Aromatic Characterization of Mangoes (Mangifera indica L.) Using Solid Phase Extraction Coupled with Gas Chromatography–Mass Spectrometry and Olfactometry and Sensory Analyses
Abstract
:1. Introduction
2. Materials and Methods
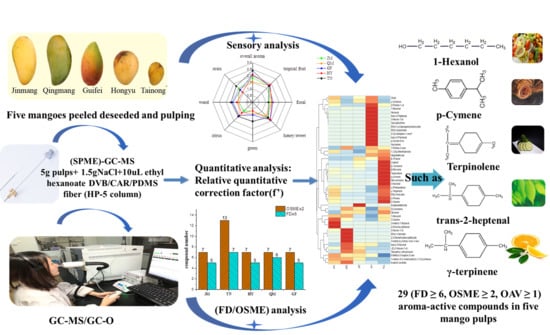
2.1. Samples Preparation
2.2. Chemicals
2.3. HS-SPME-GC-MS
2.4. Identification of Aroma-Active Compounds by GC-O
2.5. Quantitative Analysis of Aromatic Compounds
2.6. Odor Activity Value (OAV)
2.7. Sensory Panel and Aroma Profile Analysis
3. Results
3.1. Sensory Analysis of Five Mango Cultivars
3.2. Comparasion of the GC-MS Results of Five Mango Cultivars
3.3. Identification of Key Aromatic Compounds in the Pulps of Five Mango Cultivars
3.4. Odor Activity Values (OAVs) for the Pulps of Five Mango Cultivars
3.5. Comparison of GC-O(FD/OSME) and OAV Aroma-Active Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pandit, S.S.; Chidley, H.G.; Kulkarni, R.S.; Pujari, K.H.; Giri, A.P.; Gupta, V.S. Cultivar relationships in mango based on fruit volatile profiles. Food Chem. 2009, 114, 363–372. [Google Scholar] [CrossRef]
- Munafo, J.P., Jr.; Didzbalis, J.; Schnell, R.J.; Schieberle, P.; Steinhaus, M. Characterization of the major aroma-active compounds in mango (Mangifera indica L.) cultivars Haden, White Alfonso, Praya Sowoy, Royal Special, and Malindi by application of a comparative aroma extract dilution analysis. J. Agric. Food Chem. 2014, 62, 4544–4551. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.S.; Oak, P.; Chidley, H.; Deshpande, A.; Giri, A.; Gupta, V. Nutrient and flavor content of mango (Mangifera indica L.) cultivars: An appurtenance to the list of staple foods. In Nutritional Composition of Fruit Cultivars; Elsevier: Amsterdam, The Netherlands, 2016; pp. 445–467. [Google Scholar]
- Musharraf, S.G.; Uddin, J.; Siddiqui, A.J.; Akram, M.I. Quantification of aroma constituents of mango sap from different Pakistan mango cultivars using gas chromatography triple quadrupole mass spectrometry. Food Chem. 2016, 196, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 1 October 2019).
- Pino, J.A.; Mesa, J.; MUNoz, Y.; Martí, M.P.; Marbot, R. Volatile components from mango (Mangifera indica L.) cultivars. J. Agric. Food Chem. 2005, 53, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, X.W.; Zhan, R.L.; Wu, H.X.; Yao, Q.S.; Xu, W.T.; Luo, C.; Zhou, Y.G.; Liang, Q.Z.; Wang, S.B. Profiling of volatile fragrant components in a mini-core collection of mango germplasms from seven countries. PLoS ONE 2017, 12, e0187487. [Google Scholar] [CrossRef]
- Ma, X.W.; Su, M.Q.; Wu, H.X.; Zhou, Y.G.; Wang, S.B. Analysis of the Volatile Profile of Core Chinese Mango Germplasm by Headspace Solid-Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry. Molecules 2018, 23, 1480. [Google Scholar] [CrossRef] [Green Version]
- Bonneau, A.; Boulanger, R.; Lebrun, M.; Maraval, I.; Gunata, Z. Aroma compounds in fresh and dried mango fruit (Mangifera indica L. cv. Kent): Impact of drying on volatile composition. Int. J. Food Sci. Technol. 2016, 51, 789–800. [Google Scholar] [CrossRef]
- Kung, T.L.; Chen, Y.J.; Chao, L.K.; Wu, C.S.; Lin, L.Y.; Chen, H.C. Analysis of Volatile Constituents in Platostoma palustre (Blume) Using Headspace Solid-Phase Microextraction and Simultaneous Distillation-Extraction. Foods 2019, 8, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, H.; Liu, S.Q.; Xu, Y.Q.; Lassabliere, B.; Sun, J.; Yu, B. Characterising volatiles in tea (Camellia sinensis ). Part I: Comparison of headspace-solid phase microextraction and solvent assisted flavor evaporation. LWT Food Sci. Technol. 2018, 94, 178–189. [Google Scholar] [CrossRef]
- Usami, A.; Nakahashi, H.; Marumoto, S.; Miyazawa, M. Aroma evaluation of setonojigiku (Chrysanthemum japonense var. debile) by hydrodistillation and solvent-assisted flavor evaporation. Phytochem. Anal. PCA 2014, 25, 561–566. [Google Scholar] [CrossRef]
- Schuh, C.; Schieberle, P. Characterization of the key aroma compounds in the beverage prepared from Darjeeling black tea: Quantitative differences between tea leaves and infusion. J. Agric. Food Chem. 2006, 54, 916–924. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Guo, X.; Yang, Y.; Xie, Y.; Ju, F.; Guo, W. Characterization of the aromatic profile in “zijuan” and “pu-erh” green teas by headspace solid-phase microextraction coupled with GC-O and GC-MS. Anal. Methods 2016, 8, 4727–4735. [Google Scholar] [CrossRef]
- Bianchin, J.N.; Nardini, G.; Merib, J.; Dias, A.N.; Martendal, E.; Carasek, E. Screening of volatile compounds in honey using a new sampling strategy combining multiple extraction temperatures in a single assay by HS-SPME–GC–MS. Food Chem. 2014, 145, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Huang, M.; Crane, J.H.; Wang, Y. Characterization of key aroma-active compounds in lychee (Litchi chinensis Sonn.). JFDA 2018, 26, 497–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- San, A.T.; Joyce, D.C.; Hofman, P.J.; Macnish, A.J.; Webb, R.I.; Matovic, N.J.; Williams, C.M.; De Voss, J.J.; Wong, S.H.; Smyth, H.E. Stable isotope dilution assay (SIDA) and HS-SPME-GCMS quantification of key aroma volatiles for fruit and sap of Australian mango cultivars. Food Chem. 2017, 221, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.-H.; Liu, F.; Pang, X.-L.; Xiao, H.-W.; Wen, X.; Ni, Y.-Y. Effects of different osmo-dehydrofreezing treatments on the volatile compounds, phenolic compounds and physicochemical properties in mango (Mangifera indica L.). Int. J. Food Sci. Technol. 2016, 51, 1441–1448. [Google Scholar] [CrossRef]
- Pang, X.; Guo, X.; Qin, Z.; Yao, Y.; Hu, X.; Wu, J. Identification of aroma-active compounds in Jiashi muskmelon juice by GC-O-MS and OAV calculation. J. Agric. Food Chem. 2012, 60, 4179–4185. [Google Scholar] [CrossRef]
- Mastello, R.B.; Janzantti, N.S.; Monteiro, M. Volatile and odoriferous compounds changes during frozen concentrated orange juice processing. Food Res. Int. 2015, 77, 591–598. [Google Scholar] [CrossRef]
- Munafo, J.P., Jr.; Didzbalis, J.; Schnell, R.J.; Steinhaus, M. Insights into the Key Aroma Compounds in Mango (Mangifera indica L. Haden) Fruits by Stable Isotope Dilution Quantitation and Aroma Simulation Experiments. J. Agric. Food Chem. 2016, 64, 4312–4318. [Google Scholar] [CrossRef]
- Matheis, K.; Granvogl, M. Characterization of Key Odorants Causing a Fusty/Musty Off-Flavor in Native Cold-Pressed Rapeseed Oil by Means of the Sensomics Approach. J. Agric. Food Chem. 2016, 64, 8168–8178. [Google Scholar] [CrossRef]
- Bonneau, A.; Boulanger, R.; Lebrun, M.; Maraval, I.; Valette, J.; Guichard, E.; Gunata, Z. Impact of fruit texture on the release and perception of aroma compounds during in vivo consumption using fresh and processed mango fruits. Food Chem. 2018, 239, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, J. GC-O-MS technique and its applications in food flavor analysis. Food Res. Int. 2018, 114, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Shi, D.; Sun, J.; Li, A.; Sun, B.; Zhao, M.; Chen, F.; Sun, X.; Li, H.; Huang, M.; et al. Characterization of key aroma compounds in Gujinggong Chinese Baijiu by gas chromatography-olfactometry, quantitative measurements, and sensory evaluation. Food Res. Int. 2018, 105, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, L.; Xiao, Z.; Niu, Y. Characterization of the key aroma compounds in mulberry fruits by application of gas chromatography-olfactometry (GC-O), odor activity value (OAV), gas chromatography-mass spectrometry (GC-MS) and flame photometric detection (FPD). Food Chem. 2018, 245, 775–785. [Google Scholar] [CrossRef]
- Chalier, P.; Angot, B.; Delteil, D.; Doco, T.; Gunata, Z. Interactions between aroma compounds and whole mannoprotein isolated from Saccharomyces cerevisiae strains. Food Chem. 2007, 100, 22–30. [Google Scholar] [CrossRef]
- Kühn, J.; Considine, T.; Singh, H. Interactions of milk proteins and volatile flavor compounds: Implications in the development of protein foods. J. Food Sci. 2006, 71, R72–R82. [Google Scholar] [CrossRef]
- Xu, J.; He, Z.; Zeng, M.; Li, B.; Qin, F.; Wang, L.; Wu, S.; Chen, J. Effect of xanthan gum on the release of strawberry flavor in formulated soy beverage. Food Chem. 2017, 228, 595–601. [Google Scholar] [CrossRef]
- Van den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, P.; Lao, F.; Liu, J.; Liao, X.; Wu, J. Characterization of the major aroma-active compounds in Keitt mango juice: Comparison among fresh, pasteurization and high hydrostatic pressure processing juices. Food Chem. 2019, 289, 215–222. [Google Scholar] [CrossRef]
- Goodner, K.L. Practical retention index models of OV-101, DB-1, DB-5, and DB-Wax for flavor and fragrance compounds. LWT Food Sci. Technol. 2008, 41, 951–958. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef] [Green Version]
- An, K.; Liu, H.; Fu, M. Identification of the cooked off-flavor in heat-sterilized lychee (Litchi chinensis Sonn.) juice by means of molecular sensory science. Food Chem. 2019, 301, 125282. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A.; Mesa, J. Contribution of volatile compounds to mango (Mangifera indica L.) aroma. Flavor Fragr. J. 2006, 21, 207–213. [Google Scholar] [CrossRef]
- Van Gemert, L. Compilations of Odour Threshold Values in Air, Water and Other Media; BACIS: Zeist, The Netherlands, 2003. [Google Scholar]








| Code | Compounds | RI 1 | Identification 2 | RQCF (fi′) 3 | Concentration (μg/kg Mango Must) 4 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HP-5 | Reference | JM | RSD (%) | QM | RSD (%) | GF | RSD (%) | HY | RSD (%) | TN | RSD (%) | ||||
| Alcohols | |||||||||||||||
| A1 | 2-Penten-1-ol | 761 | 769 | RI, Std, MS, | 1.25 | 1.92 b5 | 0.02 | - 8 | - | - | - | 5.45 a | 0.22 | 1.87 c | 0.07 |
| A2 | (E)-3-Hexen-1-ol | 844 | 852 | RI, Std, MS, | 0.52 | 41.50 c | 0.03 | 132.56 a | 0.12 | 76.82 b | 0.12 | - | - | - | - |
| A3 | 2-Hexen-1-ol | 852 | 852 | RI, Std, MS, | 1.09 | 7.08 b | 0.23 | 35.53 a | 0.11 | - | - | - | - | - | - |
| A4 | 3-Hexen-1-ol | 854 | 857 | RI, Std, MS, | 4.3 | - | - | - | - | - | - | 6.59 | 0.11 | - | - |
| A5 | 1-Hexanol | 856 | 865 | RI, Std, MS, | 2.01 | 55.83 b | 0.08 | 59.24 a | 0.13 | 22.91 d | 0.10 | 7.71 e | 0.12 | 46.14 c | 0.17 |
| A6 | isoamyl alcohol | 921 | 910 | RI, Std, MS, | 0.74 | 38.87 | 0.03 | - | - | - | - | - | - | - | - |
| A7 | p-Cymen-8-ol | 945 | ND | RI, Std, MS, | 11.08 | - | - | - | - | - | - | 12.23 | 0.04 | - | - |
| A8 | 2-(Vinyloxy) ethanol | 1012 | ND | RI, Std, MS, | 3.34 | 3.49 | 0.06 | - | - | - | - | - | - | - | - |
| A9 | Linalool | 1103 | 1098 | RI, Std, MS, | 0.80 | - | - | - | - | 1.92 b | 0.07 | - | - | 6.90 a | 0.2 |
| Terpenes | |||||||||||||||
| B1 | α-Pinene | 918 | 916 | RI, Std, MS, | 0.36 | 4.05 b | 0.24 | 1.76 d | 0.17 | 1.93 c | 0.12 | 1.01 e | 0.17 | 7.38 a | 0.04 |
| B2 | Allo-ocimene | 947 | 950 | RI, Std, MS, | 2.56 | - | - | - | - | - | - | - | - | 0.08 | 0.12 |
| B3 | β--Pinene | 958 | 946 | RI, Std, MS, | 2.10 | - | - | - | - | 2.33 b | 0.08 | 1.34c | 0.17 | 3.54 a | 0.01 |
| B4 | β-Myrcene | 973 | 990 | RI, Std, MS, | 0.43 | 6.28 b | 0.24 | 3.83 d | 0.34 | 4.5 c | 0.07 | 1.41 e | 0.17 | 22.94 a | 0.05 |
| B5 | 2-Carene | 981 | 995 | RI, Std, MS, | 0.22 | 1.60 b | 0.31 | 0.67 c | 0.14 | 0.54 d | 0.22 | - | - | 4.44 a | 0.05 |
| B6 | Humulene | 984 | 990 | RI, Std, MS, | 0.81 | - | - | - | - | - | - | 2.93 | 0.03 | - | - |
| B7 | α-Phellandrene | 985 | 989 | RI, Std, MS, | 0.50 | 16.84 b | 0.24 | 10.84 c | 0.01 | 6.90 d | 0.07 | - | - | 30.17 a | 0.04 |
| B8 | 3-Carene | 990 | 1107 | RI, Std, MS, | 0.25 | 103.63 a | 0.24 | 60.36 c | 0.23 | 43.32 d | 0.07 | 27.67 e | 0.17 | 70.92 b | 0.01 |
| B9 | D-Limonene | 1007 | 1026 | RI, Std, MS, | 0.10 | 12.4 d | 0.24 | 32.54 a | 0.23 | 26.2 c | 0.07 | 2.56 e | 0.17 | 28.87 b | 0.04 |
| B10 | p-cymene | 1005 | 1016 | RI, Std, MS, | 1.11 | 27.43 c | 0.08 | 33.85 b | 0.14 | 19.44 d | 0.06 | 15.57 e | 0.09 | 132.96 a | 0.04 |
| B11 | β-Caryophyllene | 1018 | ND | RI, Std, MS, | 0.64 | - | - | - | - | - | - | 2.18 | 0.17 | - | - |
| B12 | 1,3-Cyclohexadiene, 1-methyl-4-(1-methylethyl) | 1021 | 1030 | RI, Std, MS, | 0.18 | 4.96 a | 0.07 | 4.84 b | 0.02 | 3.42 c | 0.06 | - | - | - | - |
| B13 | γ-Terpinene | 1036 | 1045 | RI, Std, MS, | 0.68 | 15.44 b | 0.16 | 6.73 c | 0.19 | 6.08 d | 0.26 | 1.10 e | 0.07 | 20.88 a | 0.25 |
| B14 | β-Ocimene | 1050 | 1060 | RI, Std, MS, | 1.52 | - | - | - | - | 6.97 b | 0.08 | - | - | 9.11 a | 0.04 |
| B15 | Terpinolene | 1066 | 1065 | RI, Std, MS, | 0.19 | 147.07 b | 0.24 | 59.42 c | 0.23 | 43.5 d | 0.07 | 24.90 e | 0.17 | 811.60 a | 0.04 |
| B16 | 1,3,8-p-Menthatriene | 1086 | 1110 | RI, Std, MS, | 1.37 | 11.92 c | 0.18 | 3.79 d | 0.19 | - | - | 13.58 b | 0.16 | 16.38 a | 0.14 |
| Aldehydes | |||||||||||||||
| C1 | Hexanal | 792 | 800 | RI, Std, MS, | 1.30 | 4.94 b | 0.07 | - | - | - | - | 75.54 a | 1.50 | - | - |
| C2 | Isovaleraldehyde | 837 | ND | RI, Std, MS, | 2.47 | - | - | - | - | 1.77 | 0.17 | - | - | - | - |
| C3 | trans-2-Hexenal | 840 | 844 | RI, Std, MS, | 2.20 | 8.38 c | 0.02 | 180.60 a | 0.26 | 84.03b | 0.15 | 85.64 b | 1.50 | 4.11 d | 0.26 |
| C4 | 3-hexenal | 857 | 847 | RI, Std, MS, | 11.55 | - | - | - | - | - | - | - | - | 44.13 | 0.12 |
| C5 | Heptanal | 888 | 899 | RI, Std, MS, | 0.07 | - | - | - | - | - | - | 1.65 b | 1.50 | 1.83 a | 0.44 |
| C6 | trans-2-Heptenal | 940 | 957 | RI, Std, MS, | 1.35 | - | - | - | - | - | - | 65.65 | 1.50 | - | - |
| C7 | 1-Nonanal | 1080 | 1140 | RI, Std, MS, | 0.45 | - | - | 1.46 b | 0.16 | 0.91 c | 0.28 | 8.43 a | 1.50 | 0.86 c | 0.26 |
| C8 | (E,Z)-2,6-Nonadienal | 1152 | 1152 | RI, Std, MS, | 0.64 | - | - | - | - | - | - | 0.66 | 0.10 | - | - |
| C9 | E-2-Nonenal | 1162 | 1160 | RI, Std, MS, | 1.87 | - | - | 7.60 | 0.09 | - | - | - | - | - | - |
| C10 | Decanal | 1175 | 1205 | RI, Std, MS, | 6.05 | - | - | 8.48 c | 0.06 | 8.45 c | 0.15 | - | - | 12.46 a | 0.04 |
| C11 | Citral | 1236 | 1242 | RI, Std, MS, | 0.02 | 0.18 b | 0.28 | - | - | 0.09 c | 0.05 | 1.23 a | 0.36 | - | - |
| ketones | |||||||||||||||
| D1 | 1-Penten-3-one | 652 | 687 | RI, Std, MS, | 0.05 | - | - | 0.52 a | 0.56 | - | - | 0.47 b | 0.28 | 0.31 c | 0.22 |
| D2 | 2-Cyclohepten-1-one | 673 | ND | RI, Std, MS, | 1.50 | - | - | - | - | - | - | 0.66 | 0.14 | - | - |
| D3 | 3-methylcyclohex-3-en-1-one | 967 | 986 | RI, Std, MS, | 3.15 | - | - | 0.62 | 0.17 | - | - | - | - | - | - |
| D4 | 6-Methyl-5-hepten-2-one | 969 | 985 | RI, Std, MS, | 0.42 | 0.39 a | 0.05 | 0.28 c | 0.22 | 0.30 b | 0.25 | 0.23 d | 0.32 | - | - |
| Ethers | |||||||||||||||
| E1 | Ethyl propionate | 765 | RI, Std, MS, | 1.10 | - | - | - | - | - | - | 6.39 | 0.11 | - | - | |
| E2 | Ethyl cyclopropanecarboxylate | 808 | 755 | RI, Std, MS, | 0.26 | - | - | - | - | - | - | 0.12 | 0.55 | - | - |
| E3 | Ethyl crotonate | 876 | 802 | RI, Std, MS, | 1.60 | - | - | 9.83 | 0.05 | - | - | - | - | - | - |
| E4 | Isoamyl acetate | 879 | 876 | RI, Std, MS, | 1.37 | 5.31 b | 0.09 | 7.45 a | 0.22 | 2.21 c | 0.19 | 1.28 d | 0.14 | - | - |
| E5 | Tetraethyl orthosilicate | 923 | 880 | RI, Std, MS, | 0.06 | - | - | 0.12a | 0.54 | 0.09 b | 0.93 | - | - | - | - |
| E6 | Ethyl butyrate | 978 | ND | RI, Std, MS, | 0.84 | 1.17 b | 0.06 | - | - | - | - | - | - | 1.80 a | 0.23 |
| E7 | γ-Octanoic | 1222 | 994 | RI, Std, MS, | 2.48 | 18.60 b | 0.21 | - | - | 16.49 c | 0.20 | 26.32 a | 0.11 | - | - |
| No. | Compounds | RI (Calculate) A | RI (Reference) B | Aroma description C | Identification D | Aromatic Intensity E | Frequency F | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HP-5 | Wax | HP-5 | Wax | JM | RSD (%) | TN | RSD (%) | HY | RSD (%) | QM | RSD (%) | GF | RSD (%) | JM | TN | HY | QM | GF | ||||
| 1 | 1-Penten-3-one | 652 | ND | 687 [6] | ND | mushroom | R, S, M, O | 0 | 0 | 2.25 | 10.00 | 1.67 | 5.99 | 1.33 | 11.28 | 0 | 0 | 0 | 5 | 4 | 8 | 0 |
| 2 | 2-Cyclohepten-1-one | 673 | ND | ND | ND | coffee-like | R, S, M, O | 0 | 0 | 0 | 0 | 2.00 | 0.50 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 |
| 3 | 2-Penten-1-ol | 761 | 1316 | 769 [31] | 1305 [6] | grassy, tea | R, S, M, O | 2.00 | 0.01 | 1.25 | 0.80 | 1.00 | 1.00 | 0 | 0 | 0 | 0 | 4 | 2 | 7 | 0 | 0 |
| 4 | Hexanal | 792 | 1061 | 800 [32] | 1088 [32] | grass, tallow, fat | R, S, M, O | 1.83 | 0.49 | 2.2 | 0.91 | 2.00 | 1.00 | 0 | 0 | 1.80 | 0.56 | 5 | 2 | 2 | 0 | 2 |
| 5 | Ethyl cyclopropanecarboxylate | 808 | ND | ND | ND | fruity | R, S, M, O | 1.50 | 0.23 | 1.23 | 0.20 | 1.30 | 0.90 | 0 | 2.11 | 0.56 | 0 | 6 | 6 | 8 | 0 | 8 |
| 6 | trans-2-Hexenal | 840 | 1202 | 844 [32] | 1192 [32] | green, leaf | R, S, M, O | 1.50 | 0.23 | 1.67 | 6.95 | 1.67 | 3.78 | 2.00 | 0.50 | 2.00 | 0.50 | 3 | 5 | 4 | 5 | 4 |
| 7 | 3-hexenal | 857 | ND | 857 [6] | ND | grass | R, S, M, O | 1.27 | 0.67 | 0 | 0 | 1.36 | 3.78 | 1.60 | 8.13 | 1.43 | 0.45 | 3 | 1 | 7 | 8 | 7 |
| 8 | 3-Hexen-1-ol, (E)- | 844 | 1356 | 852 [31] | 1343 [31] | grassy, tea | R, S, M, O | 1.00 | 0.01 | 0 | 0 | 0 | 0 | 1.33 | 0.75 | 1.75 | 5.71 | 4 | 2 | 0 | 2 | 3 |
| 9 | 1-Hexanol | 856 | 1360 | 865 [32] | 1362 [32] | resin, flower, green | R, S, M, O | 1.60 | 0.28 | 1.83 | 2.73 | 1.23 | 0.56 | 2.12 | 0.34 | 2.13 | 0.67 | 5 | 2 | 4 | 4 | 3 |
| 10 | Heptanal | 888 | 1174 | 899 [6] | 1184 [32] | fruity | R, S, M, O | 0 | 0 | 1.5 | 0.67 | 1.67 | 2.34 | 1.00 | 1.00 | 0 | 0 | 0 | 5 | 4 | 0 | 0 |
| 11 | α-Pinene | 918 | 1032 | 916 [31] | 1056 [23] | pine-like | R, S, M, O | 1.10 | 0.34 | 1.80 | 10.00 | 2.20 | 20.00 | 1.00 | 1.00 | 1.5 | 0.67 | 2 | 4 | 2 | 2 | 2 |
| 12 | trans-2-Heptenal | 940 | 1300 | 957 [31] | 1307 [31] | grassy, irritant | R, S, M, O | 0 | 0 | 0 | 0 | 0 | 0 | 1.25 | 1.60 | 1.56 | 0.9 | 0 | 2 | 1 | 3 | 3 |
| 13 | Allo-ocimene | 947 | 1125 | ND | 1135 [1] | floral | R, S, M, O | 0 | 0 | 0 | 0 | 1.00 | 1.00 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 |
| 14 | β-Pinene | 958 | 1113 | 946 [23] | 1108 [23] | grass | R, S, M, O | 0 | 0 | 1.56 | 0.80 | 1.23 | 1.50 | 1.67 | 0.60 | 0 | 0 | 0 | 5 | 4 | 7 | 0 |
| 15 | 3-methylcyclohex-3-en-1-one | 967 | ND | 986 [1] | ND | grass | R, S, M, O | 0 | 0 | 0 | 0 | 0 | 0 | 2.67 | 2.25 | 0 | 0 | 0 | 1 | 0 | 3 | 0 |
| 16 | 6-Methyl-5-hepten-2-one | 969 | ND | 985 [23] | ND | fruit, grass | R, S, M, O | 1.67 | 0.32 | 2.00 | 0.50 | 1.33 | 4.51 | 1.56 | 0.78 | 1.75 | 3.43 | 2 | 4 | 2 | 2 | 3 |
| 17 | β-Myrcene | 973 | 1137 | 990 [32] | 1158 [32] | light balsam, wood | R, S, M, O | 1.80 | 1.20 | 2.00 | 0.50 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 6 | 0 | 0 | 0 |
| 18 | Ethyl butyrate | 978 | 1070 | 994 [31] | 1076 [31] | fruity, apple | R, S, M, O | 1.23 | 5.79 | 1.67 | 6.95 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 5 | 0 | 0 | 0 |
| 19 | 2-Carene | 981 | ND | 995 [23] | ND | sweet, rosin | R, S, M, O | 1.89 | 0.98 | 2.20 | 2.27 | 0 | 0 | 1.54 | 0.56 | 1.23 | 0.44 | 5 | 6 | 0 | 4 | 2 |
| 20 | β-Phellandrene | 985 | 1166 | 989 [31] | 1171 [31] | citrus like | R, S, M, O | 4.10 | 1.34 | 3.53 | 6.67 | 3.00 | 0.33 | 2.10 | 0.90 | 2.50 | 0.67 | 7 | 6 | 2 | 5 | 4 |
| 21 | 3-Carene | 990 | 1148 | 1007 [31] | 1153 [31] | sweet, rosin | R, S, M, O | 1.50 | 0.87 | 2.00 | 0.50 | 1.14 | 0.34 | 1.45 | 0.76 | 1.23 | 1.56 | 3 | 4 | 2 | 2 | 2 |
| 22 | p-Cymene | 1005 | 1275 | 1026 [32] | 1274 [32] | citrus, green | R, S, M, O | 1.00 | 0.29 | 8.70 | 0.45 | 1.67 | 6.95 | 2.00 | 15.0 | 1.80 | 6.67 | 5 | 6 | 6 | 6 | 6 |
| 23 | D-Limonene | 1028 | 1205 | 1030 [32] | 1208 [32] | citrus-like, sweet | R, S, M, O | 0 | 0 | 2 | 0.5 | 0 | 0 | 2.00 | 0.50 | 0 | 0 | 0 | 5 | 0 | 4 | 0 |
| 24 | (E)-beta-ocimene | 1050 | 1241 | 1048 [33] | 1250 [33] | floral and green | R, S, M, O | 2.33 | 0.01 | 0 | 0 | 2.50 | 0.40 | 0 | 0 | 0 | 0 | 3 | 0 | 3 | 0 | 0 |
| 25 | γ-Terpinene | 1057 | 1249 | 1060 [33] | 1245 [33] | citrus, lemon | R, S, M, O | 2.33 | 0.02 | 2.50 | 1.60 | 2.33 | 1.72 | 1.67 | 6.95 | 2.00 | 2.50 | 8 | 8 | 5 | 6 | 8 |
| 26 | Terpinolene | 1066 | 1275 | 1065 [31] | 1276 [31] | rosin-like | R, S, M, O | 2.30 | 0.02 | 2.28 | 2.19 | 2.20 | 3.18 | 1.33 | 0.75 | 2.33 | 9.31 | 6 | 8 | 7 | 8 | 7 |
| 27 | 1-Nonanal | 1080 | 1328 | 1104 [31] | 1349 [31] | cucumber-like | R, S, M, O | 1.67 | 0.26 | 1.50 | 3.33 | 0 | 0 | 0 | 0 | 1.40 | 7.14 | 5 | 4 | 0 | 0 | 3 |
| 28 | 1,3,8-p-Menthatriene | 1086 | ND | 1110 [34] | ND | minty-like | R, S, M, O | 1.00 | 0.01 | 2.00 | 0.50 | 2.20 | 0.45 | 0 | 0 | 2.25 | 4.44 | 5 | 4 | 4 | 0 | 4 |
| 29 | (E,Z)-2,6-Nonadienal | 1152 | 1460 | 1152 [31] | 1469 [6] | fresh, green, cucumber | R, S, M, O | 0 | 0 | 0 | 0 | 1.67 | 0.60 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| 30 | E-2-Nonenal | 1162 | 1416 | 1160 [31] | 1436 [31] | cucumber-like | R, S, M, O | 1.20 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| 31 | Citral | 1236 | 1700 | 1242 [34] | 1679 [33] | lemon-like | R, S, M, O | 2.00 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| 32 | Decanal | 1175 | 1835 | 1205 [34] | 1846 [34] | fruity, citrus, orange | R, S, M, O | 0 | 0 | 3 | 0.33 | 0 | 0 | 3.00 | 0.33 | 0 | 0 | 0 | 2 | 0 | 2 | 0 |
| 33 | γ-Octanoic | 1222 | 1721 | 1236 [31] | 1733 [31] | floral, violet | R, S, M, O | 2.00 | 0.01 | 0 | 0 | 1.50 | 2.00 | 0 | 0 | 2.00 | 0.50 | 7 | 0 | 4 | 0 | 3 |
| No | Compounds | Threshold a (μg kg−1) | Source b | OAV c | ||||
|---|---|---|---|---|---|---|---|---|
| JM | QM | GF | HY | TN | ||||
| 1 | 1-Penten-3-one | 1.00 [35] | mango | 0 | 0.52 | 0 | 0.47 | 0.31 |
| 2 | 2-Cyclohepten-1-one | 0.14 [35] | new | 0 | 0 | 0 | 4.71 | 0 |
| 3 | 2-Penten-1-ol | 720.00 [23] | mango | <0.01 | 0 | 0 | <0.01 | <0.01 |
| 4 | Hexanal | 4.50 [23] | mango | 1.10 | 0 | 0.29 | 16.79 | 0.40 |
| 5 | Ethyl cyclopropanecarboxylate | 0.12 [23] | new | 2.17 | 0 | 0 | 0 | 1.00 |
| 6 | trans-2-Hexenal | 400.00 [23] | mango | 0.02 | 0.45 | 0.21 | 0.21 | 0.01 |
| 7 | 3-hexenal | 550.00 [23] | mango | 0 | 0 | 0 | 0 | 0.08 |
| 8 | 3-Hexen-1-ol, (E)- | 110.00 [23] | mango | 0.38 | 1.21 | 0.70 | 0 | 0 |
| 9 | 1-Hexanol | 9.00 [26] | mango | 6.20 | 6.58 | 2.55 | 0.86 | 5.13 |
| 10 | Heptanal | 1.00 [23] | mango | 0 | 0 | 0 | 1.65 | 1.83 |
| 11 | α-Pinene | 6.00 [35] | mango | 0.68 | 0.29 | 0.32 | 0.17 | 1.23 |
| 12 | trans-2-Heptenal | 13.00 [35] | mango | 0 | 0 | 0 | 5.05 | 0 |
| 13 | Allo-ocimene | 140.00 [35] | mango | 0 | 0 | 0.07 | 0.04 | 0.10 |
| 14 | β-Pinene | 100.00 [23] | mango | 0 | 0 | 0 | <0.01 | 0 |
| 15 | 3-methylcyclohex-3-en-1-one | 7.00 [36] | mango | 0 | 0.09 | 0 | 0 | 0 |
| 16 | 6-Methyl-5-hepten-2-one | 50.00 [23] | mango | <0.01 | <0.01 | <0.01 | <0.01 | 0 |
| 17 | β-Myrcene | 20.00 [23] | mango | 0.31 | 0.19 | 0.23 | 0.07 | 1.15 |
| 18 | Ethyl butyrate | 0.75 [23] | mango | 1.56 | 0 | 0 | 0 | 2.40 |
| 19 | 2-Carene | 4.00 [23] | mango | 0.40 | 0.17 | 0.14 | 0 | 1.11 |
| 20 | β-Phellandrene | 7.00 [23] | mango | 2.41 | 1.55 | 1.00 | 0 | 4.31 |
| 21 | 3-Carene | 50.00 [23] | mango | 2.07 | 1.21 | 0.87 | 0.55 | 1.42 |
| 22 | p-Cymene | 18.50 [23] | mango | 1.48 | 1.83 | 1.05 | 0.84 | 7.19 |
| 23 | D-Limonene | 26.00 [23] | mango | 0.48 | 1.25 | 1.01 | 0.10 | 1.11 |
| 24 | (E)-beta-ocimene | 6.70 [23] | mango | 0 | 0 | 1.04 | 0 | 1.36 |
| 25 | γ-Terpinene | 2.00 [23] | mango | 7.72 | 3.37 | 3.04 | 0.55 | 10.44 |
| 26 | Terpinolene | 140.00 [23] | mango | 1.05 | 0.42 | 0.31 | 0.18 | 5.80 |
| 27 | 1-Nonanal | 0.15 [23] | mango | 0 | 9.73 | 6.07 | 56.20 | 5.73 |
| 28 | 1,3,8-p-Menthatriene | 6.80 [23] | Lychee | 1.75 | 0.56 | 0 | 2.00 | 2.41 |
| 29 | (E,Z)-2,6-Nonadienal | 4.50 [23] | mango | 0.57 | 0 | 0 | 0 | 0.02 |
| 30 | E-2-Nonenal | 50.00 [36] | mango | 0 | 0.15 | 0 | 0 | 0 |
| 31 | Citral | 1.00 [23] | Lychee | 0.18 | 0 | 0.09 | 1.23 | 0 |
| 32 | Decanal | 6.00 [23] | mango | 0 | 1.41 | 1.41 | 0 | 2.08 |
| 33 | γ-Octanoic | 7.00 [35] | mango | 2.66 | 0 | 2.36 | 3.76 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; An, K.; Su, S.; Yu, Y.; Wu, J.; Xiao, G.; Xu, Y. Aromatic Characterization of Mangoes (Mangifera indica L.) Using Solid Phase Extraction Coupled with Gas Chromatography–Mass Spectrometry and Olfactometry and Sensory Analyses. Foods 2020, 9, 75. https://doi.org/10.3390/foods9010075
Liu H, An K, Su S, Yu Y, Wu J, Xiao G, Xu Y. Aromatic Characterization of Mangoes (Mangifera indica L.) Using Solid Phase Extraction Coupled with Gas Chromatography–Mass Spectrometry and Olfactometry and Sensory Analyses. Foods. 2020; 9(1):75. https://doi.org/10.3390/foods9010075
Chicago/Turabian StyleLiu, Haocheng, Kejing An, Siqi Su, Yuanshan Yu, Jijun Wu, Gengsheng Xiao, and Yujuan Xu. 2020. "Aromatic Characterization of Mangoes (Mangifera indica L.) Using Solid Phase Extraction Coupled with Gas Chromatography–Mass Spectrometry and Olfactometry and Sensory Analyses" Foods 9, no. 1: 75. https://doi.org/10.3390/foods9010075
APA StyleLiu, H., An, K., Su, S., Yu, Y., Wu, J., Xiao, G., & Xu, Y. (2020). Aromatic Characterization of Mangoes (Mangifera indica L.) Using Solid Phase Extraction Coupled with Gas Chromatography–Mass Spectrometry and Olfactometry and Sensory Analyses. Foods, 9(1), 75. https://doi.org/10.3390/foods9010075