A Review of Factors Affecting Anthocyanin Bioavailability: Possible Implications for the Inter-Individual Variability
Abstract
1. Introduction
2. Methodology
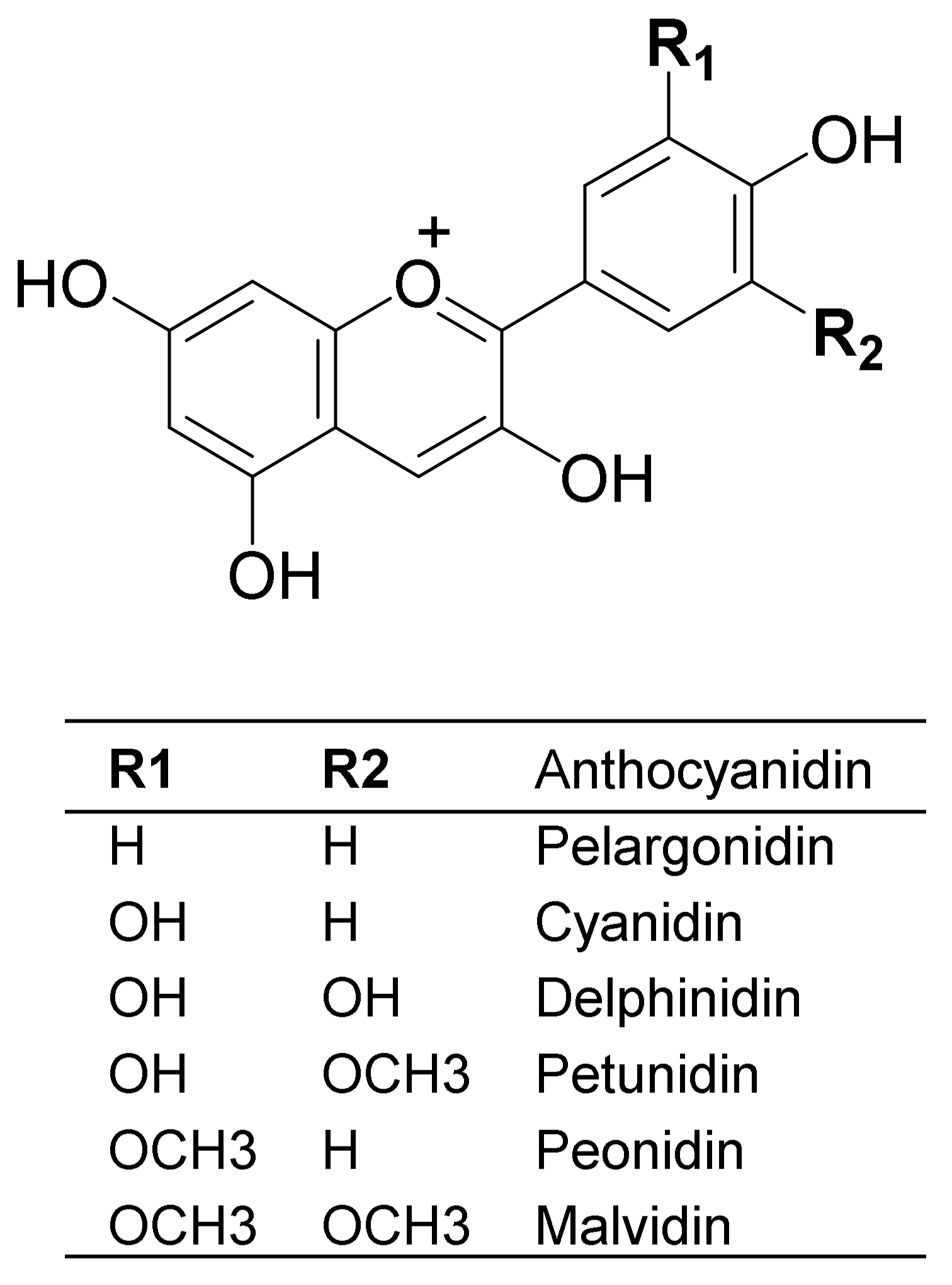
3. Anthocyanin Structure and Distribution
4. Effects of Food Processing and Food Matrix
5. Effect of Enzymatic Variability
6. Effect of the Microbiota on Inter-Individual Variability of Anthocyanin Bioavailability
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Pascual-Teresa, S.; Moreno, D.A.; García-Viguera, C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Kent, K.; Charlton, K.E.; Netzel, M.; Fanning, K. Food-based anthocyanin intake and cognitive outcomes in human intervention trials: A systematic review. J. Hum. Nutr. Diet. 2017, 30, 260–274. [Google Scholar] [CrossRef]
- Fairlie-Jones, L.; Davison, K.; Fromentin, E.; Hill, A.M. The effect of anthocyanin-rich foods or extracts on vascular function in adults: A systematic review and meta-analysis of randomised controlled trials. Nutrients 2017, 9, 908. [Google Scholar] [CrossRef]
- García-Conesa, M.T.; Chambers, K.; Combet, E.; Pinto, P.; Garcia-Aloy, M.; Andrés-Lacueva, C.; de Pascual-Teresa, S.; Mena, P.; Ristic, A.K.; Hollands, W.J.; et al. Meta-analysis of the effects of foods and derived products containing ellagitannins and anthocyanins on cardiometabolic biomarkers: Analysis of factors influencing variability of the individual responses. Int. J. Mol. Sci. 2018, 19, 694. [Google Scholar] [CrossRef]
- Clifford, M.N. Anthocyanins—Nature, occurrence and dietary sources. J. Sci. Food Agric. 2000, 80, 1063–1072. [Google Scholar] [CrossRef]
- Mazza, G.; Miniati, E. Anthocyanins in Fruits, Vegetables, and Grains; CRC Press: Boca Raton, FL, USA, 1993; ISBN 9781315890609. [Google Scholar]
- Andersen, Ø.M.; Jordheim, M. The Anthocyanins. Chemistry, Biochemistry and Applications; Andersen, Ø.M., Markham, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 471–553. ISBN 9780849320217. [Google Scholar]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; De La Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-chemistry, foodomics and health effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef]
- Jennings, A.; Welch, A.A.; Fairweather-Tait, S.J.; Kay, C.; Minihane, A.M.; Chowienczyk, P.; Jiang, B.; Cecelja, M.; Spector, T.; Macgregor, A.; et al. Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am. J. Clin. Nutr. 2012, 96, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, R.N.; Santos, D.T.; Meireles, M.A.A. Non-thermal stabilization mechanisms of anthocyanins in model and food systems—An overview. Food Res. Int. 2011, 44, 499–509. [Google Scholar] [CrossRef]
- Charron, C.S.; Kurilich, A.C.; Clevidence, B.A.; Simon, P.W.; Harrison, D.J.; Britz, S.J.; Baer, D.J.; Novotny, J.A. Bioavailability of anthocyanins from purple carrot juice: Effects of acylation and plant matrix. J. Agric. Food Chem. 2009, 57, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Novotny, J.A.; Clevidence, B.A.; Kurilich, A.C. Anthocyanin kinetics are dependent on anthocyanin structure. Br. J. Nutr. 2012, 107, 504–509. [Google Scholar] [CrossRef]
- Felgines, C.; Texier, O.; Besson, C.; Lyan, B.; Lamaison, J.L.; Scalbert, A. Strawberry pelargonidin glycosides are excreted in urine as intact glycosides and glucuronidated pelargonidin derivatives in rats. Br. J. Nutr. 2007, 98, 1126–1131. [Google Scholar] [CrossRef]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of glucosinolates and their breakdown products: Impact of processing. Front. Nutr. 2016, 3, 1–12. [Google Scholar] [CrossRef]
- Celli, G.B.; Brooks, M.S.L. Impact of extraction and processing conditions on betalains and comparison of properties with anthocyanins—A current review. Food Res. Int. 2017, 100, 501–509. [Google Scholar] [CrossRef]
- Kurilich, A.C.; Clevidence, B.A.; Britz, S.J.; Simon, P.W.; Novotny, J.A. Plasma and urine responses are lower for acylated vs nonacylated anthocyanins from raw and cooked purple carrots. J. Agric. Food Chem. 2005, 53, 6537–6542. [Google Scholar] [CrossRef]
- Hollands, W.; Brett, G.M.; Radreau, P.; Saha, S.; Teucher, B.; Bennett, R.N.; Kroon, P.A. Processing blackcurrants dramatically reduces the content and does not enhance the urinary yield of anthocyanins in human subjects. Food Chem. 2008, 108, 869–878. [Google Scholar] [CrossRef]
- Del Bo’, C.; Riso, P.; Brambilla, A.; Gardana, C.; Rizzolo, A.; Simonetti, P.; Bertolo, G.; Klimis-Zacas, D.; Porrini, M. Blanching improves anthocyanin absorption from highbush blueberry (Vaccinium corymbosum L.) purée in healthy human volunteers: A pilot study. J. Agric. Food Chem. 2012, 60, 9298–9304. [Google Scholar] [CrossRef]
- Tomas, M.; Toydemir, G.; Boyacioglu, D.; Hall, R.; Beekwilder, J.; Capanoglu, E. The effects of juice processing on black mulberry antioxidants. Food Chem. 2015, 186, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Rudloff, S.; Asseburg, H.; Borsch, C.; Fröhling, B.; Unger, F.; Dold, S.; Spengler, B.; Römpp, A.; Kunz, C. Uptake and bioavailability of anthocyanins and phenolic acids from grape/blueberry juice and smoothie in vitro and in vivo. Br. J. Nutr. 2015, 113, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Wiczkowski, W.; Szawara-Nowak, D.; Romaszko, J. The impact of red cabbage fermentation on bioavailability of anthocyanins and antioxidant capacity of human plasma. Food Chem. 2016, 190, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Vadillo, C.; Nau, F.; Guerin-Dubiard, C.; Jardin, J.; Lechevalier, V.; Sanz-Buenhombre, M.; Guadarrama, A.; Tóth, T.; Csavajda, É.; Hingyi, H.; et al. The food matrix affects the anthocyanin profile of fortified egg and dairy matrices during processing and in vitro digestion. Food Chem. 2017, 214, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Koo, S.I.; Song, W.O.; Chun, O.K. Food matrix affecting anthocyanin bioavailability: Review. Curr. Med. Chem. 2011, 18, 291–300. [Google Scholar] [CrossRef]
- Nielsen, I.L.F.; Dragsted, L.O.; Ravn-haren, G.; Freese, R.; Rasmussen, S.E. Absorption and excretion of black currant anthocyanins in humans and watanabe heritable hyperlipidemic rabbits. J. Agric. Food Chem. 2003, 51, 2813–2820. [Google Scholar] [CrossRef]
- Mullen, W.; Edwards, C.A.; Serafini, M.; Crozier, A. Bioavailability of pelargonidin-3-O-glucoside and its metabolites in humans following the ingestion of strawberries with and without cream. J. Agric. Food Chem. 2008, 56, 713–719. [Google Scholar] [CrossRef]
- Serafini, M.; Bugianesi, R.; Maiani, G.; Valtuena, S.; De Santis, S.; Crozier, A. Plasma antioxidants from chocolate. Nature 2003, 424, 1013. [Google Scholar] [CrossRef]
- Bub, A.; Watzl, B.; Heeb, D.; Rechkemmer, G.; Briviba, K. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur. J. Nutr. 2001, 40, 113–120. [Google Scholar] [CrossRef]
- Mülleder, U.; Murkovic, M.; Pfannhauser, W. Urinary excretion of cyanidin glycosides. J. Biochem. Biophys. Methods 2002, 53, 61–66. [Google Scholar] [CrossRef]
- Walton, M.C.; Lentle, R.G.; Reynolds, G.W.; Kruger, M.C.; McGhie, T.K. Anthocyanin absorption and antioxidant status in pigs. J. Agric. Food Chem. 2006, 54, 7940–7946. [Google Scholar] [CrossRef] [PubMed]
- Frank, T.; Netzel, M.; Strass, G.; Bitsch, R.; Bitsch, I. Bioavailability of anthocyanidin-3-glucosides following consumption of red wine and red grape juice. Can. J. Physiol. Pharmacol. 2003, 81, 423–435. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Assessing potential bioavailability of raspberry anthocyanins using an in vitro digestion system. J. Agric. Food Chem. 2005, 53, 5896–5904. [Google Scholar] [CrossRef] [PubMed]
- Sengul, H.; Surek, E.; Nilufer-Erdil, D. Investigating the effects of food matrix and food components on bioaccessibility of pomegranate (Punica granatum) phenolics and anthocyanins using an in-vitro gastrointestinal digestion model. Food Res. Int. 2014, 62, 1069–1079. [Google Scholar] [CrossRef]
- Ribnicky, D.M.; Roopchand, D.E.; Oren, A.; Grace, M.; Poulev, A.; Lila, M.A.; Havenaar, R.; Raskin, I. Effects of a high fat meal matrix and protein complexation on the bioaccessibility of blueberry anthocyanins using the TNO gastrointestinal model (TIM-1). Food Chem. 2014, 142, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, S.; Simsek, S.; Eker, A.T.; Pineda-Vadillo, C.; Dupont, D.; Perez, B.; Viadel, B.; Sanz-Buenhombre, M.; Rodriguez, A.G.; Kertész, Z.; et al. Stability and bioaccessibility of anthocyanins in bakery products enriched with anthocyanins. Food Funct. 2016, 7, 3488–3496. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230–242. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef]
- Wang, D.; Xia, M.; Yan, X.; Li, D.; Wang, L.; Xu, Y.; Jin, T.; Ling, W. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ. Res. 2012, 111, 967–981. [Google Scholar] [CrossRef]
- Pimpão, R.C.; Ventura, M.R.; Ferreira, R.B.; Williamson, G.; Santos, C.N. Phenolic sulfates as new and highly abundant metabolites in human plasma after ingestion of a mixed berry fruit purée. Br. J. Nutr. 2015, 113, 454–463. [Google Scholar] [CrossRef]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Kulkarni, K.; Basu, S.; Zhang, S.; Hu, M. First-pass metabolism via UDP-glucuronosyltransferase: A barrier to oral bioavailability of phenolics. J. Pharm. Sci. 2011, 100, 3655–3681. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Bioavailability of anthocyanins and derivatives. J. Funct. Foods 2014, 7, 54–66. [Google Scholar] [CrossRef]
- Court, M.H. Interindividual variability in hepatic drug glucuronidation: Studies into the role of age, sex, enzyme inducers, and genetic polymorphism using the human liver bank as a model system. Drug Metab. Rev. 2010, 42, 202–217. [Google Scholar] [CrossRef]
- Strassburg, C.P.; Strassburg, A.; Kneip, S.; Barut, A.; Tukey, R.H.; Rodeck, B.; Manns, M.P. Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut 2002, 50, 259–265. [Google Scholar] [CrossRef]
- Bolling, B.W.; Court, M.H.; Blumberg, J.B.; Chen, C.Y.O. The kinetic basis for age-associated changes in quercetin and genistein glucuronidation by rat liver microsomes. J. Nutr. Biochem. 2010, 21, 498–503. [Google Scholar] [CrossRef]
- Buckley, D.B.; Klaassen, C.D. Tissue- and gender-specific mRNA expression of UDP-glucuronosyltransferases (UGTs) in mice. Drug Metab. Dispos. 2007, 35, 121–127. [Google Scholar] [CrossRef]
- Navarro, S.L.; Peterson, S.; Chen, C.; Makar, K.W.; Schwarz, Y.; King, I.B.; Li, S.S.; Li, L.; Kestin, M.; Lampe, J.W. Cruciferous vegetable feeding alters UGT1A1 activity: Diet- and genotype-dependent changes in serum bilirubin in a controlled feeding trial. Cancer Prev. Res. 2009, 2, 345–352. [Google Scholar] [CrossRef]
- Németh, K.; Plumb, G.W.; Berrin, J.G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef]
- Mallery, S.R.; Budendorf, D.E.; Larsen, M.P.; Pei, P.; Tong, M.; Holpuch, A.S.; Larsen, P.E.; Stoner, G.D.; Fields, H.W.; Chan, K.K.; et al. Effects of human oral mucosal tissue, saliva and oral microflora on intraoral metabolism and bioactivation of black raspberry anthocyanins. Cancer Prev. Res. 2011, 4, 1209–1221. [Google Scholar] [CrossRef]
- Riches, Z.; Stanley, E.L.; Bloomer, J.C.; Coughtrie, M.W.H. Quantitative evaluation of the expression and activity of five major sulfotransferases (SULTs) in human tissues: The SULT pie. Drug Metab. Dispos. 2009, 37, 2255–2261. [Google Scholar] [CrossRef]
- Bai, H.W.; Shim, J.Y.; Yu, J.; Bao, T.Z. Biochemical and molecular modeling studies of the O-methylation of various endogenous and exogenous catechol substrates catalyzed by recombinant human soluble and membrane-bound catechol-O-methyltransferases. Chem. Res. Toxicol. 2007, 20, 1409–1425. [Google Scholar] [CrossRef]
- Miller, R.J.; Jackson, K.G.; Dadd, T.; Mayes, A.E.; Brown, A.L.; Lovegrove, J.A.; Minihane, A.M. The impact of the catechol-O-methyltransferase genotype on vascular function and blood pressure after acute green tea ingestion. Mol. Nutr. Food Res. 2012, 56, 966–975. [Google Scholar] [CrossRef]
- Sánchez-Patán, F.; Cueva, C.; Monagas, M.; Walton, G.E.; Gibson, G.R.; Quintanilla-López, J.E.; Lebrón-Aguilar, R.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Bartolomé, B. In vitro fermentation of a red wine extract by human gut microbiota: Changes in microbial groups and formation of phenolic metabolites. J. Agric. Food Chem. 2012, 60, 2136–2147. [Google Scholar] [CrossRef]
- Stalmach, A.; Edwards, C.A.; Wightman, J.D.; Crozier, A. Colonic catabolism of dietary phenolic and polyphenolic compounds from Concord grape juice. Food Funct. 2013, 4, 52–62. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Mena, P.; Calani, L.; Borges, G.; Pereira-Caro, G.; Bresciani, L.; Del Rio, D.; Lean, M.E.J.; Crozier, A. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Radic. Biol. Med. 2015, 89, 758–769. [Google Scholar] [CrossRef]
- Burgos-Edwards, A.; Jiménez-Aspee, F.; Theoduloz, C.; Schmeda-Hirschmann, G. Colonic fermentation of polyphenols from Chilean currants (Ribes spp.) and its effect on antioxidant capacity and metabolic syndrome-associated enzymes. Food Chem. 2018, 258, 144–155. [Google Scholar] [CrossRef]
- Hanske, L.; Engst, W.; Loh, G.; Sczesny, S.; Blaut, M.; Braune, A. Contribution of gut bacteria to the metabolism of cyanidin 3-glucoside in human microbiota-associated rats. Br. J. Nutr. 2013, 109, 1433–1441. [Google Scholar] [CrossRef]
- Cheng, J.R.; Liu, X.M.; Chen, Z.Y.; Zhang, Y.S.; Zhang, Y.H. Mulberry anthocyanin biotransformation by intestinal probiotics. Food Chem. 2016, 213, 721–727. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, H.; He, S.; Lou, Q.; Yu, M.; Tang, M.; Tu, L. Metabolism and prebiotics activity of anthocyanins from black rice (Oryza sativa L.) in vitro. PLoS ONE 2018, 13, e0195754. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, P.; Zhu, Y.; Lou, Q.; He, S. Antioxidant and prebiotic activity of five peonidin-based anthocyanins extracted from purple sweet potato (Ipomoea batatas (L.) Lam.). Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Trikas, E.D.; Melidou, M.; Papi, R.M.; Zachariadis, G.A.; Kyriakidis, D.A. Extraction, separation and identification of anthocyanins from red wine by-product and their biological activities. J. Funct. Foods 2016, 25, 548–558. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, M.; He, S.; Cao, X.; Sun, H.; Chen, X.; Xie, Y.; Lou, Q.; Wang, X.; Ye, Y. Extraction and probiotic properties of New anthocyanins from purple sweet potato (Solanum tuberosum). Curr. Top. Nutraceuticals Res. 2016, 14, 153–160. [Google Scholar]
- López De Las Hazas, M.C.; Mosele, J.I.; Macià, A.; Ludwig, I.A.; Motilva, M.J. Exploring the colonic metabolism of grape and strawberry anthocyanins and their in vitro apoptotic effects in HT-29 Colon Cancer Cells. J. Agric. Food Chem. 2017, 65, 6477–6487. [Google Scholar] [CrossRef]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.E.; Gibson, G.R.; de Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef]
- Flores, G.; Ruiz del Castillo, M.L.; Costabile, A.; Klee, A.; Bigetti Guergoletto, K.; Gibson, G.R. In vitro fermentation of anthocyanins encapsulated with cyclodextrins: Release, metabolism and influence on gut microbiota growth. J. Funct. Foods 2015, 16, 50–57. [Google Scholar] [CrossRef]
- Aura, A.M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142. [Google Scholar] [CrossRef]
- Guergoletto, K.B.; Costabile, A.; Flores, G.; Garcia, S.; Gibson, G.R. In vitro fermentation of juçara pulp (Euterpe edulis) by human colonic microbiota. Food Chem. 2016, 196, 251–258. [Google Scholar] [CrossRef]
- González-Barrio, R.; Edwards, C.A.; Crozier, A. Colonic catabolism of ellagitannins, ellagic acid, and raspberry anthocyanins: In vivo and in vitro studies. Drug Metab. Dispos. 2011, 39, 1680–1688. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macià, A.; Romero, M.P.; Motilva, M.J. Stability and metabolism of Arbutus unedo bioactive compounds (phenolics and antioxidants) under in vitro digestion and colonic fermentation. Food Chem. 2016, 201, 120–130. [Google Scholar] [CrossRef]
- Vendrame, S.; Guglielmetti, S.; Riso, P.; Arioli, S.; Klimis-Zacas, D.; Porrini, M. Six-week consumption of a wild blueberry powder drink increases Bifidobacteria in the human gut. J. Agric. Food Chem. 2011, 59, 12815–12820. [Google Scholar] [CrossRef]
- Boto-Ordóñez, M.; Urpi-Sarda, M.; Queipo-Ortuño, M.I.; Tulipani, S.; Tinahones, F.J.; Andres-Lacueva, C. High levels of Bifidobacteria are associated with increased levels of anthocyanin microbial metabolites: A randomized clinical trial. Food Funct. 2014, 5, 1932–1938. [Google Scholar] [CrossRef]
- Khanal, R.; Howard, L.R.; Prior, R.L. Urinary excretion of phenolic acids in rats fed cranberry, blueberry, or black raspberry powder. J. Agric. Food Chem. 2014, 62, 3987–3996. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Q.; Zhao, T.; Zhang, Z.; Mao, G.; Feng, W.; Wu, X.; Yang, L. Biotransformation and metabolism of three mulberry anthocyanin monomers by rat gut microflora. Food Chem. 2017, 237, 887–894. [Google Scholar] [CrossRef]
- Forester, S.C.; Waterhouse, A.L. Identification of cabernet sauvignon anthocyanin gut microflora metabolites. J. Agric. Food Chem. 2008, 56, 9299–9304. [Google Scholar] [CrossRef]
- Overall, J.; Bonney, S.A.; Wilson, M.; Beermann, A.; Grace, M.H.; Esposito, D.; Lila, M.A.; Komarnytsky, S. Metabolic effects of berries with structurally diverse anthocyanins. Int. J. Mol. Sci. 2017, 18, 422. [Google Scholar] [CrossRef]
- Paturi, G.; Butts, C.A.; Monro, J.A.; Hedderley, D. Effects of blackcurrant and dietary fibers on large intestinal health biomarkers in rats. Plant Foods Hum. Nutr. 2018, 73, 54–60. [Google Scholar] [CrossRef]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef]
- Pan, P.; Lam, V.; Salzman, N.; Huang, Y.W.; Yu, J.; Zhang, J.; Wang, L.S. Black raspberries and their anthocyanin and fiber fractions alter the composition and diversity of gut microbiota in F-344 rats. Nutr. Cancer 2017, 69, 943–951. [Google Scholar] [CrossRef]
- Garcia-Mazcorro, J.F.; Lage, N.N.; Mertens-Talcott, S.; Talcott, S.; Chew, B.; Dowd, S.E.; Kawas, J.R.; Noratto, G.D. Effect of dark sweet cherry powder consumption on the gut microbiota, short-chain fatty acids, and biomarkers of gut health in obese db/db mice. Peer J. 2018, 2018, 1–31. [Google Scholar] [CrossRef]
- Lacombe, A.; Li, R.W.; Klimis-Zacas, D.; Kristo, A.S.; Tadepalli, S.; Krauss, E.; Young, R.; Wu, V.C.H. Lowbush wild blueberries have the potential to modify gut microbiota and xenobiotic metabolism in the rat colon. PLoS ONE 2013, 8, e67497. [Google Scholar] [CrossRef]
- Faria, A.; Fernandes, I.; Norberto, S.; Mateus, N.; Calhau, C. Interplay between anthocyanins and gut microbiota. J. Agric. Food Chem. 2014, 62, 6898–6902. [Google Scholar] [CrossRef]
- Healey, G.R.; Murphy, R.; Brough, L.; Butts, C.A.; Coad, J. Interindividual variability in gut microbiota and host response to dietary interventions. Nutr. Rev. 2017, 75, 1059–1080. [Google Scholar] [CrossRef]

| Technology and Processing Conditions | Food | Retention | Effect on Bioaccessibility/Bioavailability | Reference |
|---|---|---|---|---|
| Microwave cooking (900 W, 12–20 min) | Purple carrots (Daucus carota) | ↓ (23% total ACs) | ↓ 11-14-fold ↓ACs urine and 8-10-fold d↓ ACs in plasma | [20] |
| Conservation, jam, squeeze | Blackcurrant fruits and products | ↓ (0.05–10.3%) | ↓ urine (fruit, 0.053%; drink, 0.036) | [21] |
| Steam-blanching (3 min) | Blueberry puree (Vaccinium corymbosum) | ↑ ACs * | ↑ | [22] |
| Juice processing (milling, mashing, pressing, pasteurization) | Black mulberries Grape/blue berry | ↑ (213.8%) | ↓ | [23,24] |
| Fermentation (at 18–24 °C, pH 3.80, 14 days) | Red cabbage (Brassica oleracea) | ↓ (15%) | ↓ | [25] |
| Domestic cooking (45 s–2.5 min, at 250 °C) | ACs enriched food matrices (milkshake, custard dessert, pancake, and omellette) | Very high recovery | ≈ | [26] |
| Enzyme (or Family) | Isoforms | Metabolites | Variability | Reference |
|---|---|---|---|---|
| Beta-glucosidases | LPH, cytosolic β-glucosidase | Glucuronides, sulfates | Small intestine | [51] |
| GBA1, GBA2, GBA3-1, GBA3-2 | Aglycons | Microbiota | [52] | |
| Sulphotransferases (SULT) | SULT1A1, SULT1A3/4, SULT1B1, SULT1E1 and SULT2A1 | Sulphates | Tissue | [53] |
| Uridine diphosphate (UDP)-glucuronosyltransferases (UGT) | UGT1A, UGT2A, UGT2B, UGT3, or UGT8 families | Glucuronates | Age, smoking | [48] |
| UGT1A1, UGT1A2, UGT 1A5, UGT1A6 | Glucuronates | Sex, tissue | [49] | |
| Catechol-O-methyltransferases (COMTs) | MB-COMT, S-COMT | Methyl substitution | Addictions | [54] |
| AA genotype, GG genotype | Methyl substitution | Genetic | [55] |
| Species | Anthocyanins | Model | Metabolites Found | Bacterial Species | Ef | Reference |
|---|---|---|---|---|---|---|
| Bac | C3G (blackcurrant) | in vitro | 3,4-dihydroxybenzoic acid, 2,4,6-trihydroxybenzaldehyde | Clostridium saccharogumia Eubacterium ramulus | [60] | |
| Bac | C3G, C3R (mulberry) | in vitro | caffeic acid, ferulic acid, protocatechuic acid, chlorogenic acid, cryptochlorogenic acid, | Lactobacillus plantarum, Streptococcus thermophiles | ↑ | [61] |
| Bac | C3G (black rice) | in vitro | phenyllactic acid, benzoic acid, phenylacetic acid, 2,4,6-trihydroxybenzoic, 4-hydroxyphenylethanol, 4-hydroxybenzoic acid, 4-hydroxyphenylacetic acid, 3-methoxy-4-hydroxybenzoic acid | Bifidobacteria Lactobacilli | ↑ ↑ | [62] |
| Bac | Pn deriv (purple sweet potato) | in vitro | Bifidobacterium bifidum B. adolescentis B. infantis L. acidophilus Staphylococcus aureus Salmonella typhimurium | ↑ ↑ ↑ ↑ ↓ ↓ | [63] | |
| Bac | Mv deriv (red grape) | in vitro | E. coli | ↓ | [64] | |
| Human | Cy and Pn deriv (purple sweet potato) | anaerobic culture | protocatechuic, phloroglucinol aldehyde, syringic acid, phloroglucinol aldehyde | Bifidobacterium and Lactobacillus/EnterococcusBacteroides-Prevotella Clostridium histolyticum | ↑ ↓ | [65] |
| Human | P3G (Strawberry) M3G (red grape) | anaerobic culture | p-hydroxybenzoic acid tyrosol, Hydroxyphenylacetic. Syringic, vanillic, Hydroxyphenylpropionic acid | [66] | ||
| Human | M3G (Red wine) | anaerobic culture | syringic acid | Bifidobacterium spp., Lactobacillus spp. total number | ↑ | [67] |
| Human | C3G, D3R, M3G | anaerobic culture | ferulic, gallic, syringic gallic acid | C. histolyticum total number | ↓ ↑ | [68] |
| Human | C3G, C3R | anaerobic culture | Protocatechuic acid (3,4-dihydroxybenzoic acid), cyanidin | [69] | ||
| Human | C3R, C3G, M3G, P3R, P3G (jucara pulp) | anaerobic culture | gallic acid, syringic acid, benzoic acid | Bifidobacterium, Eubacterium rectale/ Clostridium coccoides, Bacteroides/Prevotella group | ↑ | [70] |
| Human | C3G, C3GR (raspberry) | anaerobic culture | 3,4-Dihydroxybenzoic acid, tyrosol, catechol, resorcinol, pyrogallol | [71] | ||
| Human | M3G, Pn3G, Pt3G (red wine) | anaerobic culture | dihydroxylated benzene, catechol/pyrocatechol, syringic acid | Bifidobacterium spp. Lactobacillus/Enterococcus Bacteroides | NC NC | [56] |
| Human | C3Ga, C3A (Arbutus unedo) | anaerobic culture | 3,4-(Dihydroxyphenyl)-acetic acid | [72] | ||
| Human | Pn3G, MGa (blueberry) | In vivo | Bifidobacterium Lactobacillus acidophilus | ↑ ↑ | [73] | |
| Human | M3G (red wine) | In vivo | syringic acid, p-coumaric acid, 4-hydroxybenzoic, homovanillic acid | Bifidobacterium | ↑ | [74] |
| Species | Anthocyanins | Model | Metabolites Found | Reference |
|---|---|---|---|---|
| Pig | D3G, PT3G, P3G, M3G (red grape) | anaerobic culture | 3-O-methylgallic acid, syringic acid, 2,4,6-trihydroxybenzaldehyde | [77] |
| Rat | C3G, C3R, D3R (mulberry) | anaerobic culture | protocatechuic, vanillic, p-coumaric acid, 2,4,6-trihydroxybenzaldehyde, gallic acid, syringic acid, 2,4,6-triOHbenzaldehyde | [76] |
| Rat | Cy deriv (Black raspberry) | In vivo | 3-OHphenylpropionic, 3-hydroxybenzoic, 3-OHcinnamic acids | [75] |
| Rat | C3G | In vivo | protocatechuic acid | [41] |
| Species | Anthocyanins | Model | Bacterial species | Ef | Reference |
|---|---|---|---|---|---|
| Rat | D3G, D3R, C3G, C3R, Pt3R (blackcurrant) | In vivo | Bacteroides-Prevotella-Porphyromonas group, Lactobacillus spp. Bifidobacterium spp., Clostridium perfringens | ↑ ↓ | [79] |
| Rat | Mv deriv, Pt deriv, D deriv (blueberry) D deriv, Cy deriv (blackcurrant) Mv deriv, Pt deriv, D deriv (red grape) Cy deriv (black raspberry) Cy deriv (black berry) | In vivo | Actinobacteria, Bacteroidetes Actinobacteria, Bacteroidetes Actinobacteria Actinobacteria, Bacteroidetes Actinobacteria, Bacteroidetes | ↑ ↑ ↑ NC NC | [78] |
| Rat | Pn3Ga (cranberry) | In vivo | Verrucomicrobia Akkermansia | ↑ | [80] |
| Rat | Cy deriv (black raspberry) | In vivo | Anaerostipes, Ruminococcus, Akkermansia, Coprobacillus Acetivibrio Anaerovorax, Dorea Bifidobacterium, Lactococcus Anaerotruncus, Coprobacillus, Desulfovibrio, Victivallis, Mucispirilum, Streptococcus, Turicibacter, Acetivibrio | ↑ ↓ ↑ ↓ ↑ ↓ | [81] |
| Rat | C3R, C3G, C3G (dark sweet cherry) | In vivo | Akkermansia, Bacteroidaceae, Lactobacillus | ↑ | [82] |
| Rat | M3Ga, Pt3G (blueberries) | In vivo | Proteobacteria Actinobacteria Actinomycetales Coriobacteriaceae Enterococcus, Lactobacillus | ↓ ↑ ↑ ↑ ↓ | [83] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eker, M.E.; Aaby, K.; Budic-Leto, I.; Rimac Brnčić, S.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; de Pascual-Teresa, S. A Review of Factors Affecting Anthocyanin Bioavailability: Possible Implications for the Inter-Individual Variability. Foods 2020, 9, 2. https://doi.org/10.3390/foods9010002
Eker ME, Aaby K, Budic-Leto I, Rimac Brnčić S, El SN, Karakaya S, Simsek S, Manach C, Wiczkowski W, de Pascual-Teresa S. A Review of Factors Affecting Anthocyanin Bioavailability: Possible Implications for the Inter-Individual Variability. Foods. 2020; 9(1):2. https://doi.org/10.3390/foods9010002
Chicago/Turabian StyleEker, Merve Eda, Kjersti Aaby, Irena Budic-Leto, Suzana Rimac Brnčić, Sedef Nehir El, Sibel Karakaya, Sebnem Simsek, Claudine Manach, Wieslaw Wiczkowski, and Sonia de Pascual-Teresa. 2020. "A Review of Factors Affecting Anthocyanin Bioavailability: Possible Implications for the Inter-Individual Variability" Foods 9, no. 1: 2. https://doi.org/10.3390/foods9010002
APA StyleEker, M. E., Aaby, K., Budic-Leto, I., Rimac Brnčić, S., El, S. N., Karakaya, S., Simsek, S., Manach, C., Wiczkowski, W., & de Pascual-Teresa, S. (2020). A Review of Factors Affecting Anthocyanin Bioavailability: Possible Implications for the Inter-Individual Variability. Foods, 9(1), 2. https://doi.org/10.3390/foods9010002







