Peptide–Mineral Complexes: Understanding Their Chemical Interactions, Bioavailability, and Potential Application in Mitigating Micronutrient Deficiency
Abstract
1. Introduction
2. Methods for the Determination of the Mineral-Chelating Activity of Peptides
2.1. Iron-Chelating Activity
2.2. Zinc-Chelating Activity
2.3. Calcium-Chelating Activity
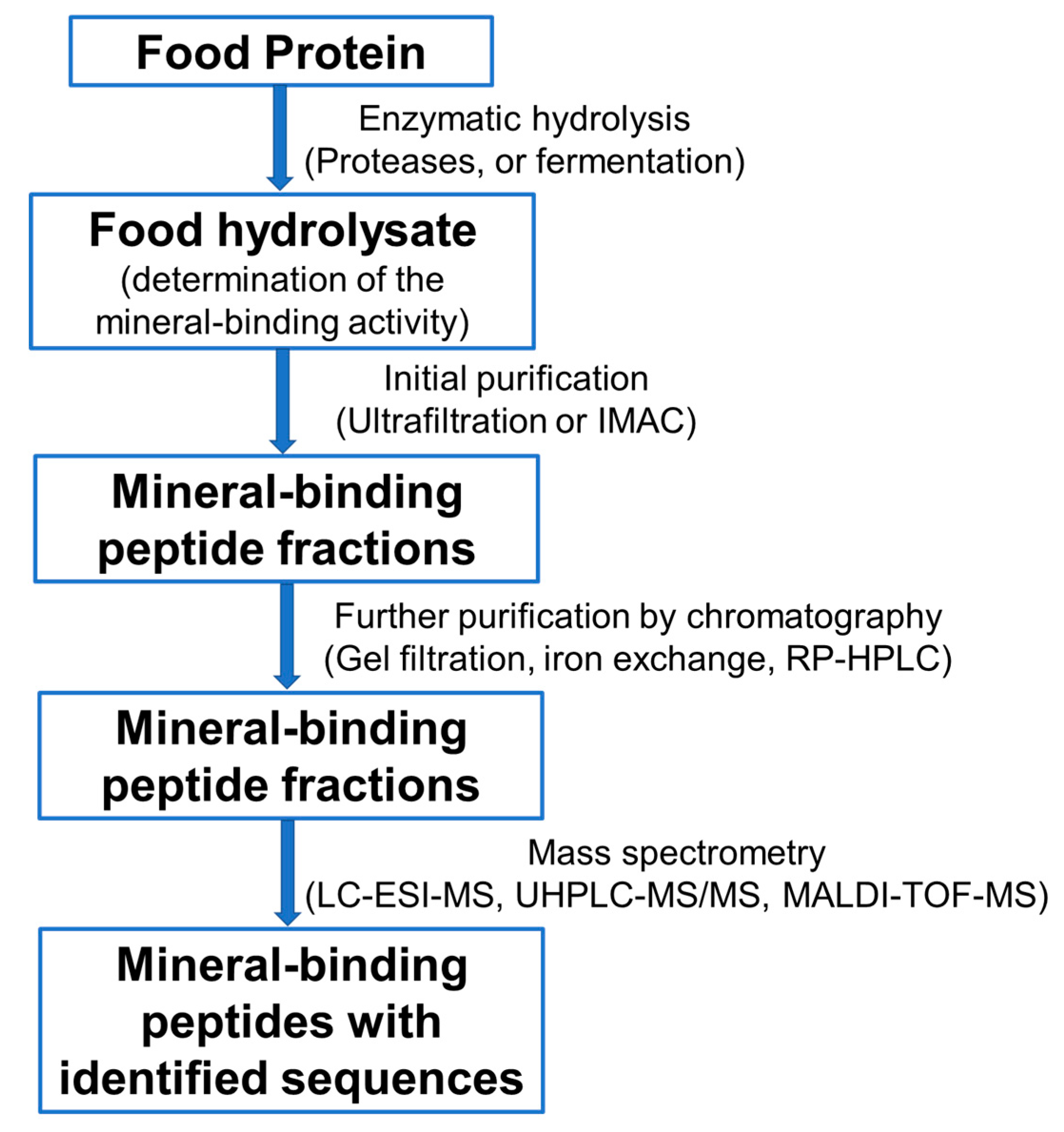
3. Preparation, Purification, and Identification of Mineral-Binding Peptides from Food
4. Structure–Activity Relationship and Stability of Peptide–Mineral Complexes
4.1. Amino Acid Composition and Peptide Sequence
4.2. Molecular Weight, Hydrophobicity, and Peptide Charge
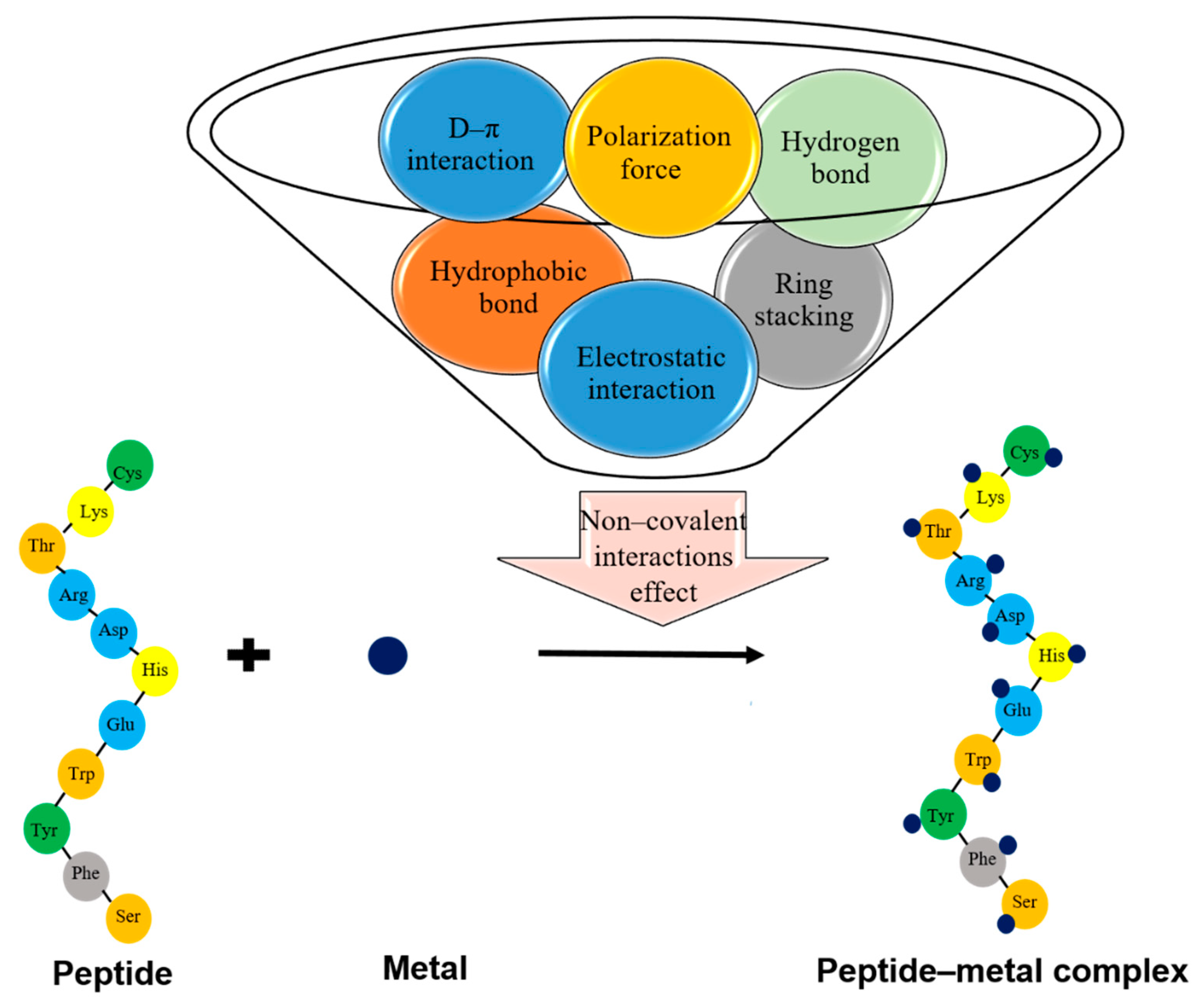
4.3. Chemical Interactions Involving in Peptide–Mineral Complex Stability
5. Solubility, Bioavailability and Absorption of Peptide–Mineral Complexes
6. Sustainable Production of Mineral-Binding Peptides
7. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Silva, C.S.; Moutinho, C.; Ferreira da Vinha, A.; Matos, C. Trace minerals in human health: Iron, zinc, copper, manganese and fluorine. IGSRM 2019, 13, 57–80. [Google Scholar]
- Walters, M.E.; Esfandi, R.; Tsopmo, A. Potential of Food Hydrolyzed Proteins and Peptides to Chelate Iron or Calcium and Enhance their Absorption. Foods 2018, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Allergies. Scientific opinion on dietary reference values for iron. EFSA J. 2015, 13, 4254. [Google Scholar] [CrossRef]
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef]
- Caetano-Silva, M.E.; Netto, F.M.; Bertoldo-Pacheco, M.T.; Alegría, A.; Cilla, A. Peptide-metal complexes: Obtention and role in increasing bioavailability and decreasing the pro-oxidant effect of minerals. Crit. Rev. Food Sci. Nutr. 2020, 1–20. [Google Scholar] [CrossRef]
- Gupta, U.; Gupta, S.C. Sources and Deficiency Diseases of Mineral Nutrients in Human Health and Nutrition: A Review. Pedosphere 2014, 24, 30–38. [Google Scholar] [CrossRef]
- Kumar, S.; Anukiruthika, T.; Dutta, S.; Kashyap, A.; Moses, J.A.; Anandharamakrishnan, C. Iron deficiency anemia: A comprehensive review on iron absorption, bioavailability and emerging food fortification approaches. Trends. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Allergies. Scientific opinion on dietary reference values for zinc. EFSA J. 2014, 12, 3844. [Google Scholar] [CrossRef]
- Udechukwu, M.C.; Collins, S.A.; Udenigwe, C.C. Prospects of enhancing dietary zinc bioavailability with food-derived zinc-chelating peptides. Food Funct. 2016, 7, 4137–4144. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Ntoupa, P.-S.A.; Spiliopoulou, C.A.; Stefanidou, M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020, 94, 1443–1460. [Google Scholar] [CrossRef]
- Prasad, A.S. Lessons Learned from Experimental Human Model of Zinc Deficiency. J. Immunol. Res. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mader, T.; Liu, Z.; Lanner, J.T. Calcium channels linked to altered cellular function and disease. Curr. Opin. Physiol. 2020. [Google Scholar] [CrossRef]
- Cormick, G.; Belizán, J.M. Calcium Intake and Health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef] [PubMed]
- Dary, O.; Hurrell, R. Guidelines on Food Fortification with Micronutrients; World Health Organization, Food and Agricultural Organization of the United Nations: Geneva, Switzerland, 2006. [Google Scholar]
- Cardoso, R.V.C.; Fernandes, Â.; Gonzaléz-Paramás, A.M.; Barros, L.; Ferreira, I.C.F.R. Flour fortification for nutritional and health improvement: A review. Food Res. Int. 2019, 125, 108576. [Google Scholar] [CrossRef]
- Jahan, T.A.; Vandenberg, A.; Glahn, R.P.; Tyler, R.T.; Reaney, M.J.T.; Tar’An, B. Iron Fortification and Bioavailability of Chickpea (Cicer arietinum L.) Seeds and Flour. Nutrients 2019, 11, 2240. [Google Scholar] [CrossRef]
- Castro-Alba, V.; Lazarte, C.E.; Perez-Rea, D.; Carlsson, N.; Almgren, A.; Bergenståhl, B.; Granfeldt, Y. Fermentation of pseudocereals quinoa, canihua, and amaranth to improve mineral accessibility through degradation of phytate. J. Sci. Food Agric. 2019, 99, 5239–5248. [Google Scholar] [CrossRef]
- Guo, L.; Harnedy, P.A.; Li, B.-F.; Hou, H.; Zhang, Z.; Zhao, X.; Fitzgerald, R.J. Food protein-derived chelating peptides: Biofunctional ingredients for dietary mineral bioavailability enhancement. Trends Food Sci. Technol. 2014, 37, 92–105. [Google Scholar] [CrossRef]
- Bovell-Benjamin, A.C.; Viteri, F.E.; Allen, L.H. Iron absorption from ferrous bisglycinate and ferric trisglycinate in whole maize is regulated by iron status. Am. J. Clin. Nutr. 2000, 71, 1563–1569. [Google Scholar] [CrossRef]
- Wu, W.; Yang, Y.; Sun, N.; Bao, Z.; Lin, S. Food protein-derived iron-chelating peptides: The binding mode and promotive effects of iron bioavailability. Food Res. Int. 2020, 131, 108976. [Google Scholar] [CrossRef]
- Cui, P.; Lin, S.; Jin, Z.; Zhu, B.-W.; Song, L.; Sun, N. In vitro digestion profile and calcium absorption studies of a sea cucumber ovum derived heptapeptide–calcium complex. Food Funct. 2018, 9, 4582–4592. [Google Scholar] [CrossRef]
- Li, B.; He, H.; Shi, W.; Hou, T. Effect of duck egg white peptide-ferrous chelate on iron bioavailabilityin vivoand structure characterization. J. Sci. Food Agric. 2018, 99, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Guo, S.-T.; Tako, E.; Glahn, R.P. Hydrolysis of Soybean Protein Improves Iron Bioavailability by Caco-2 Cell. J. Food Nutr. Res. 2014, 2, 162–166. [Google Scholar] [CrossRef][Green Version]
- Udechukwu, M.C.; Downey, B.; Udenigwe, C.C. Influence of structural and surface properties of whey-derived peptides on zinc-chelating capacity, and in vitro gastric stability and bioaccessibility of the zinc-peptide complexes. Food Chem. 2018, 240, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Carter, P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal. Biochem. 1971, 40, 450–458. [Google Scholar] [CrossRef]
- Le Vo, T.D.; Pham, K.T.; Van Le, V.M.; Lam, H.H.; Huynh, O.N.; Vo, B.C. Evaluation of iron-binding capacity, amino acid composition, functional properties of Acetes japonicus proteolysate and identification of iron-binding peptides. Process Biochem. 2020, 91, 374–386. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Gong, M.; Mohan, A.; Udechukwu, M.C. Role of surface charge of hydrolysed bovine caseins in their iron (II)-binding affinity and antioxidative capacity in iron (II)-facilitated β-carotene and glutathione oxidation. J. Food Nutr. Res. 2017, 56, 149–154. [Google Scholar]
- Saywell, L.G.; Cunningham, B.B. Determination of Iron: Colorimetric o-Phenanthroline Method. Ind. Eng. Chem. Anal. Ed. 1937, 9, 67–69. [Google Scholar] [CrossRef]
- Lee, S.-H.; Bin Song, K. Purification of an iron-binding nona-peptide from hydrolysates of porcine blood plasma protein. Process. Biochem. 2009, 44, 378–381. [Google Scholar] [CrossRef]
- Kim, S.; Seo, I.; Khan, M.A.; Ki, K.; Lee, W.; Lee, H.; Shin, H.; Kim, H. Enzymatic Hydrolysis of Heated Whey: Iron-Binding Ability of Peptides and Antigenic Protein Fractions. J. Dairy Sci. 2007, 90, 4033–4042. [Google Scholar] [CrossRef]
- Chaud, M.V.; Izumi, C.; Nahaal, Z.; Shuhama, T.; Bianchi, M.D.L.P.; De Freitas, O. Iron Derivatives from Casein Hydrolysates as a Potential Source in the Treatment of Iron Deficiency. J. Agric. Food Chem. 2002, 50, 871–877. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, W.; Chen, F.; Zou, J.; Chen, W.; Huang, G. Purification of an iron-binding peptide from scad (Decapterus maruadsi) processing by-products and its effects on iron absorption by Caco-2 cells. J. Food Biochem. 2019, 43, e12876. [Google Scholar] [CrossRef] [PubMed]
- Udechukwu, M.C.; Tsopmo, A.; Mawhinney, H.; He, R.; Kienesberger, P.C.; Udenigwe, C.C. Inhibition of ADAM17/TACE activity by zinc-chelating rye secalin-derived tripeptides and analogues. RSC Adv. 2017, 7, 26361–26369. [Google Scholar] [CrossRef]
- Wang, C.; Li, B.; Ao, J. Separation and identification of zinc-chelating peptides from sesame protein hydrolysate using IMAC-Zn2+ and LC–MS/MS. Food Chem. 2012, 134, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, J.; Tong, P.; Mao, X. Zinc-binding capacity of yak casein hydrolysate and the zinc-releasing characteristics of casein hydrolysate-zinc complexes. J. Dairy Sci. 2011, 94, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Gitelman, H.J. An improved automated procedure for the determination of calcium in biological specimens. Anal. Biochem. 1967, 18, 521–531. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Q.; Huang, S.; Lin, J.; Wang, S.; Huang, Y.; Hong, J.; Rao, P. Novel Peptide with a Specific Calcium-Binding Capacity from Whey Protein Hydrolysate and the Possible Chelating Mode. J. Agric. Food Chem. 2014, 62, 10274–10282. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Shindo, M.; Gunshin, H.; Noguchi, T.; Naito, H. Characterization of phosphopeptide derived from bovine β-casein: An inhibitor to intra-intestinal precipitation of calcium phosphate. Biochim. Biophys. Acta 1991, 1077, 413–415. [Google Scholar] [CrossRef]
- Bingtong, L.; Yongliang, Z.; Sun, L. Identification and characterization of the peptides with calcium-binding capacity from tilapia (Oreochromis niloticus) skin gelatin enzymatic hydrolysates. J. Food Sci. 2019, 85, 114–122. [Google Scholar] [CrossRef]
- Hou, H.; Wang, S.; Zhu, X.; Li, Q.; Fan, Y.; Cheng, N.; Li, B.-F. A novel calcium-binding peptide from Antarctic krill protein hydrolysates and identification of binding sites of calcium-peptide complex. Food Chem. 2018, 243, 389–395. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R. Food Protein-Derived Bioactive Peptides: Production, Processing, and Potential Health Benefits. J. Food Sci. 2011, 77, R11–R24. [Google Scholar] [CrossRef]
- Acquah, C.; Chan, Y.W.; Pan, S.; Agyei, D.; Udenigwe, C.C. Structure-informed separation of bioactive peptides. J. Food Biochem. 2019, 43, e12765. [Google Scholar] [CrossRef]
- De La Hoz, L.; Ponezi, A.N.; Milani, R.F.; Da Silva, V.S.N.; De Souza, A.S.; Pacheco, M.T.B. Iron-binding properties of sugar cane yeast peptides. Food Chem. 2014, 142, 166–169. [Google Scholar] [CrossRef]
- Miao, J.; Liao, W.; Pan, Z.; Wang, Q.; Duan, S.; Xiao, S.; Yang, Z.; Cao, Y. Isolation and identification of iron-chelating peptides from casein hydrolysates. Food Funct. 2019, 10, 2372–2381. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.-X.; Wang, X.-P.; Guo, X.-N. Isolation and characterization of zinc-chelating peptides from wheat germ protein hydrolysates. J. Funct. Foods 2015, 12, 23–32. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Z.; Huang, W.; Zhao, Y.; Dong, S.; Zeng, M. Purification and characterisation of a zinc-binding peptide from oyster protein hydrolysate. J. Funct. Foods 2013, 5, 689–697. [Google Scholar] [CrossRef]
- Liao, W.; Liu, S.; Liu, X.; Duan, S.; Xiao, S.; Yang, Z.; Cao, Y.; Miao, J. The purification, identification and bioactivity study of a novel calcium-binding peptide from casein hydrolysate. Food Funct. 2019, 10, 7724–7732. [Google Scholar] [CrossRef]
- Liu, F.-R.; Wang, L.; Wang, R.; Chen, Z.-X. Calcium-Binding Capacity of Wheat Germ Protein Hydrolysate and Characterization of Peptide–Calcium Complex. J. Agric. Food Chem. 2013, 61, 7537–7544. [Google Scholar] [CrossRef]
- Lv, Y.; Wei, K.; Meng, X.; Huang, Y.; Zhang, T.; Li, Z. Separation and identification of iron-chelating peptides from defatted walnut flake by nanoLC-ESI–MS/MS and de novo sequencing. Process. Biochem. 2017, 59, 223–228. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Alaiz, M.; Vioque, J. Affinity purification and characterisation of chelating peptides from chickpea protein hydrolysates. Food Chem. 2011, 129, 485–490. [Google Scholar] [CrossRef]
- Wang, C.; Li, B.; Li, H. Zn(II) chelating with peptides found in sesame protein hydrolysates: Identification of the binding sites of complexes. Food Chem. 2014, 165, 594–602. [Google Scholar] [CrossRef]
- Sun, X.; Udenigwe, C.C. Chemistry and Biofunctional Significance of Bioactive Peptide Interactions with Food and Gut Components. J. Agric. Food Chem. 2020, 159, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Cui, P.; Jin, Z.; Wu, H.; Wang, Y.; Lin, S. Contributions of molecular size, charge distribution, and specific amino acids to the iron-binding capacity of sea cucumber (Stichopus japonicus) ovum hydrolysates. Food Chem. 2017, 230, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Antonilli, M.; Bottari, E.; Festa, M.R.; Gentile, L. Complex formation between arginine and calcium (II) and magnesium (II). Chem. Speciat. Bioavailab. 2009, 21, 33–40. [Google Scholar] [CrossRef]
- Clarke, E.; Martell, A. Metal chelates of arginine and related ligands. J. Inorg. Nucl. Chem. 1970, 32, 911–926. [Google Scholar] [CrossRef]
- Caetano-Silva, M.E.; Pacheco, M.T.B.; Leme, A.F.P.; Netto, F.M. Iron-binding peptides from whey protein hydrolysates: Evaluation, isolation and sequencing by LC–MS/MS. Food Res. Int. 2015, 71, 132–139. [Google Scholar] [CrossRef]
- Cruz-Huerta, E.; Martínez-Maqueda, D.; De La Hoz, L.; Da Silva, V.S.N.; Pacheco, M.T.B.; Amigo, L.; Recio, I. Short communication: Identification of iron-binding peptides from whey protein hydrolysates using iron (III)-immobilized metal ion affinity chromatographyand reversed phase-HPLC-tandem mass spectrometry. J. Dairy Sci. 2016, 99, 77–82. [Google Scholar] [CrossRef]
- Zong, H.; Peng, L.; Zhang, S.; Lin, Y.; Feng, F.-Q. Effects of molecular structure on the calcium-binding properties of phosphopeptides. Eur. Food Res. Technol. 2012, 235, 811–816. [Google Scholar] [CrossRef]
- Sun, N.; Jin, Z.; Li, D.; Yin, H.; Lin, S. An Exploration of the Calcium-Binding Mode of Egg White Peptide, Asp-His-Thr-Lys-Glu, and In Vitro Calcium Absorption Studies of Peptide–Calcium Complex. J. Agric. Food Chem. 2017, 65, 9782–9789. [Google Scholar] [CrossRef]
- Eckert, E.; Lu, L.; Unsworth, L.D.; Chen, L.; Xie, J.; Xu, R. Biophysical and in vitro absorption studies of iron chelating peptide from barley proteins. J. Funct. Foods 2016, 25, 291–301. [Google Scholar] [CrossRef]
- Zhang, K.; Li, J.; Hou, H.; Zhang, H.; Li, B. Purification and characterization of a novel calcium-biding decapeptide from Pacific cod (Gadus Macrocephalus) bone: Molecular properties and calcium chelating modes. J. Funct. Foods 2019, 52, 670–679. [Google Scholar] [CrossRef]
- Chen, M.; Ji, H.; Zhang, Z.; Zeng, X.; Su, W.; Liu, S. A novel calcium-chelating peptide purified from Auxis thazard protien hydrolysate and its binding properties with calcium. J. Funct. Foods 2019, 60, 103447. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Alaiz, M.; Vioque, J. Iron-chelating activity of chickpea protein hydrolysate peptides. Food Chem. 2012, 134, 1585–1588. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Huang, J.; Li, B.; Cheng, J.; Wang, Z.; Yin, J.; Yan, X. Affinity purification and characterisation of zinc chelating peptides from rapeseed protein hydrolysates: Possible contribution of characteristic amino acid residues. Food Chem. 2015, 173, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, B.; Hou, H.; Zhang, H.; Zhao, X. Identification of iron-chelating peptides from Pacific cod skin gelatin and the possible binding mode. J. Funct. Foods 2017, 35, 418–427. [Google Scholar] [CrossRef]
- Eckert, E.; Bamdad, F.; Chen, L. Metal solubility enhancing peptides derived from barley protein. Food Chem. 2014, 159, 498–506. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, Q.; Bao, X.; Tang, W.; Yang, B.; Guo, S.-T. Identification and Characteristics of Iron-Chelating Peptides from Soybean Protein Hydrolysates Using IMAC-Fe3+. J. Agric. Food Chem. 2009, 57, 4593–4597. [Google Scholar] [CrossRef]
- Wang, X.; Ai, T.; Meng, X.; Zhou, J.; Mao, X. In vitro iron absorption of α-lactalbumin hydrolysate-iron and β-lactoglobulin hydrolysate-iron complexes. J. Dairy Sci. 2014, 97, 2559–2566. [Google Scholar] [CrossRef]
- Wang, B.; Xie, N.; Li, B. Influence of peptide characteristics on their stability, intestinal transport, and in vitro bioavailability: A review. J. Food Biochem. 2018, 43, e12571. [Google Scholar] [CrossRef]
- O’Loughlin, I.B.; Kelly, P.M.; Murray, B.A.; Fitzgerald, R.J.; Brodkorb, A. Molecular Characterization of Whey Protein Hydrolysate Fractions with Ferrous Chelating and Enhanced Iron Solubility Capabilities. J. Agric. Food Chem. 2015, 63, 2708–2714. [Google Scholar] [CrossRef]
- Lee, S.H.; Bin Song, K. Isolation of a Calcium-binding Peptide from Enzymatic Hydrolysates of Porcine Blood Plasma Protein. J. Korean Soc. Appl. Boil. Chem. 2009, 52, 290–294. [Google Scholar] [CrossRef]
- Yamauchi, O.; Odani, A.; Takani, M. Metal–amino acid chemistry. Weak interactions and related functions of side chain groups. J. Chem. Soc. Dalton Trans. 2002, 64, 3411–3421. [Google Scholar] [CrossRef]
- Rossi, M.; Tkatchenko, A.; Rempe, S.B.; Varma, S. Role of methyl-induced polarization in ion binding. Proc. Natl. Acad. Sci. USA 2013, 110, 12978–12983. [Google Scholar] [CrossRef]
- Shimazaki, Y.; Takani, M.; Yamauchi, O. Metal complexes of amino acids and amino acid side chain groups. Structures and properties. Dalton Trans. 2009, 40, 7854. [Google Scholar] [CrossRef]
- Sóvágó, I.; Kállay, C.; Várnagy, K. Peptides as complexing agents: Factors influencing the structure and thermodynamic stability of peptide complexes. Coord. Chem. Rev. 2012, 256, 2225–2233. [Google Scholar] [CrossRef]
- Sato, R.; Noguchi, T.; Naito, H. Casein phosphopeptide (CPP) enhances calcium absorption from the ligated segment of rat small intestine. J. Nutr. Sci. Vitaminol. 1986, 32, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Caetano-Silva, M.E.; Cilla, A.; Bertoldo-Pacheco, M.T.; Netto, F.M.; Alegria, A. Evaluation of in vitro iron bioavailability in free form and as whey peptide-iron complexes. J. Food Compos. Anal. 2018, 68, 95–100. [Google Scholar] [CrossRef]
- Scheers, N.; Sandberg, A.-S. Ascorbic acid uptake affects ferritin, Dcytb and Nramp2 expression in Caco-2 cells. Eur. J. Nutr. 2008, 47, 401–408. [Google Scholar] [CrossRef]
- Miquel, E.; Farré, R. Effects and future trends of casein phosphopeptides on zinc bioavailability. Trends Food Sci. Technol. 2007, 18, 139–143. [Google Scholar] [CrossRef]
- Cámara-Martos, F.; Amaro, M.; Barbera, R.; Clemente, G. Bioaccessibility of minerals in school meals: Comparison between dialysis and solubility methods. Food Chem. 2005, 92, 481–489. [Google Scholar] [CrossRef]
- Bryszewska, M.A.; Tomás-Cobos, L.; Gallego, E.; Villalba, M.P.; Rivera, D.; Taneyo-Saa, D.L.; Gianotti, A. In vitro bioaccessibility and bioavailability of iron from breads fortified with microencapsulated iron. LWT 2019, 99, 431–437. [Google Scholar] [CrossRef]
- Sun, N.; Wu, H.; Du, M.; Tang, Y.; Liu, H.; Fu, Y.; Zhu, B.-W. Food protein-derived calcium chelating peptides: A review. Trends Food Sci. Technol. 2016, 58, 140–148. [Google Scholar] [CrossRef]
- Sun, X.; Acquah, C.; Aluko, R.E.; Udenigwe, C.C. Considering food matrix and gastrointestinal effects in enhancing bioactive peptide absorption and bioavailability. J. Funct. Foods 2020, 64, 103680. [Google Scholar] [CrossRef]
- Wada, Y.; Lönnerdal, B. Bioactive peptides derived from human milk proteins—Mechanisms of action. J. Nutr. Biochem. 2014, 25, 503–514. [Google Scholar] [CrossRef] [PubMed]
- García-Nebot, M.J.; Barberá, R.; Alegría, A. Iron and zinc bioavailability in Caco-2 cells: Influence of caseinophosphopeptides. Food Chem. 2013, 138, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Frazer, D.M.; Anderson, G.J. The regulation of iron transport. BioFactors 2013, 40, 206–214. [Google Scholar] [CrossRef]
- Porath, J. Amino acid side chain interaction with chelate-liganded crosslinked dextran, agarose and TSK gel. A mini review of recent work. J. Mol. Recognit. 1990, 3, 123–127. [Google Scholar] [CrossRef]
- Chen, Q.; Guo, L.; Du, F.; Chen, T.; Hou, H.; Li, B. The chelating peptide (GPAGPHGPPG) derived from Alaska pollock skin enhances calcium, zinc and iron transport in Caco-2 cells. Int. J. Food Sci. Technol. 2017, 52, 1283–1290. [Google Scholar] [CrossRef]
- Zhang, K.; Li, B.-F.; Chen, Q.; Zhang, Z.; Zhao, X.; Hou, H. Functional Calcium Binding Peptides from Pacific Cod (Gadus macrocephalus) Bone: Calcium Bioavailability Enhancing Activity and Anti-Osteoporosis Effects in the Ovariectomy-Induced Osteoporosis Rat Model. Nutrients 2018, 10, 1325. [Google Scholar] [CrossRef]
- Xiao, C.; Lei, X.; Wang, Q.; Du, Z.; Jiang, L.; Chen, S.; Zhang, M.; Zhang, H.; Ren, F. Effects of a Tripeptide Iron on Iron-Deficiency Anemia in Rats. Boil. Trace Element Res. 2015, 169, 211–217. [Google Scholar] [CrossRef]
- Megías, C.; Pedroche, J.; Yust, M.M.; Calle, J.G.; Alaiz, M.; Millan, F.; Vioque, J. Production of copper-chelating peptides after hydrolysis of sunflower proteins with pepsin and pancreatin. LWT 2008, 41, 1973–1977. [Google Scholar] [CrossRef]
| 1 | Cell culture |
| 2 | In vivo |
| Peptide Sequences | Metal Ions | Source | Location | Net Charge (pH 7.0) * | Molecular Weight * | References |
|---|---|---|---|---|---|---|
| Asp-Ala-Asp-Ser-Val-Asn-Phe-Pro-Val-His-Gly-Leu | Iron | Acetes japonicus | n/a | −1.9 | 1270 | [26] |
| Phe-Lys-Val-Gly-Gln-Glu-Asn-Thr-Pro-Ile-Leu-Lys | Iron | Acetes japonicus | n/a | 1 | 1374 | [26] |
| Cys-Gln-Val | Zinc | Rye secalin | 324–326 | −0.1 | 348 | [33] |
| Gln-Cys-Ala | Zinc | Rye secalin | 343–345 | −0.1 | 320 | [33] |
| Leu-Ala-Gly-Asn-Pro-(Asp)2-Glu-Phe-Arg-Pro-Gln | Iron | Defatted walnut flake | n/a | −2 | 1358 | [49] |
| Val-Gln-Asp-Glu-Leu-Val-Ala-(Val)2 | Iron | Defatted walnut flake | n/a | −2 | 971 | [49] |
| Ser-Met | Iron, Zinc | Sesame | n/a | 0 | 236 | [51] |
| Asn-Cys-Ser | Iron, Zinc | Sesame | n/a | −0.1 | 322 | [51] |
| Tyr-Val-(Glu)2-Leu-Lys-Pro-Thr-Pro-Glu-Gly-Asp-Leu-Glu-Ile-Leu | Iron | Bovine β-lactoglobulin | 42–57 | −4 | 1845 | [56] |
| Arg-Thr-Pro-Glu-Val-(Asp)2-Glu-Ala-Leu-Glu-Lys | Iron | Bovine β-lactoglobulin | 124–135 | −3 | 1401 | [56] |
| Phe-Lys-Asp-Leu-Gly-(Glu)2-His | Iron | Bovine serum albumin | 11–18 | −1.9 | 974 | [56] |
| Lys-(Asp)2-Ser-Pro-Asp-Leu-Pro-Lys | Iron | Bovine serum albumin | 106–114 | −1 | 1014 | [56] |
| (Asp)3-Leu-Thr-(Asp)2-Ile | Iron | Bovine α-lactalbumin | 82–89 | −5 | 921 | [56,57] |
| Thr-Pro-Glu-Val-(Asp)2-Glu | Iron | Bovine β-lactoglobulin | 125–131 | −4 | 889 | [57] |
| Ser(P)3-(Glu)2 | Iron, Zinc, Calcium | Bovine β-casein | 18–21 | −2 | 777 | [58] |
| Asp-His-Thr-Lys-Glu | Calcium | Chicken egg white | n/a | −0.9 | 629 | [59] |
| Ser-Val-Asn-Val-Pro-Leu-Tyr | Iron | Barley B1-hordein | 275–281 | 0 | 791 | [60] |
| Lys-Gly-Asp-Pro-Gly-Leu-Ser-Pro-Gly-Lys | Calcium | Pacific cod bone | n/a | 1 | 955 | [61] |
| Glu-Pro-Ala-His | Calcium | Auxis thazard | n/a | −0.9 | 452 | [62] |
| Type of Study | Peptide/Hydrolysate | Mineral | Treatment Prior Bioavailability Assay | Bioavailability Assay/Markers of Bioavailability | Effect on Bioavailability/Absorption | Reference |
|---|---|---|---|---|---|---|
| In vitro using Caco-2 cells | NDEELNK (from trypsin hydrolysis of sea cucumber ovum) | Calcium | In vitro digestion | Calcium absorption | Increased calcium absorption | [21] |
| In vitro using Caco-2 cells and HT-29 | Sea cucumber ovum hydrolysate (trypsin, Alcalase, Neutrase, papain, Flavourzyme) | Calcium | In vitro digestion | Calcium solubility intracellular calcium concentration | Higher calcium solubility intracellular calcium concentration in complexes | |
| In vitro using Caco-2 cells | α-Lactalbumin hydrolysate β-Lactoglobulin hydrolysate (Alcalase, β-LGH ) | Iron | In vitro digestion | Ferritin content Iron absorption | β-Lactoglobulin hydrolysate–iron complexes significantly improved iron absorption and ferritin | [37] |
| In vitro using Caco-2 cells | SVNVPLY | Iron | In vitro digestion | Ferritin formation | Cell uptake increased 4 times after pepsin–pancreatin digestion | [60] |
| In vitro using Caco-2 cells | Whey protein isolate fractionates (pancreatin hydrolysis) | Iron | In vitro digestion | Ferritin synthesis in cell culture model | Ferritin synthesis in complexes with low-molecular weight (<5 kDa) | [77] |
| In vitro using Caco-2 cells | Caseinophosphopeptides (CPPs) (β-CN(1–25)4P, αs1-CN(64–74)4P and αs2-CN(1–19)4P) | IronZinc | In vitro digestion | Ferritin synthesis | Increased ferritin synthesis Increased zinc uptake | [85] |
| In vitro using Caco-2 cells | GPAGPHGPPG from Alaska pollock skin | Calcium Iron Zinc | Hydrolysis with pepsin | Transport in Caco-2 cell monolayer | 112.7% increase in calcium transport 27.7% increase iron 32.3% in zinc transport | [88] |
| In vivo (iron-deficiency anemia male rats) | Duck egg white peptides (neutrase) | Iron | Feeding | Hematological test, serum iron, serum ferritin | Hematology levels increased to the normal levels by peptide–iron complexes | [22] |
| In vivo (iron-deficiency anemia female rats) | β-Lactoglobulin hydrolysate (Alcalase) | Iron | Feeding | Hematological test Serum ferritin and transferrin | β-Lactoglobulin hydrolysate–iron complex significantly improved serum iron level, total iron-binding capacity and transferrin saturation, serum ferritin | [68] |
| In situ single-pass intestinal perfusion (in Wistar rats) | Pacific Cod (Gadus macrocephalus) Bone calcium binding peptides (trypsin and neutral protease) | Calcium | Single-pass intestinal perfusion | Calcium absorption Calcium retention | Increased calcium absorption and serum calcium | [89] |
| In vivo (iron-deficiency anemia male rats) | Tripeptide REE | Iron | Feeding | Hematological test Serum ferritin and transferrin Serum iron Hepcidin mRNA expression | Increase in hematological parameters to normal levels Restoration of renal coefficient, total iron-binding capacity, and transferrin, liver hepcidin mRNA to normal levels | [90] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Sarteshnizi, R.A.; Boachie, R.T.; Okagu, O.D.; Abioye, R.O.; Pfeilsticker Neves, R.; Ohanenye, I.C.; Udenigwe, C.C. Peptide–Mineral Complexes: Understanding Their Chemical Interactions, Bioavailability, and Potential Application in Mitigating Micronutrient Deficiency. Foods 2020, 9, 1402. https://doi.org/10.3390/foods9101402
Sun X, Sarteshnizi RA, Boachie RT, Okagu OD, Abioye RO, Pfeilsticker Neves R, Ohanenye IC, Udenigwe CC. Peptide–Mineral Complexes: Understanding Their Chemical Interactions, Bioavailability, and Potential Application in Mitigating Micronutrient Deficiency. Foods. 2020; 9(10):1402. https://doi.org/10.3390/foods9101402
Chicago/Turabian StyleSun, Xiaohong, Roghayeh Amini Sarteshnizi, Ruth T. Boachie, Ogadimma D. Okagu, Raliat O. Abioye, Renata Pfeilsticker Neves, Ikenna Christian Ohanenye, and Chibuike C. Udenigwe. 2020. "Peptide–Mineral Complexes: Understanding Their Chemical Interactions, Bioavailability, and Potential Application in Mitigating Micronutrient Deficiency" Foods 9, no. 10: 1402. https://doi.org/10.3390/foods9101402
APA StyleSun, X., Sarteshnizi, R. A., Boachie, R. T., Okagu, O. D., Abioye, R. O., Pfeilsticker Neves, R., Ohanenye, I. C., & Udenigwe, C. C. (2020). Peptide–Mineral Complexes: Understanding Their Chemical Interactions, Bioavailability, and Potential Application in Mitigating Micronutrient Deficiency. Foods, 9(10), 1402. https://doi.org/10.3390/foods9101402








