Cyanogenesis in Macadamia and Direct Analysis of Hydrogen Cyanide in Macadamia Flowers, Leaves, Husks, and Nuts Using Selected Ion Flow Tube–Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Buffer Preparation
2.3. Optimization of Heating Temperature and Heating Time
2.4. Optimization of pH-Buffering Solution
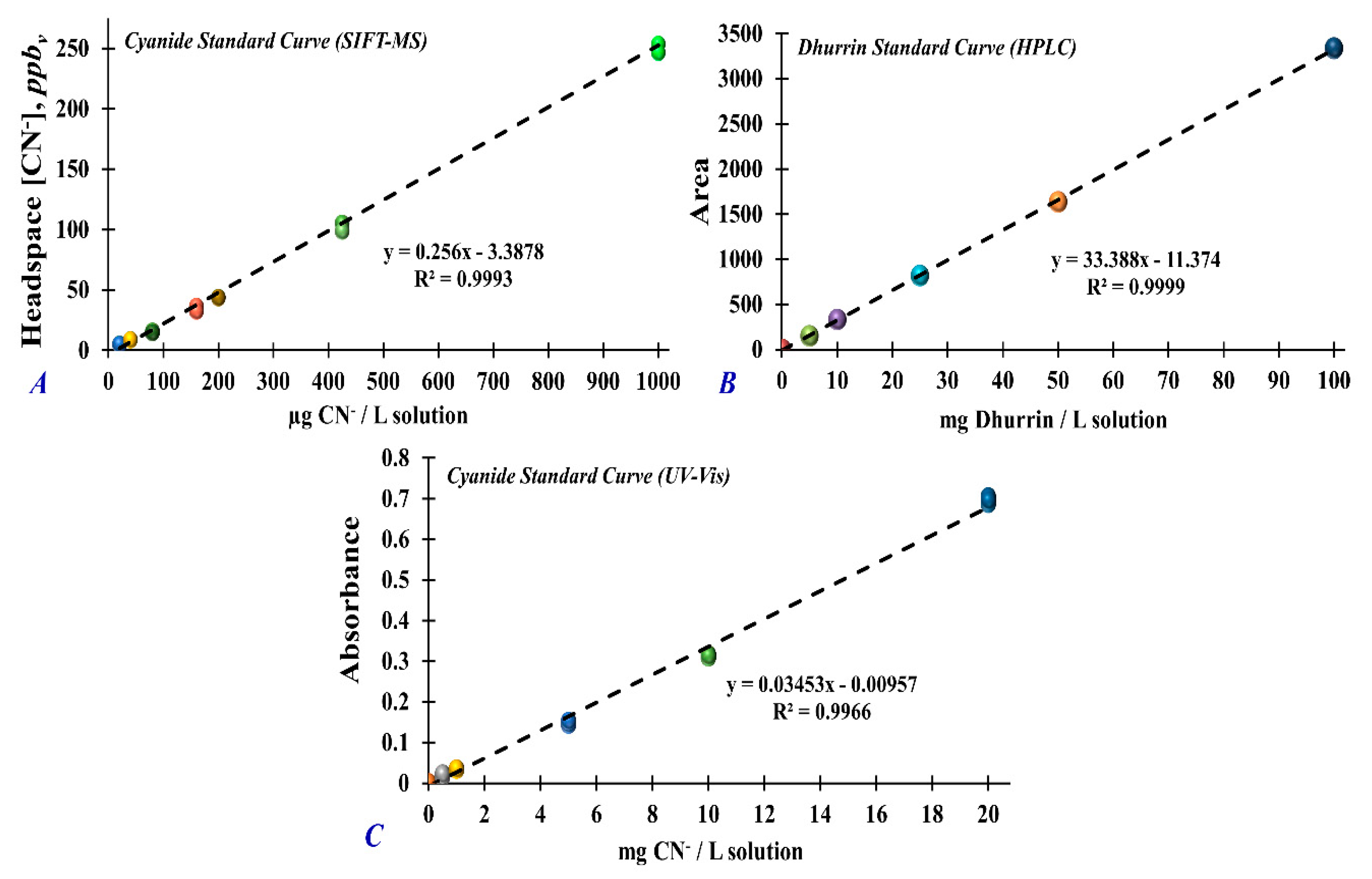
2.5. Headspace Cyanide Analysis Using SIFT-MS
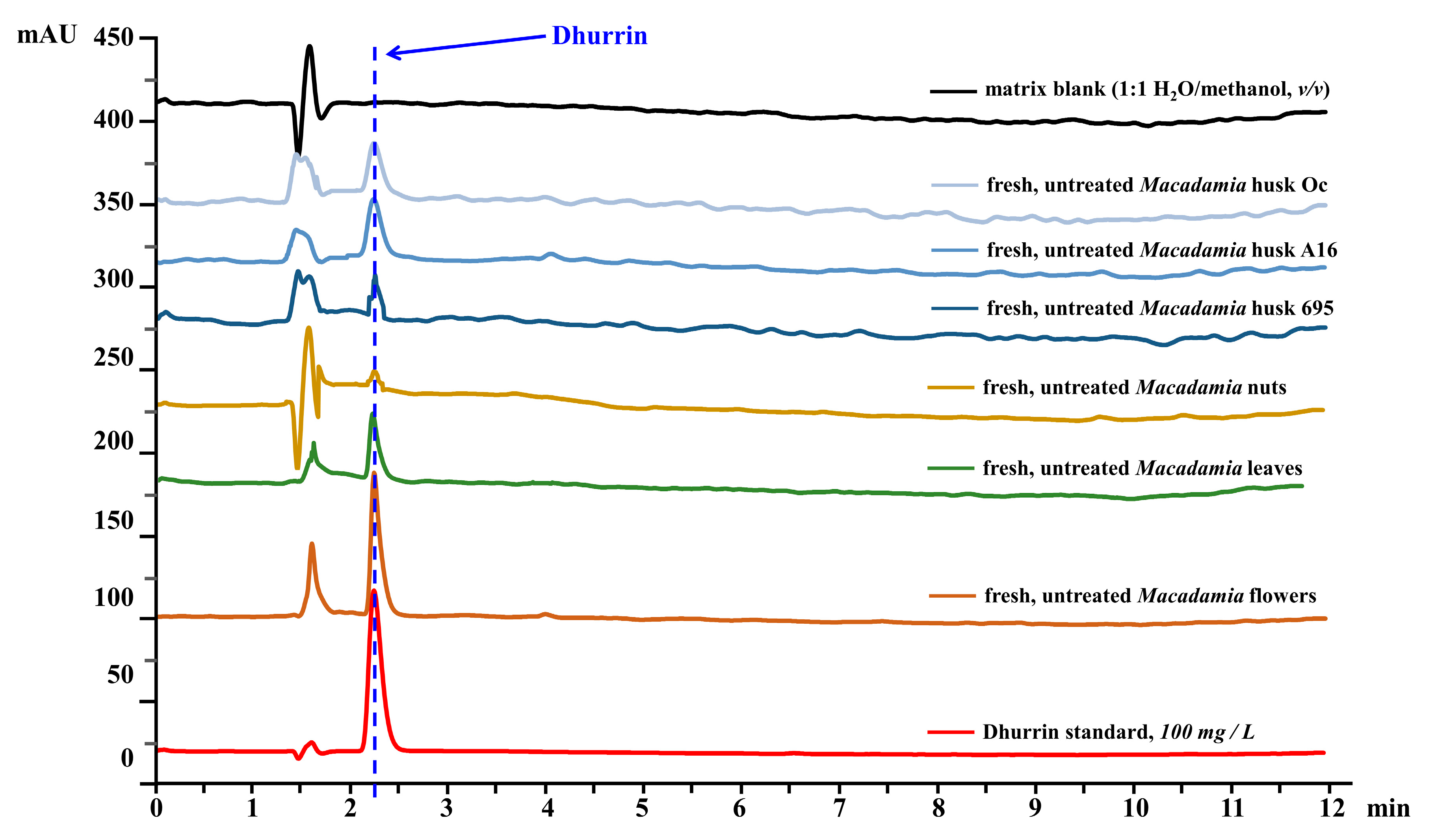
2.6. Dhurrin Analysis in Plant Extracts Using HPLC
2.7. Total Cyanide Analysis in Plant Extracts Using UV–Vis Spectrophotometer
2.8. Statistical Analysis
3. Results and Discussion
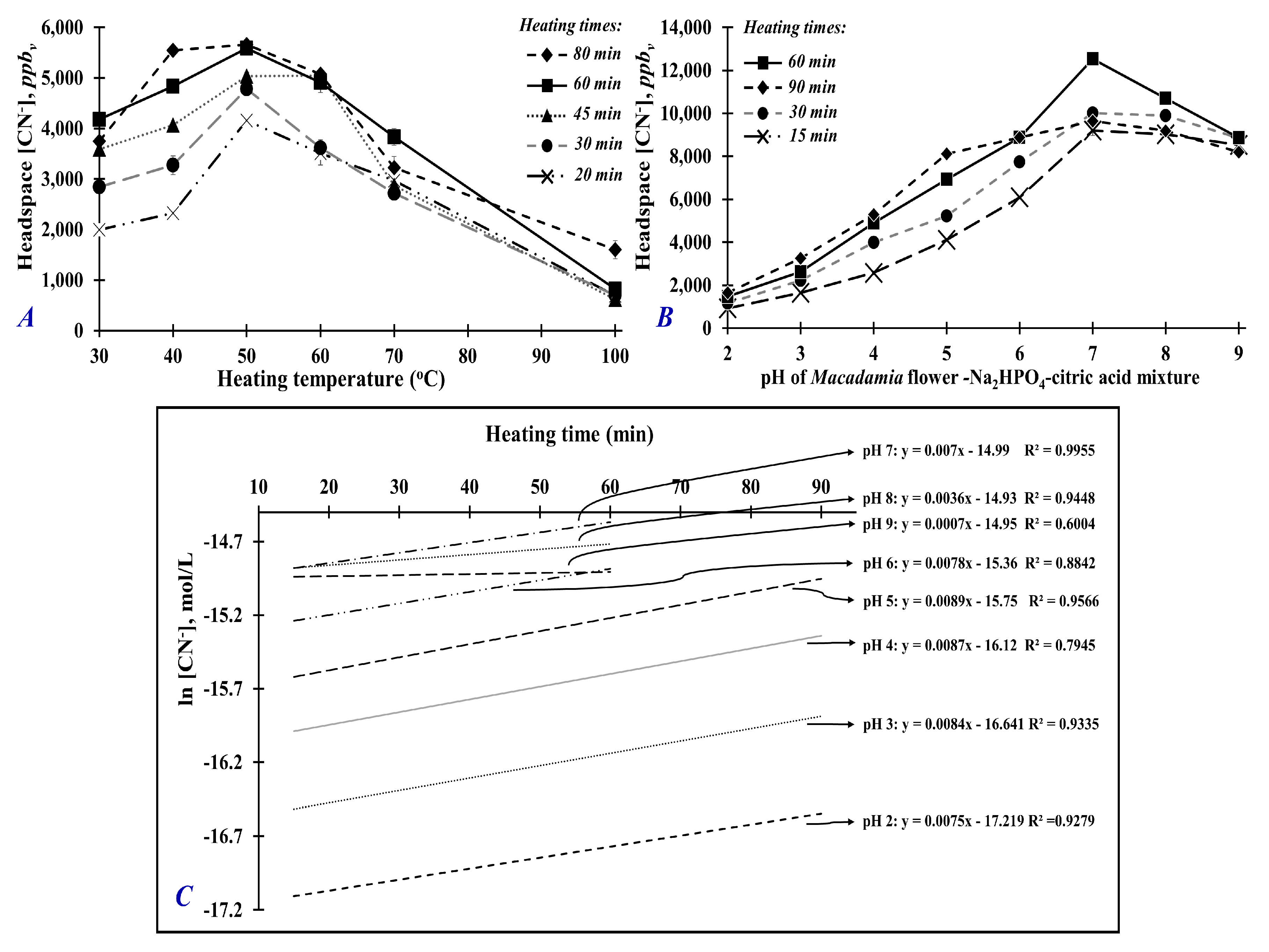
3.1. Optimization of Heating Temperature and Time for Maximum Generation of Hydrogen Cyanide
3.2. Optimization of Mixture’s pH for Maximum Generation of Hydrogen Cyanide
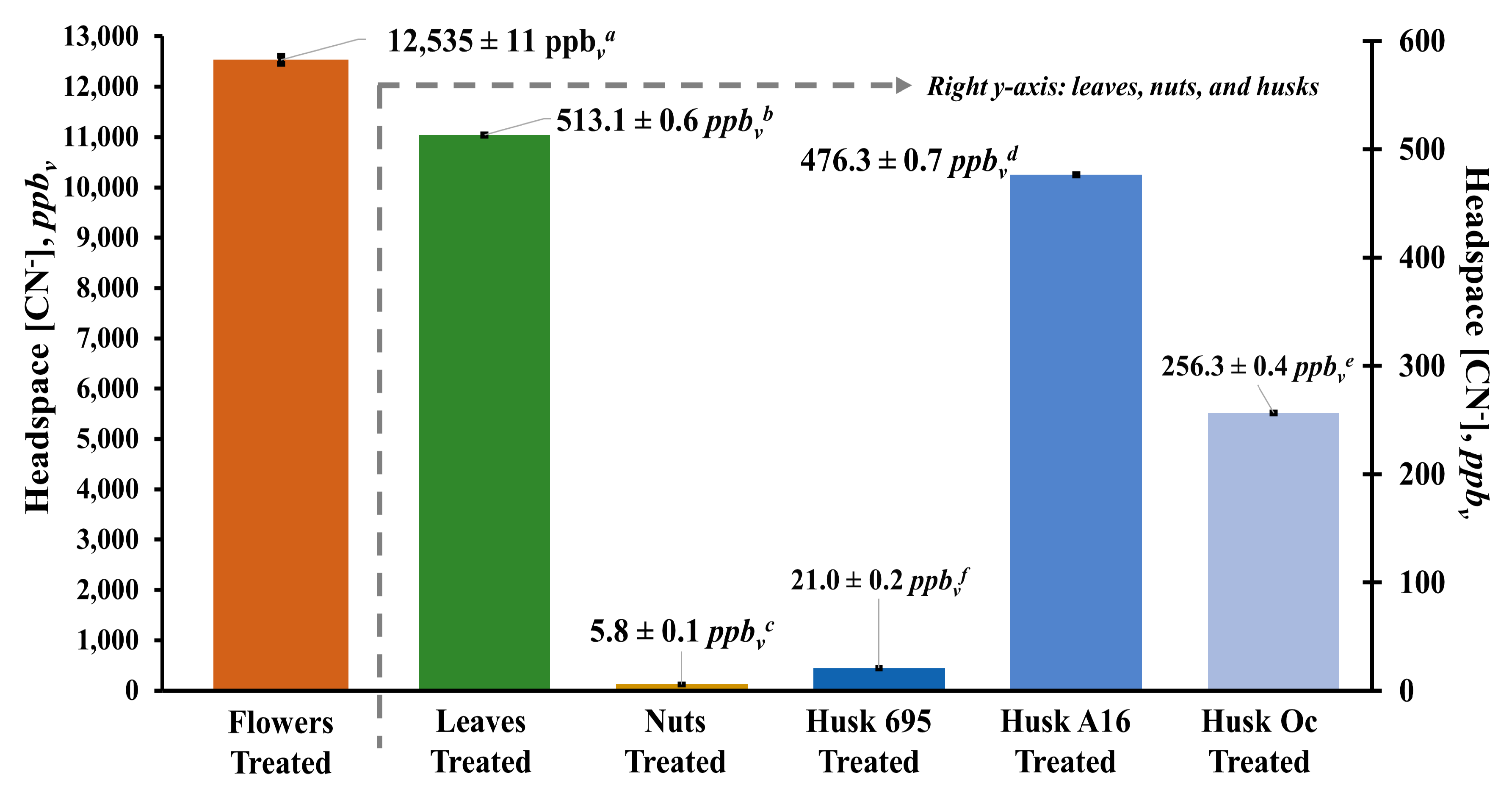
3.3. Headspace Concentration of Hydrogen Cyanide above Macadamia Flowers, Leaves, Nuts, and Husks
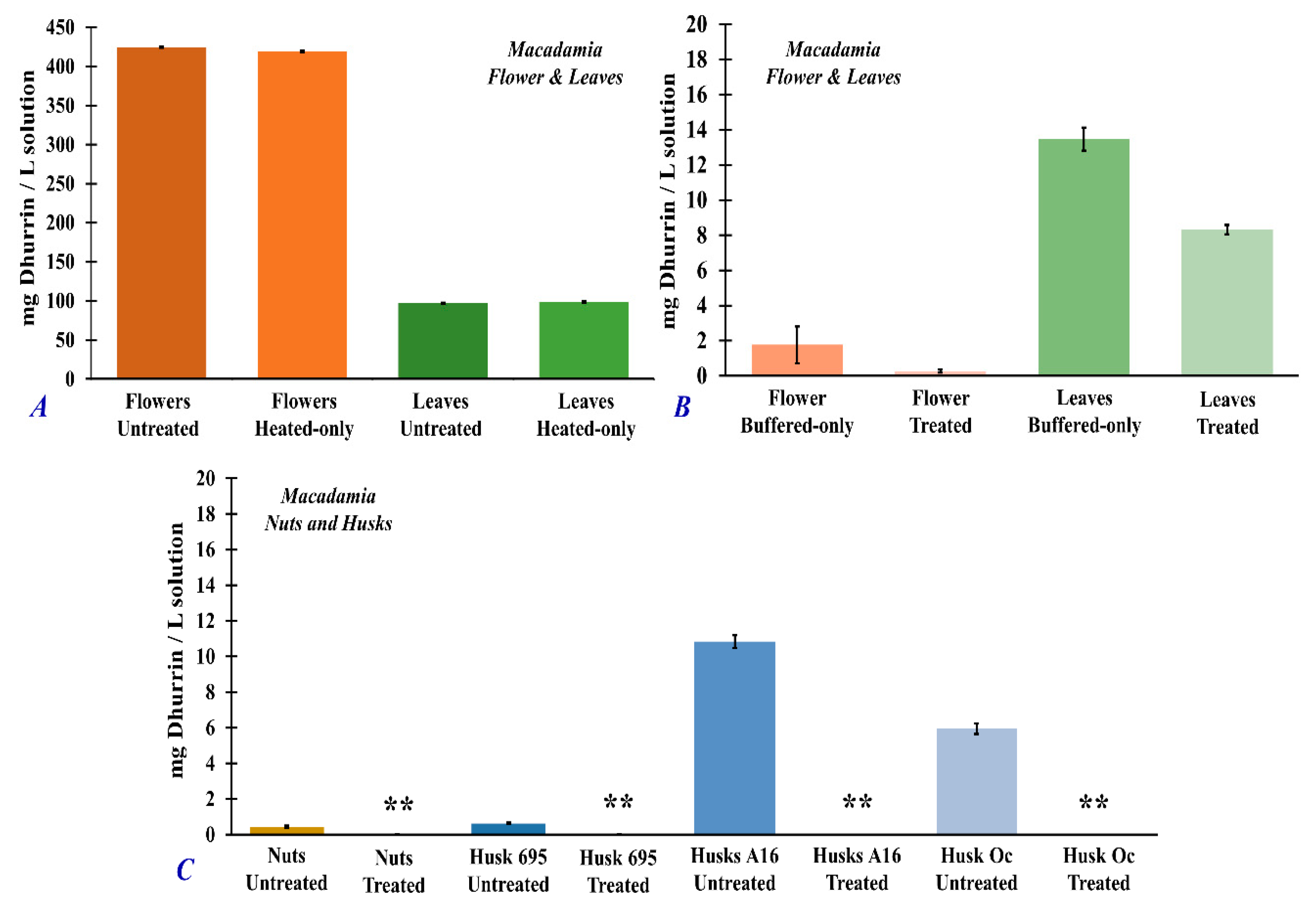
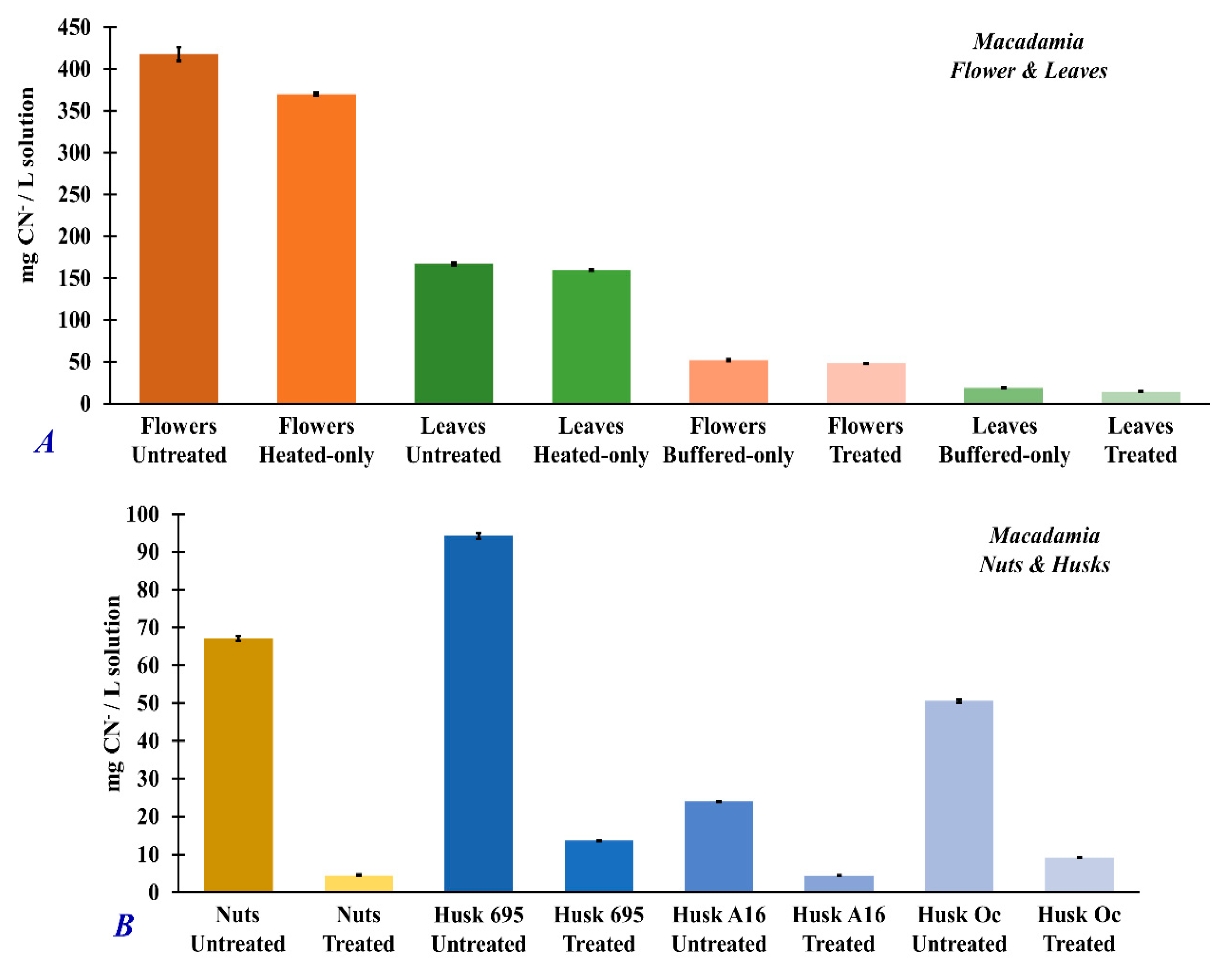
3.4. Dhurrin and Total Cyanide Concentrations in Untreated and Treated Macadamia Plant Part Extracts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quinn, L.A.; Tang, H.H. Antioxidant properties of phenolic compounds in Macadamia nuts. J. Am. Oil Chem. Soc. 1996, 73, 1585–1588. [Google Scholar] [CrossRef]
- Lim, T.K. Macadamia integrifolia. In Edible Medicinal and Non-Medicinal Plants; Lim, T.K., Ed.; Springer: Dordrecht, The Netherlands, 2013; Volume 5, pp. 494–501. [Google Scholar]
- Dailey, A.; Vuong, Q.V. Effect of extraction solvents on recovery of bioactive compound and antioxidant properties from Macadamia (Macadamia tetraphylla) skin waste. Cogent Food Agric. 2015, 1, 1115646. [Google Scholar] [CrossRef]
- Wood, L.G.; Garg, M.L. Macadamia nuts (M. Integrifolia and M. Tetraphylla) and their use in hypercholesterolemic subjects. In Nuts and Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Elsevier: London, UK, 2011; pp. 717–725. [Google Scholar]
- Aquino-Bolaños, E.N.; Mapel-Velazco, L.; Martin-del-Campo, S.T.; Chavez-Servia, J.L.; Martinez, A.J.; Verdalet-Guzman, I. Fatty acids profile of oil from nine varieties of Macadamia nut. Int. J. Food Prop. 2017, 20, 1262–1269. [Google Scholar] [CrossRef] [Green Version]
- Dahler, J.M.; McConchie, C.A.; Turnbull, C.G.N. Quantification of cyanogenic glycosides in seedlings of three Macadamia (Proteaceae) species. Aust. J. Bot. 1995, 43, 619–628. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Orfila, C.; Morgan, R.A. Amygdalin content of seeds, kernels, and food products commercially available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Poulton, J.E. Cyanogenesis in plants. Plant Physiol. 1990, 94, 401–405. [Google Scholar] [CrossRef] [Green Version]
- Møller, B.L. Functional diversifications of cyanogenic glucosides. Curr. Opin. Plant Biol. 2010, 13, 337–346. [Google Scholar] [CrossRef]
- Seigler, D.S. Cyanide and cyanogenic glycosides. In Herbivores: Their Interactions with Secondary Plant Metabolites; Rosenthal, G.A., Berenbaum, M.R., Eds.; Academic Press: San Diego, CA, USA, 1991; pp. 35–77. [Google Scholar]
- Miller, R.E.; Jensen, R.; Woodrow, I.E. Frequency of cyanogenesis in tropical rainforests of Far North Queensland, Australia. Ann. Bot. 2006, 97, 1017–1044. [Google Scholar] [CrossRef] [Green Version]
- Thayer, S.S.; Conn, E.E. Subcellular localization of dhurrin β-glucosidase and hydroxynitrile lyase in the mesophyll cells of Sorghum leaf blades. Plant Physiol. 1981, 67, 617–622. [Google Scholar] [CrossRef] [Green Version]
- Hickel, A.; Hasslacher, M.; Griengl, H. Hydroxynitrile lyases: Functions and properties. Physiol. Plant. 1996, 98, 891–898. [Google Scholar] [CrossRef]
- Halkier, B.A.; Møller, B.L. Biosynthesis of the cyanogenic glucoside dhurrin in seedlings of Sorghum bicolor (L.) Moench and partial purification of the enzyme system involved. Plant Physiol. 1989, 90, 1552–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conn, E.E. The metabolism of a natural product: Lessons learned from cyanogenic glycosides. Planta Med. 1991, 57, S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Nahrstedt, A. Cyanogenesis and Foodplants. In Phytochemistry and Agriculture; van Beek, T.A., Breteler, H., Eds.; Clarendon Press: Oxford, UK, 1993; pp. 107–129. [Google Scholar]
- Johansen, H.; Rasmussen, L.H.; Olsen, C.E.; Hansen, H.C.B. Rate of hydrolysis and degradation of the cyanogenic glycoside—Dhurrin—In soil. Chemosphere 2007, 67, 259–266. [Google Scholar] [CrossRef]
- Mao, C.-H.; Anderson, L. Partial purification and characterization of two β-glucosidases from Sorghum tissues. Phytochemistry 1967, 6, 473–483. [Google Scholar] [CrossRef]
- Egekeze, J.O.; Oehme, F.W. Cyanides and their toxicity. A literature review. Vet. Quart. 1980, 2, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Onojah, P.K.; Odin, E.M. Cyanogenic glycoside in food plants. Int. J. Innov. Sci. Math. 2015, 3, 2347–9051. [Google Scholar]
- Geller, R.J.; Barthold, C.; Saiers, J.A.; Hall, A.H. Pediatric cyanide poisoning: Causes, manifestations, management, and unmet needs. Rev. Pediatr. 2006, 118, 2146–2158. [Google Scholar] [CrossRef]
- Miller, R.E.; Tuck, K.L. The rare cyanogenic proteacin, and dhurrin, from foliage of Polyscias australiana, Araliaceae. Phytochemistry 2013, 93, 210–215. [Google Scholar] [CrossRef]
- Kriedemann, P.E. Cyanogenesis in Sorghum almum in relation to nitrogen and phosphorus nutrition. Aust. J. Exp. Agric. Anim. Husb. 1964, 4, 15–16. [Google Scholar] [CrossRef]
- Jones, D.A. Cyanogenic glycosides and their function. In Phytochemical Ecology; Harbone, J.B., Ed.; Academic Press: London, UK, 1972; pp. 103–124. [Google Scholar]
- Bradbury, J.H.; Egan, S.V.; Lynch, M.J. Analysis of cyanide in cassava using acid hydrolysis of cyanogenic glucosides. J. Sci. Food Agric. 1991, 55, 277–290. [Google Scholar] [CrossRef]
- Gleadow, R.M.; Woodrow, I.E. Temporal and spatial variation in cyanogenic glycosides in Eucalyptus cladoclyx. Tree Physiol. 2000, 20, 591–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Dasgupta, P.K. Recent developments in cyanide detection: A review. Anal. Chim. Acta 2010, 673, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, L.J.; Stuart, P.; Picmanova, M.; Rasmussen, S.; Olsen, C.E.; Harholt, J.; Møller, B.L.; Bjarnholt, N. Dhurrin metabolism in the developing grain of Sorghum bicolor (L.) Moench investigated by metabolite profiling and novel clustering analyses of time-resolved transcriptomic data. BMC Genom. 2016, 17, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castada, H.Z.; Barringer, S.B.; Wick, M. Gas-phase chemical ionization of 4-akyl branched-chain carboxylic acids and 3-methylindole using H3O+, NO+, and O2+ ions. Rapid Commun. Mass Spectrom. 2017, 31, 1641–1650. [Google Scholar] [CrossRef]
- Castada, H.Z.; Polentz, V.; Barringer, S.B.; Wick, M. Temperature-dependent Henry’s law constants of 4-alkyl-branched chain fatty acids and 3-methylindole in an oil-air matrix and analysis of volatile in lamb fat using selected ion flow tube mass spectrometry (SIFT-MS). Rapid Commun. Mass Spectrom. 2017, 31, 2135–2145. [Google Scholar] [CrossRef]
- Castada, H.Z.; Barringer, S.B. Online, real-time, and direct use of SIFT-MS to measure garlic breath deodorization: A Review. Flavour Fragr. J. 2019, 34, 299–306. [Google Scholar] [CrossRef]
- Castada, H.Z.; Hanas, K.; Barringer, S.B. Swiss cheese flavor variability based on correlations of volatile flavor compounds, descriptive sensory attributes, and consumer preference. (Special Issue—Cheese: Technology, Compositional, Physical and Biofunctional Properties). Foods 2019, 8, 78. [Google Scholar] [CrossRef] [Green Version]
- Castada, H.Z.; Sun, Z.; Barringer, S.B.; Huang, X. Thermal degradation of p-Hydroxybenzoic acid in macadamia nut oil, olive oil, and corn oil. J. Am. Oil Chem. Soc. 2020, in press. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Castada, H.Z.; Huang, X.; Barringer, S.B. Comparison of encapsulation of garlic oil with α-, β- and γ-cyclodextrin using Selected Ion Flow Tube-Mass Spectrometry (SIFT-MS). J. Food Process. Preserv. 2019, 43, e13865. [Google Scholar] [CrossRef]
- De Nicola, G.R.; Leoni, O.; Malaguti, L.; Bernardi, R.; Lazzeri, L. A simple analytical method for dhurrin content evaluation in cyanogenic plants for their utilization in fodder and biofumigation. J. Agric. Food Chem. 2011, 59, 8065–8069. [Google Scholar] [CrossRef]
- Sarkiyayi, S.; Agar, T.M. Comparative analysis on the nutritional and anti-nutritional contents of the sweet and bitter cassava varieties. Adv. J. Food Sci. Technol. 2010, 2, 328–334. [Google Scholar]
- Omar, N.F.; Hassan, S.A.; Yusoff, U.K.; Abdullah, N.A.P.; Wahab, P.E.M.; Sinniah, U.R. Phenolics, flavonoids, antioxidant activity and cyanogenic glycosides of organic and mineral-base fertilized cassava tubers. Molecules 2012, 17, 2378–2387. [Google Scholar] [CrossRef] [PubMed]
- Browne, R.W.; Whitcomb, B.W. Procedures for determination of detection limits: Application to high-performance liquid chromatography analysis of fat-soluble vitamins in human serum. Epidemiology 2010, 21, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantification of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Conn, E.E. Cyanogenic glycosides. J. Agric. Food Chem. 1969, 17, 519–526. [Google Scholar] [CrossRef]
- Conn, E.E. Cyanogenic compounds. Ann. Rev. Plant Physiol. 1980, 31, 433–451. [Google Scholar] [CrossRef]
- Davis, R.H. Cyanogens. In Toxic Substances in Crop Plants; D’Mello, J.P.F., Duffus, C.M., Duffus, J.H., Eds.; The Royal Society of Chemistry: Cambridge, UK, 1991; pp. 202–225. [Google Scholar]
- Ballhorn, D.J. Cyanogenic glycosides in nuts and seeds. In Nuts & Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Petel, V.B., Eds.; Elsevier Inc.: Oxford, UK, 2011; pp. 129–136. [Google Scholar]
- Newton, R.J.; Baltuskonis, D.A.; Goeschl, J.D.; Meckenstock, D.H.; Miller, F.R. Distribution and transformation of soluble carbohydrates during germination growth of sorghum. Crop Sci. 1980, 20, 265–268. [Google Scholar] [CrossRef]
- Lang, A.; Nitsch, J.P. Flower and fruit formation. In Differentiation and Development/Differenzierung und Entwicklung. Encyclopedia of Plant Physiology/Handbuch der Pflanzenphysiologie; Allsopp, A., Bloch, R., Bonner, J.T., Bopp, M., Brachet, J., Brown, R., Bunning, E., Buvat, R., Cantino, E.C., Champagnat, P., et al., Eds.; Springer: Berlin, Germany, 1965; pp. 1380–1647. [Google Scholar]
- Domingos, S.; Fino, J.; Paulo, O.S.; Oliveira, C.M.; Goulao, L.F. Molecular candidates for early-stage flower-to-fruit transition in stenospermocarpic table grape (Vitis vinifera L.) inflorescences ascribed by differential transcriptome and metabolome profiles. Plant Sci. 2016, 24, 40–56. [Google Scholar] [CrossRef] [Green Version]
- Borghi, M.; Fernie, A.R. Floral metabolism of sugars and amino acids: Implications for pollinators’ preferences and seed and fruit set. Plant Physiol. 2017, 175, 1510–1524. [Google Scholar] [CrossRef] [Green Version]
- Stafford, H.A. Changes in phenolic compounds & related enzymes in young plants of sorghum. Phytochemistry 1969, 8, 743–752. [Google Scholar]
- Bough, W.A.; Gander, J.E. Exogenous L-tyrosine metabolism and dhurrin turnover in sorghum seedlings. Phytochemistry 1971, 10, 67–77. [Google Scholar] [CrossRef]
- Dement, W.A.; Mooney, H.A. Seasonal variation in the production of tannins and cyanogenic glucosides in the chaparral shrub, Heteromeles arbutifolia. Oecologia 1974, 15, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Akinsanmi, O.A.; Drenth, A. Characterisation of husk rot in Macadamia. Ann. Appl. Biol. 2017, 170, 104–115. [Google Scholar] [CrossRef]
- Lamont, B.B. Injury-induced cyanogenesis in vegetative and reproductive parts of two Grevillea species and their F1 hybrid. Ann. Bot. 1993, 71, 537–542. [Google Scholar] [CrossRef]
| pH of Macadamia Flower–Na2HPO4–Citric Acid Buffered Mixture | Empirical Rate of [HCN] Production at 50 °C (d[HCN]/dt), M·L−1·min−1 |
|---|---|
| 2 | 4.61 × 10−10 ± 0.2 × 10−10 |
| 3 | 8.25 × 10−10 ± 0.2 × 10−10 |
| 4 | 1.95 × 10−09 ± 0.07 × 10−09 |
| 5 | 2.36 × 10−09 ± 0.08 × 10−09 |
| 6 | 2.34 × 10−09 ± 0.08 × 10−09 |
| 7 | 2.79 × 10−10 ± 2 × 10−10 |
| 8 | 1.40 × 10−09 ± 0.2 × 10−09 |
| 9 | 2.78 × 10−10 ± 4 × 10−10 |
| Samples | mg Dhurrin/g Sample | % Treatment Efficiency | Total Cyanide (mg/g sample) | % Treatment Efficiency |
|---|---|---|---|---|
| Macadamia Flower Untreated | 37.14 (±0.05) | --- | 4.18 (±8.0 × 10−2) | --- |
| Flowers (heated only) | 36.68 (±0.09) | 1.2 (±0.5) | 3.70 (±7.5 × 10−3) | 11.5 (±0.2) |
| Flowers (buffered only) | 0.15 (±0.09) | 99.6 (±0.5) | 0.52 (±1.1 × 10−2) | 87.6 (±2.9) |
| Flowers Treated | 0.02 (±0.09) | 99.9 (±0.5) | 0.48 (±3.6 × 10−4) | 88.5 (±2.9) |
| Macadamia Leaves Untreated | 8.50 (±0.05) | --- | 1.67 (±1.8 × 10−2) | --- |
| Leaves (heated only) | 8.60 (±0.09) | 1.2 (±0.5) | 1.59 (±7.5 × 10−3) | 4.6 (±0.2) |
| Leaves (buffered only) | 1.18 (±0.06) | 86.1 (±0.3) | 0.19 (±3.3 × 10−3) | 88.7 (±1.6) |
| Leaves Treated | 0.73 (±0.09) | 93.1 (±0.5) | 0.15 (±1.6 × 10−3) | 91.3 (±1.6) |
| Macadamia Nuts Untreated | 0.04 (±0.07) | --- | 0.67 (±6.3 × 10−3) | --- |
| Nuts Treated | 0.00 (±0.09) | 100.0 (±0.5) | 0.05 (±2.2 × 10−3) | 93.2 (±1.4) |
| Macadamia Husks 695 Untreated | 0.05 (±0.07) | --- | 0.94 (±7.4 × 10−3) | --- |
| Husks 695 Treated | 0.00 (±0.09) | 100.0 (±0.5) | 0.14 (±2.9 × 10−4) | 85.5 (±1.2) |
| Macadamia Husks A16 Untreated | 0.97 (±0.07) | --- | 0.24 (±1.2 × 10−3) | --- |
| Husks A16 Treated | 0.00 (±0.09) | 100.0 (±0.5) | 0.04 (±3.6 × 10−4) | 81.3 (±0.9) |
| Macadamia Husks Oc Untreated | 0.53 (±0.07) | --- | 0.51 (±4.1 × 10−3) | --- |
| Husks Oc Treated | 0.00 (±0.09) | 100.0 (±0.5) | 0.09 (±3.1 × 10−4) | 81.7 (±1.3) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castada, H.Z.; Liu, J.; Ann Barringer, S.; Huang, X. Cyanogenesis in Macadamia and Direct Analysis of Hydrogen Cyanide in Macadamia Flowers, Leaves, Husks, and Nuts Using Selected Ion Flow Tube–Mass Spectrometry. Foods 2020, 9, 174. https://doi.org/10.3390/foods9020174
Castada HZ, Liu J, Ann Barringer S, Huang X. Cyanogenesis in Macadamia and Direct Analysis of Hydrogen Cyanide in Macadamia Flowers, Leaves, Husks, and Nuts Using Selected Ion Flow Tube–Mass Spectrometry. Foods. 2020; 9(2):174. https://doi.org/10.3390/foods9020174
Chicago/Turabian StyleCastada, Hardy Z., Jinyi Liu, Sheryl Ann Barringer, and Xuesong Huang. 2020. "Cyanogenesis in Macadamia and Direct Analysis of Hydrogen Cyanide in Macadamia Flowers, Leaves, Husks, and Nuts Using Selected Ion Flow Tube–Mass Spectrometry" Foods 9, no. 2: 174. https://doi.org/10.3390/foods9020174
APA StyleCastada, H. Z., Liu, J., Ann Barringer, S., & Huang, X. (2020). Cyanogenesis in Macadamia and Direct Analysis of Hydrogen Cyanide in Macadamia Flowers, Leaves, Husks, and Nuts Using Selected Ion Flow Tube–Mass Spectrometry. Foods, 9(2), 174. https://doi.org/10.3390/foods9020174






