Assessment of Volatiles and Polyphenol Content, Physicochemical Parameters and Antioxidant Activity in Beers with Dotted Hawthorn (Crataegus punctata)
Abstract
1. Introduction
2. Materials and Research Methods
2.1. Reagents and Standards
2.2. Biological Material
2.3. Research Material
2.4. Brewing Technology
2.5. Physicochemical Parameters
2.6. Determination of Carbohydrate Profile, Ethanol and Glycerol Content
2.7. Analysis of Total Polyphenol Content and Antioxidative Activity
2.7.1. Analysis of Total Polyphenol Content
2.7.2. Free-Radical-Scavenging Ability by the Use of a DPPH• Radical Assay
2.7.3. Ferric Reducing/Antioxidant Power (FRAP) Assay
2.7.4. Free-Radical-Scavenging Ability by the Use of an ABTS+• Radical Cation
2.8. Adsorption of Volatile Compounds Using Solid Phase Microextraction (SPME)
2.9. Gas Chromatography and Mass Spectrometry of Compounds Adsorbed on Fibre
2.10. Sensory Analysis
2.11. Data Analysis
3. Results and Discussion
3.1. Concentration of Carbohydrates, Glycerol, Ethanol and pH Value of Tested Beers
3.2. Concentration of Total Polyphenols and Antioxidative Activity
3.3. Concentration of Volatile Compounds in Tested Beers
3.4. Organoleptic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Callemien, D.; Collin, S. Structure, Organoleptic Properties, Quantification Methods, and Stability of Phenolic Compounds in Beer—A Review. Food Rev. Int. 2009, 26, 1–84. [Google Scholar] [CrossRef]
- Habschied, K.; Lončarić, A.; Mastanjević, K. Screening of Polyphenols and Antioxidative Activity in Industrial Beers. Foods 2020, 9, 238. [Google Scholar] [CrossRef] [PubMed]
- Koren, D.; Kun, S.; Hegyesné Vecseri, B.; Kun-Farkas, G. Study of antioxidant activity during the malting and brewing process. J. Food Sci. Technol. 2009, 56, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Brien, S.E.; Ronksley, P.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: Systematic review and meta-analysis of interventional studies. BMJ 2011, 342, d636. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Caspi, A.; Libman, I.; Leontowicz, H.; Leontowicz, M.; Tashma, Z.; Trakhtenberg, S. Bioactivity of beer and its influence on human metabolism. Int. J. Food Sci. Nutr. 2007, 58, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Castaneda-Ovando, A.; De Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Quifer-Rada, P.; Vallverdú-Queralt, A.; Martínez-Huélamo, M.; Chiva-Blanch, G.; Jáuregui, O.; Estruch, R.; Lamuela-Raventós, R. A comprehensive characterisation of beer polyphenols by high resolution mass spectrometry (LC–ESI-LTQ-Orbitrap-MS). Food Chem. 2015, 169, 336–343. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy effects of plant polyphenols: Molecular mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef]
- Dack, R.E.; Black, G.W.; Koutsidis, G.; Usher, S.J. The effect of Maillard reaction products and yeast strain on the synthesis of key higher alcohols and esters in beer fermentations. Food Chem. 2017, 232, 595–601. [Google Scholar] [CrossRef]
- Witrick, K.T.; Duncan, S.E.; Hurley, K.E.; O’Keefe, S.F. Acid and Volatiles of Commercially-Available Lambic Beers. Beverages 2017, 3, 51. [Google Scholar] [CrossRef]
- Cela, N.; Condelli, N.; Caruso, M.C.; Perretti, G.; Di Cairano, M.; Tolve, R.; Galgano, F. Gluten-Free Brewing: Issues and Perspectives. Fermentation 2020, 6, 53. [Google Scholar] [CrossRef]
- Richter, T.; Silcock, P.; Algarra, A.; Eyres, G.T.; Capozzi, V.; Bremer, P.; Biasioli, F. Evaluation of PTR-ToF-MS as a tool to track the behavior of hop-derived compounds during the fermentation of beer. Food Res. Int. 2018, 111, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Jurić, A.; Ćorić, N.; Odak, A.; Herceg, Z.; Tišma, M. Analysis of total polyphenols, bitterness and haze in pale and dark lager beers produced under different mashing and boiling conditions. J. Inst. Brew. 2015, 121, 541–547. [Google Scholar] [CrossRef]
- Ditrych, M.; Kordialik-Bogacka, E.; Czyżowska, A. Antiradical and reducing potential of commercial beers. Czech. J. Food Sci. 2015, 33, 261–266. [Google Scholar] [CrossRef]
- Aquilani, B.; Laureti, T.; Poponi, S.; Secondi, L. Beer choice and consumption determinants when craft beers are tasted: An exploratory study of consumer preferences. Food Qual. Prefer. 2015, 41, 214–224. [Google Scholar] [CrossRef]
- Adadi, P.; Kovaleva, E.G.; Glukhareva, T.V.; Shatunova, S.A.; Petrov, A.S. Production and analysis of non-traditional beer supplemented with sea buckthorn. Agron. Res. 2017, 15, 1831–1845. [Google Scholar]
- Nardini, M.; Garaguso, I. Characterization of bioactive compounds and antioxidant activity of fruit beers. Food Chem. 2020, 305, 125437. [Google Scholar] [CrossRef]
- Martínez, A.; Vegara, S.; Martí, N.; Valero, M.; Saura, D. Physicochemical characterization of special persimmon fruit beers using bohemian pilsner malt as a base. J. Inst. Brew. 2017, 123, 319–327. [Google Scholar] [CrossRef]
- Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Prorok, P.; Piórecki, N. Physicochemical and antioxidative properties of Cornelian cherry beer. Food Chem. 2019, 281, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Patraşcu, L.; Banu, I.; Bejan, M.; Aprodu, I. Quality parameters of fruit beers available on romanian market. Stud. Cercet. Stiint. Chim. Ing. Chim. Biotehnol. Ind. Aliment. (Univ. Bacau.) 2018, 19, 323–335. [Google Scholar]
- Horincar, G.; Enachi, E.; Bolea, C.; Râpeanu, G.; Aprodu, I. Value-Added Lager Beer Enriched with Eggplant (Solanum melongena L.) Peel Extract. Molecules 2020, 25, 731. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, T.A.; Talent, N. Polyploidy in Crataegus and Mespilus (Rosaceae, Maloideae): Evolutionary inferences from flow cytometry of nuclear DNA amounts. Botany 2005, 83, 1268–1304. [Google Scholar]
- Дейнека, В.И.; Макаревич, С.Л.; Дейнека, Л.А.; Фирсoв, Г.А.; Сoрoкoпудoв, В.Н.; Третьякoв, М.Ю.; Бакшутoв, С.А. Anthocyanins of some hawthorn species (Crataegus L. Rosaceae) fruits. Chem. Plant. Raw Mater. 2005, 1, 119–124. [Google Scholar]
- Suwen, L.; Xuan, Z.; Lu, Y.; Zhaoyuan, G.; Xuedong, C. Changes in anthocyanin profile, color, and antioxidant capacity of hawthorn wine (Crataegus pinnatifida L.) during storage by pretreatments. LWT 2018, 95, 179–186. [Google Scholar]
- Pietrzak, W.; Kawa-Rygielska, J.; Król, B.; Lennartsson, P.R.; Taherzadeh, M.J. Ethanol, feed components and fungal biomass production from field bean (Vicia faba var. equina) seeds in an integrated process. Bioresour. Technol. 2016, 216, 69–76. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Chen, H.Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Lech, K.; Szumny, A.; Figiel, A.; Carbonell-Barrachina, A.A. Volatile composition of sweet basil essential oil (Ocimum basilicum L.) as affected by drying method. Food Res. Int. 2012, 48, 217–225. [Google Scholar] [CrossRef]
- Liu, P.; Kallio, H.; Lu, D.; Zhou, C.; Ou, S.; Yang, B. Acids, sugars, and sugar alcohols in Chinese hawthorn (Crataegus spp.) fruits. J. Agric. Food Chem. 2010, 58, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.G. Saccharomyces species in the Production of Beer. Beverages 2016, 2, 34. [Google Scholar] [CrossRef]
- Tang, X.R.; Kalviainen, N.; Tuorila, H. Sensory and hedonic characteristics of juice of sea buckthorn (Hippophae rhamnoides L.) origins and hybrids. Lebensm. Wiss. Technol. 2001, 34, 102–110. [Google Scholar] [CrossRef]
- Humia, B.V.; Santos, K.S.; Barbosa, A.M.; Sawata, M.; Mendonça, M.; Padilha, F.F. Beer Molecules and Its Sensory and Biological Properties: A Review. Molecules 2019, 24, 1568. [Google Scholar] [CrossRef]
- Adamenko, K.; Kawa-Rygielska, J.; Kucharska, A. Characteristics of Cornelian cherry sour non-alcoholic beers brewed with the special yeast Saccharomycodes ludwigii. Food Chem. 2019, 312, 125968. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Hiralal, L.; Mokoena, M.P.; Pillay, B. Flavour-active volatile compounds in beer: Production, regulation and control. J. Inst. Brew. 2017, 123, 13–23. [Google Scholar] [CrossRef]
- Zapata, P.J.; Martínez-Esplá, A.; Gironés-Vilaplana, A.; Santos-Lax, D.; Noguera-Artiaga, L.; Carbonell-Barrachina, Á.A. Phenolic, volatile, and sensory profiles of beer enriched by macerating quince fruits. LWT 2019, 103, 139–146. [Google Scholar] [CrossRef]
- Gao, T.; Han, S.; Liu, Y. Analysis of Volatile Components in Fresh Hawthorn by Solid-Phase Microextraction Combined with Gas Chromatography-Mass Spectrometry. Food Sci. 2013, 34, 144–147. [Google Scholar]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef]
- Holt, S.; Miks, M.H.; De Carvalho, B.T.; Foulquié-Moreno, M.R.; Thevelein, J.M. The molecular biology of fruity and floral aromas in beer and other alcoholic beverages. FEMS Microbiol. Rev. 2019, 43, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.S.; Guo, W.F.; Lu, Y.; Jiang, Y.X. Flavor Characteristics of Lapsang Souchong and Smoked Lapsang Souchong, a Special Chinese Black Tea with Pine Smoking Process. J. Agric. Food Chem. 2005, 53, 8688–8693. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Jin, G.-J.; Mei, W.-C.; Li, T.; Tao, Y.-S. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 2017, 239, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Ciani, E.; Galli, E.; Comitini, F.; Ciani, M. Evolution of Aromatic Profile of Torulaspora delbrueckii Mixed Fermentation at Microbrewery Plant. Fermentation 2020, 6, 7. [Google Scholar] [CrossRef]
- Mosciano, G. Organoleptic characteristics of flavor materials. Perfum. Flavor. 1997, 6, 41. [Google Scholar]
- Kucharska, A.; Piórecki, N.; Łyczko, J.; Szumny, A.; Wińska, K.; Sokół-Łętowska, A. Olejek eteryczny z owoców głogu oraz zastosowanie olejku eterycznego z głogu. Patent 430680, 2019. [Google Scholar]
- Zhang, W.; Chi, L.; Wu, Y.; Zhang, L.; Xu, C. Quality Comparison of Hawthorn Wines Fermented by Saccharomyces cerevisiae with and without Pulp Contact and Pectase Treatment. J. Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Haslbeck, K.; Bub, S.; Von Kamp, K.; Michel, M.; Zarnkow, M.; Hutzler, M.; Coelhan, M. The influence of brewing yeast strains on monoterpene alcohols and esters contributing to the citrus flavour of beer. J. Inst. Brew. 2018, 124, 403–415. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Sun, G.M.; Liu, Y.G.; Lv, L.L.; Yang, W.X.; Zhao, W.F.; Wei, C.B. Aroma volatile compounds from two fresh pineapple varieties in China. Int. J. Mol. Sci. 2012, 13, 7383–7392. [Google Scholar] [CrossRef] [PubMed]
- Arn, H.; Acree, T.E. Flavornet: A database of aroma compounds based on odor potency in natural products. Dev. Food Sci. 1998, 40, 27. [Google Scholar]
- Riu-Aumatell, M.; Miró, P.; Serra-Cayuela, A.; Buxaderas, S.; López-Tamames, E. Assessment of the aroma profiles of low alcohol beers using HS-SPME–GC-MS. Food Res. Int. 2014, 57, 196–202. [Google Scholar] [CrossRef]
- Błażewicz, J.; Anioł, M.; Tobola, D.; Stompor, M. Xanthohumol content in Polish beers. Przem. Chem. 2014, 93, 1447–1450. [Google Scholar]
- Yan, D.; Wong, Y.F.; Shellie, R.A.; Marriott, P.J.; Whittock, S.P.; Koutoulis, A. Assessment of the phytochemical profiles of novel hop (Humulus lupulus L.) cultivars: A potential route to beer crafting. Food Chem. 2019, 275, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Pistelli, L.; Ferri, B.; Cioni, P.L.; Koziara, M.; Agacka, M.; Skomra, U. Aroma profile and bitter acid characterization of hop cones (Humulus lupulus L.) of five healthy and infected Polish cultivars. Ind. Crops Prod. 2018, 124, 653–662. [Google Scholar] [CrossRef]
- Wang, S.C.; Tseng, T.Y.; Huanga, C.M.; Tsaic, T.H. Gardenia herbal active constituents: Applicable separation procedures. J. Chromatogr. B Biomed. Appl. 2004, 812, 193–202. [Google Scholar] [CrossRef]
- Lukinac, J.; Mastanjević, K.; Mastanjević, K.; Nakov, G.; Jukić, M. Computer Vision Method in Beer Quality Evaluation—A Review. Beverages 2019, 5, 38. [Google Scholar] [CrossRef]
| Beer Type 1 | Ethanol | Glycerol | Maltose | Maltotriose | Dextrin | Glucose | pH |
|---|---|---|---|---|---|---|---|
| [g/L] | [g/L] | [g/L] | [g/L] | [g/L] | [g/L] | ||
| BC | 41.63 ± 0.02 a | 2.23 ± 0.01 a | 1.05 ± 0.002 a | 1.13 ± 0.003 a | 17.58 ± 0.01 a | n.d. | 3.87 ± 0.02 a |
| BJ | 35.42 ± 0.2 c | 1.95 ± 0.01 c | 0.8 ± 0.015 c | 0.92 ± 0.007 c | 14.21 ± 0.05 c | n.d. | 3.5 ± 0.02 c |
| BF | 37.23 ± 0.03 b | 2.07 ± 0.04 b | 0.9 ± 0.001 b | 0.98 ± 0.015 b | 15.77 ± 0.3 b | n.d. | 3.7 ± 0.01 b |
| Analysed Sample 1 | Polyphenol Concentration | ABTS+• | DPPH• | FRAP |
|---|---|---|---|---|
| mg GAE/L | mmol TE/L | mmol TE/L | mmol TE/L | |
| BC | 200.5 ± 1.9 d | 0.936 ± 0.09 d | 0.352 ± 0.03 c | 0.512 ± 0.01 d |
| BJ | 410.1 ± 11.8 b | 2.041 ± 0.12 b | 2.175 ± 0.01 b | 1.35 ± 0.02 b |
| BF | 279.6 ± 2 c | 1.356 ± 0.11 c | 0.443 ± 0.04 c | 0.869 ± 0.01 c |
| J | 2633.9 ± 27.9 a | 11.03 ± 0.32 a | 17.22 ± 0.29 a | 8.52 ± 0.13 a |
| Kovats Indices | Concentration in Beer µg/100 ML 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound Name | Retention Time | Chemical Family | KI Exp. | KI NIST | CAS Number | BJ | BF | BC | |
| 1 | 1-Butanol, 3-methyl- | 4.208 | Alcohols | 724 | 735 | 123-51-3 | 5.7104 ± 2.8229 c | 11.9594 ± 4.9811 b | 18.4103 ± 9.6497 a |
| 2 | Isobutyl acetate | 5.145 | Esters | 763 | 771 | 110-19-0 | 0.0257 ± 0.0133 b | 0.2048 ± 0.0954 a | 0.0328 ± 0.0147 b |
| 3 | Butanoic acid. ethyl ester | 5.852 | Esters | 799 | 802 | 105-54-4 | 0.0812 ± 0.0622 b | 0.5324 ± 0.3472 a | 0.0492 ± 0.0251 b |
| 4 | 1-Hexanol | 7.899 | Alcohols | 872 | 868 | 111-27-3 | 0.0327 ± 0.0142 b | 0.0819 ± 0.0533 a | 0.0164 ± 0.0086 c |
| 5 | 1-Butanol, 3-methyl-, acetate | 8.095 | Esters | 872 | 876 | 123-92-2 | 3.0275 ± 1.3892 b | 18.4305 ± 6.4922 a | 1.6409 ± 0.7294 b |
| 6 | 5-Hepten-2-one, 6-methyl- | 11.656 | Ketones | 987 | 986 | 110-93-0 | 0.0139 ± 0.0068 b | 0.0819 ± 0.0412 a | 0.0153 ± 0.0089 b |
| 7 | β-Myrcene | 11.782 | Monoterpenes | 994 | 991 | 123-35-3 | 0.0137 ± 0.0101 b | 0.1229 ± 0.0844 a | 0.0164 ± 0.0114 b |
| 8 | Hexanoic acid, ethyl ester | 12.034 | Esters | 1001 | 1000 | 123-66-0 | 1.3959 ± 0.9982 b | 11.2221 ± 6.4921 a | 0.9681 ± 0.06742 b |
| 9 | Acetic acid, hexyl ester | 12.508 | Esters | 1017 | 1011 | 123-35-3 | 0.0059 ± 0.0036 b | 0.1229 ± 0.0712 a | Trace |
| 10 | Propanoic acid, 2-methyl-, 2-methylbutyl ester | 12.577 | Esters | 1019 | 1016 | 2445-69-4 | 0.0042 ± 0.0021 b | 0.6963 ± 0.3152 a | Trace |
| 11 | p-Cymene | 12.83 | Aromatic hydrocarbons | 1028 | 1025 | 99-87-6 | 0.0079 ± 0.0023 c | 0.6144 ± 0.2197 a | 0.0328 ± 0.0094 b |
| 12 | Limonene | 12.955 | Monoterpenes | 1032 | 1030 | 5989-54-8 | 0.0218 ± 0.0074 b | 0.1638 ± 0.0852 a | Trace |
| 13 | Butanoic acid, 3-methylbutyl ester | 13.937 | Esters | 1062 | 1056 | 106-27-4 | 0.0041 ± 0.0019 c | 0.1229 ± 0.0622 a | 0.0164 ± 0.0072 b |
| 14 | Ethyl 5-methylhexanoate | 14.035 | Esters | 1066 | 1072 | 10236-10-9 | 0.0119 ± 0.0041 a | 0.0819 ± 0.0332 a | Trace |
| 15 | 1-Octanol | 14.316 | Alcohols | 1075 | 1071 | 111-87-5 | 0.0972 ± 0.0386 c | 7.4541 ± 2.8264 a | 0.5743 ± 0.2848 b |
| 16 | 2-Nonanone | 14.96 | Ketones | 1096 | 1092 | 821-55-6 | 0.0119 ± 0.0049 c | 41.5231 ± 11.9227 a | 2.7894 ± 1.0048 b |
| 17 | Linalool | 15.2 | Alcohols | 1099 | 1099 | 78-70-6 | 0.8554 ± 0.2652 b | 1.1468 ± 0.4392 a | 0.0824 ± 0.0312 c |
| 18 | 2-Nonen-1-ol | 15.311 | Alcohols | 1099 | 1105 | 22104-79-6 | 0.1584 ± 0.0466 c | 43.4859 ± 13.5984 a | 2.7894 ± 0.9982 b |
| 19 | Phenylethyl Alcohol | 15.607 | Esters | 1114 | 1116 | 1960-12-08 | 3.0156 ± 0.9954 a | 1.1877 ± 0.05572 b | 0.0492 ± 0.0235 c |
| 20 | Octanoic acid, methyl ester | 15.945 | Esters | 1127 | 1126 | 106-32-1 | 0.0396 ± 0.0178 b | 0.2431 ± 0.0861 a | trace |
| 21 | Endo-Borneol * | 17.199 | Monoterpenes | 1177 | 1167 | 464-43-7 | 0.0158 ± 0.0084 c | 0.2457 ± 0.0973 a | 0.0656 ± 0.0221 b |
| 22 | Benzoic acid, ethyl ester | 17.311 | Esters | 1180 | 1171 | 93-89-0 | 0.0976 ± 0.0394 c | 10.1163 ± 3.8572 a | 0.7548 ± 0.2362 b |
| 23 | Octanoic acid | 17.674 | Organic acids | 1192 | 1180 | 124-07-2 | 1.3425 ± 0.5283 a | 0.5734 ± 0.2271 b | 0.0492 ± 0.0198 c |
| 24 | α-Terpineol | 17.772 | Monoterpene | 1194 | 1190 | 98-55-5 | 5.7758 ± 2.2853 b | 73.1488 ± 23.0739 a | 0.1019 ± 0.0476 c |
| 25 | Octanoic acid, ethyl ester | 17.858 | Esters | 1196 | 1196 | 106-32-1 | 8.3716 ± 2.3497 a | 0.2457 ± 0.0612 b | 0.0656 ± 0.0227 c |
| 26 | Decanal | 18.028 | Aldehydes | 1207 | 1206 | 112-31-2 | 0.1465 ± 0.0381 b | 1.2287 ± 0.4776 a | 0.0164 ± 0.0048 c |
| 27 | Acetic acid, octyl ester | 18.126 | Esters | 1217 | 1211 | 112-14-1 | 0.0178 ± 0.0061 c | 4.0547 ± 1.1286 a | 0.3774 ± 0.0982 b |
| 28 | Citronellol | 18.46 | Alcohols | 1238 | 1229 | 106-22-9 | 0.5307 ± 0.2163 a | Tr ace | Trace |
| 29 | Benzeneacetic acid, ethyl ester | 18.709 | Esters | 1254 | 1246 | 101-97-3 | 0.0139 ± 0.0047 c | 20.3555 ± 4.3992 a | 2.0182 ± 0.8642 b |
| 30 | Isopentyl hexanoate | 18.752 | Esters | 1256 | 1252 | 2198-61-0 | 0.0079 ± 0.0032 b | 0.0287 ± 0.0092 a | trace |
| 31 | Acetic acid, 2-phenylethyl ester | 18.879 | Esters | 1263 | 1258 | 103-45-7 | 2.9661 ± 1.0873 a | 0.3277 ± 0.0932 b | 0.0164 ± 0.0052 c |
| 32 | 1-Decanol | 19.077 | Alcohols | 1277 | 1273 | 112-31-2 | 0.0812 ± 0.0272 a | 0.0416 ± 0.0138 b | 0.0328 ± 0.0098 b |
| 33 | 2,4-Heptadienoic acid, 6-methyl-, ethyl ester | 19.146 | Esters | 1282 | 1293 | 10236-06-03 | 0.1327 ± 0.4921 b | 0.2457 ± 0.0733 a | 0.0164 ± 0.0043 b |
| 34 | Methyl geranate | 19.728 | Esters | 1339 | 1326 | 214-712-6 | 0.4396 ± 0.0963 b | 1.4744 ± 0.3896 a | 0.3282 ± 0.1158 b |
| 35 | Octanoic acid, 2-methylpropyl ester | 19.938 | Esters | 1352 | 1348 | 5461-06-03 | 0.2198 ± 0.0624 b | 0.3686 ± 0.1374 a | 0.1641 ± 0.0556 b |
| 36 | Citronellol acetate | 20.023 | Esters | 1357 | 1354 | 150-84-5 | 0.0973 ± 0.0372 b | 0.3277 ± 0.0992 a | 0.0985 ± 0.0313 b |
| 37 | Unknown sesquiterpene | 20.149 | Sesquiterpenes | 1369 | 1.8197 ± 0.6294 a | 0.6553 ± 0.3278c | 1.2799 ± 0.3775 b | ||
| 38 | Ethyl (4E)-4-decenoate | 20.291 | Esters | 1384 | 1377 | 76649-16-6 | 0.1267 ± 0.0352 b | 0.4505 ± 0.0966 a | 0.0985 ± 0.0217 b |
| 39 | Ethyl trans-2-decenoate | 20.347 | Esters | 1389 | 1389 | 7367-88-6 | 0.7702 ± 0.2432 b | 7.5366 ± 2.8836 a | 0.5087 ± 0.1764 b |
| 40 | Decanoic acid, ethyl ester | 20.418 | Esters | 1396 | 1396 | 110-38-3 | 4.1086 ± 1.2883 a | 1.6792 ± 0.4732 b | 0.5127 ± 0.2296 c |
| 41 | Dodecanal | 20.561 | Aldehydes | 1412 | 1409 | 112-54-9 | 0.0396 ± 0.0084 b | 0.3277 ± 0.0912 a | 0.0492 ± 0.0068 b |
| 42 | β-Caryophyllene | 20.786 | Sesquiterpenes | 1440 | 1419 | 87-44-5 | 0.0772 ± 0.0252 b | 0.3686 ± 0.0992 a | 0.0476 ± 0.0118 b |
| 43 | Octanoic acid, 3-methylbutyl ester | 20.87 | Esters | 1453 | 1446 | 2035-99-6 | 0.1148 ± 0.0326 b | 1.4335 ± 0.3774 a | 0.0985 ± 0.0264 b |
| 44 | (E)-β-Famesene | 20.984 | Sesquiterpenes | 1464 | 1457 | 18794-84-8 | 0.0436 ± 0.0092 b | 1.6383 ± 0.4694 a | 0.0492 ± 0.0111 b |
| 45 | Humulene | 21.069 | Sesquiterpenes | 1474 | 1454 | 6753-98-6 | 0.4772 ± 0.0958 b | 1.9659 ± 0.5312 a | 0.5118 ± 0.1322 b |
| 46 | α-Muurolene | 21.224 | Sesquiterpenes | 1493 | 1485 | 10208-80-7 | 0.0178 ± 0.0054 b | 0.2457 ± 0.0618 a | 0.0164 ± 0.0051 b |
| 47 | Pentadecane | 21.296 | Hydrocarbons | 1500 | 1500 | 629-62-9 | 0.0139 ± 0.0058 c | 0.3686 ± 0.1372 a | 0.0492 ± 0.0126 b |
| 48 | δ-Cadinene | 21.561 | Sesquiterpenes | 1538 | 1524 | 483-76-1 | 0.1267 ± 0.0344 b | 0.9838 ± 0.2258 a | 0.1149 ± 0.0376 b |
| 49 | Unknown compound | 21.687 | 1557 | 0.0317 ± 0.0076 a | 0.0023 ± 0.0017 b | Trace | |||
| 50 | β-Calacorene | 21.744 | Sesquiterpenes | 1565 | 1563 | 50277-34-4 | 0.0475 ± 0.0122 b | 0.0819 ± 0.0315 a | 0.0328 ± 0.0087 b |
| 51 | Nerolidol | 21.786 | Sesquiterpenes | 1574 | 1565 | 7212-44-4 | 0.0297± 0.0102 b | 0.4505 ± 0.0936 a | 0.0245 ± 0.0097 b |
| 52 | Dodecanoic acid, ethyl ester | 21.955 | Esters | 1590 | 1595 | 106-33-2 | 0.9722 ± 0.2318 b | 14.2939 ± 3.5972 a | 0.6563 ± 0.2532 b |
| 53 | Humulene epoxide I | 22.167 | Sesquiterpenes | 1607 | 1604 | 19888-33-6 | 0.2812 ± 0.0676 b | 3.1127 ± 0.9136 a | 0.2969 ± 0.0754 b |
| Total | 43.8884 | 287.8529 | 35.9348 | ||||||
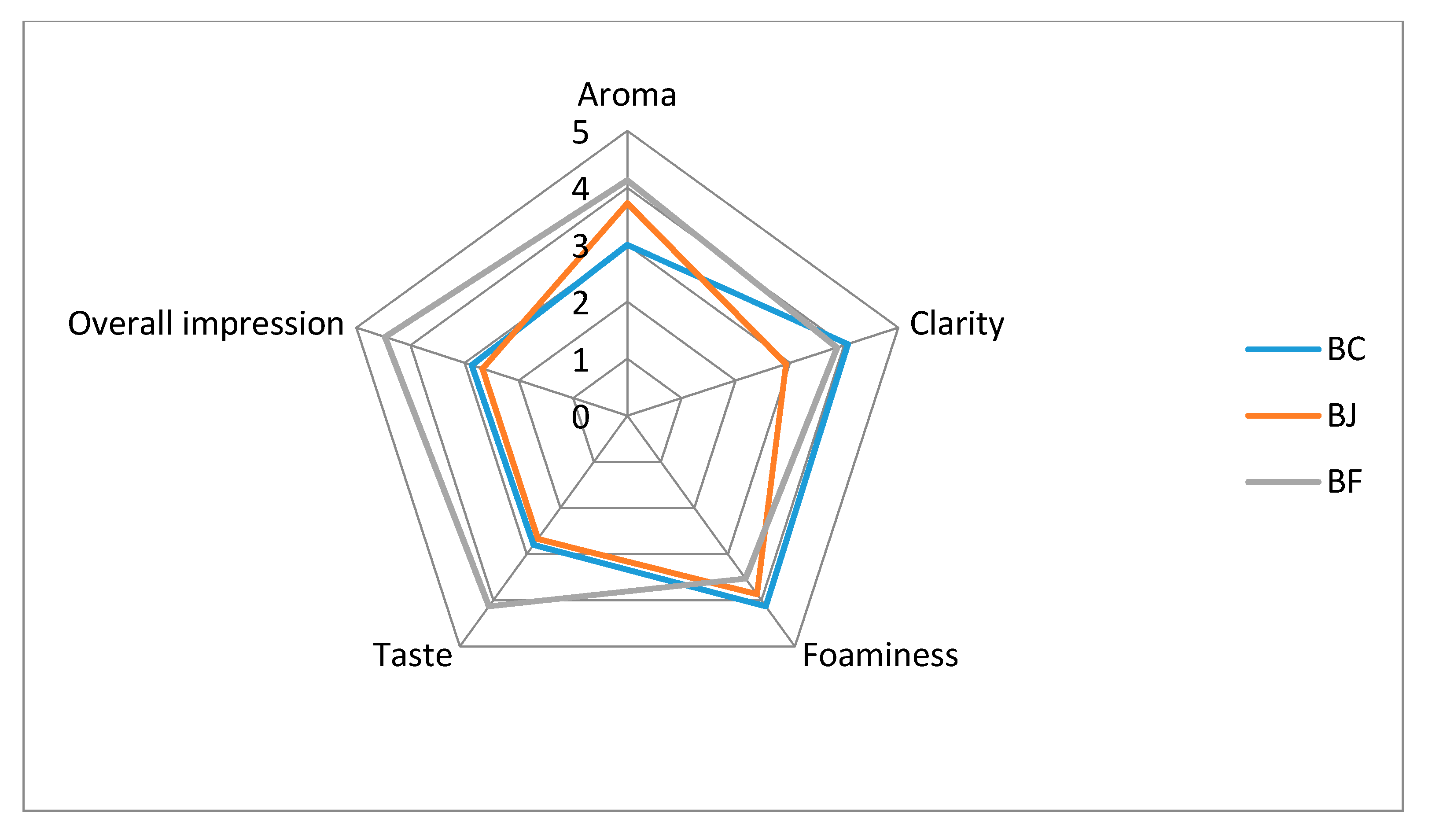
| Beer Type 1 | Aroma | Clarity | Foaminess | Taste | Overall Impression |
|---|---|---|---|---|---|
| BC | 3.02 ± 0.2 c | 4.07 ± 0.21 a | 4.13 ± 0.19 a | 2.81 ± 0.17 b | 2.87 ± 0.19 b |
| BJ | 3.73 ± 0.15 b | 2.93 ± 0.18 b | 3.87 ± 0.19 ab | 2.67 ± 0.21 b | 2.67 ± 0.19 b |
| BF | 4.13 ± 0.19 a | 3.87 ± 0.24 a | 3.53 ± 0.13 b | 4.13 ± 0.19 a | 4.47 ± 0.17 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasiński, A.; Kawa-Rygielska, J.; Szumny, A.; Gąsior, J.; Głowacki, A. Assessment of Volatiles and Polyphenol Content, Physicochemical Parameters and Antioxidant Activity in Beers with Dotted Hawthorn (Crataegus punctata). Foods 2020, 9, 775. https://doi.org/10.3390/foods9060775
Gasiński A, Kawa-Rygielska J, Szumny A, Gąsior J, Głowacki A. Assessment of Volatiles and Polyphenol Content, Physicochemical Parameters and Antioxidant Activity in Beers with Dotted Hawthorn (Crataegus punctata). Foods. 2020; 9(6):775. https://doi.org/10.3390/foods9060775
Chicago/Turabian StyleGasiński, Alan, Joanna Kawa-Rygielska, Antoni Szumny, Justyna Gąsior, and Adam Głowacki. 2020. "Assessment of Volatiles and Polyphenol Content, Physicochemical Parameters and Antioxidant Activity in Beers with Dotted Hawthorn (Crataegus punctata)" Foods 9, no. 6: 775. https://doi.org/10.3390/foods9060775
APA StyleGasiński, A., Kawa-Rygielska, J., Szumny, A., Gąsior, J., & Głowacki, A. (2020). Assessment of Volatiles and Polyphenol Content, Physicochemical Parameters and Antioxidant Activity in Beers with Dotted Hawthorn (Crataegus punctata). Foods, 9(6), 775. https://doi.org/10.3390/foods9060775






