Bioshell Calcium Oxide-Containing Liquids as a Sanitizer for the Reduction of Histamine Production in Raw Japanese Pilchard, Japanese Horse Mackerel, and Chub Mackerel
Abstract
:1. Introduction
2. Materials and Methods
2.1. BiSCaO Powder, Chemicals and Species of Blue-Skinned Fishes
2.2. BiSCaO Suspensions, BiSCaO Dispersion with Na2HPO4, BiSCaO Colloidal Dispersion with NapolyPO4 (PP) and BiSCaO Water
2.3. Removal of Histamine and Bactericidal Activity by BiSCaO Suspension, Dispersion, Colloidal Dispersion, and Water
2.4. Reduction of Histamine Using Decomposing Raw Japanese Pilchard, Japanese Horse Mackerel and Chub Mackerel, and Reduction of Bactericidal Activity by BiSCaO Suspension, Dispersion, Colloidal Dispersion, and BiSCaO Water
3. Results
3.1. Removal of Histamine and Bactericidal Activity Using BiSCaO Suspension, Dispersion, Colloidal Dispersion, SSP-Ca(OH)2, or BiSCaO Water
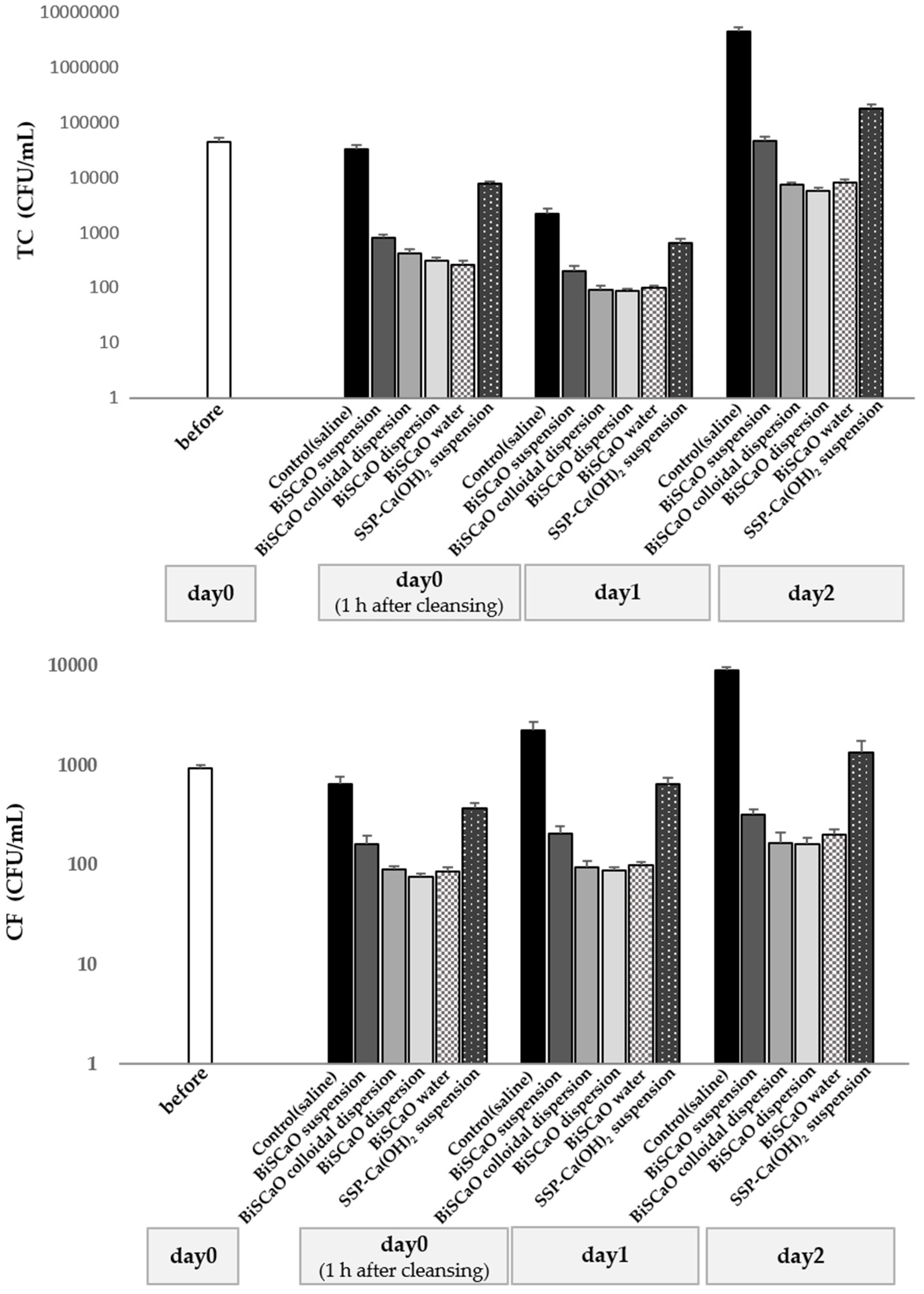
3.2. Microbicidal Efficacy of BiSCaO Suspension, Dispersions, Colloidal Dispersion, and BiSCaO Water
3.3. Reduction of Histamine in Decomposing Raw Japanese Pilchard, Japanese Horse Mackerel and Chub Mackerel, and Bactericidal Activity of BiSCaO Suspension, Dispersion, Colloidal Dispersion and BiSCaO Water
3.4. Antimicrobial Efficacy of BiSCaO Suspension, Dispersions, Colloidal Dispersion and BiSCaO Water Determined Using pre-Incubated Japanese Horse Mackerel Slices
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schirone, M.; Visciano, P.; Tofalo, R.; Suzzi, G. Histamine Food Poisoning. Handb. Exp. Pharmacol. 2017, 241, 217–235. [Google Scholar] [PubMed]
- Feng, C.; Teuber, S.; Gershwin, M.E. Histamine (Scombroid) Fish Poisoning: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Velut, G.; Delon, F.; Mérigaud, J.P.; Tong, C.; Duflos, G.; Boissan, F.; Watier-Grillot, S.; Boni, M.; Derkenne, C.; Dia, A.; et al. Histamine food poisoning: A sudden, large outbreak linked to fresh yellowfin tuna from Reunion Island, France, April 2017. Eurosurveillance 2019, 24, 1800405. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, U.T.; Inan, D. Quantification of histamine in various fish samples using square wave stripping voltammetric method. J. Food Sci. Technol. 2015, 52, 6671–6678. [Google Scholar] [CrossRef] [Green Version]
- Visciano, P.; Schirone, M.; Tofalo, R.; Suzzi, G. Biogenic amines in raw and processed seafood. Front. Microbiol. 2012, 3, 188. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Capillas, C.; Herrero, A.M. Impact of biogenic amines on food quality and safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Gonzaga, V.E.; Lescano, A.G.; Huaman, A.A.; Salmon-Mulanovich, M.; Blazes, D.L. Histamine levels in fish from markets in Lima, Peru. J. Food Prot. 2009, 72, 1112–1115. [Google Scholar] [CrossRef]
- Ferran, M.; Yebenes, M. Flushing associated with scombroid fish poisoning. Dermatol. Online J. 2006, 12, 15. [Google Scholar]
- Demoncheaux, J.P.; Michel, R.; Mazenot, C.; Duflos, G.; Iacini, C.; de Laval, F.; Delaval, F.; Saware, E.M.; Renard, J.C. A large outbreak of acombroid fish poisoning associated with eating yellowfin tuna (Thunnus albacares) at a military mass catering in Dakar, Senegal. Epidemiol. Infect. 2012, 140, 1008–1012. [Google Scholar] [CrossRef]
- Wiegand, C.; Abel, M.; Ruth, P.; Elsner, P.; Hipler, U.C. PH influence on antibacterial efficacy of common antiseptic substances. Skin Pharmacol. Physiol. 2015, 28, 147–158. [Google Scholar] [CrossRef]
- Hungerford, J.M.; Arefyev, A.A. Flow-injection assay of enzyme inhibition in fish using immobilized diamine oxidase. Anal. Chim. Acta 1992, 261, 351–359. [Google Scholar] [CrossRef]
- Gardini, F.; Özogul, Y.; Suzzi, G.; Tabanelli, G.; Özogul, F. Technological Factors Affecting Biogenic Amine Content in Foods: A Review. Front. Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hungerford, J.M. Scombroid poisoning: A review. Toxicon 2010, 56, 231–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, Y.; Ishihara, M.; Nakamura, S.; Fukuda, K.; Takayama, T.; Hiruma, S.; Murakami, K.; Fujita, M.; Yokoe, H. Preparation and application of bioshell calcium oxide (BiSCaO) nanoparticles-dispersions with bactericidal activity. Molecules 2019, 24, 3415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawai, J. Antimicrobial characteristics of heated scallop shell powder and its application. Biocontrol Sci. 2011, 16, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Ono, T.; Yamashita, K.; Murayama, T.; Sato, T. Microbicidal effect of weak acid hypochlorous solution on various microorganisms. Biocontrol Sci. 2012, 17, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, M.; Murakami, K.; Fukuda, K.; Nakamura, S.; Kuwabara, M.; Hattori, H.; Fujita, M.; Kiyosawa, T.; Yokoe, H. Stability of weak acidic hypochlorous acid solution with microbicidal activity. Biocontrol Sci. 2017, 22, 223–227. [Google Scholar] [CrossRef] [Green Version]
- Kuwabara, M.; Ishihara, M.; Fukuda, K.; Nakamura, S.; Murakami, K.; Sato, Y.; Yokoe, H.; Kiyosawa, T. Disinfection by Hypochlorous Acid for Pseudomonas aeruginosa-Infected Wounds in Diabetic db/db Mice. Wound Med. 2018, 23, 1–5. [Google Scholar] [CrossRef]
- Fukuzaki, S.; Urano, H.; Yamada, S. Effects of pH on the efficacy of sodium hypochlorite solution as cleaning and bactericidal agents. Surf. Sci. 2007, 58, 465–469. [Google Scholar] [CrossRef]
- Fukuzaki, S. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci. 2006, 11, 147–157. [Google Scholar] [CrossRef]
- Sato, Y.; Ishihara, M.; Nakamura, S.; Fukuda, K.; Kuwabara, M.; Takayama, T.; Murakami, K.; Fujita, M.; Yokoe, H. Comparison of various disinfectants on bactericidal activity under organic matter contaminated water. Biocontrol Sci. 2019, 24, 103–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, K.; Sato, Y.; Ishihara, M.; Nakamura, S.; Takayama, T.; Murakami, K.; Fujita, M.; Yokoe, H. Cleansing technique for skin wound with disinfectant using high-velocity steam-air micro mist jet spray. Biocontrol Sci. 2020, 25, 35–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, Y.; Ohata, H.; Inoue, A.; Ishihara, M.; Nakamura, S.; Fukuda, K.; Takayama, T.; Murakami, K.; Hiruma, S.; Yokoe, H. Application of colloidal dispersions of bioshell calcium oxide (BiSCaO) for disinfection. Polymers 2019, 11, 1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, K.; Ishihara, M.; Murakami, K.; Nakamura, S.; Sato, Y.; Kuwabara, M.; Fujita, M.; Kiyosawa, T.; Yokoe, H. Cleaning technique using high-velocity steam-air micromist jet spray. J. Med. Eng. Technol. 2017, 41, 522–528. [Google Scholar] [CrossRef]
- Sato, Y.; Ishihara, M.; Fukuda, K.; Nakamura, S.; Murakami, K.; Fujita, M.; Yokoe, H. Behavior of nitrate nitorogen and nitrite nitrogen in drinking waters. Biocontrol Sci. 2018, 23, 139–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visciano, P.; Schirone, M.; Tofalo, R.; Suzzi, G. Histmine poisoning and control measures in fish and fishery products. Front. Microbiol. 2014, 5, 500. [Google Scholar] [CrossRef] [Green Version]
- Harmelin, Y.; Hubiche, T.; Pharaon, N.; Del Giudice, P. Three cases of scombroid poisoning. Ann. Dermatol. Venereol. 2018, 145, 29–32. [Google Scholar] [CrossRef]
- Kinoda, J.; Ishihara, M.; Hattori, H.; Nakamura, S.; Fukuda, K.; Yokoe, H. Cytotoxicity of silver nanoparticle and chitin-nanofiber sheet composites caused by oxidative stress. Nanomaterials 2016, 6, 189. [Google Scholar] [CrossRef]
- McCauley, R.L.; Linares, H.A.; Pelligrini, V.; Herndon, D.N.; Robson, M.C.; Heggers, J.P. In vitro toxicity of topical antimicrobial agents to human fibroblasts. J. Surg. Res. 1989, 46, 267–274. [Google Scholar] [CrossRef]
- Ishihara, M.; Nguyen, V.Q.; Mori, Y.; Nakamura, S.; Hattori, H. Adsorption of silver nanoparticles onto different surface structures of chitin/chitosan and correlations with antimicrobial activities. Int. J. Mol. Sci. 2015, 16, 13973–13988. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.Q.; Ishihara, M.; Kinoda, J.; Hattori, H.; Nakamura, S.; Ono, T.; Miyahira, M.; Matsui, T. Development of antimicrobial biomaterials produced from chitin-nanofiber sheet/silver nanoparticle composites. J. Nanobiotechnol. 2014, 12, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, Y.; Tagawa, T.; Fujita, M.; Kuno, T.; Suzuki, S.; Matsui, T.; Ishihara, M. Simple and environmentally friendly preparation and size control of silver nanoparticles using an inhomogeneous system with silver-containing glass powder. J. Nanopart. Res. 2011, 13, 2799–2806. [Google Scholar] [CrossRef]

| Reagent Concentration | |||||||
|---|---|---|---|---|---|---|---|
| 1 wt.% | 0.2 wt.% | 0.04 wt.% | |||||
| Test Sample | PW | CS | PW | CS | PW | CS | |
| BiSCaO suspension | 47.5 ± 3.5 12.6 ± 0.03 | 31.3 ± 0.9 12.5 ± 0.02 | 51.2 ± 3.7 12.3 ± 0.01 | 35.2 ± 2.7 12.2 ± 0.01 | 76.2 ± 8.5 11.5 ± 0.01 | 67.1 ± 7.0 11.2 ± 0.02 | (ppm) pH |
| BiSCaO colloidal dispersion | 21.9 ± 1.8 12.6 ± 0.02 | 18.1 ± 1.0 12.4 ± 0.02 | 29.2 ± 2.4 12.5 ± 0.02 | 24 ± 1.3 12.2 ± 0.01 | 67.1± 4.7 11.7 ± 0.02 | 62.5 ± 6.0 11.5 ± 0.01 | (ppm) pH |
| BiSCaO dispersion | 26.8 ± 2.6 12.6 ± 0.02 | 21.9 ± 1.4 12.3 ± 0.01 | 42.1 ± 3.9 12.3 ± 0.02 | 32.6 ± 3.4 12.0 ± 0.01 | 70.3 ± 6.9 11.5 ± 0.01 | 66.8 ± 7.6 11.1 ± 0.02 | (ppm) pH |
| SSP-Ca(OH)2 suspension | 71.5 ± 7.3 12.2 ± 0.01 | 68.2 ± 6.9 11.9 ± 0.01 | 85.1 ± 8.4 12.0 ± 0.01 | 73.7 ± 6.4 11.8 ± 0.02 | 91.5 ± 9.3 11.5 ± 0.01 | 88.8 ± 8.3 11.2 ± 0.01 | (ppm) pH |
| SSP-Ca(OH)2 colloidal dispersion | 59.5 ± 3.7 12.2 ± 0.01 | 35.5 ± 3.6 11.9 ± 0.01 | 74.1 ± 7.1 12.0 ± 0.01 | 61.1 ± 6.3 11.8 ± 0.01 | 88.7 ± 8.2 11.5 ± 0.01 | 76.3 ± 6.5 11.2 ± 0.01 | (ppm) pH |
| SSP-Ca(OH)2 dispersion | 61.9 ± 6.1 12.4 ± 0.01 | 41.3 ± 3.7 12.2 ± 0.01 | 75.2 ± 7.7 12.0 ± 0.01 | 64.4 ± 6.9 11.6 ± 0.01 | 90.2 ± 9.0 11.3 ± 0.01 | 81.6 ± 8.0 10.8 ± 0.01 | (ppm) pH |
| Reagent Concentrations | |||||||
| Undiluted | 2-fold diluted | 4-fold diluted | |||||
| Test sample | PW | CS | PW | CS | PW | CS | |
| BiSCaO water | 50.9 ± 5.0 12.7 ± 0.03 | 27.7 ± 2.4 12.6 ± 0.02 | 55.1 ± 4.1 12.6 ± 0.02 | 35.3 ± 2.9 12.5 ± 0.02 | 72.6 ± 8.8 12.2 ± 0.03 | 68.5 ± 7.4 12.0 ± 0.01 | (ppm) pH |
| Reagent Concentration | ||||||
|---|---|---|---|---|---|---|
| 1 wt.% | 0.2 wt.% | 0.04 wt.% | ||||
| Test Sample | TC | CF | TC | CF | TC | CF |
| BiSCaO suspension | 0 | 0 | 84 ± 12 | 15 ± 5 | 68 ± 17 (×103) | 25 ± 6 (×103) |
| BiSCaO colloidal dispersion | 0 | 0 | 16 ± 5 | 3 ± 2 | 72 ± 21 (×102) | 18 ± 5 (×102) |
| BiSCaO dispersion | 0 | 0 | 44 ± 10 | 6 ± 2 | 12 ± 4 (×103) | 42 ± 11 (×102) |
| SSP-Ca(OH)2 suspension | 0 | 0 | 9 ± 3 (×102) | 21 ± 5 (×10) | 25 ± 6 (×105) | 48 ± 12 (×104) |
| SSP-Ca(OH)2 colloidal dispersion | 0 | 0 | 32 ± 7 (×10) | 56 ± 16 | 68 ± 16 (×104) | 81 ± 32 (×103) |
| SSP-Ca(OH)2 dispersion | 0 | 0 | 48 ± 9 (×10) | 62 ± 18 | 87 ± 23 (×104) | 12 ± 5 (×104) |
| Reagent Concentration | ||||||
| Undiluted | 2-fold diluted | 4-fold diluted | ||||
| Test sample | CF | TC | CF | TC | CF | TC |
| BiSCaO water | 0 | 0 | 0 | 0 | 88 ± 21 (×10) | 27 ± 7 (×10) |
| Day 1 | |||
| Histamine (ppm) | |||
| Test sample | Japanese pilchard | Japanese horse mackerel | Chub mackerel |
| Control (saline) | 40 ± 5 | 25 ± 3 | 19 ± 2 |
| BiSCaO suspension | 12 ± 2 | 9 ± 1 | 8 ± 1 |
| BiSCaO colloidal dispersion | 10 ± 2 | 7 ± 1 | 4 ± 1 |
| BiSCaO dispersion | 9 ± 1 | 6 ± 1 | 5 ± 1 |
| BiSCaO water | 11 ± 2 | 8 ± 2 | 5 ± 1 |
| SSP-Ca(OH)2 suspension | 19 ± 5 | 14 ± 3 | 10 ± 2 |
| Day 2 | |||
| Histamine (ppm) | |||
| Test sample | Japanese pilchard | Japanese horse mackerel | Chub mackerel |
| Control (saline) | 168 ± 21 | 95 ± 22 | 43 ± 10 |
| BiSCaO suspension | 31 ± 6 | 26 ± 6 | 18 ± 6 |
| BiSCaO colloidal dispersion | 15 ± 3 | 18 ± 5 | 11 ± 2 |
| BiSCaO dispersion | 14 ± 3 | 18 ± 5 | 11 ± 2 |
| BiSCaO water | 15 ± 3 | 20 ± 6 | 12 ± 2 |
| SSP-Ca(OH)2 suspension | 56 ± 12 | 65 ± 15 | 23 ± 6 |
| Day 4 | |||
| Histamine (ppm) | |||
| Test sample | Japanese pilchard | Japanese horse mackerel | Chub mackerel |
| Control (saline) | 880 ± 180 | 460 ± 68 | 235 ± 36 |
| BiSCaO suspension | 92 ± 19 | 70 ± 16 | 59 ± 9 |
| BiSCaO colloidal dispersion | 32 ± 5 | 55 ± 8 | 27 ± 6 |
| BiSCaO dispersion | 38 ± 7 | 57 ± 8 | 28 ± 8 |
| BiSCaO water | 41 ± 8 | 59 ± 9 | 27 ± 7 |
| SSP-Ca(OH)2 suspension | 232 ± 56 | 207 ± 35 | 90 ± 17 |
| 1 h after Cleansing | |||
| Histamine (ppm) | |||
| Test sample | Japanese pilchard | Japanese horse mackerel | Chub mackerel |
| Control (saline) | 195 ± 31 | 140 ± 22 | 108 ± 12 |
| BiSCaO suspension | 115 ± 22 | 97 ± 11 | 66 ± 11 |
| BiSCaO colloidal dispersion | 96 ± 17 | 86 ± 10 | 45 ± 9 |
| BiSCaO dispersion | 93 ± 15 | 84 ± 11 | 44 ± 10 |
| BiSCaO water | 102 ± 23 | 80 ± 9 | 50 ± 8 |
| SSP-Ca(OH)2 suspension | 136 ± 18 | 110 ± 19 | 57 ± 12 |
| 1 day after Cleansing | |||
| Histamine (ppm) | |||
| Test sample | Japanese pilchard | Japanese horse mackerel | Chub mackerel |
| Control (saline) | 451 ± 32 | 305 ± 18 | 212 ± 15 |
| BiSCaO suspension | 145 ± 25 | 121 ± 16 | 92 ± 15 |
| BiSCaO colloidal dispersion | 112 ± 16 | 95 ± 10 | 65 ± 9 |
| BiSCaO dispersion | 110 ± 10 | 91 ± 7 | 61 ± 8 |
| BiSCaO water | 125 ± 21 | 98 ± 8 | 66 ± 10 |
| SSP-Ca(OH)2 suspension | 265 ± 25 | 210 ± 15 | 142 ± 16 |
| 2 day after Cleansing | |||
| Histamine (ppm) | |||
| Test sample | Japanese pilchard | Japanese horse mackerel | Chub mackerel |
| Control (saline) | 920 ± 180 | 790 ± 87 | 458 ± 58 |
| BiSCaO suspension | 311 ± 56 | 210 ± 22 | 167 ± 9 |
| BiSCaO colloidal dispersion | 145 ± 27 | 111 ± 24 | 105 ± 12 |
| BiSCaO dispersion | 140 ± 28 | 105 ± 15 | 106 ± 11 |
| BiSCaO water | 161 ± 31 | 121 ± 12 | 110 ± 14 |
| SSP-Ca(OH)2 suspension | 468 ± 98 | 432 ± 78 | 335 ± 23 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiruma, S.; Ishihara, M.; Nakamura, S.; Sato, Y.; Asahina, H.; Fukuda, K.; Takayama, T.; Murakami, K.; Yokoe, H. Bioshell Calcium Oxide-Containing Liquids as a Sanitizer for the Reduction of Histamine Production in Raw Japanese Pilchard, Japanese Horse Mackerel, and Chub Mackerel. Foods 2020, 9, 964. https://doi.org/10.3390/foods9070964
Hiruma S, Ishihara M, Nakamura S, Sato Y, Asahina H, Fukuda K, Takayama T, Murakami K, Yokoe H. Bioshell Calcium Oxide-Containing Liquids as a Sanitizer for the Reduction of Histamine Production in Raw Japanese Pilchard, Japanese Horse Mackerel, and Chub Mackerel. Foods. 2020; 9(7):964. https://doi.org/10.3390/foods9070964
Chicago/Turabian StyleHiruma, Sumiyo, Masayuki Ishihara, Shingo Nakamura, Yoko Sato, Haruka Asahina, Koichi Fukuda, Tomohiro Takayama, Kaoru Murakami, and Hidetaka Yokoe. 2020. "Bioshell Calcium Oxide-Containing Liquids as a Sanitizer for the Reduction of Histamine Production in Raw Japanese Pilchard, Japanese Horse Mackerel, and Chub Mackerel" Foods 9, no. 7: 964. https://doi.org/10.3390/foods9070964
APA StyleHiruma, S., Ishihara, M., Nakamura, S., Sato, Y., Asahina, H., Fukuda, K., Takayama, T., Murakami, K., & Yokoe, H. (2020). Bioshell Calcium Oxide-Containing Liquids as a Sanitizer for the Reduction of Histamine Production in Raw Japanese Pilchard, Japanese Horse Mackerel, and Chub Mackerel. Foods, 9(7), 964. https://doi.org/10.3390/foods9070964





