UV-C light assisted by mild heat and addition of Yerba Mate (Ilex paraguariensis) extract. Food Chem. 2018, 269, 567–576. [Google Scholar] [CrossRef]
- La Cava, E.; Sgroppo, S. Combined effect of UV-C light and mild heat on microbial quality and antioxidant capacity of grapefruit juice by flow continuous reactor. Food Bioproc. Tech. 2019, 12, 645–653. [Google Scholar] [CrossRef]
- Fenoglio, D.; Ferrario, M.; Schenk, M.; Guerrero, S. Effect of pilot-scale UV-C light treatment assisted by mild heat on E. coli, L. plantarum, and S. cerevisiae inactivation in clear and turbid fruit juices. Storage study of surviving populations. Int. J. Food Microbiol. 2020, 332, 108767. [Google Scholar] [CrossRef]
- Gouma, M.; Álvarez, I.; Condón, S.; Gayán, E. Pasteurization of carrot juice by combining UV-C and mild heat: Impact on shelf-life and quality compared to conventional thermal treatment. Innov. Food Sci. Emerg. Technol. 2020, 64, 102362. [Google Scholar] [CrossRef]
- Valerga, J.; Reta, M.; Lanari, M. Polyphenol input to the antioxidant activity of yerba mate (Ilex paraguariensis) extracts. LWT–Food Sci. Technol. 2012, 45, 28–35. [Google Scholar] [CrossRef]
- Rząsa-Duran, E.; Kryczyk-Poprawa, A.; Drabicki, D.; Podkowa, A.; Sułkowska-Ziaja, K.; Szewczyk, A.; Kała, K.; Opoka, W.; Zięba, P.; Fidurski, M. Yerba Mate as a Source of Elements and Bioactive Compounds with Antioxidant Activity. Antioxidants 2022, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Smith, A. Effects of caffeine on human behaviour. Food Chem. Toxicol. 2002, 40, 1243–1255. [Google Scholar] [CrossRef]
- Yang, S.Z.; Dong, Z.Q.; Yin, C.C.; Yue, H.J.; Gao, W.W.; Yang, F.K. Green synthesis of caffeine based on methylating reagent dimethyl carbonate and environmental friendly separating method. J. Chin. Chem. Soc. 2020, 67, 1715–1720. [Google Scholar] [CrossRef]
- Fenoglio, D.; Ferrario, M.; Andreone, A.; Guerrero, S. Development of an orange-tangerine juice treated by assisted pilot-scale UV-C light and loaded with yerba mate: Microbiological, physicochemical, and dynamic sensory studies. Food Bioproc. Tech. 2022, 15, 915–932. [Google Scholar] [CrossRef]
- Giraldo Pineda, C.; Yamul, D.K.; Navarro, A.S. Effect of flaxseed (Linum usitatissimum L.) flour and yerba mate (Ilex paraguariensis) extract on physicochemical and sensory properties of a gluten-free corn-based snack. J. Food Sci. Technol. 2021, 58, 3890–3901. [Google Scholar] [CrossRef]
- Neis, E.R.; Covinich, M.M.; Scipioni, G.P. Polyphenol content, colour and acceptability of carrot pickles added with yerba mate powder extract. Braz. J. Food Technol. 2022, 25, e2021013. [Google Scholar] [CrossRef]
- Rocha Saraiva, B.; Vital, A.C.; Anjo, F.A.; Rocha Ribas, J.C.; Matumoto Pintro, P.T. Effect of yerba mate (Ilex paraguariensis A. St.-Hil.) addition on the functional and technological characteristics of fresh cheese. J. Food Sci. Tech. 2019, 56, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Kozono, M.L.; Andreone, A.; Schenk, M.; Rivero, R.; Taravini, I.R.; Guerrero, S.N. Ultrasound-assisted extraction of yerba mate leaves for the development of active additives in sports nutrition products. J. Food Process Eng. 2024, 47, e14670. [Google Scholar] [CrossRef]
- Schenk, M.; Ferrario, M.; Schmalko, M.; Rivero, R.; Taravini, I.R.; Guerrero, S.N. Development of extracts obtained from yerba mate leaves with different industrial processing steps: Antimicrobial capacity, antioxidant properties, and induced damage. J. Food Process Preserv. 2021, 45, e15482. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Mena, P.; Moreno, D.A.; García-Viguera, C. Evaluation of sensorial, phytochemical and biological properties of new isotonic beverages enriched with lemon and berries during shelf life. J. Sci. Food Agric. 2014, 94, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Guleria, P.; Chand, P.; Kaushik, A.; Dhawan, A. Role of nutrients and sports drinks on sports performance: A review. Int. J. Physiol. Nutr. Phys. Educ. 2018, 3, 184–189. [Google Scholar]
- U.S. Food and Drug Administration. Code 21CFR179.39 Title 21—Food and Drugs (page 438) Part 179—Irradiation in the Production, Processing and Handling of Food. Subpart B—Radiation and Radiation Sources. Sec. 179.39. Ultraviolet Radiation for the Processing and Treatment of Food. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-179 (accessed on 15 March 2025).
- Rahn, R.O. Potassium iodide as a chemical actinometer for 254 nm radiation: Use of iodate as an electron scavenger. Photochem. Photobiol. 1997, 66, 450–455. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Code 21CFR120.24, Title 21—Food and Drugs, Part 120—Hazard Analysis and Critical Control Point (HACCP) Systems. Subchapter B—Pathogen Reduction. Sec. 120.24. Process Controls. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-120 (accessed on 15 March 2025).
- Kozono, M.L.; Fenoglio, D.; Ferrario, M.; Guerrero, S.N. Inactivation of Alicyclobacillus acidoterrestris spores, single or composite Escherichia coli and native microbiota in isotonic fruit-flavoured sports drinks processed by UV-C light. Int. J. Food Microbiol. 2023, 386, 110024. [Google Scholar] [CrossRef]
- Andriolli Ribeiro, J.; Magri, E.; Gonçalves, I.L.; Paese, K.; Roman, J.; Valduga, A.T. Photoprotector effect of emulsions with Yerba-Mate (Ilex paraguariensis) extract. Sci. Pharm. 2023, 91, 22. [Google Scholar] [CrossRef]
- ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms Intended for the Sensory Analysis of Products. ISO: Geneva, Switzerland, 2007.
- Lawless, H.; Heymann, H. Consumer field tests and questionnaire design. In Sensory Evaluation of food Principles and Practices, 2nd ed.; Lawless, H.T., Heymann, H., Eds.; Springer: New York, NY, USA, 2010; pp. 349–378. [Google Scholar]
- Vidal, L.; Ares, G.; Hedderley, D.I.; Meyners, M.; Jaeger, S.R. Comparison of rate-all-that-apply (RATA) and check-all-that-apply (CATA) questions across seven consumer studies. Food Qual. Prefer. 2018, 67, 49–58. [Google Scholar] [CrossRef]
- Bruzzone, F.; Vidal, L.; Antúnez, L.; Giménez, A.; Deliza, R.; Ares, G. Comparison of intensity scales and CATA questions in new product development: Sensory characterization and directions for product reformulation of milk desserts. Food Qual. 2015, 44, 183–193. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Ravento’s, R.M. Analysis of total phenols and other oxidation substances and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Laouini, S.E.; Segni, L.; Ridha, O.M. Comparative study of the antioxidant activity and phytochemical composition of leaves extract between three varieties of date palm tree. Int. Lett. Chem. Phys. Astron. 2013, 14, 162–171. [Google Scholar] [CrossRef]
- Rattanathanalerk, M.; Chiewchan, N.; Srichumpoung, W. Effect of thermal processing on the quality loss of pineapple juice. J. Food Eng. 2005, 66, 259–265. [Google Scholar] [CrossRef]
- Liu, C.; Bhattarai, M.; Mikkonen, K.S.; Heinonen, M. Effects of enzymatic hydrolysis of fava bean protein isolate by alcalase on the physical and oxidative stability of oil-in-water emulsions. J. Agric. Food Chem. 2019, 67, 6625–6632. [Google Scholar] [CrossRef]
- Brouns, F.; Kovacs, E. Functional drinks for athletes. Trends Food Sci. Technol. 1997, 8, 414–421. [Google Scholar] [CrossRef]
- Fenoglio, D.; Ferrario, M.; García Carrillo, M.; Schenk, M.; Guerrero, S. Characterization of microbial inactivation in clear and turbid juices processed by short-wave ultraviolet light. J. Food Process. Pres. 2020, 44, e14452. [Google Scholar] [CrossRef]
- Gómez-Sánchez, D.L.; Antonio-Gutiérrez, O.; López-Díaz, A.S.; Palou, E.; López-Malo, A.; Ramírez-Corona, N. Performance of combined technologies for the inactivation of Saccharomyces cerevisiae and Escherichia coli in pomegranate juice: The effects of a continuous-flow UV-Microwave system. J. Food Process Eng. 2020, 43, e13565. [Google Scholar] [CrossRef]
- Steyn, C.E.; Cameron, M.; Witthuhn, R.C. Occurrence of Alicyclobacillus in the fruit processing environment—A review. Int. J. Food Microbiol. 2011, 147, 1–11. [Google Scholar] [CrossRef]
- Sauceda-Gálvez, J.N.; Tió-Coma, M.; Martínez-García, M.; Hernández-Herrero, M.M.; Gervilla, R.; Roig-Sagués, A.X. Effect of single and combined UV-C and ultra-high pressure homogenization treatments on inactivation of Alicyclobacillus acidoterrestris spores in apple juice. Innov. Food Sci. Emerg. Technol. 2020, 60, 102299. [Google Scholar] [CrossRef]
- Tremarin, A.; Brandão, T.R.; Silva, C.L. Application of ultraviolet radiation and ultrasound treatments for Alicyclobacillus acidoterrestris spores inactivation in apple juice. LWT 2017, 78, 138–142. [Google Scholar] [CrossRef]
- Ferreira, T.V.; Mizuta, A.G.; de Menezes, J.L.; Dutra, T.V.; Bonin, E.; Castro, J.C.; de Abreu Filho, B.A. Effect of ultraviolet treatment (UV–C) combined with nisin on industrialized orange juice in Alicyclobacillus acidoterrestris spores. LWT 2020, 133, 109911. [Google Scholar] [CrossRef]
- Van Luong, T.S.; Moir, C.; Chandry, P.S.; Pinfold, T.; Olivier, S.; Broussolle, V.; Bowman, J.P. Combined high pressure and heat treatment effectively disintegrates spore membranes and inactivates Alicyclobacillus acidoterrestris spores in acidic fruit juice beverage. Innov. Food Sci. Emerg. Technol. 2020, 66, 102523. [Google Scholar] [CrossRef]
- Health Protection Agency. Guidelines for Assessing the Microbiological Safety of Ready-to-Eat Foods; Health Protection Agency: London, UK, 2009. Available online: https://assets.publishing.service.gov.uk/media/5a7efde0e5274a2e8ab497a4/Guidelines_for_assessing_the_microbiological_safety_of_ready-to-eat_foods_on_the_market.pdf (accessed on 18 June 2024).
- Santana-Jiménez, A.Z.; Quintero-Ramos, A.; Sánchez-Madrigal, M.Á.; Meléndez-Pizarro, C.O.; Valdez-Cárdenas, M.d.C.; Orizaga-Heredia, M.d.R.; Méndez-Zamora, G.; Talamás-Abbud, R. Effects of UV-C irradiation and thermal processing on the microbial and physicochemical properties of Agave tequilana weber var. azul extracts at various pH values. Processes 2020, 8, 841. [Google Scholar] [CrossRef]
- Türkmen, F.U.; Takci, H.A. Ultraviolet-C and ultraviolet-B lights effect on black carrot (Daucus carota ssp. sativus) juice. J. Food Meas. Charact. 2018, 12, 1038–1046. [Google Scholar] [CrossRef]
- Porfírio, M.C.; Gonçalves, M.S.; Borges, M.V.; Leite, C.X.; Santos, M.R.; Da Silva, A.G.; Fontan, G.C.; Leão, D.J.; De Jesus, R.M.; Gualberto, S.A.; et al. Development of isotonic beverage with functional attributes based on extract of Myrciaria jabuticaba (Vell) Berg. Food Sci. Technol. 2020, 40, 614–620. [Google Scholar] [CrossRef]
- Ros-Polski, V.; Popović, V.; Koutchma, T. Effect of ultraviolet-C light treatment on hydroxymethylfurfural (5-HMF) content in high fructose corn syrup (HFCS) and model syrups. J. Food Eng. 2016, 179, 78–87. [Google Scholar] [CrossRef]
- Choudhary, A.; Kumar, V.; Kumar, S.; Majid, I.; Aggarwal, P.; Suri, S. 5-Hydroxymethylfurfural (HMF) formation, occurrence and potential health concerns: Recent developments. Toxin Rev. 2021, 40, 545–561. [Google Scholar] [CrossRef]
- AIJN. Code of Practice for Evaluation of Fruit and Vegetable Juices. Association of the Industry of Juices and Nectars from Fruits and Vegetables of the European Union; AIJN: Brussels, Belgium, 2001. [Google Scholar]
- Yıkmış, S.; Gök, S.B.; Levent, O.; Kombak, E. Moderate temperature and UV-C light processing of Uruset apple juice: Optimization of bioactive components and evaluation of the impact on volatile profile, HMF and colour. J. Food Process Eng. 2021, 44, e13893. [Google Scholar] [CrossRef]
- Santhirasegaram, V.; Razali, Z.; Somasundram, C. Effects of sonication and ultraviolet-C treatment as a hurdle concept on quality attributes of Chokanan mango (Mangifera indica L.). juice. Food Sci. Technol. Int. 2015, 21, 232–241. [Google Scholar] [CrossRef]
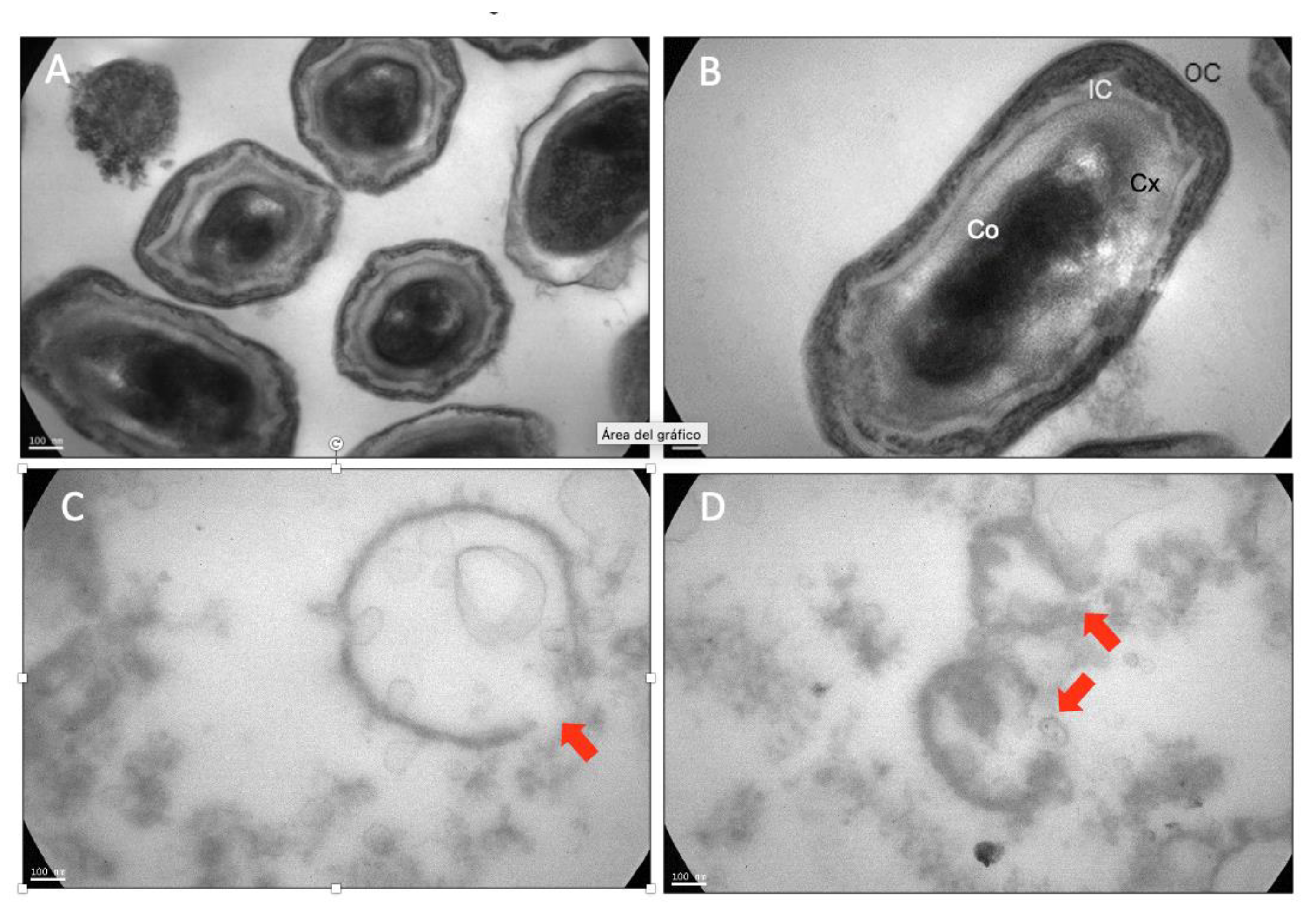
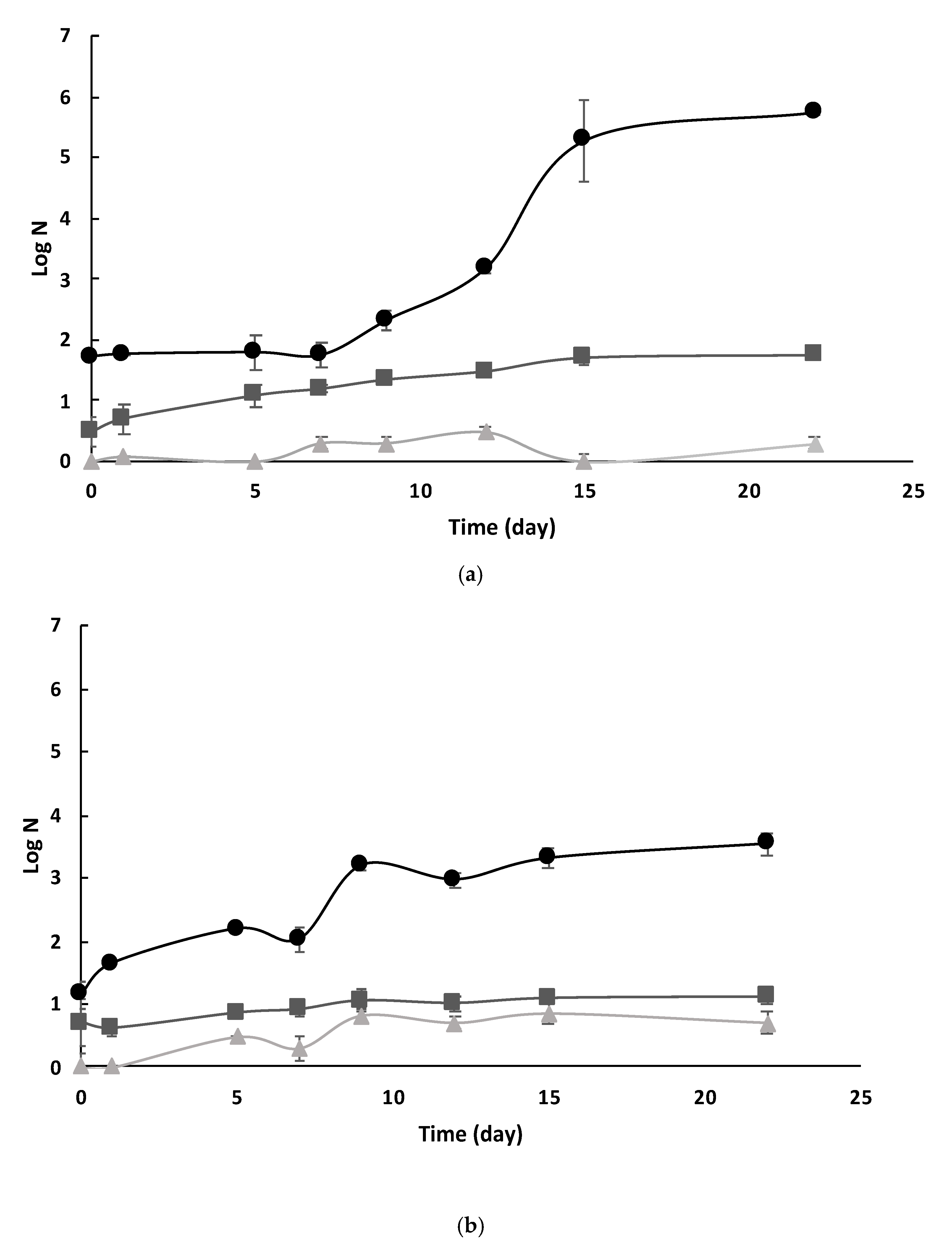

| (a) | ||||||||
| System | Storage Time (Day) | pH | TSS (°Brix) | Turbidity (NTU) | L* | a* | b* | General Aspect |
| ISD-C | 0 | 3.77 ± 0.01 a | 6.9 ± 0.1 a | 1494 ± 93 a | 36.30 ± 0.19 a,b | −2.93 ± 0.44 a | 27.94 ± 1.04 a |  |
| 5 | 3.84 ± 0.01 a | 6.8 ± 0.2 a | 1469 ± 426 a | 39.88 ± 0.22 a | −4.29 ± 0.17 b | 26.36 ± 0.91 a,b | ||
| 9 | 3.87 ± 0.01 a | 6.8 ± 0.1 a | 1834 ± 118 a | 39.75 ± 0.20 a | −4.29 ± 0.09 b | 26.40 ± 0.18 a,b | ||
| 15 | 3.82 ± 0.01 a | 6.4 ± 0.2 b | 1574 ± 73 a | 38.17 ± 0.09 a,b | −3.80 ± 0.01 b | 26.72 ± 0.18 a,b | ||
| 23 | 3.80 ± 0.01 a | 6.6 ± 0.2 a,b | 1682 ± 70 a | 35.76 ± 0.15 b | −2.83 ± 0.29 a | 25.49 ± 0.19 b | ||
| ISD-UVC/H | 0 | 3.76 ± 0.01 a | 6.7 ± 0.2 a,b | 1584 ± 165 a | 36.26 ± 0.18 b | −3.20 ± 0.21 a | 26.66 ± 0.32 a |  |
| 5 | 3.84 ± 0.01 a | 7.0 ± 0.2 a | 1599 ± 142 a | 39.72 ± 0.18 a | −4.04 ± 0.07 b | 26.07 ± 0.35 a | ||
| 9 | 3.85 ± 0.02 a | 6.4 ± 0.2 b,c | 1575 ± 296 a | 39.50 ± 0.16 a | −4.18 ± 0.10 b | 24.99 ± 1.49 a | ||
| 15 | 3.80 ± 0.01 a | 6.1 ± 0.5 a,b,c | 1449 ± 382 a | 38.04 ± 0.29 a,b | −3.56 ± 0.12 a,b | 26.68 ± 0.48 a | ||
| 23 | 3.79 ± 0.01 a | 6.0 ± 0.1 c | 1676 ± 15 a | 35.75 ± 0.21 a,b | −2.89 ± 0.14 a | 25.04 ± 0.21 a | ||
| ISD-UVC/H+YME | 0 | 3.86 ± 0.01 a | 7.1 ± 0.2 a | 2151 ± 106 a | 34.68 ± 0.22 a,b | −2.52 ± 0.18 a | 24.45 ± 0.12 a |  |
| 5 | 3.97 ± 0.02 a | 6.9 ± 0.4 a,b | 1665 ± 158 b | 38.21 ± 0.12 a,b | −3.52 ± 0.11 b | 25.73 ± 0.31 a | ||
| 9 | 4.00 ± 0.03 a | 6.7 ± 0.2 a,b | 2116 ± 337 a | 38.75 ± 0.26 a,b | −3.42 ± 0.18 b | 24.39 ± 1.36 a | ||
| 15 | 3.88 ± 0.01 a | 6.6 ± 0.1 b | 2005 ± 30 a | 36.88 ± 0.09 a,b | −3.13 ± 0.06 b | 25.52 ± 0.84 a | ||
| 23 | 3.89 ± 0.02 a | 6.1 ± 0.4 b | 1951 ± 73 a,b | 33.73 ± 0.55 b | −2.53 ± 0.77 a | 22.64 ± 0.73 b | ||
| (b) | ||||||||
| Sample | Time (Day) | TPC (mg GAE/mL) | TAADPPH (mg Trolox Eq/mL) | TAAABTS (mg Trolox Eq/mL) | FC (mg Catechin Eq/mL) | HMF (mg HMF/L) | ||
| ISD-C | 0 | 0.308 ± 0.001 a | 0.099 ± 0.025 a | 1.348 ± 0.046 a | 0.332 ± 0.014 a | 0.290 ± 0.096 a | ||
| 5 | 0.379 ± 0.005 a | 0.063 ± 0.023 a | 5.026 ± 0.026 b | 0.300 ± 0.052 a | 0.061 ± 0.069 b | |||
| 9 | 0.367 ± 0.015 a | 0.076 ± 0.012 a | 2.710 ± 0.007 c | 0.294 ± 0.058 a | 0.070 ± 0.001 b | |||
| 15 | 0.276 ± 0.016 a | 0.067 ± 0.073 a | 4.170 ± 0.072 d | 0.283 ± 0.070 a | 0.441 ± 0.011 c | |||
| 23 | 0.290 ± 0.024 a | 0.066 ± 0.075 a | 0.834 ± 0.025 e | 0.370 ± 0.029 a | 0.456 ± 0.096 c | |||
| ISD-UVC/H | 0 | 0.401 ± 0.048 a | 0.254 ± 0.077 b | 1.208 ± 0.026 a | 0.335 ± 0.005 a | 0.842 ± 0.169 d | ||
| 5 | 0.399 ± 0.035 a | 0.374 ± 0.007 b | 1.730 ± 0.036 a | 0.273 ± 0.022 a | 0.140 ± 0.107 a | |||
| 9 | 0.444 ± 0.021 a | 0.342 ± 0.015 b | 3.959 ± 0.114 d | 0.288 ± 0.025 a | 0.212 ± 0.051 a | |||
| 15 | 0.347 ± 0.007 a | 0.276 ± 0.020 b | 1.453 ± 0.077 a | 0.173 ± 0.007 a | 0.593 ± 0.051 c | |||
| 23 | 0.358 ± 0.018 a | 0.303 ± 0.041 b | 1.292 ± 0.023 a,e | 0.361 ± 0.007 a | 0.568 ± 0.034 c | |||
| ISD-UVC/H+YME | 0 | 1.149 ± 0.092 b | 2.380 ± 0.068 d | 8.963 ± 0.016 f | 5.541 ± 0.589 b | 1.428 ± 0.271 e | ||
| 5 | 1.420 ± 0.145 c | 1.637 ± 0.255 e | 9.741 ± 0.051 g | 2.197 ± 0.060 c | 1.184 ± 0.304 d,e | |||
| 9 | 1.306 ± 0.349 b,c | 1.477 ± 0.035 e | 9.119 ± 0.028 g | 1.907 ± 0.596 c | 1.096 ± 0.316 d,e | |||
| 15 | 1.080 ± 0.033 b | 0.655 ± 0.058 f | 6.318 ± 0.028 h | 3.756 ± 0.054 d | 0.407 ± 0.086 c | |||
| 23 | 1.416 ± 0.114 c | 1.493 ± 0.124 e | 7.843 ± 0.016 i | 4.434 ± 0.007 d | 0.798 ± 0.186 d | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozono, M.L.; Durán Cassiet, M.; Andreone, A.; Schenk, M.L.; Guerrero, S.N. A Comprehensive Study on the Development of an Isotonic Sports Drink Preserved by UV-C Light Assisted by Mild Heat and Loaded with Yerba Mate (Ilex paraguariensis St. Hill.) Extract. Foods 2025, 14, 1494. https://doi.org/10.3390/foods14091494
Kozono ML, Durán Cassiet M, Andreone A, Schenk ML, Guerrero SN. A Comprehensive Study on the Development of an Isotonic Sports Drink Preserved by UV-C Light Assisted by Mild Heat and Loaded with Yerba Mate (Ilex paraguariensis St. Hill.) Extract. Foods. 2025; 14(9):1494. https://doi.org/10.3390/foods14091494
Chicago/Turabian StyleKozono, María Luz, Magdalena Durán Cassiet, Antonella Andreone, Marcela L. Schenk, and Sandra N. Guerrero. 2025. "A Comprehensive Study on the Development of an Isotonic Sports Drink Preserved by UV-C Light Assisted by Mild Heat and Loaded with Yerba Mate (Ilex paraguariensis St. Hill.) Extract" Foods 14, no. 9: 1494. https://doi.org/10.3390/foods14091494
APA StyleKozono, M. L., Durán Cassiet, M., Andreone, A., Schenk, M. L., & Guerrero, S. N. (2025). A Comprehensive Study on the Development of an Isotonic Sports Drink Preserved by UV-C Light Assisted by Mild Heat and Loaded with Yerba Mate (Ilex paraguariensis St. Hill.) Extract. Foods, 14(9), 1494. https://doi.org/10.3390/foods14091494






