Influence of Protein Type on the Antimicrobial Activity of LAE Alone or in Combination with Methylparaben
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Stock Solutions
2.3. Cultivation of Bacteria
2.4. Preparation of the (Modified) Model Systems
2.5. Determination of Viable Cell Counts and Minimal Lethal Concentrations
2.6. Characterization of Interactions Between Proteins and LAE
2.7. Statistical Analysis
3. Results and Discussion
3.1. Impact of Protein Interactions on the Antimicrobial Efficacy of LAE or Methylparaben
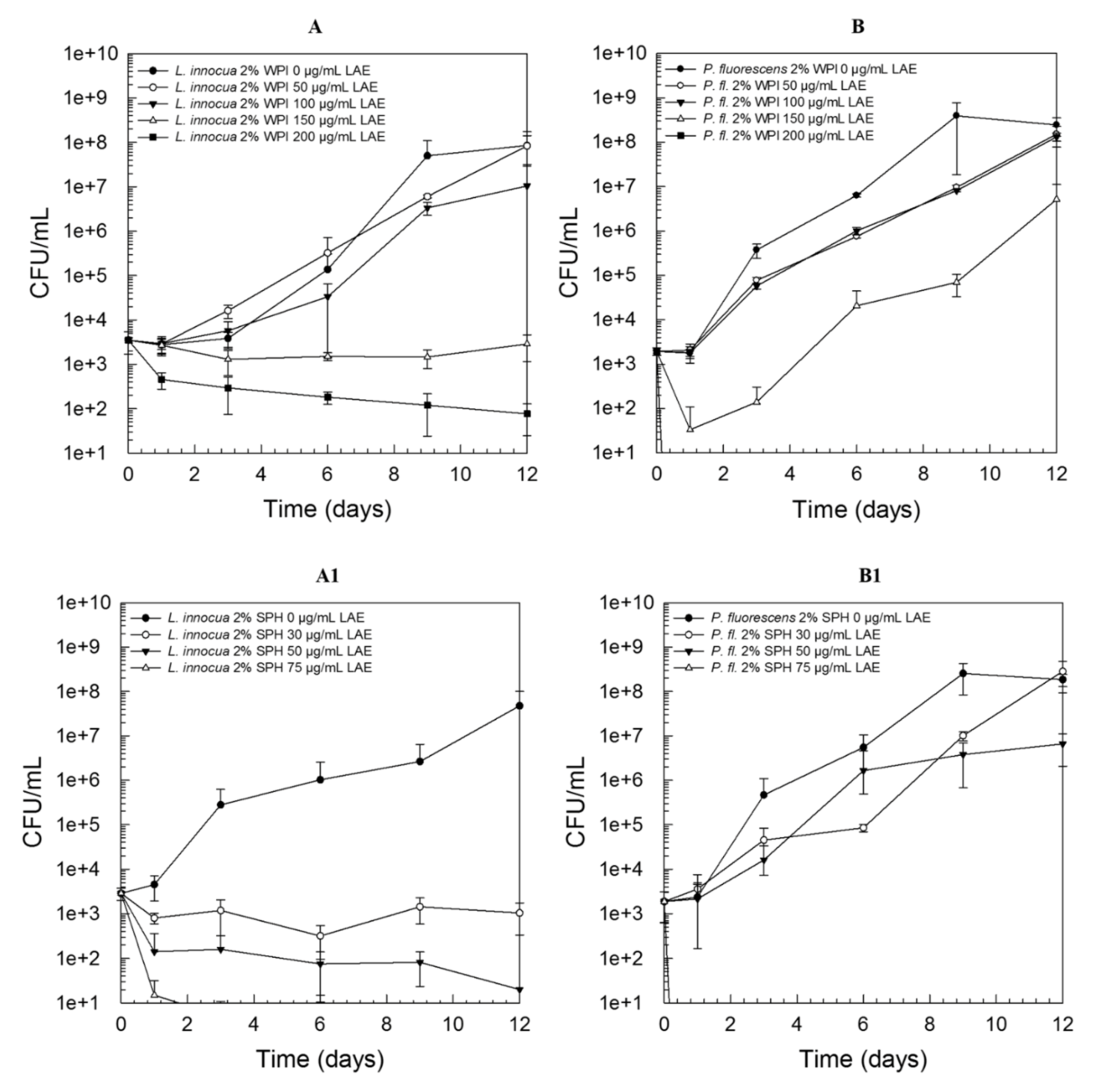
3.2. Solution Behaviour of Combinations of LAE and Proteins
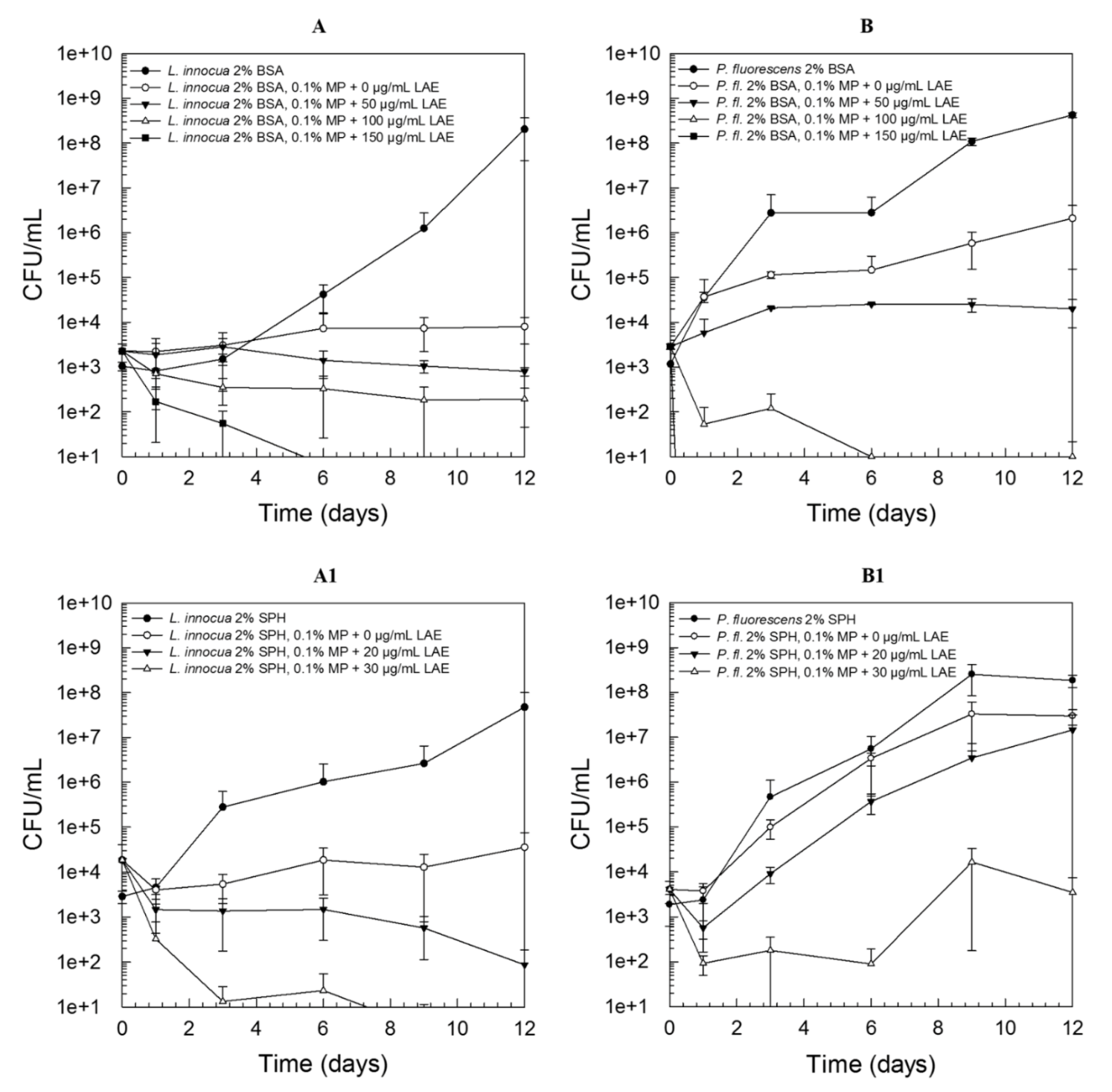
3.3. Combination of Amphiphilic Cationic LAE with Hydrophobic Methylparaben
3.4. Mechanistic Insights
- (i)
- Due to its positively charged head group, LAE easily forms complexes with negatively charged proteins, whereas methylparaben is not involved in complex formations but may be able to slightly interact with hydrophobic regions of the proteins. However, in the case of globular proteins, these regions are predominantly presented in the protein core to guarantee sufficient water solubility.
- (ii)
- With increasing LAE concentrations, sufficient high numbers of cationic LAE molecules (possibly in the form of micelles) bind to the proteins causing a shift from a highly negative charged complex to a (more) positively charged one. Even though, LAE molecules (micelles) are bound to the complexes, the availability of free positively charged LAE head groups or even free LAE molecules (micelles) enables now electrostatic interactions with bacterial cells.
- (iii)
- There are some advantages resulting from the combined application of LAE and methylparaben. In the case of Gram-positive bacteria, both components attack the cytoplasmic membrane and other targets at the microbial cell, such as key enzyme systems. This multi-target attack causes several damages at the microbial cell and therefore minimal lethal concentrations were found to be very low. In the case of Gram-negative bacteria, both components are known to initially interact with the outer membrane. Methylparaben was reported to cause the same effects as Gram-positive bacteria, however due to an initial binding in the outer membrane the physical uptake needed to cause an effect at the cytoplasmic membrane may take longer. In the case of LAE, the main antimicrobial target is the outer membrane, leading to an alteration of cell integrity due to morphological changes through to disruption. Therefore, methylparaben uptake may be facilitated.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Banerjee, M.; Sarkar, P.K. Antibiotic resistance and susceptibility to some food preservative measures of spoilage and pathogenic microorganisms from spices. Food Microbiol. 2004, 21, 335–342. [Google Scholar] [CrossRef]
- Davidson, P.M.; Harrison, M.A. Resistance and adaptation to food antimicrobials, sanitizers, and other process controls. Food Technol. 2002, 56, 69–78. [Google Scholar]
- Loeffler, M.; Beiser, S.; Suriyarak, S.; Gibis, M.; Weiss, J. Antimicrobial efficacy of emulsified essential oil components against weak acid adapted spoilage yeasts in clear and cloudy apple juice. J. Food Prot. 2014, 77, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Suksathit, S.; Tangwatcharin, P. Activity of organic acid salts in combination with Lauric arginate against Listeria monocytogenes and Salmonella Rissen. Sci. Asia 2013, 39, 346–355. [Google Scholar] [CrossRef] [Green Version]
- Becerril, R.; Manso, S.; Nerin, C.; Gómez-Lus, R. Antimicrobial activity of Lauroyl Arginate Ethyl (LAE), against selected food-borne bacteria. Food Control 2013, 32, 404–408. [Google Scholar] [CrossRef]
- Ma, Q.; Davidson, P.M.; Zhong, Q. Properties and potential food applications of Lauric arginate as a cationic antimicrobial. Int. J. Food Microbiol. 2020, 315, 108417. [Google Scholar] [CrossRef]
- Bonnaud, M.; Weiss, J.; McClements, D.J. Interaction of a food-grade cationic surfactant (Lauric arginate) with food-grade biopolymers (pectin, carrageenan, xanthan, alginate, dextran, and chitosan). J. Agric. Food Chem. 2010, 58, 9770–9777. [Google Scholar] [CrossRef]
- Loeffler, M.; McClements, D.J.; McLandsborough, L.; Terjung, N.; Chang, Y.; Weiss, J. Electrostatic interactions of cationic Lauric arginate with anionic polysaccharides affect antimicrobial activity against spoilage yeasts. J. Appl. Microbiol. 2014, 117, 28–39. [Google Scholar] [CrossRef] [Green Version]
- Terjung, N.; Loeffler, M.; Gibis, M.; Salminen, H.; Hinrichs, J.; Weiss, J. Impact of Lauric arginate application form on its antimicrobial activity in meat emulsions. Food Biophys. 2014, 9, 88–98. [Google Scholar] [CrossRef]
- Leistner, L. Basic aspects of food preservation by hurdle technology. Int. J. Food Microbiol. 2000, 55, 181–186. [Google Scholar] [CrossRef]
- Hilbig, J.; Ma, Q.; Davidson, P.M.; Weiss, J.; Zhong, Q. Physical and antimicrobial properties of cinnamon bark oil co-nanoemulsified by Lauric arginate and Tween 80. Int. J. Food Microbiol. 2016, 233, 52–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manrique, Y.; Gibis, M.; Schmidt, H.; Weiss, J. Influence of application sequence and timing of eugenol and lauric arginate (LAE) on survival of spoilage organisms. Food Microbiol. 2017, 64, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Soni, M.G.; Carabin, I.G.; Burdock, G.A. Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem. Toxicol. 2005, 43, 985–1015. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.M.; Sofos, J.N.; Branen, A.L. (Eds.) Antimicrobials in Food, 3rd ed.; Taylor & Francis: Boca Raton, FL, USA, 2005; p. 706. [Google Scholar]
- Magrinyà, N.; Terjung, N.; Loeffler, M.; Gibis, M.; Bou, R.; Weiss, J. Influence of fat addition on the antimicrobial activity of sodium lactate, Lauric arginate and methylparaben in minced meat. Int. J. Food Microbiol. 2015, 215, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.M.; Taylor, T.M.; Schmidt, S.E. Chemical preservatives and natural antimicrobial compounds. In Food Microbiology: Fundamentals and Frontiers, 4th ed.; American Society for Microbiology: Washington, DC, USA, 2013; pp. 765–801. [Google Scholar]
- Fukahori, M.; Akatsu, S.; Sato, H.; Yotsuyanagi, T. Relationship between uptake of p-hydroxybenzoic acid esters by Escherichia coli and antibacterial activity. Chem. Pharm. Bull. 1996, 44, 1567–1570. [Google Scholar] [CrossRef]
- Soni, M.G.; Taylor, S.L.; Greenberg, N.A.; Burdock, G.A. Evaluation of the health aspects of methyl paraben: A review of the published literature. Food Chem. Toxicol. 2002, 40, 1335–1373. [Google Scholar] [CrossRef]
- Lund, B.; Baird-Parker, A.C.; Baird-Parker, T.C.; Gould, G.W. Microbiological Safety and Quality of Food, 1st ed.; US 978-0-8342-1323-4, Springer: Berlin/Heidelberg, Germany, 2000; p. 2024. [Google Scholar]
- Rodriguez, E.; Seguer, J.; Rocabayera, X.; Manresa, A. Cellular effects of monohydrochloride of l-arginine, Nalpha-lauroyl ethylester (LAE) on exposure to Salmonella Typhimurium and Staphylococcus aureus. J. Appl. Microbiol. 2004, 96, 903–912. [Google Scholar] [CrossRef]
- Pattanayaiying, R.; H-Kittikun, A.; Cutter, C.N. Effect of Lauric arginate, nisin Z, and a combination against several food-related bacteria. Int. J. Food Microbiol. 2014, 188, 135–146. [Google Scholar] [CrossRef]
- Down, S. What Exactly Is in Soybean Protein Hydrolysate? Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2014; Available online: www.spectroscopynow.com (accessed on 28 February 2020).
- Jimenez-Ruiz, E.I.; Calderon de la Barca, A.M.; Sotelo-Mundo, R.R.; Arteaga-Mackinney, G.E.; Valenzuela-Melendez, M.; Pena-Ramos, E.A. Partial characterization of ultrafiltrated soy protein hydrolysates with antioxidant and free radical scavenging activities. J. Food Sci. 2013, 78, C1152–C1158. [Google Scholar] [CrossRef]
- Balkani, S.; Shamekhi, S.; Raoufinia, R.; Parvan, R.; Abdolalizadeh, J. Purification and characterization of bovine serum albumin using chromatographic method. Adv. Pharm. Bull. 2016, 6, 651–654. [Google Scholar] [CrossRef] [Green Version]
- Madureira, A.R.; Pereira, C.I.; Gomes, A.M.P.; Pintado, M.E.; Xavier Malcata, F. Bovine whey proteins—Overview on their main biological properties. Food Res. Int. 2007, 40, 1197–1211. [Google Scholar] [CrossRef]
- Weiss, J.; Loeffler, M.; Terjung, N. The antimicrobial paradox: Why preservatives loose activity in foods. Curr. Opin. Food Sci. 2015, 4, 69–75. [Google Scholar] [CrossRef]
- Kun, R.; Kis, L.; Dékány, I. Hydrophobization of bovine serum albumin with cationic surfactants with different hydrophobic chain length. Colloids Surf. B Biointerfaces 2010, 79, 61–68. [Google Scholar] [CrossRef]
- Miller, R.; Alahverdjieva, V.S.; Fainerman, V.B. Thermodynamics and rheology of mixed protein–surfactant adsorption layers. Soft Matter 2008, 4, 1141–1146. [Google Scholar] [CrossRef]
- Otzen, D.E.; Sehgal, P.; Westh, P. α-Lactalbumin is unfolded by all classes of surfactants but by different mechanisms. J. Colloid Interface Sci. 2009, 329, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Otzen, D. Protein–surfactant interactions: A tale of many states. Biochim. Et Biophys. Acta BBA Proteins Proteom. 2011, 1814, 562–591. [Google Scholar] [CrossRef]
- Close, J.A.; Neilsen, P.A. Resistance of a strain of Pseudomonas cepacia to esters of p-hydroxybenzoic acid. Appl. Environ. Microbiol. 1976, 31, 718–722. [Google Scholar] [CrossRef] [Green Version]
- Bargiota, E.; Rico-Munoz, E.; Davidson, P.M. Lethal effect of methyl and propyl parabens as related to Staphylococcus aureus lipid composition. Int. J. Food Microbiol. 1987, 4, 257–266. [Google Scholar] [CrossRef]
- Lingbeck, J.M.; Cordero, P.; O’Bryan, C.A.; Johnson, M.G.; Ricke, S.C.; Crandall, P.G. Temperature effects on the antimicrobial efficacy of condensed smoke and Lauric arginate against Listeria and Salmonella. J. Food Prot. 2014, 77, 934–940. [Google Scholar] [CrossRef]
- Razavilar, V.; Genigeorgis, C. Prediction of Listeria spp. growth as affected by various levels of chemicals, pH, temperature and storage time in a model broth. Int. J. Food Microbiol. 1998, 40, 149–157. [Google Scholar] [CrossRef]
- Dai, Y.; Normand, M.D.; Weiss, J.; Peleg, M. Modeling the efficacy of triplet antimicrobial combinations: Yeast suppression by Lauric arginate, cinnamic acid, and sodium benzoate or potassium sorbate as a case study. J. Food Prot. 2010, 73, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Davidson, P.M.; Zhong, Q. Antimicrobial properties of Lauric arginate alone or in combination with essential oils in tryptic soy broth and 2% reduced fat milk. Int. J. Food Microbiol. 2013, 166, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Davidson, P.M.; Critzer, F.; Zhong, Q. Antimicrobial activities of Lauric arginate and cinnamon oil combination against foodborne pathogens: Improvement by ethylenediaminetetraacetate and possible mechanisms. LWT Food Sci. Technol. 2016, 72, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Clare, B.; Guo, W.; Martinac, B. The effects of parabens on the mechanosensitive channels of E. coli. Eur. Biophys. J. 2005, 34, 389–395. [Google Scholar] [CrossRef]
- PubChem Compound Database. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/7456 (accessed on 28 February 2020).
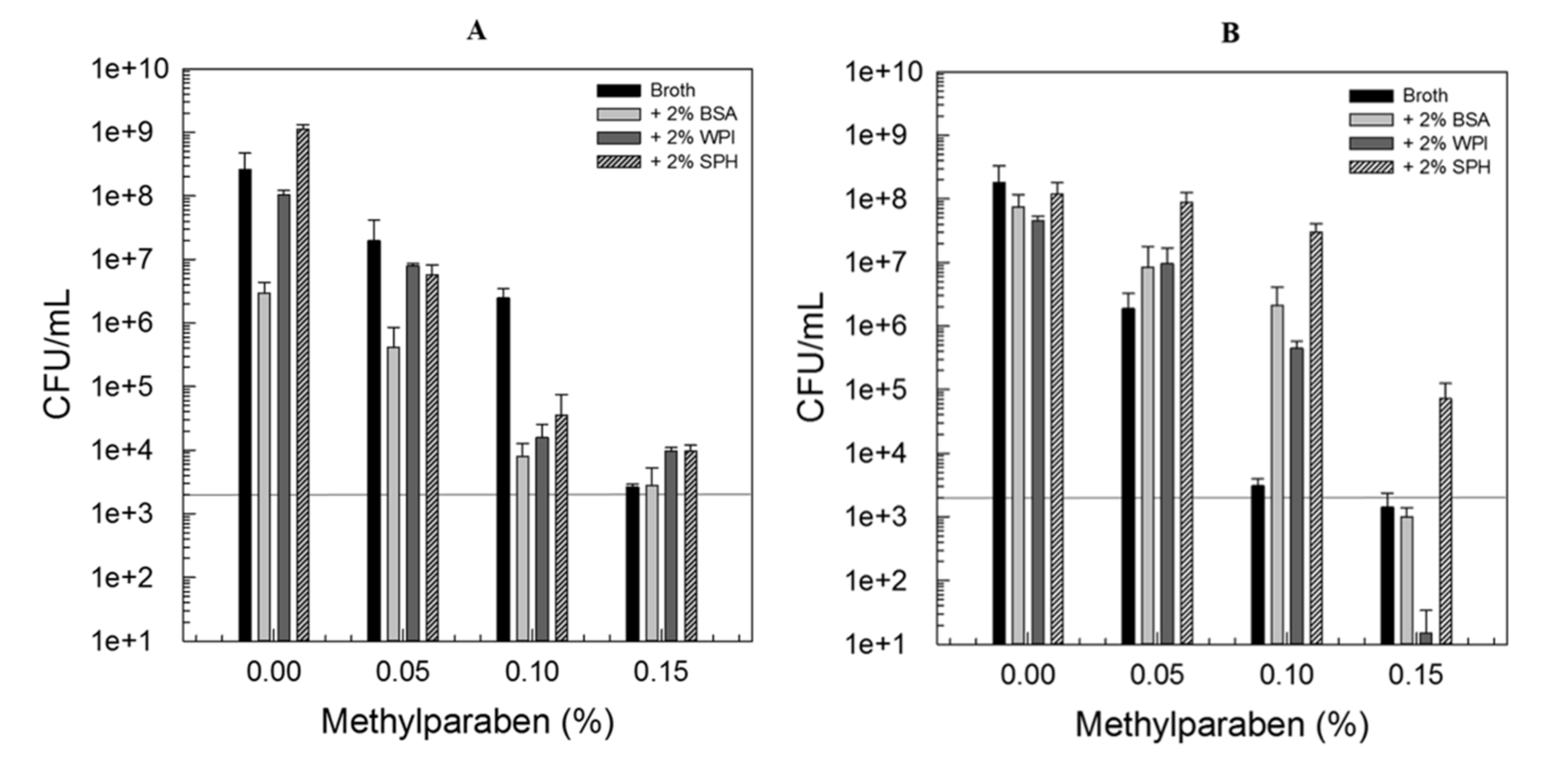



| 0% Methylparaben LAE (µg/mL) | 0.1% Methylparaben LAE (µg/mL) | |
|---|---|---|
| L. innocua LTH3096 | ||
| Nutrient broth | 20 A | |
| 2% BSA | 300 a,B | 175 b,A |
| 2% WPI | 250 a,C | 175 b,A |
| 2% SPH | 75 a,D | 35 ± 5 b,B |
| P. fluorescens DSM50091 | ||
| Nutrient broth | 15 A | |
| 2% BSA | 200 a,B | 125 b,A |
| 2% WPI | 175 a,C | 175 a,B |
| 2% SPH | 75 a,D | 50 b,C |
| BSA | WPI | SPH | |
|---|---|---|---|
| Isoelectric point | 4.7 | 4.5–5.5 | 4.5–5.5 |
| Type of protein | globular | globular | hydrolysate |
| Molecular weight | 66.400 kDa | β-lactoglobulin: 18,277 kDa α-lactalbumin: 14,175 kDa | Mixture of oligopeptides (di-tri-tetrapeptides), free amino acids MW << globular proteins |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loeffler, M.; Schwab, V.; Terjung, N.; Weiss, J.; McClements, D.J. Influence of Protein Type on the Antimicrobial Activity of LAE Alone or in Combination with Methylparaben. Foods 2020, 9, 270. https://doi.org/10.3390/foods9030270
Loeffler M, Schwab V, Terjung N, Weiss J, McClements DJ. Influence of Protein Type on the Antimicrobial Activity of LAE Alone or in Combination with Methylparaben. Foods. 2020; 9(3):270. https://doi.org/10.3390/foods9030270
Chicago/Turabian StyleLoeffler, Myriam, Verena Schwab, Nino Terjung, Jochen Weiss, and D. Julian McClements. 2020. "Influence of Protein Type on the Antimicrobial Activity of LAE Alone or in Combination with Methylparaben" Foods 9, no. 3: 270. https://doi.org/10.3390/foods9030270
APA StyleLoeffler, M., Schwab, V., Terjung, N., Weiss, J., & McClements, D. J. (2020). Influence of Protein Type on the Antimicrobial Activity of LAE Alone or in Combination with Methylparaben. Foods, 9(3), 270. https://doi.org/10.3390/foods9030270






