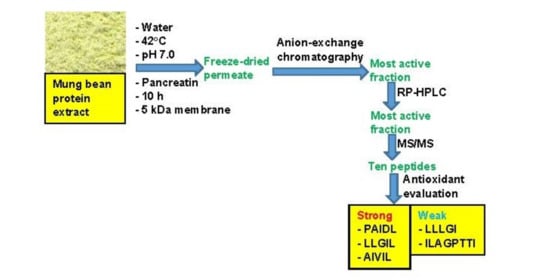
Structure and Function of Mung Bean Protein-Derived Iron-Binding Antioxidant Peptides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Mung Bean Protein Hydrolysate (MBPH) Using Continuous Enzymatic Membrane Reactor (cEMR)
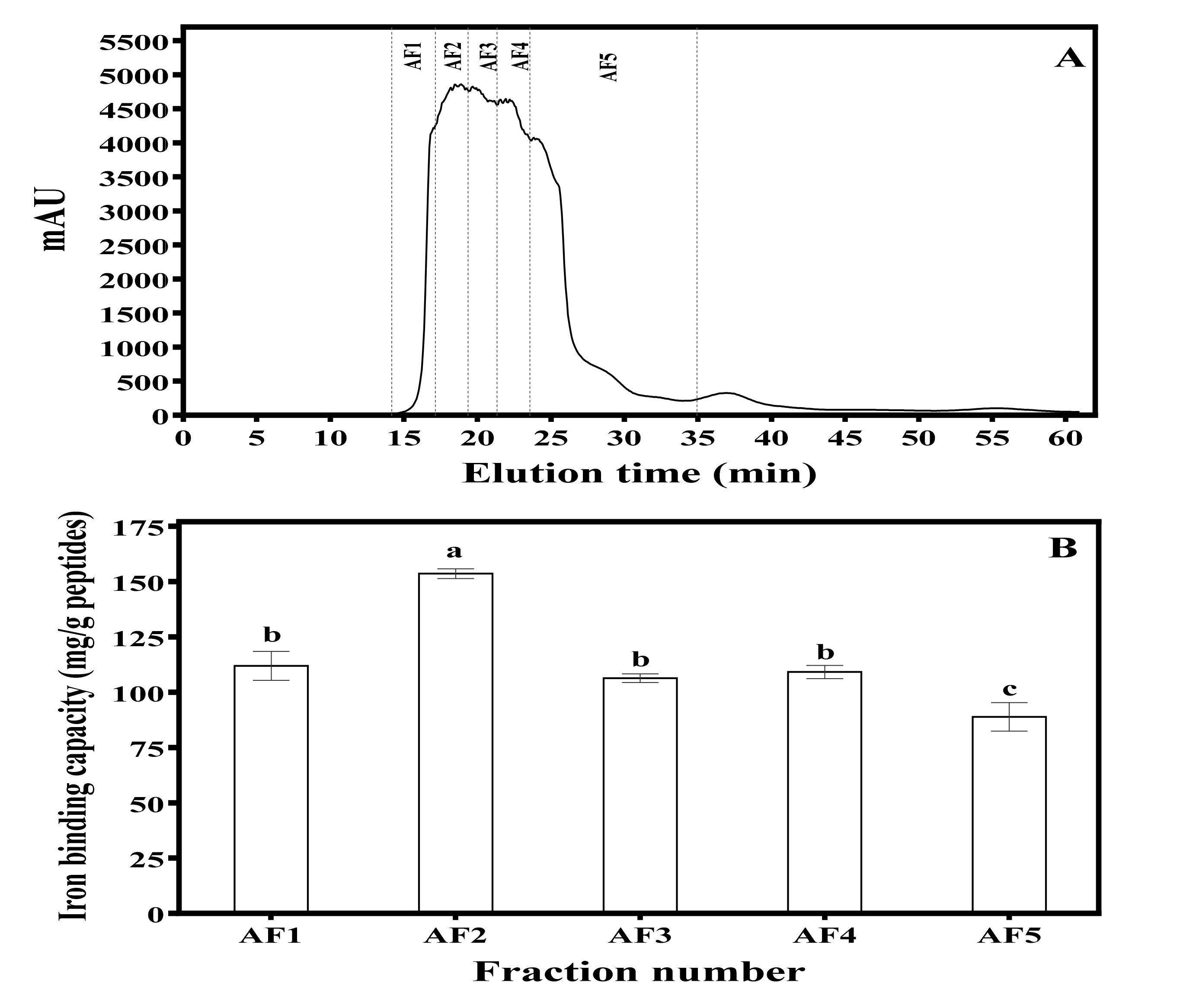
2.3. Anion-Exchange Chromatography Separation of MBPH
2.4. Determination of the Molecular Weight Distribution
2.5. Amino Acid Composition
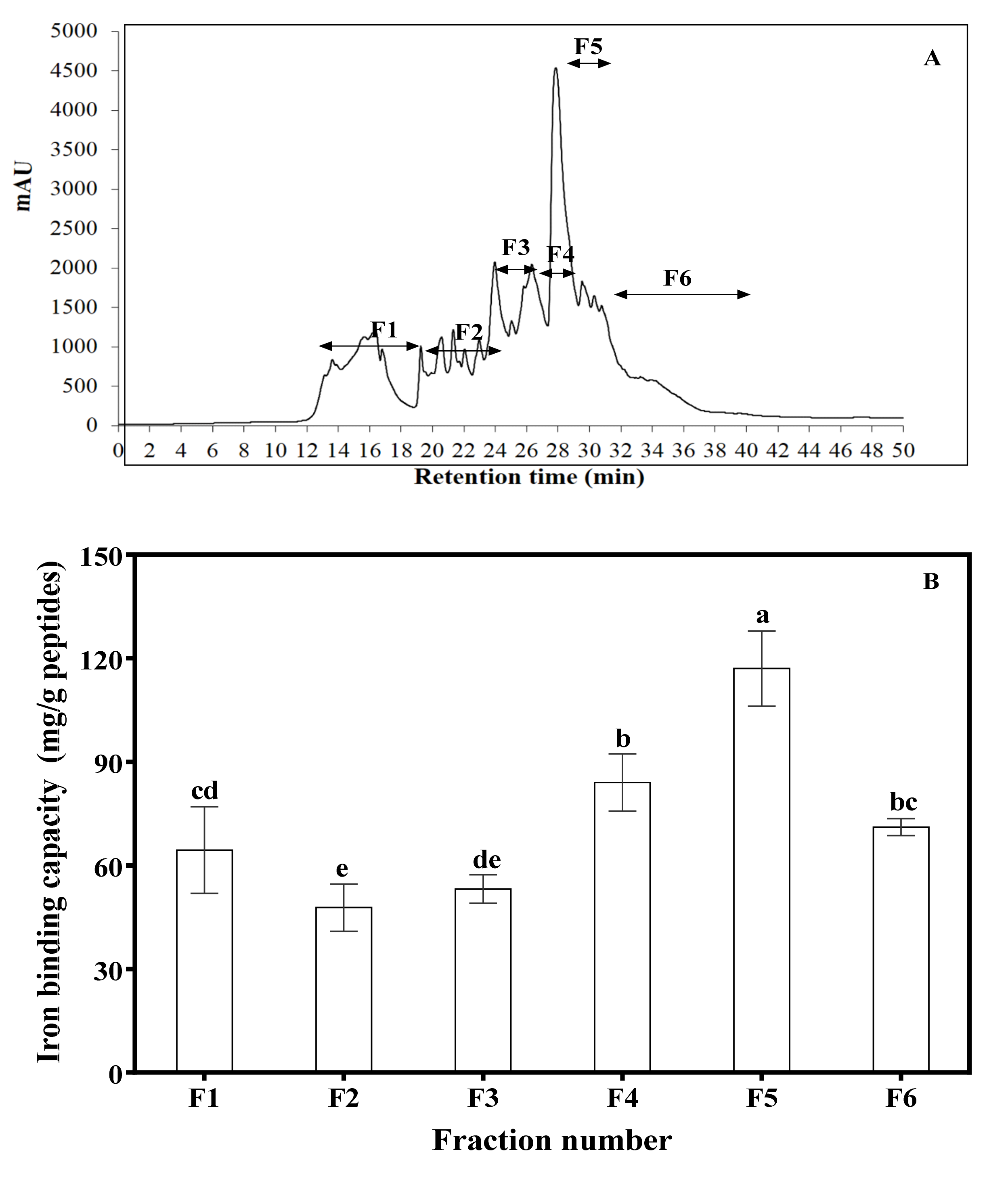
2.6. Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC)
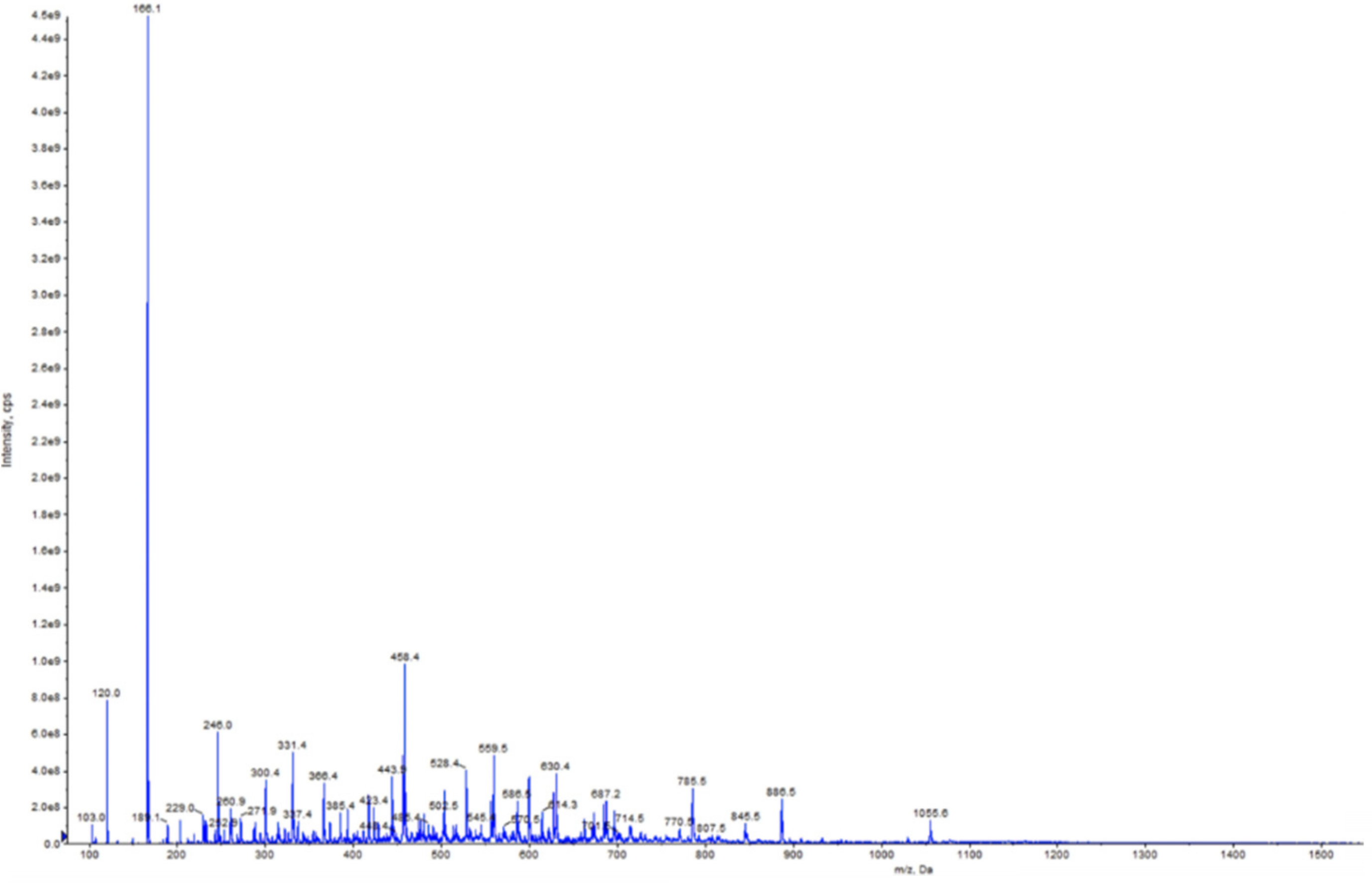
2.7. Peptide Identification by Mass Spectroscopy
2.8. Metal Chelating Activity
2.9. Ferric Reducing Antioxidant Power Activity (FRAP)
2.10. DPPH Radical Scavenging Activity
2.11. Hydroxyl Radical Scavenging Activity (HRSA)
2.12. Superoxide Radical Scavenging Activity (SRSA)
2.13. Statistical Analysis
3. Results
3.1. Iron-Binding Capacity of MBPH and the Anion-Exchange Column Chromatography Fractions (AF)
3.2. Molecular Weight (MW) Profile
3.3. RP-HPLC Fractionation of AF2 and Amino Acid Composition of Mung Bean Protein and Peptides
3.4. Peptide Identification by Mass Spectrometry
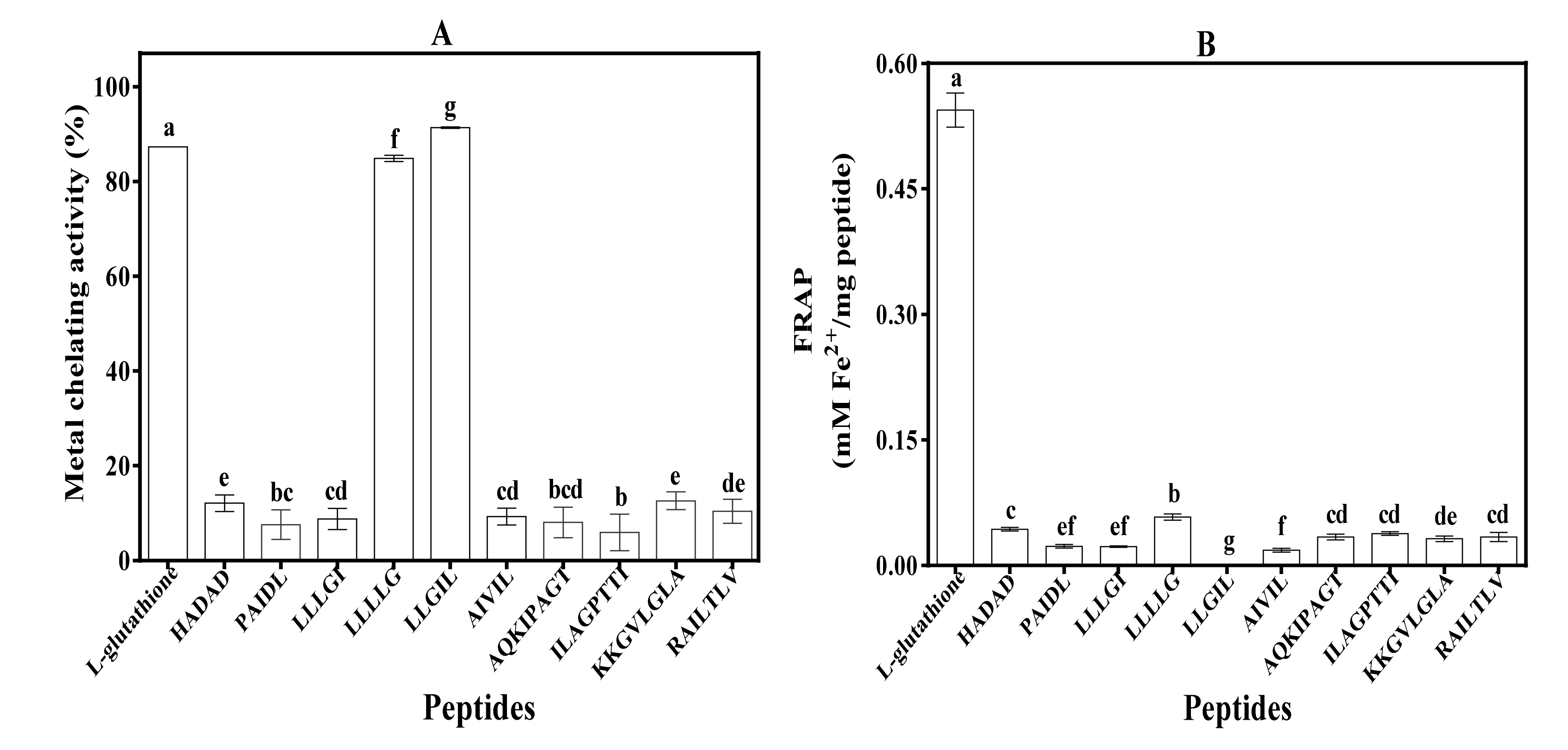
3.5. Metal Chelating Activity and Ferric Reducing Antioxidant Power (FRAP)
3.6. DPPH Radical Scavenging Activity
3.7. Hydroxyl Radical Scavenging Activity (HRSA)
3.8. Superoxide Radical Scavenging Activity (SRSA)
4. Discussions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Caetano-Silva, M.E.; Bertoldo-Pacheco, M.T.; Paes-Leme, A.F.; Netto, F.M. Iron-binding peptides from whey protein hydrolysates: Evaluation, isolation and sequencing by LC–MS/MS. Food Res. Int. 2015, 71, 132–139. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Alaiz, M.; Vioque, J. Iron-chelating activity of chickpea protein hydrolysate peptides. Food Chem. 2012, 134, 1585–1588. [Google Scholar] [CrossRef]
- MacKenzie, E.L.; Iwasaki, K.; Tsuji, Y. Intracellular iron transport and storage: From molecular mechanisms to health implications. Antioxid. Redox Signal. 2008, 10, 997–1030. [Google Scholar] [CrossRef] [PubMed]
- Beard, J. Iron deficiency alters brain development and functioning. J. Nutr. 2003, 133, 1468S–1472S. [Google Scholar] [CrossRef] [PubMed]
- Dostal, A.; Chassard, C.; Hilty, F.M.; Zimmermann, M.B.; Jaeggi, T.; Rossi, S.; Lacroix, C. Iron depletion and repletion with ferrous sulfate or electrolytic iron modifies the composition and metabolic activity of the gut microbiota in rats. J. Nutr. 2012, 142, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Goddard, A.F.; James, M.W.; McIntyre, A.S.; Scott, B.B. Guidelines for the management of iron deficiency anaemia. Gut 2011, 60, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.; Dignass, A.U. Management of iron deficiency anemia in inflammatory bowel disease—A practical approach. Ann. Gastroenterol. 2013, 26, 104–113. [Google Scholar] [PubMed]
- Kou, X.; Gao, J.; Xue, Z.; Zhang, Z.; Wang, H.; Wang, X. Purification and identification of antioxidant peptides from chickpea (Cicer arietinum L.) albumin hydrolysates. LWT Food Sci. Technol. 2013, 50, 591–598. [Google Scholar] [CrossRef]
- Stadtman, E.R. Protein oxidation and aging. Science 1992, 257, 1220–1224. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef]
- Ajibola, C.F.; Fashakin, J.B.; Fagbemi, T.N.; Aluko, R.E. Effect of peptide size on antioxidant properties of African yam bean seed (Sphenostylis stenocarpa) protein hydrolysate fractions. Int. J. Mol. Sci. 2011, 12, 6685–6702. [Google Scholar] [CrossRef] [PubMed]
- Torres-Fuentes, C.; Contreras, M.D.M.; Recio, I.; Alaiz, M.; Vioque, J. Identification and characterization of antioxidant peptides from chickpea protein hydrolysates. Food Chem. 2015, 180, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Abbas, M.; Shah, H. Proximate and mineral composition of mung bean. Sarhad J. Agric. 2007, 23, 463–466. [Google Scholar]
- Li, W.; Shu, C.; Yan, S.; Shen, Q. Characteristics of sixteen mung bean cultivars and their protein isolates. Int. J. Food Sci. Technol. 2010, 45, 1205–1211. [Google Scholar] [CrossRef]
- Mendoza, E.M.T.; Adachi, M.; Bernardo, A.E.N.; Utsumi, S. Mungbean (Vigna radiata (L.) Wilczek) globulins: Purification and characterization. J. Agric. Food Chem. 2001, 49, 1552–1558. [Google Scholar] [CrossRef]
- Li, G.H.; Wan, J.Z.; Le, G.W.; Shi, Y.H. Novel angiotensin I-converting enzyme inhibitory peptides isolated from Alcalase hydrolysate of mung bean protein. J. Peptide Sci. 2006, 12, 509–514. [Google Scholar] [CrossRef]
- Viernes, L.; Garcia, R.; Torio, M.; Angelia, M. Antihypertensive peptides from vicilin, the major storage protein of mung bean (Vigna radiata (L.) R. Wilczek). J. Biol. Sci. 2012, 12, 393. [Google Scholar]
- Kusumah, J.; Real Hernandez, L.M.; de Mejia, E.G. Antioxidant potential of mung bean (Vigna radiata) albumin peptides produced by enzymatic hydrolysis analyzed by biochemical and in silico methods. Foods 2020, 9, 1241. [Google Scholar] [CrossRef]
- Xie, J.; Ye, H.; Du, M.; Yu, Q.; Chen, Y.; Shen, M. Mung bean protein hydrolysates protect mouse liver cell line nctc-1469 cell from hydrogen peroxide-induced cell injury. Foods 2020, 9, 14. [Google Scholar] [CrossRef]
- Tauntong, M.; Sirinupong, N.; Youravong, W. Effect of pre-hydrolysis by Alcalase on enzymatic membrane reactor performance in production of low molecular weight peptide from nile tilapia skin gelatin. Kasetsart J. Nat. Sci. 2014, 48, 929–941. [Google Scholar]
- Thuanthong, M.; De Gobba, C.; Sirinupong, N.; Youravong, W.; Otte, J. Purification and characterization of angiotensin-converting enzyme-inhibitory peptides from Nile tilapia (Oreochromis niloticus) skin gelatine produced by an enzymatic membrane reactor. J. Funct. Foods 2017, 36, 243–254. [Google Scholar] [CrossRef]
- Lee, S.-H.; Song, K.B. Purification of an iron-binding nona-peptide from hydrolysates of porcine blood plasma protein. Process. Biochem. 2009, 44, 378–381. [Google Scholar] [CrossRef]
- Budseekoad, S.; Yupanqui, C.T.; Sirinupong, N.; Alashi, A.M.; Aluko, R.E.; Youravong, W. Structural and functional characterization of calcium and iron-binding peptides from mung bean protein hydrolysate. J. Funct. Foods 2018, 49, 333–341. [Google Scholar] [CrossRef]
- Markwell, M.A.C.; Haas, S.M.; Biebar, L.L.; Tolbert, N.E. A modification of the Lowry procedure to simplify protein determination in membrane and in protein samples. Anal. Biochem. 1978, 87, 206–211. [Google Scholar] [CrossRef]
- Omoni, A.; Aluko, R.E. Mechanism of the inhibition of calmodulin-dependent neuronal nitric oxide synthase by flaxseed protein hydrolysates. J. Am. Oil Chem. Soc. 2006, 83, 335–340. [Google Scholar] [CrossRef]
- Malomo, S.A.; Onuh, J.O.; Girgih, A.T.; Aluko, R.E. Structural and antihypertensive properties of enzymatic hemp seed protein hydrolysates. Nutrients 2015, 7, 7616–7632. [Google Scholar] [CrossRef]
- Bidlingmeyer, B.A.; Cohen, S.A.; Tarvin, T.L. Rapid analysis of amino acids using pre-column derivatization. J. Chrom. B 1984, 336, 93–104. [Google Scholar] [CrossRef]
- Gehrke, C.W.; Wall, L., Sr.; Absheer, J.; Kaiser, F.; Zumwalt, R. Sample preparation for chromatography of amino acids: Acid hydrolysis of proteins. J. Assoc. Off. Anal. Chem. 1985, 68, 811–821. [Google Scholar] [CrossRef]
- Landry, J.; Delhaye, S. Simplified procedure for the determination of tryptophan of foods and feedstuffs from barytic hydrolysis. J. Agric. Food Chem. 1992, 40, 776–778. [Google Scholar] [CrossRef]
- Girgih, A.T.; Udenigwe, C.C.; Aluko, R.E. Reverse-phase HPLC separation of hemp seed (Cannabis sativa L.) protein hydrolysate produced peptide fractions with enhanced antioxidant capacity. Plant. Foods Hum. Nutr. 2013, 68, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Malomo, S.A.; Aluko, R.E. Kinetics of acetylcholinesterase inhibition by hemp seed protein-derived peptides. J. Food Biochem. 2019, 43, e12897. [Google Scholar] [CrossRef] [PubMed]
- Arise, A.K.; Alashi, A.M.; Nwachukwu, I.D.; Ijabadeniyi, O.A.; Aluko, R.E.; Amonsou, E.O. Antioxidant activities of bambara groundnut (Vigna subterranea) protein hydrolysates and their membrane ultrafiltration fractions. Food Funct. 2016, 7, 2431–2437. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhao, M.; Shi, J.; Wang, J.; Jiang, Y.; Cui, C.; Kakuda, Y.; Xue, S.J. Purification and identification of antioxidant peptides from grass carp muscle hydrolysates by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem. 2008, 108, 727–736. [Google Scholar] [CrossRef]
- Kim, S.-B.; Ku, M.-J.; Cho, W.-M.; Ki, K.-S.; Kim, H.-S.; Nam, M.-S. Production of iron-binding peptides from colostral whey by enzymatic hydrolysis. Korean J. Food Sci. Anim. Res. 2010, 30, 923–929. [Google Scholar] [CrossRef]
- Zhang, M.-N.; Huang, G.-R.; Jiang, J.-X. Effects of chemical modification and molecular weight distribution on iron binding ability of phytate-removal soybean protein isolate hydrolysate. Adv. J. Food Sci. Technol. 2012, 4, 78–83. [Google Scholar]
- Lv, Y.; Wei, K.; Meng, X.; Huang, Y.; Zhang, T.; Li, Z. Separation and identification of iron-chelating peptides from defatted walnut flake by nano LC-ESI–MS/MS and de novo sequencing. Process. Biochem. 2017, 59, 223–228. [Google Scholar] [CrossRef]
- Carrasco-Castilla, J.; Hernandez-Alvarez, A.J.; Jimenez-Martinez, C.; Jacinto-Hernandez, C.; Alaiz, M.; Giron-Calle, J.; Vioque, J.; Davila-Ortiz, G. Antioxidant and metal chelating activities of peptide fractions from phaseolin and bean protein hydrolysates. Food Chem. 2012, 135, 1789–1795. [Google Scholar] [CrossRef]
- Guo, L.; Hou, H.; Li, B.; Zhang, Z.; Wang, S.; Zhao, X. Preparation, isolation and identification of iron-chelating peptides derived from Alaska pollock skin. Process. Biochem. 2013, 48, 988–993. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Miao, M.; Jiang, B. Purification and characterisation of a new antioxidant peptide from chickpea (Cicer arietium L.) protein hydrolysates. Food Chem. 2011, 128, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Adebooye, O.C.; Alashi, A.M.; Aluko, R.E. A brief review on emerging trends in global polyphenol research. J. Food Biochem. 2018, 42, e12519. [Google Scholar] [CrossRef]
- Xia, Y.; Bamdad, F.; Gänzle, M.; Chen, L. Fractionation and characterization of antioxidant peptides derived from barley glutelin by enzymatic hydrolysis. Food Chem. 2012, 134, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Pownall, T.L.; Udenigwe, C.C.; Aluko, R.E. Effects of cationic property on the in vitro antioxidant activities of pea protein hydrolysate fractions. Food Res. Int. 2011, 44, 1069–1074. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Chemometric analysis of the amino acid requirements of antioxidant food protein hydrolysates. Int. J. Mol. Sci. 2011, 12, 3148–3161. [Google Scholar] [CrossRef]
- Chen, N.; Yang, H.; Sun, Y.; Niu, J.; Liu, S. Purification and identification of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates. Peptides 2012, 38, 344–349. [Google Scholar] [CrossRef]
- Yan, Q.J.; Huang, L.H.; Sun, Q.; Jiang, Z.Q.; Wu, X. Isolation, identification and synthesis of four novel antioxidant peptides from rice residue protein hydrolyzed by multiple proteases. Food Chem. 2015, 179, 290–295. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Byun, H.-G.; Kim, S.-K. Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci. 2005, 77, 2166–2178. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant. Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Zou, T.-B.; He, T.-P.; Li, H.-B.; Tang, H.-W.; Xia, E.-Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Najafian, L.; Babji, A.S. Isolation, purification and identification of three novel antioxidative peptides from patin (Pangasius sutchi) myofibrillar protein hydrolysates. LWT Food Sci. Technol. 2015, 60, 452–461. [Google Scholar] [CrossRef]
- Gluvić, A.; Ulriha, N.P. Peptides derived from food sources: Antioxidative activities and interactions with model lipid membranes. Food Chem. 2019, 287, 324–332. [Google Scholar] [CrossRef] [PubMed]

| Amino Acids | MBPE | MBPH | AF1 | AF2 | AF3 | AF4 | AF5 |
|---|---|---|---|---|---|---|---|
| Asx | 12.35 | 11.48 | 9.80 | 10.04 | 11.62 | 13.73 | 12.06 |
| Thr | 3.35 | 3.40 | 3.61 | 3.60 | 3.14 | 2.90 | 2.53 |
| Ser | 6.20 | 5.90 | 5.16 | 5.04 | 5.71 | 6.06 | 5.46 |
| Glx | 18.73 | 16.87 | 13.32 | 13.26 | 16.36 | 19.42 | 18.45 |
| Pro | 5.44 | 4.87 | 9.90 | 5.37 | 4.18 | 3.82 | 5.66 |
| Gly | 3.48 | 3.32 | 3.42 | 3.41 | 3.43 | 3.50 | 3.42 |
| Ala | 4.19 | 4.03 | 3.89 | 4.30 | 4.18 | 4.04 | 3.67 |
| Cys | 0.38 | 0.31 | 4.17 | 0.66 | 0.38 | 0.82 | 2.53 |
| Val | 4.38 | 5.66 | 4.69 | 6.02 | 6.07 | 5.71 | 6.23 |
| Met | 1.32 | 1.42 | 3.47 | 1.30 | 1.22 | 0.66 | 2.89 |
| Ile | 3.83 | 5.21 | 4.13 | 6.09 | 5.33 | 4.30 | 3.58 |
| Leu | 8.19 | 9.52 | 6.14 | 10.67 | 10.54 | 8.03 | 5.91 |
| Tyr | 3.18 | 3.88 | 0.52 | 0.88 | 2.84 | 3.80 | 5.66 |
| Phe | 6.47 | 7.71 | 2.25 | 5.11 | 8.95 | 8.58 | 8.11 |
| His | 3.51 | 3.41 | 2.72 | 3.17 | 3.61 | 3.45 | 2.89 |
| Lys | 7.27 | 6.97 | 12.48 | 11.92 | 6.43 | 4.12 | 3.58 |
| Arg | 6.78 | 5.01 | 8.44 | 8.76 | 5.27 | 3.85 | 3.79 |
| Trp | 0.95 | 1.04 | 1.88 | 0.40 | 0.76 | 3.18 | 3.58 |
| AAA | 10.61 | 12.63 | 4.64 | 6.40 | 12.55 | 15.56 | 17.35 |
| BCAA | 16.40 | 20.39 | 14.96 | 22.78 | 21.94 | 18.05 | 15.72 |
| HAA | 30.83 | 34.03 | 35.64 | 37.16 | 34.95 | 30.06 | 31.36 |
| PCAA | 17.55 | 15.40 | 23.64 | 23.85 | 15.31 | 11.42 | 10.26 |
| NCAA | 40.63 | 37.65 | 31.89 | 31.94 | 36.83 | 42.11 | 38.50 |
| SCAA | 1.70 | 1.72 | 7.65 | 1.96 | 1.60 | 1.48 | 5.42 |
| Observed Mass (m/z) | Peptide Sequence | Position | Parent Proteins | Iron-Binding Capacity (mg/g Peptide) 1 | Anti-Oxidant of Radical Scavenging Activities | ||
|---|---|---|---|---|---|---|---|
| DPPH (%) * | HRSA (EC50 = mM) ** | SRSA (EC50 = mM) *** | |||||
| 528.400 | HADAD | f 100–104 | 8 S globulin β isoform | 745.73 ± 120.85 d | 9.06 ± 1.43 d | 1.08 ± 0.19 c | 0.89 ± 0.02 b |
| f 102–106 | 8 S globulin α isoform | ||||||
| f 102–106 | β-conglycinin, β-chain like | ||||||
| PAIDL | f 358–362 | basic 7 S globulin 2-like | 9058.65 ± 1409.72 a | 46.63 ± 0.50 b | 0.09 ± 0.02 f | 0.07 ± 0.00 f | |
| LLLGI | f 10–14 | 8 S globulin α isoform | 288.29 ± 6.87 f | −6.04 ± 4.1g | 2.75 ± 0.07 a | 5.09 ± 0.17 a | |
| f 8–12 | 8 S globulin β isoform | ||||||
| f 10–14 | β-conglycinin, β-chain like | ||||||
| LLLLG | f 9–13 | 8 S globulin α isoform | 940.71 ± 202.72 d | 10.47 ± 3.94 d | 0.86 ± 0.16 c | 0.89 ± 0.07 b | |
| f 9–13 | β-conglycinin, β-chain like | ||||||
| LLGIL | f 11–15 | 8 S globulin α isoform | 2139.68 ± 243.06 b | 81.27 ± 0.00 a | 0.37 ± 0.04 e | 0.36 ± 0.00 e | |
| f 9–13 | 8 S globulin β isoform | ||||||
| f 11–15 | β-conglycinin, β-chain like | ||||||
| AIVIL | f 312–316 | 8 S globulin α isoform | 1439.62 ± 35.57 c | 28.80 ± 0.56 c | 0.55 ± 0.01 d | 0.07 ± 0.00 f | |
| f 309–313 | 8 S globulin β isoform | ||||||
| f 312–316 | β-conglycinin, β-chain like | ||||||
| 785.500 | AQKIPAGT | f 136–143 | 8 S globulin α isoform | 353.62 ± 70.59 f | 4.03 ± 0.96 e | 1.55 ± 0.35 b | 0.57 ± 0.02 d |
| f 134–141 | 8 S globulin β isoform | ||||||
| f 136–143 | β-conglycinin, β-chain like | ||||||
| ILAGPTTI | f 99–106 | Mung bean seed albumin | 349.37 ± 48.91 f | −1.21 ± 0.17 g | 1.54 ± 0.20 b | 0.75 ± 0.11 c | |
| KKGVLGLA | f 188–195 | basic 7 S globulin 2-like | 310.92 ± 46.98 f | 1.01 ± 0.27 f | 1.73 ± 0.25 b | 0.58 ± 0.03 d | |
| RAILTLV | f 113–119 | 8 S globulin β isoform | 597.17 ± 65.06 e | 1.71 ± 3.66 f | 0.90 ± 0.10 c | 0.62 ± 0.01 d | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chunkao, S.; Youravong, W.; Yupanqui, C.T.; Alashi, A.M.; Aluko, R.E. Structure and Function of Mung Bean Protein-Derived Iron-Binding Antioxidant Peptides. Foods 2020, 9, 1406. https://doi.org/10.3390/foods9101406
Chunkao S, Youravong W, Yupanqui CT, Alashi AM, Aluko RE. Structure and Function of Mung Bean Protein-Derived Iron-Binding Antioxidant Peptides. Foods. 2020; 9(10):1406. https://doi.org/10.3390/foods9101406
Chicago/Turabian StyleChunkao, Siriporn, Wirote Youravong, Chutha T. Yupanqui, Adeola M. Alashi, and Rotimi E. Aluko. 2020. "Structure and Function of Mung Bean Protein-Derived Iron-Binding Antioxidant Peptides" Foods 9, no. 10: 1406. https://doi.org/10.3390/foods9101406
APA StyleChunkao, S., Youravong, W., Yupanqui, C. T., Alashi, A. M., & Aluko, R. E. (2020). Structure and Function of Mung Bean Protein-Derived Iron-Binding Antioxidant Peptides. Foods, 9(10), 1406. https://doi.org/10.3390/foods9101406