Analytical and Sample Preparation Techniques for the Determination of Food Colorants in Food Matrices
Abstract
:1. Introduction
2. Natural Food Colorants
3. Synthetic Food Colorants
4. Toxicological Aspects and Regulatory Framework
5. Analytical Methodologies for the Determination of Food Colorants
5.1. Analytical Techniques in the Use of Natural Food Colorants Determinations
5.2. Sample Preparation for Natural Colorant Analysis
5.3. Analytical Techniques in the Use of Synthetic Food Colorants Determinations
5.4. Sample Preparation for the Determination of Synthetic Colorants in Foods
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Codex Alimentarius. Available online: http://www.codexalimentarius.org/standards/gsfa/ (accessed on 18 September 2018).
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trans Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Amchova, P.; Kotolova, H.; Ruda-Kucerova, J. Health safety issues of synthetic food colorants. Reg. Toxic. Pharm. 2015, 73, 914–922. [Google Scholar] [CrossRef]
- Aberamound, A. A Review Article on Edible Pigments Properties and Sources as Natural Biocolorants in Foodstuff and Food Industry. World J. Dairy Food Sci. 2011, 6, 71–78. [Google Scholar]
- Burrows, A.J.D. Palette of Our Palates: A Brief History of Food Coloring and Its Regulation. Compreh. Rev. Food Sci. Food Saf. 2009, 9, 394–408. [Google Scholar] [CrossRef]
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C. Food colorants: Challenges, opportunities and current desires of agro-industries to en-sure consumer expectations and regulatory practices. Trends Food Sci. Technol. 2016, 52, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Amaya, D.B. Natural food pigments and colorants. Curr. Opin. Food Sci. 2016, 7, 20–26. [Google Scholar] [CrossRef]
- Tumolo, T.; Lanfer-Marquez, U.M. Copper chlorophyllin: A food colorant with bioactive properties? Food Res. Int. 2012, 46, 451–459. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, Y.; Kokot, S. Simultaneous kinetic spectrophotometric analysis of five synthetic food colorants with the aid of chemometrics. Talanta 2009, 78, 432–441. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural colorants: food colorants from natural sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef]
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef]
- Dias, M.I.; Ferreira, I.C.; Barreiro, M.F. Microencapsulation of bioactives for food applications. Food Funct. 2015, 6, 1035–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patakova, P. Monascus secondary metabolites: Production and biological activity. J. Ind. Microbiol. Biotechnol. 2013, 40, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, D.; Chen, M.; Wang, Y.; Li, Z.; Li, F. Stimulatory effects of blue light on the growth, monascin and ankaflavin production in Monascus. Biotechnol. Lett. 2015, 37, 1043–1048. [Google Scholar] [CrossRef]
- Dias, M.G.; Camoes, M.F.G.; Oliveira, L. Carotenoids in traditional Portuguese fruits and vegetables. Food Chem. 2009, 113, 808–815. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Masone, D.; Chanforan, C. Study on the interaction of artificial and natural food colorants with human serum albumin: A computational point of view. Comput. Biol. Chem. 2015, 56, 152–158. [Google Scholar] [CrossRef]
- Oplatowska-Stachowiak, M.; Elliottt, C.T. Food colors: Existing and emerging food safety concerns. Crit. Rev. Food Sci. Nutr. 2017, 57, 524–548. [Google Scholar] [CrossRef]
- Feng, J.; Cerniglia, C.E.; Chen, H. Toxicological significance of azo dye metabolism by human intestinal microbiota. Front. Biosci. 2012, 4, 568–586. [Google Scholar] [CrossRef]
- Golka, K.; Kopps, S.; Myslak, Z.W. Carcinogenicity of azo colorants: Influence of solubility and bioavailability. Toxicol. Lett. 2004, 151, 203–210. [Google Scholar] [CrossRef]
- Vojdani, A.; Vojdani, C. Immune reactivity to food coloring. Altern. Ther. Health Med. 2015, 21, 52–62. [Google Scholar]
- Mpountoukas, P.; Pantazaki, A.; Kostareli, E.; Christodoulou, P.; Kareli, D.; Poliliou, S.; Mourelatos, C.; Lambropoulou, V.; Lialiaris, T. Cytogenetic evaluation and DNA interaction studies of the food colorants amaranth, erythrosine and tartrazine. Food Chem. Toxic. 2010, 48, 2934–2944. [Google Scholar] [CrossRef]
- Pan, X.; Qin, P.; Liu, R.; Wang, J. Characterizing the Interaction between tartra-zine and two serum albumins by a hybrid spectroscopic approach. J. Agric. Food Chem. 2011, 59, 6650–6656. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Kumar, G.S. Study on the interaction of the toxic food additive Carmoisine with serum albumins: A microcalorimetric investigation. J. Hazard Mater. 2014, 273, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Mahapatra, N.; Halder, M. pH-insensitive electrostatic interaction of carmoisine with two serum proteins: A possible caution on its uses in food and pharmaceutical industry. J. Photochem. Photobiol. B 2013, 124, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, G.; Wang, Y. Binding properties of food colorant Allura Red with human serum albumin in vitro. Mol. Biol. Rep. 2014, 41, 3381–3391. [Google Scholar] [CrossRef]
- Wu, D.; Yan, J.; Wang, J.; Wang, Q.; Li, H. Characterisation of interaction between food colourant A Allura red AC and human serum albumin: Multi-spectroscopic analyses and docking simulations. Food Chem. 2015, 170, 423–429. [Google Scholar] [CrossRef]
- Tellier, F.; Steibel, J.; Chabrier, R.; Ble, F.X.; Tubaldo, H.; Rasata, R.; Chambron, J.; Duportail, G.; Simon, H.; Rodier, J.F.; et al. Sentinel lymph nodes fluorescence detection and imaging using Patent Blue V bound to human serum albumin. Biomed. Opt. Exp. 2012, 3, 2306–2316. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wei, D.; Yang, H.; Yang, Y.; Xing, W.; Li, Y. Development of a highly sensitive and specific monoclonal antibody-based enzyme-linked immunosorbent assay (ELISA) for detection of Sudan I in food samples. Talanta 2009, 77, 1783–1789. [Google Scholar] [CrossRef]
- Moller, P.; Wallin, H. Genotoxic hazards of azo pigments and other colorants related to 1-phenylazo-2-hydroxynaphthalene. Mutat. Res. 2000, 462, 13–30. [Google Scholar] [CrossRef]
- Stiborova, M.; Martinek, V.; Rydlova, H.; Hodek, P.; Frei, E. Sudan I is a Potential Carcinogen for Humans: Evidence for Its Metabolic Activation and Detoxication by Human Recombinant Cytochrome P450 1A1 and Liver Microsomes. Cancer Res. 2002, 62, 5678–5684. [Google Scholar]
- European Food Safety Authority. Available online: http://www.efsa.europa.eu (accessed on 15 September 2018).
- Lehto, S.; Buchweitz, M.; Klimm, A.; Straßburger, R.; Bechtold, C.; Ulberth, F. Comparison of food colour regulations in the EU and the US: A review of cur-rent provisions. Food Addit. Contam. 2017, 34, 335–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dykes, L.; Rooney, W.L.; Rooney, L.W. Evaluation of phenolics and antioxidant activity of black sorghum hybrids. J. Cereal Sci. 2013, 58, 278–283. [Google Scholar] [CrossRef]
- Sivakumar, V.; Vijaeeswarri, J.; Anna, J.L. Effective natural dye extraction from different plant materials using ultrasound. Ind. Crop. Prod. 2011, 33, 116–122. [Google Scholar] [CrossRef]
- Sagdic, O.; Ekici, L.; Ozturk, I.; Tekinay, T.; Polat, B.; Tastemur, B.; Senturk, B. Cytotoxic and bioactive properties of different color tulip flowers and degradation kinetic of tulip flower anthocyanins. Food Chem. Toxicol. 2013, 58, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Longo, L.; Scardino, A.; Vasapollo, G. Identification and quantification of anthocyanins in the berries of Pistacia lentiscus L., Phillyrea latifolia L. and Rubia peregrina L. Innov. Food Sci. Emerg. Technol. 2007, 8, 360–364. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Schieber, A.; Carle, R. Betacyanins in fruits from red-purple pitaya, Hylocereus polyrhizus (Weber) Britton & Rose. Food Chem. 2002, 77, 101–106. [Google Scholar]
- Ravichandran, K.; Saw, N.M.M.T.; Mohdaly, A.A.; Gabr, A.M.; Kastell, A.; Riedel, H.; Cai, Z.-Z.; Knorr, D.; Smetanska, I. Impact of processing of red beet on betalain content and antioxidant activity. Food Res. Int. 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Drioli, E. Physico-chemical parameters of cactus pear (Opuntia ficus-indica) juice clarified by microfiltration and ultrafiltration processes. Desalination 2010, 250, 1101–1104. [Google Scholar] [CrossRef]
- Otalora, M.C.; Carriazo, J.G.; Iturriaga, L.; Nazareno, M.A.; Osorio, C. Micro-encapsulation of betalains obtained from cactus fruit (Opuntia ficus- indica) by spray drying using cactus cladode mucilage and maltodextrin as encapsulating agents. Food Chem. 2015, 187, 174–181. [Google Scholar] [CrossRef]
- Khan, M.I.; Giridhar, P. Enhanced chemical stability, chromatic properties and regeneration of betalains in Rivina humilis L. berry juice. LWT—Food Sci. Technol. 2014, 58, 649–657. [Google Scholar] [CrossRef]
- Sun, M.; Temelli, F. Supercritical carbon dioxide extraction of carotenoids from carrot using canola oil as a continuous co-solvent. J. Supercrit. Fluids 2006, 37, 397–408. [Google Scholar] [CrossRef]
- Sobral, D.; Costa, R.G.B.; Machado, G.M.; Paula, J.C.J.; Teodoro, V.A.M.; Nunes, N.M.; Pinto, M.S. Can lutein replace annatto in the manufacture of Prato cheese? LWT—Food Sci. Technol. 2016, 68, 349–355. [Google Scholar] [CrossRef]
- Khalil, M.; Raila, J.; Ali, M.; Islam, K.M.S.; Schenk, R.; Krause, J.-P.; Schweigert, F.H.; Rawel, H. Stability and bioavailability of lutein ester supplements from Tagetes flower prepared under food processing conditions. J. Funct. Foods 2012, 4, 602–610. [Google Scholar] [CrossRef]
- Grewe, C.; Menge, S.; Griehl, C. Enantioselective separation of all-E- astaxanthin and its determination in microbial sources. J. Chromatogr. A 2007, 1166, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Kido, H.; Ohyama, K.; Ichibangase, T.; Kishikawa, N.; Ohba, Y.; Nakashima, M.N.; Kuroda, N.; Nakashima, K. Chemiluminescent screening of quenching effects of natural colorants against reactive oxygen species: Evaluation of grape seed, monascus, gardenia and red radish extracts as multi-functional food additives. Food Chem. 2007, 101, 980–986. [Google Scholar] [CrossRef]
- Thalhamer, B.; Buchberger, W. Adulteration of beetroot red and paprika extract based food colorant with Monascus red pigments and their detection by HPLC-QTof MS analyses. Food Control 2019, 105, 58–63. [Google Scholar] [CrossRef]
- Minioti, K.S.; Sakellariou, C.F.; Thomaidis, N.S. Determination of 13 synthetic food colorants in water-soluble foods by reversed-phase high- performance liquid chromatography coupled with diode-array detector. Anal. Chim. Acta 2007, 583, 103–110. [Google Scholar] [CrossRef]
- Ai, Y.-J.; Wu, Y.-X.; Dong, Q.-M.; Li, J.-B.; Xu, B.-J.; Yu, Z.; Ni, D. Rapid qualitative and quantitative determination of food colorants by both T Raman spectra and Surface-enhanced Raman Scattering (SERS). Food Chem. 2018, 241, 427–433. [Google Scholar] [CrossRef]
- Al-Degs, Y.S. Determination of three dyes in commercial soft drinks using HLA/GO and liquid chromatography. Food Chem. 2009, 117, 485–490. [Google Scholar] [CrossRef]
- Bonan, S.; Fedrizzi, G.; Menotta, S.; Elisabetta, C. Simultaneous determination of synthetic dyes in foodstuffs and beverages by high-performance liquid chromatography coupled with diode-array detector. Dyes Pigment. 2013, 99, 36–40. [Google Scholar] [CrossRef]
- Chen, X.H.; Zhao, Y.G.; Shen, H.Y.; Zhou, L.X.; Pan, S.D.; Jin, M.C. Fast determination of seven synthetic pigments from wine and soft drinks using magnetic dispersive solid-phase extraction followed by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2014, 1346, 123–128. [Google Scholar] [CrossRef]
- De Andrade, F.I.; Guedes, M.I.F.; Vieira, Í.G.; Mendes, F.N.P.; Rodrigues, P.A.S.; Maia, C.S.C.; Ávila, M.M.M.; Ribeiro, L.d.M. Determination of synthetic food dyes in commercial soft drinks by TLC and ion-pair HPLC. Food Chem. 2014, 157, 193–198. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, A.H.; Al-Degs, Y.S. Spectrophotometric determination of food dyes in soft drinks by second order multivariate calibration of the absorbance spectra-pH data matrices. Dyes Pigments 2013, 97, 330–339. [Google Scholar] [CrossRef]
- Gosetti, F.; Gianotti, V.; Polati, S.; Gennaro, M.C. HPLC–MS degradation study of E110 Sunset Yellow FCF in a commercial beverage. J. Chromatogr. A 2005, 1090, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Gosetti, F.; Frascarolo, P.; Mazzucco, E.; Gianotti, V.; Bottaro, M.; Gennaro, M.C. Photodegradation of E110 and E122 dyes in a commercial aperitif: A high performance liquid chromatography–diode array–tandem mass spectrometry study. J. Chromatogr. A 2008, 1202, 58–63. [Google Scholar] [CrossRef]
- Gosetti, F.; Chiuminatto, U.; Mazzucco, E.; Calabrese, G.; Gennaro, M.C.; Marengo, E. Identification of photodegradation products of Allura Red AC (E129) in a beverage by ultra-high performance liquid chromatography–quadrupole- time-of-flight mass spectrometry. Anal. Chim. Acta 2012, 746, 84–89. [Google Scholar] [CrossRef]
- Gosetti, F.; Chiuminatto, U.; Mazzucco, E.; Calabrese, G.; Gennaro, M.C.; Marengo, E. Non-target screening of Allura Red AC photodegradation products in a beverage through ultra-high performance liquid chromatography coupled with hybrid triple quadrupole/linear ion trap mass spectrometry. Food Chem. 2013, 136, 617–623. [Google Scholar] [CrossRef]
- Harp, B.P.; Miranda-Bermudez, E.; Barrows, J.N. Determination of seven certified color additives in food products using liquid chromatography. J. Agric. Food Chem. 2013, 61, 3726–3736. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Shih, Y.-C.; Chen, Y.-C. Determining eight colorants in milk beverages by capillary electrophoresis. J. Chromatogr. A 2002, 959, 317–325. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Chiu, C.-W.; Sue, S.-L.; Cheng, C.-F. Analysis of food colorants by capillary electrophoresis with large- volume sample stacking. J. Chromatogr. A 2003, 995, 29–36. [Google Scholar] [CrossRef]
- Karanikolopoulos, G.; Gerakis, A.; Papadopoulou, K.; Mastrantoni, I. Determination of synthetic food colorants in fish products by an HPLC-DAD method. Food Chem. 2015, 177, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Khanavi, M.; Hajimahmoodi, M.; Ranjbar, A.M.; Oveisi, M.R.; Ardekani, M.R.S.; Mogaddam, G. Development of a green chromatographic method for simultaneous determination of food colorants. Food Anal. Methods 2012, 5, 408–415. [Google Scholar] [CrossRef]
- Kirschbaum, J.; Krause, C.; Brückner, H. Liquid chromatographic quantification of synthetic colorants in fish roe and caviar. Eur. Food Res. Technol. 2006, 222, 572–579. [Google Scholar] [CrossRef]
- Kong, C.; Fodjo, E.K.; Li, D.; Cai, Y.; Huang, D.; Wang, Y.; Shen, X. Chitosan-based ad-sorption and freeze deproteinization: Improved extraction and purifica-tion of synthetic colorants from protein-rich food samples. Food Chem. 2015, 188, 240–247. [Google Scholar] [CrossRef]
- Liao, Q.G.; Li, W.H.; Luo, L.G. Applicability of accelerated solvent extraction for synthetic colorants analysis in meat products with ultrahigh performance liquid chromatography–photodiode array detection. Anal. Chim. Acta 2012, 716, 128–132. [Google Scholar] [CrossRef]
- Liu, F.-J.; Liu, C.-T.; Li, W.; Tang, A.-N. Dispersive solid-phase micro- extraction and capillary electrophoresis separation of food colorants in beverages using diamino moiety functionalized silica nanoparticles as both extractant and pseudostationary phase. Talanta 2015, 132, 366–372. [Google Scholar] [CrossRef]
- Ma, M.; Luo, X.; Chen, B.; Su, S.; Yao, S. Simultaneous determination of water-soluble and fat-soluble synthetic colorants in foodstuff by high- perfo-mance liquid chromatography-diode array detection-electrospray mass spectrometry. J. Chromatogr. A 2006, 1103, 170–176. [Google Scholar] [CrossRef]
- Ma, K.; Yang, Y.N.; Jiang, X.X.; Zhao, M.; Cai, Y.Q. Simultaneous determination of 20 food additives by high performance liquid chromatography with photo-diode array detector. Chin. Chem. Lett. 2012, 23, 492–495. [Google Scholar] [CrossRef]
- Medeiros, R.A.; Lourencao, B.C.; Rocha-Filho, R.C.; Fatibello-Filho, O. Flow injection simultaneous determination of synthetic colorants in food using multiple pulse amperometric detection with a boron-doped diamond electrode. Talanta 2012, 99, 883–889. [Google Scholar] [CrossRef]
- Medeiros, R.A.; Lourencao, B.C.; Rocha-Filho, R.C.; Fatibello-Filho, O. Simultaneous voltammetric determination of synthetic colorants in food using a cathodically pretreated boron-doped diamond electrode. Talanta 2012, 97, 291–297. [Google Scholar] [CrossRef]
- Ryvolova, M.; Taborský, P.; Vrabel, P.; Krasenský, P.; Preisler, J. Sensitive determination of erythrosine and other red food colorants using capillary electrophoresis with laser-induced fluorescence detection. J. Chromatogr. A 2007, 1141, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.A.; Boas, L.F.V.; Bronze, M.R.; Godoy, H.T. Validation of methodology for simultaneous determination of synthetic dyes in alcoholic beverages by capillary electrophoresis. J. Chromatogr. A 2006, 1136, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, X.; Prinyawiwatkul, W.; Xu, Z. Simultaneous determination of red and yellow artificial food colourants and carotenoid pigments in food products. Food Chem. 2014, 157, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Sorouraddin, M.H.; Rostami, A.; Saadati, M.A. simple and portable multi-colour light emitting diode based photocolourimeter for the analysis of mixtures of five common food dyes. Food Chem. 2011, 127, 308–313. [Google Scholar] [CrossRef]
- Soylak, M.; Unsal, Y.E.; Tuzen, M. Spectrophotometric determination of trace levels of allura red in water samples after separation and pre-concentration. Food Chem. Toxicol. 2011, 49, 1183–1187. [Google Scholar] [CrossRef]
- Sun, H.; Sun, N.; Li, H.; Zhang, J.; Yang, Y. Development of multiresidue analysis for 21 synthetic colorants in meat by microwave-assisted extraction–solid-phase extraction–reversed-phase ultrahigh performance liquid chromatography. Food Anal. Methods 2013, 6, 1291–1299. [Google Scholar] [CrossRef]
- Tang, B.; Xi, C.; Zou, Y.; Wang, G.; Li, X.; Zhang, L.; Chen, D.; Zhang, J. Simultaneous determination of 16 synthetic colorants in hotpot condiment by high performance liquid chromatography. J. Chromatogr. B 2014, 960, 87–91. [Google Scholar] [CrossRef]
- Turak, F.; Ozgur, M.U. Simultaneous determination of allura red and ponceau 4R in drinks with the use of four derivative spectrophotometric methods and comparison with high-performance liquid chromatography. J. AOAC Int. 2013, 96, 1377–1386. [Google Scholar] [CrossRef]
- Vidotti, E.C.; Costa, W.F.; Oliveira, C.C. Development of a green chromatographic method for determination of colorants in food samples. Talanta 2006, 68, 516–521. [Google Scholar] [CrossRef]
- Wu, H.; Guo, J.B.; Du, L.M.; Tian, H.; Hao, C.X.; Wang, Z.F.; Wang, J.Y. A rapid shaking-based ionic liquid dispersive liquid phase micro extraction for the simultaneous determination of six synthetic food colourants in soft drinks, sugar-and gelatin-based confectionery by high-performance liquid chromatography. Food Chem. 2013, 141, 182–186. [Google Scholar] [CrossRef]
- Xie, Y.; Li, Y.; Niu, L.; Wang, H.; Qian, H.; Yao, W. A novel surface-enhanced Raman scattering sensor to detect prohibited colorants in food by graphene/silver nanocomposite. Talanta 2012, 100, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Meng, M.; Xue, H.; Zhang, T.; Yin, Y.; Xi, R. Development of a polyclonal antibody-based enzyme-linked immunosorbent assay (ELISA) for detection of sunset yellow FCF in food samples. Talanta 2012, 99, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, N.; Ichihashi, K. Determination of 40 synthetic food colors in drinks and candies by high- performance liquid chromatography using a short column with photodiode array detection. Talanta 2008, 74, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; He, P.; Yasen, A.; Li, Z. Determination of seven synthetic dyes in animal feeds and meat by high performance liquid chromatography with diode array and tandem mass detectors. Food Chem. 2013, 138, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-F.; Kuo, C.-H.; Shih, D.Y.-C. Determination of 20 synthetic dyes in chili powders and syrup-preserved fruits by liquid chromatography/tandem mass spectrometry. J. Food Drug Anal. 2015, 23, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Périat, A.; Bieri, S.; Mottier, N. SWATH-MS screening strategy for the determination of food dyes in spices by UHPLC-HRMS. Food Chem. X 2019, 1, 100009. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhang, Q.H.; Ma, K.; Li, H.M.; Guo, Z. Identification and determination of 34 water-soluble synthetic dyes in foodstuff by high performance liquid chromatography–diode array detection–ion trap time-of-flight tandem mass spectrometry. Food Chem. 2015, 182, 316–326. [Google Scholar] [CrossRef]
- Yamjala, K.; Nainar, M.S.; Ramisetti, N.R. Methods for the analysis of azo dyes employed in food industry—A review. Food Chem. 2016, 192, 813–824. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar]

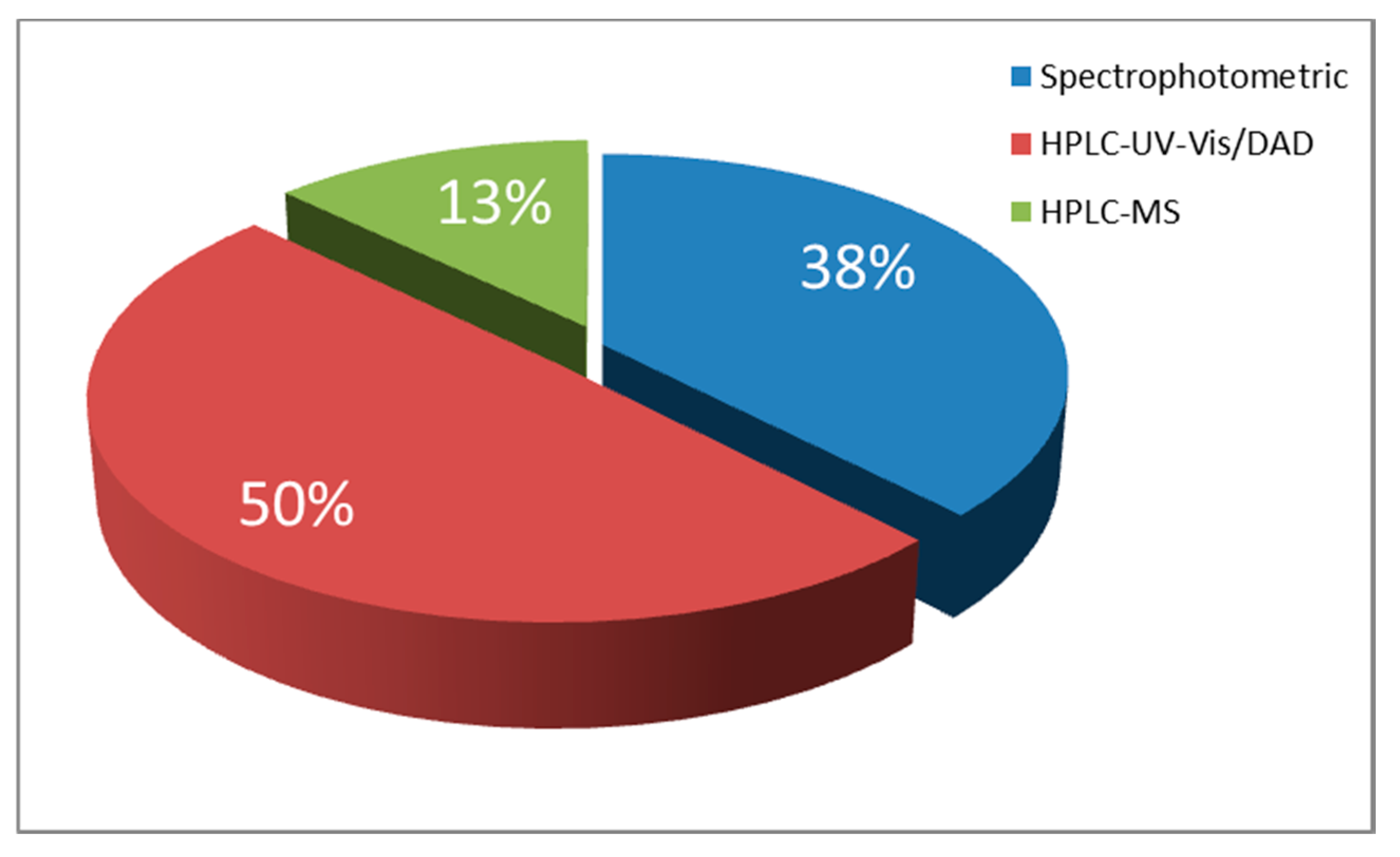
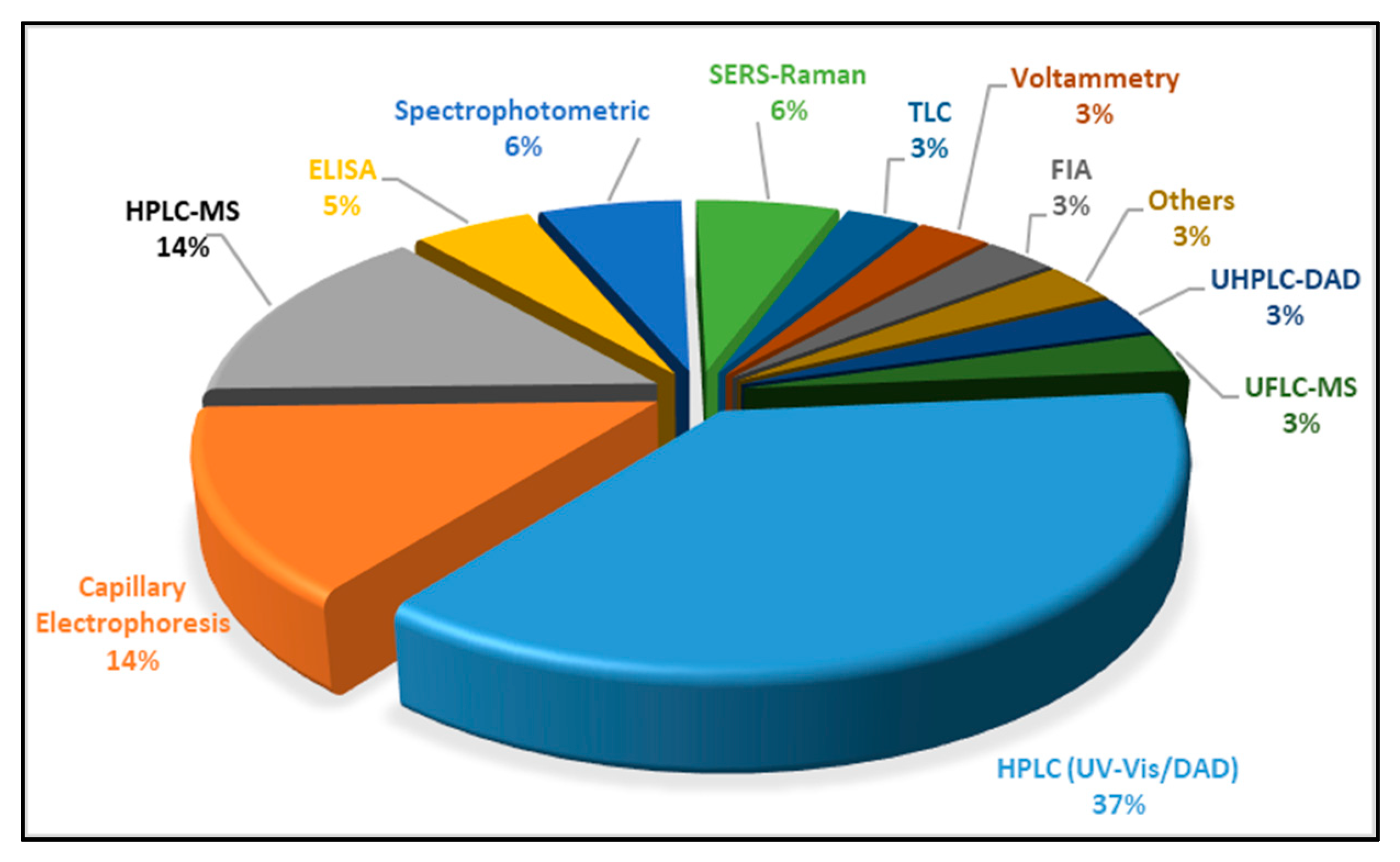
| Food Colorant | Food Matrix | Analytical Technique | Detection | Detection Settings (i.e., λ, Ionisation) | Column | Elution | Mobile Phase | Inj. Volume | Figures of Merit (LOD, LOQ, Linear Range) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 3-Deoxy-anthocyanidins | Sorghum bicolor (L.) Moench seeds | High Pressure Liquid Chromato-graphy (HPLC) | Diode Array Detection (DAD) | 485 nm | Luna C18 column (150 × 4.6 mm, 5 mm) | Gradient | 4% HCOOH in H2O (v/v) (Solvent A) and acetonitrile (Solvent B) | 20 μL | n/a | [34] |
| Anthocyanin-derived extracts | Acacia decurrens Willd. Bark | Spectrophotometric analysis | UV-Vis | 400–800 nm | n/a | n/a | n/a | n/a | [35] | |
| -Tulipa gesneriana L. | Spectrophotometric analysis | UV-Vis | 765 nm | n/a | n/a | n/a | n/a | n/a | [36] | |
| Cyanidin 3-glucoside | -Pistacia lentiscus L. fruits; -Santalum album L. fruits | HPLC | DAD | 520 nm, 440 nm, 310 nm and 280 nm | SS Wakosil C18 (150 × 4.6 mm, 5 μm) | Gradient | 0.1% trifluoroacetic acid (TFA) in H2O (solvent A) and 0.1% TFA in acetonitrile (Solvent B) | 20 μL | n/a | [37] |
| Cyanidin 3-glucoside | -Pistacia lentiscus L. fruits; -Santalum album L. fruits | HPLC | ESI-MS | SS Wakosil C18 (150 × 4.6 mm, 5 μm) | Gradient | 0.1% TFA in H2O (solvent A) and 0.1% TFA in acetonitrile (Solvent B | 20 μL | n/a | [37] | |
| Betacyanins | -Hylocereus polyrhizus | HPLC | MS | ESI (+) | AQUA C18-reversed phase column, 5 μm | Gradient | (A) 2% (v/v) CH3COOH in H2O and (B)0.5% CH3COOH in H2O/acetonitrile (50/50, v/v) | n/a | [38] | |
| Betalains | Beta vulgaris L. roots | HPLC | UV-Vis | 538 nm; 480 nm | Lichrocart 250 × 4 RP-18 (5 μm) | Gradient | H2O (A) and acetonitrile (B). | 20 μL | n/a | [39] |
| Betalains | Opuntia ficus-indica [L.] | HPLC | UV-Vis | 245 nm | Luna C18(2) column (250 × 4.6 mm, 5 µm) | Isocratic | 20 mM KH2PO4/ Acetonitrile 95:5 v/v | 20 μL | n/a | [40] |
| Betalains | Opuntia ficus-indica [L.] | Spectrophotometric analysis | UV-Vis | λ = 536 nm | n/a | n/a | n/a | n/a | n/a | [41] |
| Betalains | Rivina humilis L. fruits, juice | Spectrophotometric analysis | UV-Vis | λ = 535 nm | n/a | n/a | n/a | n/a | n/a | [42] |
| α-carotene | Daucus carota L. roots | HPLC | UV-Vis | 450 nm | Supelcosil LC-18 column (15 cm × 4.6 cm, 5 μm) | Isocratic | Methanol/10% (v/v) Acetonitrile: H2O | 50 μL | n/a | [43] |
| Lutein | Commercial/Milk | HPLC | UV-Vis | 450 nm | RP C30 YMC (250 × 4.6 mm, 5 μm) | Isocratic | ethanol, tert-butyl-methyl-ether (MTBE) as the mobile phase | 50 μL | n/a | [44] |
| Lutein | Hawaii “T. erecta”, Carmen “T. patula”. | HPLC | DAD | 450 nm | Waters-Spherisorb column SC-04 (125 × 4.0 mm, ODS2, 3.0 μm) | Gradient | (A) Acetonitrile–methanol (9:1 v/v): (B) Ethyl acetate | 100 μL | n/a | [45] |
| Astaxanthin | Microalgae and yeasts | HPLC | DAD | 470 nm | Chiralcel OD-RH column (5 μm, 150 mm × 4.6 mm) | (A) Acetonitrile and (B) phosphoric acid (3.5 mM) | n/a | n/a | [46] | |
| Crocetin, Crocin | Grape seed, monascus, gardenia, and red radish | Spectrophotometric analysis | UV-Vis | 438 nm; 462 nm | n/a | n/a | n/a | n/a | n/a | [47] |
| Monascus red pigments | Beetroot red and paprika extract | High Resolution Mass Spectrometry | HPLC-QTOF-MS | ESI (+) | Kinetex c18 column (2.6 μm, 50 mm × 4.6 mm) | Gradient | (A) Acetonitrile; (B) H2O; (C) aqueous HCOOH 1% v/v | n/a | n/a | [48] |
| Food Colorant | Extraction/Sample Preparation | Ref. |
|---|---|---|
| 3-Deoxyanthocyanidins | Ground sample, with 1% HCl in methanol, centrifugation hydrolysis; | [34] |
| Anthocyanin-derived extracts | Comparison of different extraction methods (ultrasonic and natural extraction) vs. magnetic stirring | [35] |
| Extraction with ethanol: H2O (1:1 v/v) acidified with 0.01% HCl | [36] | |
| Cyanidin 3-glucoside | Extraction with 0.1% HCl (v/v) in methanol, combination of the extracts, evaporation, and dissolution | [37] |
| Betacyanins | Mixing with water, filtration, addition of ethanol (precipitation of pectic substances and proteins) | [38] |
| Betalains | Sample dissolution in ethanol, agitated and homogenized | [39] |
| Betalains | Filtration of water extract (no pH adjustment) | [40] |
| Betalains | Lyophilization and macerated with PBS (pH 5.0) in 1:5 w/w ratio, followed by spray-drying | [41] |
| Betalains | Dilution of the juice; filtration; addition of Se4+, Zn2+, and Cu2+ | [42] |
| α-carotene | Comparison between simple extraction and Supercritical Fluid extraction (CO2); Simple extraction: Hexane/acetone; SFE: SC-CO2 (SFE) | [43] |
| Lutein | Sample dilution in 95% ethanol and extraction with acetone and petroleum ether. Evaporation and reconstitution | [44] |
| Lutein | Extraction with organic solvent (isopropanol), centrifugation and supernatant extracted with hexane | [45] |
| Astaxanthin | Extraction with ethyl acetate, filtration | [46] |
| Crocetin, Crocin | Dilution in DMSO | [47] |
| Food Colorant | MATRIX | Analytical Technique | Detection | Column | Elution | Mobile Phase | Inj. Volume | Figures of Merit (LOD, LOQ) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Brilliant blue | Liquid foods | CE | UV (λ = 220 nm) 36 cm capillary; Separation voltage (8 kV, −8 kV); | Fused-silica cappilaries of 375 od μm and 75 μm i.d | - | 30 mM PBS buffer (pH6), with 0.9 mg/mL dASNPs and 2 mM β-cyclodextrin (CD) | Electro kinetic injection | LOD = 0.36 mg/L LOQ = 0.63 mg/L | [2] |
| Amaranth, ponceau 4R, sunset yellow, tartrazine, brilliant blue | spectrophotometric kinetic method | UV-Vis λPrussian blue = 760 nm | n/a | n/a | n/a | n/a | LOD = 0.2–6.0 mg/L | [9] | |
| Sudan I | Non-alcoholic drinks, sweets, jellies | Enzyme-linked Immuno-sorbent assay (ELISA) | n/a | n/a | n/a | n/a | n/a | LOD = 0.07 ng/mL | [29] |
| Sudan I | Non-alcoholic drinks, jellies | HPLC | UV- Vis (478 nm) | C18 (250 × 4.6 mm, 5.0 μm) | Gradient | methanol/2% CH3COOH | 20 μL | LOD = 0.14 ng/mL | [29] |
| Tartrazine, quinoline Yellow, sunset yellow, Carmoisine, Amaranth, ponceau 4R, Erythrosine, Red 2G, allura Red AC, Patent Blue V, Indigo Carmine, brilliant blue, Green S | Beverages, dairy powders, jellies, candies, condiments, icings, syrups, extracts | HPLC | DAD Various wavelengths | Discovery C18 (250 mm × 4.6 mm 5 μm) | Gradient | CH3COONH4 0.13 M (pH = 7.5; NaOH)/methanol: acetonitrile 80:20 v/v | 20 μL | LOD = 1.87–22.1 μg/L | [49] |
| Tartrazine, sunset yellow, brilliant Blue, acid red | Powder | SERS-Raman | confocal microscope Raman spectrometer system | n/a | n/a | n/a | n/a | LOD = 10−7 M | [50] |
| Allura red, sunset yellow, tartrazine | Soft drinks | HPLC | DAD | n/a | Gradient | methanol (HPLC grade) and NaH2PO4/ Na2HPO4 buffer (0.10 M, pH = 7.0). | n/a | LOD = 0.06–0.30 μg/mL | [51] |
| Allura red, sunset yellow, tartrazine | Soft drinks | HLA-Go | - | - | - | - | - | - | [51] |
| Azorubine, amaranth, cochineal red A, red 2G, allura red, azocarmine B (AZO B), azocarmine G (AZO G), ponceau 2R, ponceau 6R, tartrazine, sunset yellow, quinoline yellow, orange II, metanil yellow (MY), patent blue V, indigo carmine, brilliant blue | Solid food/liquid beverages | HPLC | DAD DAD, λquant.: −620 nm (blue); −515 nm (red); −420 and 480 nm (yellow). | C8 (150 × 4.6 mm, 3 μm) | Gradient | Acetonitrile/sodium acetate (pH = 7) | 20 μL | 5–300 mg/kg (solid food samples) 5–100 mg/L (drinks) | [52] |
| Brilliant blue, Tartrazine, amaranth, carmine, sunset yellow, allura red, erythrosine | Wine and soft drinks | UFLC | ESI (−)-MS/MS | ODS II (100 mm × 2.0 mm; 2.2 μm) | Gradient | A: Acetonitrile: CH3COONH4 5.0 mM/ (B) H2O: CH3COONH4 5.0 mM | 5 μL | LOD = 0.45–1.51 μg/L LOQ = 1.51–5.00 μg/L | [53] |
| Brilliant blue, tartrazine, amaranth, sunset yellow | Wine and soft drinks | TLC-UV-Vis | UV-Vis | TLC-PET 20 × 20 silica gel | n/a | 8 mL 2-propanol and 3 mL NH4OH | 5 μL (standards) and 30 μL sample | n/a | [54] |
| Allura red, sunset yellow, tartrazine | Solid food/liquid beverages | Spectrophoto-metric BLLS/RBL | Absorbance spectra-pH data Spectral measurements (300–600 nm) at different pH | n.a | n.a | n.a | n.a | LOD = 0.54 mg/L | [55] |
| Sunset yellow | Beverage | HPLC | ESI (−)-MS | C18-ether column (150 mm × 4.6 mm, 5 μm) | Isocratic | (A) 63% aqueous solution 20 mM CH3COONH4: (B) 37% methanol | 20 μL | n/a | [56] |
| Carmoisine, sunset yellow | Beverage | HPLC | ESI (−)-MS | C18 column (250 mm × 2 mm, 4 μm) | Isocratic | (A) Methanol and (B) 10 mM HCOONH4 (45:55, v/v) | 20 μL | LOD = 10–12 μg/L | [57] |
| Allura red | Beverage | HPLC | ESI (−)-MS | HSS-T3 column (2.1 mm 100 mm, 1.8 μm) | Gradient | A: H2O: CH3COONH4 1.0 mM/ (B) Methanol: CH3COONH4 1.0 mM | 20 μL | n/a | [58,59] |
| Brilliant blue, tartrazine, allura red, amaranth, Azorubine, patent Blue V, ponceau 4R | Various food products | HPLC | DAD Various wavelengths | Xterra RP18 column (250 × 4.6 mm, 5 μm) | Gradient | A) 0.1 M CH3COONH4 in water and (B) 0.1 M CH3COONH4 in methanol | 20 μL | LOD = 0.02–1.49 mg/L | [60] |
| Brilliant blue, indigo carmine, allura red, carminic acid, ponceau 4R, sunset yellow, tartrazine | Dairy powders, color beverages, jellies, candies, condiments, icings, syrups, | CE | UV (200 nm) Condiiton with 1 M NaOH, H2O electrode polarity (25kV) | - | - | Running buffer of pH 10 (20 mM NaOH solution to 15 mM disodium tetraborate (borax) to 20 mM NaOH, until the desired pH | Large-volume injection | LODs 0.05–0.40 μg/mL | [61] |
| Brilliant blue, indigo carmine, allura red, carminic acid, ponceau 4R, sunset yellow, tartrazine, fast green FCF | Liquid foods | CE | UV (λ = 200 nm) Condiiton with 1 M NaOH, H2O electrode polarity (25kV) | - | Running buffer of pH 10 (20 mM NaOH to 15 mM disodium tetraborate (borax) to 20 mM NaOH, until desired pH | Large-volume injection | LOD = 0.002–0.026 μg/mL | [62] | |
| Sunset yellow, carmoisine, amaranth, ponceau 4R, erythrosine, red 2G, allura red | Soft drinks | HPLC | DAD Various wavelengths | Symmetry C18 (Waters, Milford, USA) column (150 mm × 4.6 mm, 5 μm) | Gradient | CH3COONH4 buffer (1% w/v) (0.13 M) (pH: 7.5) by addition of 0.1 M aq. NH3 (solvent A), methanol (solvent B) and acetonitrile (solvent C) | n/a | 0.5–1.4 μg/mL | [63] |
| Tartrazine, quinoline yellow, sunset yellow, carmoisine, ponceau 4R, allura red, indigo carmine, brilliant blue | Various foods and medicines | HPLC | DAD Various wavelengths | C18 column (250 mm × 4.6 mm, 5 μm) | Isocratic | A) Triton X-100 (0.25%, v/v) and (B) 50 mmol/L PBS (pH 7) | 20 μL | LOD = 0.05–0.44 μg/mL LOQ = 0.05–1.12 μg/mL | [64] |
| Tartrazine, sunset yellow, azorubine, amaranth, cochineal red, red 2G, allura red AC, Brilliant Black BN, brown FK, Brown HT Patent Blue V, brilliant Blue FCF, Green S | Fish roe | HPLC | DAD Various wavelengths | Xterra RP18 column (250 × 4.6 mm, 5 μm) | Gradient | (a) 100 mmol/L CH3COONa buffer (pH 7.0) and (b) Acetonitrile | 20 μL | LOD = 0.02--1.49 mg/L | [65] |
| Brilliant blue, Indigo carmine, allura red, erythrosine, ponceau 4R, sunset yellow, Lemon yellow | Protein-rich samples | HPLC | DAD Various wavelengths | RP-C18 Column | Gradient | Methanol- 20 mM of CH3COONH4 | 20 μL | LOD = 0.1–0.4 mg/kg | [66] |
| Brilliant blue, tartrazine, sunset yellow, amaranth, carminic acid, acid red, allura red | Meat products | UHPLC | PDA Various wavelengths | BEH C18 (100 × 2.1 mm, 1.7 μm | Gradient | Acetonitrile/ CH3COONH4 | 5 μL | LOD = 0.01 mg/kg LOQ = 0.05 mg/kg | [67] |
| Carminic acid, sunset yellow, tartrazine | Non-alcoholic drinks, sweets, jellies | Capillary Electrophoresis (CE) | UV (λ = 220 nm) 36 cm capillary; Separation voltage (8 kV, −8 kV); | Fused-silica capillaries of 375 od μm and 75 μm i.d | - | 30 mM PBS buffer (pH6), with 0.9 mg/mL dASNPs and 2 mM β-cyclodextrin (CD) | Electrokinetic injection | LOD = 0.03 -0.072 mg/L LOQ = 0.16–0.31 mg/L | [68] |
| Amaranth, Ponceau 4R, Sunset yellow, tartrazine, Sudan I-IV | Soft drinks/solid samples | HPLC-ESI (+)-MS | ESI (+)-MS | Spherigel C18 (250 × 4.6 mm, 5 μm) | gradient | aq. methanol 0.1% HCOOH/aqueous methanol 20 mM CH3COONH4/1% CH3COOH | 20 μL | LOD = 2.0–3.5 ng LOQ = 5.4–10.5 ng | [69] |
| Brilliant blue, allura red, amaranth, Erythrosine, ponceau 4R, sunset yellow, tartrazine | Soft drinks and processed meats | HPLC | DAD Various wavelengths | Inertsil ODS-SP column (250 × 4.6 mm, 5 μm) | Gradient | CH3COONH4 (0.1 M, pH = 7.2)—methanol-acetonitrile (9:1 v/v) | 20 μL | LOD: 0.005 μg/mL LOQ: 0.018 μg/mL | [70] |
| Brilliant blue, sunset yellow, tartrazine | Dairy powders, beverages, jellies, candies, syrups, extracts | Flow injection (FIA) | Amperometric detection (boron-doped diamondelectrode) | - | - | Edet.2 = −450 mV (100 ms duration) vs. Ag/AgCl (3.0 M KCl). | LOD = 0.8–3.5 μM | [71] | |
| Brilliant blue, sunset yellow, tartrazine | Beverages, jellies, candies, condiments, icings, syrups | Differential pulse voltammetry (DPV) | Cathodically pretreated boron-doped diamond (BDD) elctrode | - | - | 30 Hz; amplitude(a), 40 mV; for DPV scan rate (v), 0 mVs | - | LOD = 13.1–143 nM | [72] |
| Erythrosine, carmoisine, amaranth, ponceau 4R, Red 3G | Syrups | Capillary electrophoresis (CE) | Laser-induced fluorescence detection Various λexc./λemis.: (Nd:YAG laser with wavelength 532 nm and power 5 mW) | Fused silica capillary with I.D. 50 μm, O.D. 360 μm, length 30 cm | n/a | V = +17 kV (intensity of electrical field was 460 Vcm−1). | n/a | LOD = 0.2–0.4 μg/mL | [73] |
| Tartrazine, sunset yellow, azorrubine, bordeaux S, ponceau 4R, erytrosine, red no 40, patent blue V, indigo carmin, brilliant blue | Alcoholic beverages | CE | UV/vis PBS 10 mM with sodium dodecyl sulfate 10 mM, pH 11, and +25 kV of voltage | Fused silica capillary (73 cm) | n/a | Phosphate buffer solution of 10 mM with sodium dodecyl sulfate 10 mM, pH 11, and +25 kV voltage | n/a | LOD = 0.4–2.5 μg/mL LOQ = μg/mL | [74] |
| Tartazine, Amaranth, Sunset yellow, allura red, Lutein, lycopene, β-carotene | Various foodstuff | HPLC | DAD Various wavelengths | C18 column (250 mm × 4.6 mm, 5 μm) | Gradient | 1% CH3COONH4, methanol and acetone; | 20 μL | LOD = 0.2–50 ng/mL | [75] |
| Tartrazine, Quinoline yellow, Sunset Yellow, Carmoisine, Brilliant Blue | Solid foods | spectrophotometric method | UV-Vis Various wavelengths | n/a | n/a | n/a | n/a | LOQ = 1–5 μg/mL | [76] |
| Allura red | Liquid foods | spectrophotometric method | UV-Vis (506 nm) | n/a | n/a | n/a | n/a | LOD = 2.35 μg/L | [77] |
| Tartrazine, New red, Amaranth, Ponceau 4R, Sunset yellow, Allura red, Acid red, Brilliant Blue, Acid red, Erythrosine, Acid orange, Basic flavine O, Basic orange, Siperse blue 106, Crystal violet, Leucine malachite green, Leucine crystal violet | Meat | UHPLC | DAD (200–800 nm) | C18 column (2.1 mm × 50 mm, 1.7 μm) | Gradient | (A) 20 mM CH3COONH4–0.02% acetic acid (pH 5) and (B) acetonitrile | 2 μL | LOD = 0.96–2.16 μg/kg LOQ = 1.61–7.19 μg/kg | [78] |
| New red, Amaranth, Camine, Sunset yellow, Acid Red G, Allura red, Acid Scarlett GR, Erythrosine, Rhodamine B, Sudan I, Para red, Sudan II, Sudan III, Sudan red 7B, Sudab IV, Sudan Orange G | Hotpot condiment | HPLC | DAD Various wavelengths | C18 column (4.6 mm × 250 mm, 5 μm) | Gradient | (A) Methanol; (B) 0.01 M PBS (pH = 7.5) | 20 μL | LOD = 0.001–0.00 3 mg/kg | [79] |
| Allura Red, Ponceau 4R | Granulated drinks | UV–visible spectrophotometer | UV–visible spectrophotometer (ZCDS) | n/a | n/a | n/a | n/a | LOD = 0.059–0.102 μg/mL LOQ = 0.198–0.341 μg/mL | [80] |
| Brilliant Blue, Sunset Yellow, Tartrazine | Non-alcoholic drinks, sweets, jellies | HPLC | UV-Vis (630 nm, 480 nm, 430 nm) | Modified C18 column (250 × 4.6 mm, 5 μm) with a 0.25% (v/v) Triton X-100 aq. solution at pH 7 | Isocratic | 0.25 mL of Triton X-100 (Sigma) up to 100 mL with 50 mmol l−1 phosphate buffer solution at pH 7 | 20 μL | LOD = 0.143–0.080 mg/L | [81] |
| Tartrazine, Amaranth, Sunset Yellow, Allura red, Ponceau 4R, Erythrosine | Soft drinks, sugar and gelatin based confectionery | HPLC-UV | UV 430 nm, 510 nm | C18 column (250 mm × 4.6 mm, 5 μm | Gradient | (A) 0.1 mol/L ammonium acetate aqueous solution (pH 7.5, adjusted with 10 mol/L NaOH- methanol–acetonitrile (30:70, v/v) | 20 μL | LOD = 0.015–0.32 ng/mL | [82] |
| Allurea red, Amaranth, Erythrosine, Ponceau 4R, Sunset Yellow | Beverages, alcoholic drinks and fish foe | SERS-Raman | Radiation of 514.5 nm from an air-cooled argon ion laser was used for SERS excitation | n/a | n/a | n/a | n/a | LOD = 10−7–10−5 M | [83] |
| Sunset yellow | beverage, dried bean curd, braised pork | ELISA | n/a | n/a | n/a | n/a | LOD = 25 pg mL−1 | [84] | |
| (40 food colorants) Ponceau 6R, Tartrazine, Fast yellow AB, Amaranth, Indigotine, Naphthol yellow S, Chrysoine, Ponceau 4R, Sunset yellow FCF, Red 10B, Orange G, Acid violet 7, Brilliant black PN, Allura red AC, Yellow 2G, Red 2G, Uranine, Fast red E, Green S, Ponceau 2R, Azorubine, Orange I, Quinoline yellow, Martius yellow, Ponceau SX, Ponceau 3R, Fast green FCF, Eosine, Brilliant blue FCF, Orange II, Orange RN, Acid blue 1, Erythrosine, Amido black 10B, Acid red 52, Patent blue V, Acid green 9, Phloxine B, Benzyl violet 4B, Rose bengal | Drinks, syrups and candies | HPLC | HPLC–DAD | C18 column (50 mm × 4.6 mm, 1.8 μm) | Gradient | (A) 0.1 mol/L of CH3COONH4 pH 6.7 and (B) was Methanol–Acetonitrile (50:50, v/v) | 5 μL | LOD = 0.03–0.1 μg/g | [85] |
| Ponceau 4R, Sunset Yellow, Allura Red, Azophloxine, Ponceauxylidine, Erythrosine, Orange II | Animal feed and meat | LC | ESI (−)-MS | C18 column (2.1 mm × 150 mm, 5 μm) | Gradient | (A) 20 mmol/L CH3COONH4: Acetonitrile | LOD = 0.02–21.83 ng/mL | [86] | |
| New Coccine, Indigo Carmine, Erythrosine, Tartrazine, Sunset Yellow FCF, Fast Green FCF, Brilliant Blue FCF, Allura Red AC, Amaranth, Dimethyl Yellow, Fast Garnet GBC, Para Red, Sudan I, Sudan II, Sudan III, Sudan IV, Sudan Orange G, Sudan Red 7B, Sudan Red B, Sudan Red G | Chili powders; commercial syrup preserved fruits | LC | ESI (−) and ESI (+) MS/MS | Acclaim Polar Advantage C16 (3 mm, 4.6 × 150 mm) | Gradient | (A) Acetonitrile and (B) 20 mM CH3COONH4 –1.0% CH3COOH | LOQ = 0.005–1 mg/kg | [87] | |
| Multi-class (53 food colorants) | Spices | UHPLC | QTOF-MS (sequential window acquisition of all theoretical fragment-ion spectra- SWATH) | Acquity UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm,) | Gradient | (A) Acetonitrile and (B) 10 mM CH3COONH4 pH = 6.7 | n/a | n/a | [88] |
| Multi-class (34 water soluble synthetic food colorants) | Beverages, syrup, chewing gum | HPLC | DAD-IT-TOF/MS (λ = 200–700 nm) | Atlantis™ dC18 (4.6 mm × 250 mm, 5 μm) | Gradient | (A)20 mM HCOONH4 buffer; (B) methanol/acetonitrile (1:1 v/v) | 20 μL | LODs = 0.009–0.102 μg/mL; LOQs = 0.045–0.203 μg/mL | [89] |
| Food Colorant | MATRIX | Extraction/Sample Preparation | Ref. |
|---|---|---|---|
| Amaranth, Ponceau 4R, Sunset Yellow, Tartrazine and Brilliant Blue | Reduction of iron (III) in sodium acetate/hydrochloric acid solution (pH 1.71) followed by a chromogenic reaction with potassium hexacyanoferrate (III) to form the Prussian blue species | [9] | |
| Sudan I | Non-alcoholic drinks, sweets, jellies | Extraction, sonication, centrifugation, filtration. Sample extracts or liquid samples were filtrated | [29] |
| Tartrazine, quinoline Yellow, Sunset Yellow, Carmoisine, Amaranth, Ponceau 4R, Erythrosine, Red 2G, Allura Red AC, Patent Blue V, Indigo Carmine, Brilliant Blue FCF, Green S | Dairy powders, color beverages, jellies, candies, condiments, icings, syrups, extracts | Beverages: degassed by stirring Solid: dissolved in water, ultrasonication, filtration | [49] |
| Tartrazine, Sunset yellow, Brilliant Blue, Acid red | Powder | Fabrication of flower-like silver nanostructures by adding 10 mL of ultrapure water, 2 mL of PVP solution (1%) and 0.2 mL of silver nitrate solution (1 mol/L) | [50] |
| Allura Red, Sunset Yellow, and Tartrazine | Soft drinks | Food solutions were prepared with dilution with methanol–water mixture (v/v, 50/50) | [51] |
| Azorubine, amaranth, cochineal red A, red 2G, allura red, azocarmine B (AZO B), azocarmine G (AZO G), ponceau 2R, ponceau 6R, tartrazine, sunset yellow, quinoline yellow, orange II, metanil yellow (MY), patent blue V, indigo carmine and brilliant blue FCF. | Solid food/liquid beverages | 4 g solid sample +20 mL ethanol-H2O (1:1 v/v), ultrasound and shaking, centrifugation, separation, and SPE in polyamide (PA) cartridge Beverages: degas (ultrasound), diluted 1.1 with H2O, centrifuge | [52] |
| Brilliant Blue FCF, Tartrazine, Amaranth, Carmine, Sunset yellow, Allura red, Erythrosine | Wine and soft drinks | Magnetic dispersive solid-phase extraction (M-dSPE): | [53] |
| Brilliant Blue FCF, Tartrazine, Amaranth, Sunset yellow, | Wine and soft drinks | Degassing followed by SPE with Sep-Pack C18 and elution with 2-propanol | [54] |
| Allura red, sunset yellow, tartrazine | Solid food/liquid beverages | Sample dilution (0.5–2.0 g) in 100 mL H2O | [55] |
| Sunset yellow | Beverage | No extraction | [56] |
| Carmoisine, sunset yellow | Beverage | Samples were diluted with water and filtered through 0.2 μm polypropylene membrane | [57] |
| Allura red | Beverage | filtered through 0.45 μm nylon filter and diluted 1:20 (v/v) in ultrapure water | [58,59] |
| Brilliant blue, tartrazine, allura red Amaranth, Azorubine, Patent Blue V, Ponceau 4R | Various food products | Beverages: sample sonicated, addition of aq. NH3, filtration; Solid: homogenization, addition of aq. NH3, sonication, centrifugation | [60] |
| Brilliant Blue FCF, Indigo carmine, Allura red, carminic acid, Ponceau 4R, Sunset yellow, tartrazine | Dairy powders, color beverages, jellies, candies, condiments, icings, syrups, extracts | Flavored milk samples: diluted with ethanol (1:1 v/v), SPE with PA cartridge | [61] |
| Brilliant Blue FCF, Indigo carmine, Allura red, carminic acid, Ponceau 4R, Sunset yellow, tartrazine, fast green FCF | Liquid foods | Beverages: degas (ultrasound) and directly to CE Milk: diluted with ethanol (1:1 v/v), SPE with PA cartridge Jelly: blended with ethanol: H2O 1:1 v/v at 65 °C × 4 h + SPE (Polyamide cartridge) | [62] |
| Sunset yellow, Carmoisine, Amaranth, Ponceau 4R, Erythrosine., Red 2G, Allura red | Soft drinks | Extraction stage, followed by sonification, centrifugation, and concentration step + clean up via SPE on polyamide (PA) cartridges | [63] |
| Tartrazine, Quinoline Yellow, Sunset Yellow, Carmoisine, Ponceau, 4R, Allura Red, Indigo Carmine, Brilliant Blue | Various foods and medicines | Homogenization, dissolution, filtration | [64] |
| Tartrazine, Sunset Yellow FCF, Azorubine Amaranth Cochineal Red, Red 2G), Allura Red AC, Brilliant Black BN, Brown FK and Brown HT, Patent Blue V, Brilliant Blue FCF, and Green S | Fish roe | Extraction with aq. NH3, centrifugation, pH adjustment, addition of PA sorbent and extraction with methanol | [65] |
| Brilliant blue, Indigo carmine, allura red, erythrosine, ponceau 4R, sunset yellow, Lemon yellow | Protein-rich samples | Purification/deproteinization with chitosan | [66] |
| Brilliant blue, tartrazine, sunset yellow, amaranth, carmininic acid, acid red, allura red | Meat products | ASE (static) with ethanol-H2O_NH3 75:24:1 v/v/v, 85oC for 10 min | [67] |
| Carminic acid, sunset yellow, tartrazine, brilliant blue. | Non-alcoholic drinks, sweets, jellies | d-SPME with diamino-moiety functionalized silica nanoparticles (dASNPs) and β-cyclodextrin (β-CD) and pseudo-stationary phases (PSPs). Optimization of pH (2.5), sorbent, amount of dASNPs; ionic strength; extraction time and mode; desorption time; interferences (no interferences identified) | [68] |
| Amaranth, Ponceau 4R, Sunset yellow, tartrazine, Sudan I-IV | Soft drinks/solid samples | Liquid: filtration, degassing Solid: homogenization, extraction with DMSO, sanitation, centrifugation, and filtration | [69] |
| Brilliant blue FCF, Allura red, Amaranth, Erythrosine, Ponceau 4R, Sunset Yellow, Tartrazine | Soft drinks and processed meats | [70] | |
| Brilliant blue, sunset yellow, tartrazine | Dairy powders, color beverages, jellies, candies, condiments, icings, syrups, extracts | Homogenization, addition in 0.1 M H2SO4 (gelatin dissolution), ultrasonic and dilution with supporting electrolyte | [71] |
| Brilliant blue, sunset yellow, tartrazine | Dairy powders, color beverages, jellies, candies, condiments, icings, syrups, extracts | - | [72] |
| Erythrosine, carmoisine, amaranth, ponceau 4R, Red 3G | Syrups | Dilution to PBS (20 mM, pH 11) as a background electrolyte (BGE) in the ratio of 1:10 or 1:2 for CE–LIF | [73] |
| Tartrazine, sunset yellow, azorrubine, bordeaux S, ponceau 4R, erytrosine, red no 40, patent blue V, indigo carmine, brilliant blue FCF | Alcoholic beverages | degassed by mechanical agitation and filtered | [74] |
| Tartazine, Amaranth, Sunset yellow, Allura red, Lutein, Lycopene, β-carotene | Various foodstuff | Ultrasound-assisted solvent extraction: immersion to methanol, sonication, centrifugation, extraction with acetone, evaporation. Liquid: 0.5 mL + 1 mL methanol; Solid: 0.2 g + 1 mL methanol | [75] |
| Tartrazine, Quinoline yellow, Sunset Yellow, Carmoisine, Brilliant Blue | Solid foods | Solid: a portion of food was diluted in H2O, centrifuges and diluted with equal volume of CH3COOH 3 M Liquid: a portion of was diluted in a mixture of NH3: ethanol 2:73%, mix, and centrifuged. | [76] |
| Allura red | Liquid foods | Sample filtered, pH adjusted (4.0), extraction with Acetonitrile by SPE (MCI GEL CHP20P resin) | [77] |
| Tartrazine, New red, Amaranth, Ponceau 4R, Sunset yellow, Allura red, Acid red, Brilliant Blue, Acid red, Erythrosine, Acid orange, Basic flavine O, Basic orange, Siperse blue 106, Crystal violet, Leucine malachite green, Leucine crystal violet | Meat | Microwave assisted extraction: sample with methanol/H2O (95:5 v/v) followed by SPE (C18 column), evaporation to dryness and reconstitution with methanol | [78] |
| New red, Amaranth, Carmine, Sunset yellow, Acid Red G, Allura red, Acid Scarlett GR, Erythrosine, Rhodamine B, Sudan I, Para red, Sudan II, Sudan III, Sudan red 7B, Sudab IV, Sudan Orange G | Hotpot condiment | Direct solvent extraction: sample with solvent (acetone-methanol), vortex, centrifugation, evaporation, pH adjustment | [79] |
| Allura Red, Ponceau 4R | Granulated drinks | Powdered sample dissolved in distilled water, | [80] |
| Brilliant Blue, Sunset Yellow, Tartrazine | Non-alcoholic drinks, sweets, jellies | Solid: dilution in H2O and filtration | [81] |
| Tartrazine, Amaranth, Sunset Yellow, Allura red, Ponceau 4R, Erythrosine | Soft drinks, sugar and gelatin based confectionery | Ionic liquid dispersive liquid phase microextraction. Sample + ionic liquid [1-Octyl-3-methylimidazolium tetrafluoroborate ([C8MIM][BF4])] and methanol addition. | [82] |
| Allura red, Amaranth, Erythrosine, Ponceau 4R, Sunset Yellow | Beverages, alcoholic drinks and fish foe | Synthesis of the G/Ag nanoparticle composite | [83] |
| Sunset yellow | Beverage, dried bean curd, braised pork | [84] | |
| (40 food colorants) Ponceau 6R, Tartrazine, Fast yellow AB, Amaranth, Indigotine, Naphthol yellow S, Chrysoine, Ponceau 4R, Sunset yellow FCF, Red 10B, Orange G, Acid violet 7, Brilliant black PN, Allura red AC, Yellow 2G, Red 2G, Uranine, Fast red E, Green S, Ponceau 2R, Azorubine, Orange I, Quinoline yellow, Martius yellow, Ponceau SX, Ponceau 3R, Fast green FCF, Eosine, Brilliant blue FCF, Orange II, Orange RN, Acid blue 1, Erythrosine, Amido black 10B, Acid red 52, Patent blue V, Acid green 9, Phloxine B, Benzyl violet 4B, Rose bengal. | Drinks, syrups, and candies | Drinks: degas, evaporation Solid: grind, mixing with solvent and SPE with PA column with 1% NH3/ethanol solution | [85] |
| Ponceau 4R, Sunset Yellow, Allura Red, Azophloxine, Ponceauxylidine, Erythrosine Orange II | Animal feed and meat | Homogenization, extraction with ethanol:NH3:H2O (80:1:19 v/v), evaporation, reconstitution | [86] |
| New Coccine, Indigo Carmine, Erythrosine, Tartrazine, Sunset Yellow FCF, Fast Green FCF, Brilliant Blue FCF, Allura Red AC, Amaranth, Dimethyl Yellow, Fast Garnet GBC, Para Red, Sudan I, Sudan II, Sudan III, Sudan IV, Sudan Orange G, Sudan Red 7B, Sudan Red B, Sudan Red G | Chili powders; commercial syrup preserved fruits | Homogenization and extraction with Acetonitrile twice | [87] |
| Multi-class (53 food colorants) | Spices | Extraction with H2O/methanol/acetonitrile/THF, 9:1:5:5, v/v/v/v) | [88] |
| Multi-class (34 water soluble synthetic food colorants) | Beverages, syrup, chewing gum | Beverages: degassing, pH adjustment, and dilution. Syrup: dilution, sonication, and pH adjustment. Chewing gum: washing with water, pH adjustment. | [89] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntrallou, K.; Gika, H.; Tsochatzis, E. Analytical and Sample Preparation Techniques for the Determination of Food Colorants in Food Matrices. Foods 2020, 9, 58. https://doi.org/10.3390/foods9010058
Ntrallou K, Gika H, Tsochatzis E. Analytical and Sample Preparation Techniques for the Determination of Food Colorants in Food Matrices. Foods. 2020; 9(1):58. https://doi.org/10.3390/foods9010058
Chicago/Turabian StyleNtrallou, Konstantina, Helen Gika, and Emmanouil Tsochatzis. 2020. "Analytical and Sample Preparation Techniques for the Determination of Food Colorants in Food Matrices" Foods 9, no. 1: 58. https://doi.org/10.3390/foods9010058
APA StyleNtrallou, K., Gika, H., & Tsochatzis, E. (2020). Analytical and Sample Preparation Techniques for the Determination of Food Colorants in Food Matrices. Foods, 9(1), 58. https://doi.org/10.3390/foods9010058







