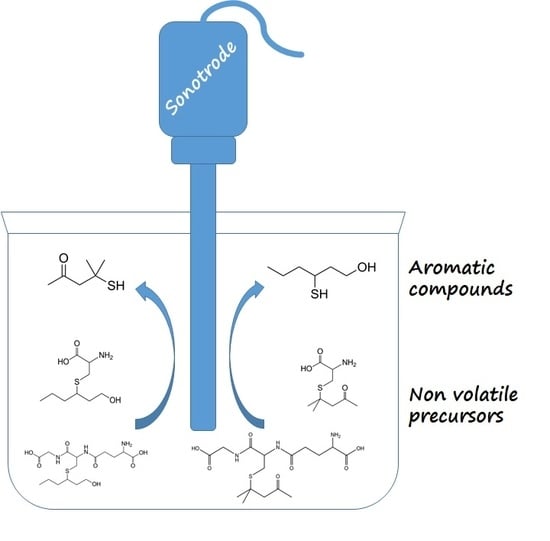
Evidence of the Possible Interaction between Ultrasound and Thiol Precursors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Precursors Concentration in Marc and Juice
2.2. Ultrasound Application in Grape Must
2.3. Ultrasound Application in Model Solution
2.4. Analytical Methods
2.4.1. Reagents
2.4.2. LC-MS/MS Analysis of Thiol Precursors
2.4.3. GC-MS/MS Analysis of Free Thiols
2.4.4. Spectrophotometric and Conductivity Analysis
2.4.5. Quality Control Parameters of Grape Juice
3. Results and Discussion
3.1. Precursors Concentration in Marc and Juice
3.2. Ultrasound Application on Grape Must
3.3. Ultrasound Application in Model Solution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tominaga, T.; Peyrot des Gachons, C.; Dubourdieu, D. A new type of flavor precursors in Vitis vinifera L. cv. Sauvignon Blanc: S-cysteine conjugates. J. Agric. Food Chem. 1998, 46, 5215–5219. [Google Scholar] [CrossRef]
- Fedrizzi, B.; Pardon, K.H.; Sefton, M.A.; Elsey, G.M.; Jeffery, D.W. First identification of 4-S-glutathionyl-4-methylpentan-2-one, a potential precursor of 4-mercapto-4-methylpentan-2-one, in Sauvignon Blanc juice. J. Agric. Food Chem. 2009, 57, 991–995. [Google Scholar] [CrossRef]
- Lund, S.T.; Bohlmann, J. The molecular basis for wine grape quality—A volatile subject. Science 2006, 311, 804–805. [Google Scholar] [CrossRef] [Green Version]
- Roland, A.; Schneider, R.; Charrier, F.; Cavelier, F.; Rossignol, M.; Razungles, A. Distribution of varietal thiol precursors in the skin and the pulp of Melon B. and Sauvignon Blanc grapes. Food Chem. 2011, 125, 139–144. [Google Scholar] [CrossRef]
- Pinu, F.R.; Jouanneau, S.; Nicolau, L.; Gardner, R.C.; Villas-Boas, S.G. Concentrations of the volatile thiol 3-mercaptohexanol in Sauvignon Blanc wines: No correlation with juice precursors. Am. J. Enol. Viticult. 2012, 63, 407–412. [Google Scholar] [CrossRef]
- Larcher, R.; Nicolini, G.; Tonidandel, L.; Román Villegas, T.; Malacarne, M.; Fedrizzi, B. Influence of oxygen availability during skin-contact maceration on the formation of precursors of 3-mercaptohexan-1-ol in Müller-Thurgau and Sauvignon Blanc grapes. Aust. J. Grape Wine Res. 2013, 19, 342–348. [Google Scholar] [CrossRef]
- Allen, T.; Herbst-Johnstone, M.; Girault, M.; Butler, P.; Logan, G.; Jouanneau, S.; Nicolau, L.; Kilmartin, P.A. Influence of grape-harvesting steps on varietal thiol aromas in Sauvignon Blanc wines. J. Agric. Food Chem. 2011, 59, 10641–10650. [Google Scholar] [CrossRef]
- Concejero, B.; Peña-Gallego, A.; Fernandez-Zurbano, P.; Hernández–Orte, P.; Ferreira, V. Direct accurate analysis of cysteinylated and glutathionylated precursors of 4-mercapto-4-methyl-2-pentanone and 3-mercaptohexan-1-ol in must by ultrahigh performance liquid chromatography coupled to mass spectrometry. Anal. Chim. Acta 2014, 812, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Villegas, T.R.; Tonidandel, L.; Fedrizzi, B.; Larcher, R.; Nicolini, G. Novel technological strategies to enhance tropical thiol precursors in winemaking by-products. Food Chem. 2016, 207, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Cordente, A.G.; Borneman, A.R.; Bartel, C.; Capone, D.; Solomon, M.; Roach, M.; Curtin, C.D. Inactivating mutations in Irc7p are common in wine yeasts, attenuating carbon-sulfur β-lyase activity and volatile sulfur compound production. Appl. Environ. Microbiol. 2019, 85, 684–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, R.; Charrier, F.; Razungles, A.; Baumes, R. Evidence for an alternative biogenetic pathway leading to 3-mercaptohexanol and 4-mercapto-4-methylpentan-2-one in wines. Anal. Chim. Acta 2006, 563, 58–64. [Google Scholar] [CrossRef]
- OIV. Resolution OIV-OENO 616-2019; OIV: Geneva, Switzerland, 2019. [Google Scholar]
- Tao, Y.; Sun, D.W. Enhancement of food processes by ultrasound: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 570–594. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Ferrara, L.; Naviglio, D. Application of ultrasound in food science and technology: A perspective. Foods 2018, 7, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacciola, V.; Batllò, I.F.; Ferraretto, P.; Vincenzi, S.; Celotti, E. Study of the ultrasound effects on yeast lees lysis in winemaking. Eur. Food Res. Technol. 2013, 236, 311–317. [Google Scholar] [CrossRef]
- Kulkarni, P.; Loira, I.; Morata, A.; Tesfaye, W.; González, M.C.; Suárez-Lepe, J.A. Use of non-Saccharomyces yeast strains coupled with ultrasound treatment as a novel technique to accelerate ageing on lees of red wines and its repercussion in sensorial parameters. LWT Food Sci. Technol. 2015, 64, 1255–1262. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Wang, T.T. Effect of ultrasound irradiation on the evolution of color properties and major phenolic compounds in wine during storage. Food Chem. 2017, 234, 372–380. [Google Scholar] [CrossRef]
- Schmid, F.; Grbin, P.; Yap, A.; Jiranek, V. Relative efficacy of high pressure hot water and high power ultrasonics for wine oak barrel sanitization. Am. J. Enol. Viticult. 2011, 62, 519–526. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Schmid, F.; Grbin, P.R.; Jiranek, V. Viability of common wine spoilage organisms after exposure to high power ultrasonics. Ultrason. Sonochem. 2012, 19, 415–420. [Google Scholar] [CrossRef]
- Ferraretto, P.; Cacciola, V.; Batlló, I.F.; Celotti, E. Ultrasounds application in winemaking: Grape maceration and yeast lysis. Ital. J. Food Sci. 2013, 25, 160. [Google Scholar]
- Morata, A.; Loira, I.; Vejarano, R.; González, C.; Callejo, M.J.; Suárez-Lepe, J.A. Emerging preservation technologies in grapes for winemaking. Trends Food Sci. Technol. 2017, 67, 36–43. [Google Scholar] [CrossRef]
- Ferraretto, P.; Celotti, E. Preliminary study of the effects of ultrasound on red wine polyphenols. CyTA J. Food 2016, 14, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Larcher, R.; Tonidandel, L.; Villegas, T.R.; Nardin, T.; Fedrizzi, B.; Nicolini, G. Pre-fermentation addition of grape tannin increases the varietal thiols content in wine. Food Chem. 2015, 166, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Method Enzym. 1999, 299, 152–178. [Google Scholar]
- Ribéreau-Gayon, P. Les dosage des composés phénoliques totaux dans les vins rouges. Chim. Anal. 1970, 52, 627–631. [Google Scholar]
- Zironi, R.; Buiatti, S.; Celotti, E. Evaluation of a new colourimetric method for the determination of catechins in musts and wines. Wein-Wissenschaft 1992, 47, 1–7. [Google Scholar]
- Bate-Smith, E.-C. Leuco-anthocyanins. I. Detection and identification of anthocyanin formed from leuco-anthocyanins in plant tissues. Biochem. J. 1954, 11, 1153–1156. [Google Scholar] [CrossRef] [Green Version]
- OIV. Compendium of International Methods of Analysis of Wines and Musts; OIV: Paris, France, 2019; ISBN 978-2-85038-003-7. [Google Scholar]
- Capone, D.L.; Sefton, M.A.; Jeffery, D.W. Application of a modified method for 3-mercaptohexan-1-ol determination to investigate the relationship between free thiol and related conjugates in grape juice and wine. J. Agric. Food Chem. 2011, 59, 4649–4658. [Google Scholar] [CrossRef]
- Román, T.; Tonidandel, T.; Larcher, R.; Celotti, E.; Nicolini, G. Importance of polyfunctional thiols on semi-industrial Gewürztraminer wines and the correlation to technological treatments. Eur. Food Res. Technol. 2018, 244, 379–386. [Google Scholar] [CrossRef]
- Des Gachons, C.P.; Tominaga, T.; Dubourdieu, D. Localization of S-cysteine conjugates in the berry: Effect of skin contact on aromatic potential of Vitis vinifera L. cv. Sauvignon Blanc must. Am. J. Enol. Viticult. 2002, 53, 144–146. [Google Scholar]
- Capone, D.L.; Jeffery, D.W. Effects of transporting and processing Sauvignon Blanc grapes on 3-mercaptohexan-1-ol precursor concentrations. J. Agric. Food Chem. 2011, 59, 4659–4667. [Google Scholar] [CrossRef]
- Lund, C.M.; Thompson, M.K.; Benkwitz, F.; Wohler, M.W.; Triggs, C.M.; Gardner, R.; Hildegarde, H.; Nicolau, L. New Zealand Sauvignon Blanc distinct flavor characteristics: Sensory, chemical, and consumer aspects. Am. J. Enol. Viticult. 2009, 60, 1–12. [Google Scholar]
- Thibon, C.; Dubourdieu, D.; Darriet, P.; Tominaga, T. Impact of noble rot on the aroma precursor of 3-sulfanylhexanol content in Vitis vinifera L. cv Sauvignon Blanc and Semillon grape juice. Food Chem. 2009, 114, 1359–1364. [Google Scholar] [CrossRef]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; van Kan, J.A. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef] [PubMed]
- González-Manzano, S.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Extraction of flavan-3-ols from grape seed and skin into wine using simulated maceration. Anal. Chim. Acta 2004, 513, 283–289. [Google Scholar] [CrossRef]
- Cheynier, V.; Owe, C.; Rigaud, J. Oxidation of grape juice phenolic compounds in model solutions. J. Food Sci. 1988, 53, 1729–1732. [Google Scholar] [CrossRef]
- Delgado-Povedano, M.M.; de Castro, M.L. A review on enzyme and ultrasound: A controversial but fruitful relationship. Anal. Chim. Acta 2015, 889, 1–21. [Google Scholar] [CrossRef]
- Kobayashi, H.; Takase, H.; Suzuki, Y.; Tanzawa, F.; Takata, R.; Fujita, K.; Konho, M.; Mochizuki, M.; Suzuki, S.; Konno, T.; et al. Environmental stress enhances biosynthesis of flavor precursors, S-3-(hexan-1-ol)-glutathione and S-3-(hexan-1-ol)-L-cysteine, in grapevine through glutathione S-transferase activation. J. Exp. Bot. 2010, 62, 1325–1336. [Google Scholar] [CrossRef]
- Henglein, A. Chemical effects of continuous and pulsed ultrasound in aqueous solutions. Ultrason. Sonochem. 1995, 2, S115–S121. [Google Scholar] [CrossRef]
- Wu, Z.; Ondruschka, B.; Stark, A. Ultrasonic cleavage of thioethers. J. Phys. Chem. A 2005, 109, 3762–3766. [Google Scholar] [CrossRef]
- Simons, J. Mechanisms for S–S and N–Cα bond cleavage in peptide ECD and ETD mass spectrometry. Chem. Phys. Lett. 2010, 484, 81–95. [Google Scholar] [CrossRef]
- Celotti, E.; Ferraretto, P. Studies for the ultrasound application in winemaking for a low impact enology. In Proceedings of the 39th World Congress of Vine and Wine 2016, Bento Gonçalves, Brazil, 24–28 October 2016; pp. 104–106, ISBN 979-10-91799-62-1. [Google Scholar]
- Petit, E.; Jacquet, R.; Pouységu, L.; Deffieux, D.; Quideau, S. Reactivity of wine polyphenols under oxidation conditions: Hemisynthesis of adducts between grape catechins or oak ellagitannins and odoriferous thiols. Tetrahedron 2019, 75, 551–560. [Google Scholar] [CrossRef]
- Nikolantonaki, M.; Chichuc, I.; Teissedre, P.L.; Darriet, P. Reactivity of volatile thiols with polyphenols in a wine-model medium: Impact of oxygen, iron, and sulfur dioxide. Anal. Chim. Acta 2010, 660, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Ugliano, M. Oxygen contribution to wine aroma evolution during bottle aging. J. Agric. Food Chem. 2013, 61, 6125–6136. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.N.; Shi, M.C. pH-affectin g sonochemical formation of hydroxyl radicals under 20 kHz ultrasonic irradiation. Environ. Eng. Manag. J. 2010, 20, 245–250. [Google Scholar]
- Winter, G.; Van Der Westhuizen, T.; Higgins, V.J.; Curtin, C.; Ugliano, M. Contribution of cysteine and glutathione conjugates to the formation of the volatile thiols 3-mercaptohexan-1-ol (3MH) and 3-mercaptohexyl acetate (3MHA) during fermentation by Saccharomyces cerevisiae. Aust. J. Grape Wine Res. 2011, 17, 285–290. [Google Scholar] [CrossRef]
- Bonnaffoux, H.; Delpech, S.; Rémond, E.; Schneider, R.; Roland, A.; Cavelier, F. Revisiting the evaluation strategy of varietal thiol biogenesis. Food Chem. 2018, 268, 126–133. [Google Scholar] [CrossRef]




| Grape Sample | °Brix | pH | Titratable Acidity (g/L) | Tartaric Acid (g/L) | Malic Acid (g/L) | Potassium (mg/L) | YAN (mg/L) | GSH-3MH (μg/kg) | Cys-3MH (μg/kg) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Juice | Marc | Juice | Marc | ||||||||
| A | 21.9 | 3.13 | 5.8 | 4.0 | 2.8 | 795 | 168 | 121 | 802 | 19 | 377 |
| B | 23.2 | 2.81 | 10.9 | 9.2 | 3.1 | 1581 | <20 | 57 | 486 | 29 | 534 |
| C | 23.6 | 2.82 | 10.5 | 8.6 | 3.5 | 1552 | <20 | 95 | 436 | 33 | 446 |
| D | 22.8 | 2.85 | 9.3 | 8.3 | 2.6 | 1386 | 41 | 84 | 1555 | 32 | 799 |
| E | 22.7 | 2.81 | 9.7 | 8.9 | 2.4 | 1479 | 24 | 100 | 1055 | 32 | 842 |
| F | 22.2 | 2.83 | 9.2 | 8.3 | 2.6 | 1352 | 23 | 122 | 2388 | 31 | 807 |
| G | 21.5 | 2.86 | 10.0 | 8.4 | 3.8 | 1417 | 120 | 120 | 2049 | 30 | 1175 |
| H | 20.1 | 2.88 | 10.4 | 8.7 | 3.9 | 1476 | 148 | 208 | 2233 | 50 | 953 |
| I | 21.2 | 2.97 | 9.6 | 8.3 | 3.9 | 1490 | 147 | 120 | 932 | 28 | 865 |
| J | 19.9 | 2.88 | 10.2 | 7.7 | 4.1 | 1134 | 131 | 129 | 2306 | 70 | 842 |
| K | 21.8 | 2.94 | 9.5 | 7.4 | 3.9 | 1181 | 144 | 155 | 2478 | 79 | 641 |
| L | 18.7 | 2.82 | 10.3 | 7.9 | 3.6 | 1126 | 90 | 106 | 1130 | 57 | 500 |
| M | 20.6 | 2.81 | 10.1 | 8.0 | 3.3 | 1114 | 60 | 92 | 1179 | 64 | 503 |
| Parameter | Control (n = 5) | 3 min (n = 5) | 5 min (n = 5) | |||
|---|---|---|---|---|---|---|
| Conductibility (mS/cm) | 2.06 ± 0.22 | b | 2.41 ± 0.26 | a | 2.61 ± 0.22 | a |
| Catechins (mg/L) | 8.4 ± 2.0 | b | 18.2 ± 5.1 | b | 37.4 ± 20.8 | a |
| Tannins (mg/L) | 17.69 ± 4.11 | 20.70 ± 4.31 | 21.96 ± 3.97 | |||
| Total polyphenols (mg/L) | 403.2 ± 44.7 | b | 510.1 ± 134.5 | ab | 624.5 ± 160.9 | a |
| Hydroxycinnamate-tartaric acids (mg/L) | 1.61 ± 0.35 | 1.82 ± 0.52 | 2.12 ± 0.67 | |||
| GSH-3MH (µg/L) | 168 ± 43 | 156 ± 36 | 149 ± 32 | |||
| Cys-3MH (µg/L) | 96 ± 44 | 95 ± 44 | 97 ± 41 | |||
| Cys-4MMP (µg/L) | 13 ± 3 | 12 ± 3 | 12 ± 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roman, T.; Tonidandel, L.; Nicolini, G.; Bellantuono, E.; Barp, L.; Larcher, R.; Celotti, E. Evidence of the Possible Interaction between Ultrasound and Thiol Precursors. Foods 2020, 9, 104. https://doi.org/10.3390/foods9010104
Roman T, Tonidandel L, Nicolini G, Bellantuono E, Barp L, Larcher R, Celotti E. Evidence of the Possible Interaction between Ultrasound and Thiol Precursors. Foods. 2020; 9(1):104. https://doi.org/10.3390/foods9010104
Chicago/Turabian StyleRoman, Tomas, Loris Tonidandel, Giorgio Nicolini, Elisabetta Bellantuono, Laura Barp, Roberto Larcher, and Emilio Celotti. 2020. "Evidence of the Possible Interaction between Ultrasound and Thiol Precursors" Foods 9, no. 1: 104. https://doi.org/10.3390/foods9010104
APA StyleRoman, T., Tonidandel, L., Nicolini, G., Bellantuono, E., Barp, L., Larcher, R., & Celotti, E. (2020). Evidence of the Possible Interaction between Ultrasound and Thiol Precursors. Foods, 9(1), 104. https://doi.org/10.3390/foods9010104