LC–MS/MS Analysis of Choline Compounds in Japanese-Cultivated Vegetables and Fruits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. General Techniques
2.3. Synthesis of PCh, BCh, LCh and EN
2.3.1. Synthesis of PCh
2.3.2. Synthesis of BCh
2.3.3. Synthesis of EN
2.4. Samples of Fresh Cultivated Vegetables and Fruits
2.5. Sample Preparation for Quantification
2.6. Quantification of Choline Compounds
2.7. Method Validation
2.7.1. Linearity
2.7.2. LOD and LOQ
2.7.3. Precision
2.7.4. Accuracy
2.8. Statistical Analysis
3. Results
3.1. Quantification of Choline Compounds in Fresh Cultivated Vegetables and Fruits
3.2. Quantification of Choline Compounds in Six Cultivars of Eggplants
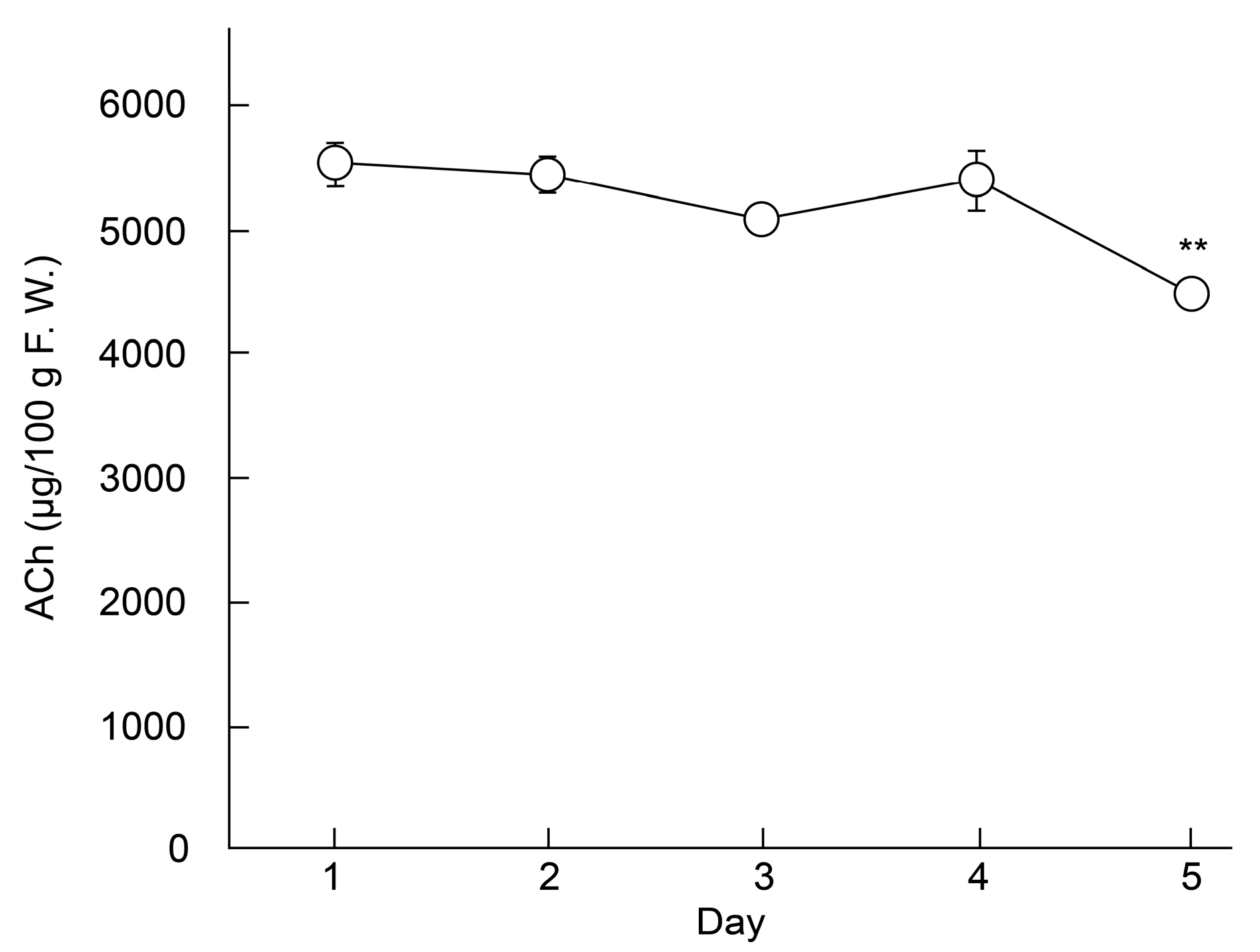
3.3. Effect of Storage on Choline Compound Content in Eggplants (′Higomurasaki′)
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Strecker, A. Über eingige neue Bestandtheile der Schweinegalle. Justus Liebigs Ann. Chem. 1862, 123, 353–360. (In German) [Google Scholar] [CrossRef]
- Zeisel, S.H.; Da Costa, K.; Franklin, P.D.; Alexander, E.A.; Lamont, J.T.; Sheard, N.F.; Beiser, A. Choline, an essential nutrient for humans. FASEB J. 1991, 5, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Choline: An essential nutrient for humans. Nutrition 2000, 16, 669–671. [Google Scholar] [CrossRef]
- Guo, W.-X.; Pye, Q.N.; Williamson, K.S.; Stewart, C.A.; Hensley, K.L.; Kotake, Y.; Floyd, R.A.; Broyles, R.H. Mitochondrial dysfunction in choline deficiency-induced apoptosis in cultured rat hepatocytes. Free Radic. Boil. Med. 2005, 39, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, S.; D’Anneo, A.; Bellavia, G.; Vassallo, B.; Lauricella, M.; De Blasio, A.; Vento, R.; Tesoriere, G. Sodium butyrate induces apoptosis in human hepatoma cells by a mitochondria/caspase pathway, associated with degradation of β-catenin, pRb and Bcl-XL. Eur. J. Cancer 2004, 40, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Mantymaa, P.; Siitonen, T.; Guttorm, T.; Saily, M.; Kinnula, V.; Savolainen, E.-R.; Koistinen, P. Induction of mitochondrial manganese superoxide dismutase confers resistance to apoptosis in acute myeloblastic leukaemia cells exposed to etoposide. Br. J. Haematol. 2000, 108, 574–581. [Google Scholar] [CrossRef]
- Nakamura, K.; Naramoto, K.; Koyama, M. Blood-pressure-lowering effect of fermented buckwheat sprouts in spontaneously hypertensive rats. J. Funct. Foods 2013, 5, 406–415. [Google Scholar] [CrossRef]
- Nakamura, K.; Okitsu, S.; Ishida, R.; Tian, S.; Igari, N.; Amano, Y. Identification of natural lactoylcholine in lactic acid bacteria-fermented food. Food Chem. 2016, 201, 185–189. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Matsumoto, K.; Koyama, M.; Tian, S.; Watanabe, M.; Takahashi, A.; Miyatake, K.; Nakamura, K. Antihypertensive effects of orally administered eggplant (Solanum melongena) rich in acetylcholine on spontaneously hypertensive rats. Food Chem. 2019, 276, 376–382. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Kimura, R.; Kato, N.; Fujii, T.; Seki, M.; Endo, T.; Kato, T.; Kawashima, K. Evolutional study on acetylcholine expression. Life Sci. 2003, 72, 1745–1756. [Google Scholar] [CrossRef]
- Yamada, T.; Fujii, T.; Kanai, T.; Amo, T.; Imanaka, T.; Nishimasu, H.; Wakagi, T.; Shoun, H.; Kamekura, M.; Kamagata, Y.; et al. Expression of acetylcholine (ACh) and ACh-synthesizing activity in Archaea. Life Sci. 2005, 77, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Ewins, A.J. Acetylcholine, a New Active Principle of Ergot. Biochem. J. 1914, 8, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Loewi, O. Über humorale übertragbarkeit der Herznervenwirkung. Pflügers Arch. Eur. J. Physiol. 1921, 189, 239–242. [Google Scholar] [CrossRef]
- Dale, H. Pharmacology and Nerve Endings. J. Nerv. Ment. Dis. 1935, 82, 457. [Google Scholar] [CrossRef]
- USDA Food Composition Databases. Nutrient Lists. 2019. Available online: https://ndb.nal.usda.gov/ndb/nutrients/report/nutrientsfrm?max=25&offset=0&totCount=0&nutrient1=421&nutrient2=&fg=9&fg=11&subset=0&sort=c&measureby=g.html (accessed on 18 May 2019).
- Zeisel, S.H.; Mar, M.-H.; Howe, J.C.; Holden, J.M. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology, Gold Book. Version 2.3.3; International Union of Pure and Applied Chemistry (IUPAC): Research Triangle Park, NC, USA, 2014; pp. 839–841. [Google Scholar]
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. Bioanalytical Method Validation, Guidance for Industry; 2018; pp. 4–10. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 30 July 2020).
- Abe, K.; Ogata, K. Effects of temperature and humidity on pitting injury of eggplant fruits during storage. J. Inst. Cold Chain 1976, 2, 104–108. [Google Scholar] [CrossRef]
- Nishimura, M.; Suzuki, M.; Takahashi, R.; Yamaguchi, S.; Tsubaki, K.; Fujita, T.; Nishihira, J.; Nakamura, K. Daily Ingestion of Eggplant Powder Improves Blood Pressure and Psychological State in Stressed Individuals: A Randomized Placebo-Controlled Study. Nutrition 2019, 11, 2797. [Google Scholar] [CrossRef]
- Trujillo, L. The Elegant Eggplant. Master Gardener J. 2003, 1, 12–14. [Google Scholar]
- Food and Agriculture Organization of the United Nations. FAOSTAT. Statistics Division. Forestry Production and Trade. Available online: http://www.fao.org/faostat/en/#data/FO (accessed on 4 April 2019).
- National Institute of Diabetes and Digestive and Kidney Diseases. NIH Nutrition Research Report, 2015 & 2016; U.S. Department of Health and Human Services, National Institutes of Health: Bethesda, MD, USA, 2017.
- Diabetes Forecast 2011. Eating Colorful Foods Has Health Benefits. Available online: http://www.diabetesforecast.org/2011/aug/eating-colorful-food-has-health-benefits.html (accessed on 30 July 2020).
- Diabetes Forecast 2015. Foods for Your Plate. Available online: http://www.diabetesforecast.org/2015/adm/diabetes-plate-method/foods-for-your-plate.html (accessed on 30 July 2020).
- Guimarães, P.; Galvão, A.; Batista, C.; Azevedo, G.; Oliveira, R.; Lamounier, R.; Freire, N.; Barros, A.; Sakurai, E.; Oliveira, J.; et al. Eggplant (Solanum melongena) infusion has a modest and transitory effect on hypercholesterolemic subjects. Braz. J. Med Boil. Res. 2000, 33, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Scorsatto, M.; Rosa, G.; Luiz, R.R.; da Rocha Pinheiro Mulder, A.; Teodoro, A.J.; de Oliveira, G.M.M. Effect of Eggplant Flour (Solanum melongena L.) associated with hypoenergetic diet on antioxidant status in overweight women—A randomised clinical trial. Int. J. Food Sci. Technol. 2019, 54, 2182–2189. [Google Scholar] [CrossRef]
- Casati, L.; Pagani, F.; Braga, P.C.; Scalzo, R.L.; Sibilia, V. Nasunin, a new player in the field of osteoblast protection against oxidative stress. J. Funct. Foods 2016, 23, 474–484. [Google Scholar] [CrossRef]
- Horie, H.; Ando, A.; Saito, T. The contents of γ-amino butyric acid in eggplant and its accumulation with heat treatment. Nippon Shokuhin Kagaku Kogaku Kaishi 2013, 66, 661–664, (In Japanese, with English abstract). [Google Scholar] [CrossRef]
- Singh, A.P.; Luthria, D.L.; Wilson, T.; Vorsa, N.; Singh, V.; Bañuelos, G.S.; Pasakdee, S. Polyphenols content and antioxidant capacity of eggplant pulp. Food Chem. 2009, 114, 955–961. [Google Scholar] [CrossRef]
- Das, S.; Raychaudhuri, U.; Falchi, M.; Bertelli, A.; Braga, P.C.; Das, D.K. Cardioprotective properties of raw and cooked eggplant (Solanum melongena L.). Food Funct. 2011, 2, 395–399. [Google Scholar] [CrossRef]
- Sudheesh, S.; Sandhya, C.; Koshy, A.S.; Vijayalakshmi, N.R. Antioxidant activity of flavonoids from Solanum melongena. Phytother. Res. 1999, 13, 393–396. [Google Scholar] [CrossRef]
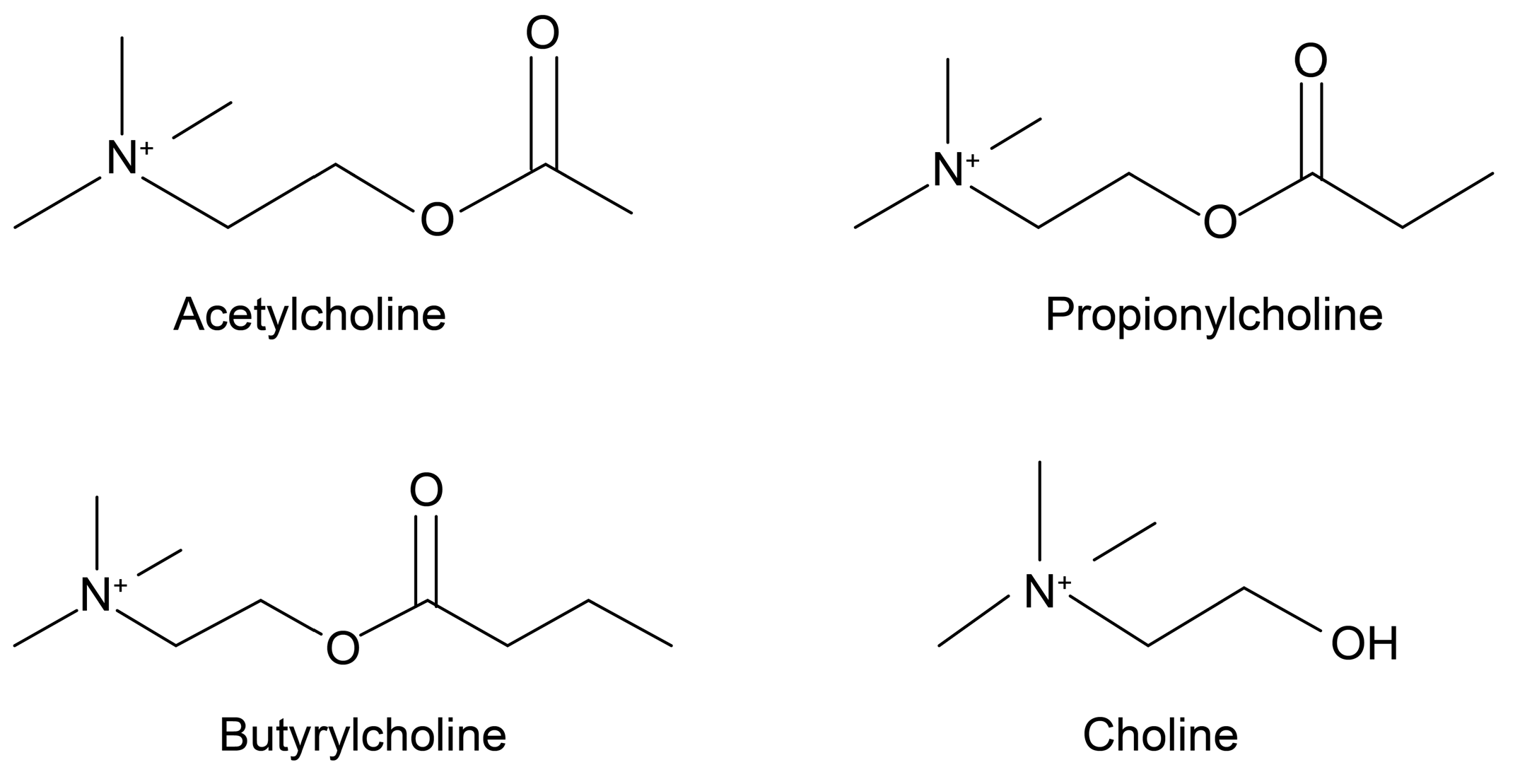
| 1H, 13C NMR in D2O (ppm) | PCh | BCh | EN | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δC | Type | δH | Multi (J in Hz) | Type | δC | Type | δH | Multi (J in Hz) | Type | δC | Type | δH | Multi (J in Hz) | Type | |
| Chemical shift | 8.0 | CH3 | 0.98 | t (7.5) | CH3 | 12.7 | CH3 | 0.74 | t (7.5) | CH3 | 26.3 | CH3 | 1.04 | s | CH3 |
| 27.1 | CH2 | 2.34 | q (7.5) | CH2 | 17.9 | CH2 | 1.45 | sextet (7.4) | CH2 | 33.6 | CH2 | 3.04 | s | CH3 | |
| 53.7 | CH3 | 3.10 | s | CH3 | 35.5 | CH2 | 2.25 | t (7.5) | CH2 | 38.4 | C | 3.34 | m | CH2 | |
| 58.2 | CH2 | 3.63 | m | CH2 | 53.7 | CH3 | 3.03 | s | CH3 | 53.2 | CH3 | 3.55 | m | CH2 | |
| 64.5 | CH2 | 4.44 | m | CH2 | 58.1 | CH2 | 3.56 | m | CH2 | 64.2 | CH2 | ||||
| 176.4 | C | 64.5 | CH2 | 4.38 | m | CH2 | 182.9 | C | |||||||
| 176.4 | C | ||||||||||||||
| MALDI–TOF MS | found | required | found | required | found | required | |||||||||
| [M]+ (m/z) | 160.1327 | 160.1332 | 174.1479 | 174.1489 | 187.1809 | 187.1805 | |||||||||
| Crop | Cultivar | Fresh Weight (g) | Dry Weight (g) | Yield (%) |
|---|---|---|---|---|
| Eggplant | Senryo No. 2 | 56.59 | 4.49 | 7.93 |
| Eggplant | Senshu mizunasu | 89.24 | 4.73 | 5.30 |
| Eggplant | Batten nasu | 61.21 | 3.78 | 6.18 |
| Eggplant | Koryo sarada nasu | 41.21 | 2.40 | 5.82 |
| Eggplant | Onaga nasu | 131.96 | 7.98 | 6.05 |
| Eggplant | Chikuyo | 64.30 | 4.13 | 6.42 |
| Eggplant | Higomurasaki | 121.78 | 6.96 | 5.72 |
| Cucumber | Zubari 163 | 114.52 | 5.90 | 5.15 |
| Tomato | Rinka 409 | 95.37 | 4.99 | 5.23 |
| Paprika | Special | 90.61 | 8.90 | 9.82 |
| Bell pepper | Bell-masari | 25.96 | 3.07 | 11.83 |
| Shishito pepper | Manganji togarashi | 22.19 | 1.99 | 8.97 |
| Asparagus | Welcome | 17.60 | 1.45 | 8.24 |
| Japanese yam | Nagaimo | 79.76 | 21.23 | 26.62 |
| Cabbage | Shinshu 868 | 129.60 | 7.64 | 5.90 |
| Lettuce | Shinano hope | 79.19 | 2.85 | 3.60 |
| Carrot | Kouyou No. 2 | 113.68 | 13.98 | 12.30 |
| Kaiware daikon | unknown | 26.99 | 1.44 | 5.34 |
| Broccoli sprout | unknown | 21.92 | 1.12 | 5.52 |
| Alfalfa bean sprout | unknown | 32.17 | 1.70 | 5.28 |
| Pea sprout | unknown | 33.47 | 2.63 | 7.86 |
| Buckwheat sprout | unknown | 15.89 | 1.12 | 7.05 |
| Apple | Shinano dolce | 71.23 | 10.07 | 14.14 |
| Japanese pear | Twentieth century | 80.59 | 10.59 | 13.14 |
| Grape | Nagano purple | 71.54 | 14.34 | 20.04 |
| Types | Choline Compounds | Linearity | Precision | Accuracy | Limit | ||||
|---|---|---|---|---|---|---|---|---|---|
| Range (μg/mL) | R2 | Intraday (%) | Interday (%) | Recovery (%) | RSD (%) | LOD (pmol/mL) | LOQ (pmol/mL) | ||
| Standard solutions | ACh | 0.0100–3.00 | 1.00 | 0.0727 | 0.521 | 96.4 | 0.471 | 38.7 | 133 |
| PCh | 0.0100–0.500 | 0.999 | 0.915 | 1.29 | 97.3 | 0.929 | 70.6 | 242 | |
| BCh | 0.0100–0.500 | 0.998 | 0.384 | 0.676 | 97.1 | 1.24 | 77.0 | 264 | |
| LCh | 0.0100–6.00 | 0.997 | 0.756 | 1.58 | 96.2 | 1.10 | 42.1 | 144 | |
| Ch | 0.0100–30.0 | 0.999 | 0.483 | 1.15 | 97.4 | 0.693 | 53.6 | 184 | |
| Eggplant samples | ACh | 0.805 | 7.28 | 84.0 | 6.30 | 6.73 × 103 | 2.31 × 104 | ||
| PCh | 1.80 | 5.39 | 95.7 | 12.4 | 3.23 | 11.1 | |||
| BCh | 3.31 | 4.18 | 91.3 | 11.6 | 8.63 | 29.5 | |||
| Ch | 3.19 | 14.5 | 81.4 | 0.240 | 2.12 × 104 | 7.27 × 104 | |||
| Crop | Cultivar | Status | Choline Compounds | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ACh | PCh | BCh | Ch | |||||||
| Cucumber | Zubari 163 | μg/100 g FW | 7.79 × 10−1 | ±0.01 ** | 10.1 | ±0.25 ** | 2.10 | ±0.05 ** | 3.24 × 104 | ±502 |
| μg/100 g DW | 15.1 | ±0.18 ** | 196 | ±4.79 ** | 40.7 | ±1.03 ** | 6.29 × 105 | ±9751 ** | ||
| Tomato | Rinka 0409 | μg/100 g FW | 8.11 × 10−1 | ±0.05 ** | ND | ND | 7.57 × 104 | ±2130 ** | ||
| μg/100 g DW | 15.5 | ±0.90 ** | 1.45 × 106 | ±40,706 ** | ||||||
| Paprika | Special | μg/100 g FW | 1.80 | ±0.04 ** | 4.31 × 10−1 | ±0.01 ** | 3.47 × 10−1 | ±0.00 | 3.40 × 104 | ±88 |
| μg/100 g DW | 18.3 | ±0.43 ** | 4.39 | ±0.10 ** | 3.53 | ±0.05 | 3.46 × 105 | ±892 | ||
| Bell pepper | Bell-masari | μg/100 g FW | 5.95 | ±0.16 ** | 1.20 | ±0.03 ** | 3.98 | ±0.03 ** | 8.40 × 104 | ±1543 ** |
| μg/100 g DW | 50.3 | ±1.33 ** | 10.1 | ±0.28 ** | 33.7 | ±0.25 ** | 7.11 × 105 | ±13,043 ** | ||
| Shishito pepper | Manganji togarashi | μg/100 g FW | 1.55 | ±0.03 ** | 2.86 | ±0.06 ** | 5.47 | ±0.24 ** | 4.31 × 104 | ±375 * |
| μg/100 g DW | 17.3 | ±0.38 ** | 31.9 | ±0.72 ** | 61.0 | ±2.67 ** | 4.81 × 105 | ±4187 | ||
| Eggplant | Senryo No. 2 | μg/100 g FW | 6.12 × 103 | ±132 | 6.25 | ±0.09 | 5.26 × 10−1 | ±0.01 | 2.91 × 104 | ±968 |
| μg/100 g DW | 7.71 × 104 | ±1668 | 78.8 | ±1.15 ** | 6.62 | ±0.12 | 3.67 × 105 | ±12,204 | ||
| Asparagus | Welcome | μg/100 g FW | 2.04 | ±0.02 ** | 15.2 | ±0.27 ** | 1.90 × 10−1 | ±0.01 | 9.69 × 104 | ±1964 ** |
| μg/100 g DW | 24.7 | ±0.19 ** | 184 | ±3.26 ** | 2.31 | ±0.07 | 1.18 × 106 | ±23,836 ** | ||
| Japanese yam | Nagaimo | μg/100 g FW | 2.87 | ±0.06 ** | 84.3 | ±0.18 ** | 3.79 | ±0.11 ** | 8.18 × 104 | ±1130 ** |
| μg/100 g DW | 10.8 | ±0.24 ** | 317 | ±0.69 ** | 14.3 | ±0.41 * | 3.07 × 105 | ±4245 | ||
| Cabbage | Shinshu 868 | μg/100 g FW | 6.83 × 10−1 | ±0.02 ** | 9.49 × 10−1 | ±0.03 ** | 3.32 × 10−1 | ±0.01 | 5.55 × 104 | ±2908 ** |
| μg/100 g DW | 11.6 | ±0.39 ** | 16.1 | ±0.59 ** | 5.64 | ±0.20 | 9.41 × 105 | ±49,337 ** | ||
| Lettuce | Shinano hope | μg/100 g FW | 3.32 × 10−1 | ±0.01 ** | 7.73 × 10−2 | ±0.00 ** | ND | 4.40 × 104 | ±790 ** | |
| μg/100 g DW | 9.22 | ±0.29 ** | 2.15 | ±0.03 ** | 1.22 × 106 | ±21,959 ** | ||||
| Carrot | Kouyou No. 2 | μg/100 g FW | 2.22 | ±0.11 ** | 7.95 × 10−1 | ±0.04 ** | 6.44 × 10−1 | ±0.04 | 1.22 × 105 | ±3068 ** |
| μg/100 g DW | 18.0 | ±0.86 ** | 6.47 | ±0.36 ** | 5.23 | ±0.29 | 9.88 × 105 | ±24,946 ** | ||
| Kaiware daikon | unknown | μg/100 g FW | 8.64 × 10−1 | ±0.03 ** | 2.51 × 10−1 | ±0.01 ** | 3.16 | ±0.14 ** | 7.72 × 104 | ±1441 ** |
| μg/100 g DW | 16.2 | ±0.52 ** | 4.70 | ±0.11 ** | 59.2 | ±1.55 ** | 1.45 × 106 | ±27,004 ** | ||
| Broccoli sprout | unknown | μg/100 g FW | 3.11 | ±0.13 ** | 7.07 × 10−2 | ±0.00 ** | 3.92 | ±0.14 ** | 8.61 × 104 | ±3868 ** |
| μg/100 g DW | 56.4 | ±2.37 ** | 1.28 | ±0.03 ** | 71.1 | ±2.46 ** | 1.56 × 106 | ±70,066 ** | ||
| Alfalfa bean sprout | unknown | μg/100 g FW | 1.70 | ±0.11 ** | 1.45 × 10−1 | ±0.01 ** | ND | 7.82 × 104 | ±751 ** | |
| μg/100 g DW | 32.2 | ±2.06 ** | 2.75 | ±0.14 ** | 1.48 × 106 | ±14,210 ** | ||||
| Pea sprout | unknown | μg/100 g FW | 1.29 | ±0.02 ** | ND | ND | 7.84 × 104 | ±1812 ** | ||
| μg/100 g DW | 16.4 | ±0.19 ** | 9.98 × 105 | ±23,062 ** | ||||||
| Buckwheat sprout | unknown | μg/100 g FW | 4.19 | ±0.05 ** | 5.64 × 10−1 | ±0.03 ** | 4.55 × 10−1 | ±0.03 ** | 7.41 × 104 | ±2693 ** |
| μg/100 g DW | 59.4 | ±0.76 ** | 8.00 | ±0.38 ** | 6.45 | ±0.36 | 1.05 × 106 | ±38,210 ** | ||
| Apple | Shinano dolce | μg/100 g FW | 2.13 | ±0.06 ** | 4.13 × 10−1 | ±0.02 ** | ND | 4.91 × 104 | ±1905 ** | |
| μg/100 g DW | 15.1 | ±0.45 ** | 2.92 | ±0.11 ** | 3.47 × 105 | ±13,472 | ||||
| Japanese pear | Twentieth century | μg/100 g FW | 2.60 | ±0.01 ** | 2.89 × 10−1 | ±0.02 ** | ND | 2.69 × 104 | ±719 | |
| μg/100 g DW | 19.8 | ±0.05 ** | 2.20 | ±0.13 ** | 2.05 × 105 | ±5470 | ||||
| Grape | Nagano purple | μg/100 g FW | 3.15 | ±0.10 ** | 4.35 × 10−1 | ±0.02 ** | ND | 6.86 × 104 | ±186 ** | |
| μg/100 g DW | 15.7 | ±0.50 ** | 2.17 | ±0.08 ** | 3.42 × 105 | ±928 | ||||
| Cultivar | ACh | PCh | BCh | Ch | ||||
|---|---|---|---|---|---|---|---|---|
| Higomurasaki | 5.53 × 103 | ±171 | 7.78 | ±0.18 | 2.85 | ±0.09 | 3.09 × 104 | ±720 |
| Chikuyo | 5.47 × 103 | ±88 | 6.97 | ±0.15 | 1.43 | ±0.04 | 3.38 × 104 | ±1419 |
| Senshu mizunasu | 5.15 × 103 | ±102 | 5.65 | ±0.04 | 0.740 | ±0.04 | 2.18 × 104 | ±743 |
| Koryo sarada nasu | 4.24 × 103 | ±107 | 3.71 | ±0.07 | 1.28 | ±0.05 | 1.96 × 104 | ±338 |
| Batten nasu | 3.06 × 103 | ±50 | 1.53 | ±0.01 | 0.604 | ±0.08 | 3.63 × 104 | ±1748 |
| Onaga nasu | 2.79 × 103 | ±112 | 5.90 | ±0.10 | 2.94 | ±0.04 | 3.56 × 104 | ±848 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Yamaguchi, S.; Koyama, M.; Tian, S.; Ino, A.; Miyatake, K.; Nakamura, K. LC–MS/MS Analysis of Choline Compounds in Japanese-Cultivated Vegetables and Fruits. Foods 2020, 9, 1029. https://doi.org/10.3390/foods9081029
Wang W, Yamaguchi S, Koyama M, Tian S, Ino A, Miyatake K, Nakamura K. LC–MS/MS Analysis of Choline Compounds in Japanese-Cultivated Vegetables and Fruits. Foods. 2020; 9(8):1029. https://doi.org/10.3390/foods9081029
Chicago/Turabian StyleWang, Wenhao, Shohei Yamaguchi, Masahiro Koyama, Su Tian, Aya Ino, Koji Miyatake, and Kozo Nakamura. 2020. "LC–MS/MS Analysis of Choline Compounds in Japanese-Cultivated Vegetables and Fruits" Foods 9, no. 8: 1029. https://doi.org/10.3390/foods9081029
APA StyleWang, W., Yamaguchi, S., Koyama, M., Tian, S., Ino, A., Miyatake, K., & Nakamura, K. (2020). LC–MS/MS Analysis of Choline Compounds in Japanese-Cultivated Vegetables and Fruits. Foods, 9(8), 1029. https://doi.org/10.3390/foods9081029





