Investigation of the Distribution and Content of Acetylcholine, a Novel Functional Compound in Eggplant
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Eggplant and Tomato Samples
2.3. Sample Preparation for Quantification
2.4. Quantification of ACh and Choline
2.5. Statistical Analysis
3. Results
3.1. Quantification of ACh in 26 Eggplant Varieties
3.2. Quantification of ACh in Eggplant (Senryo No. 2) and Tomato (Home Momotaro)
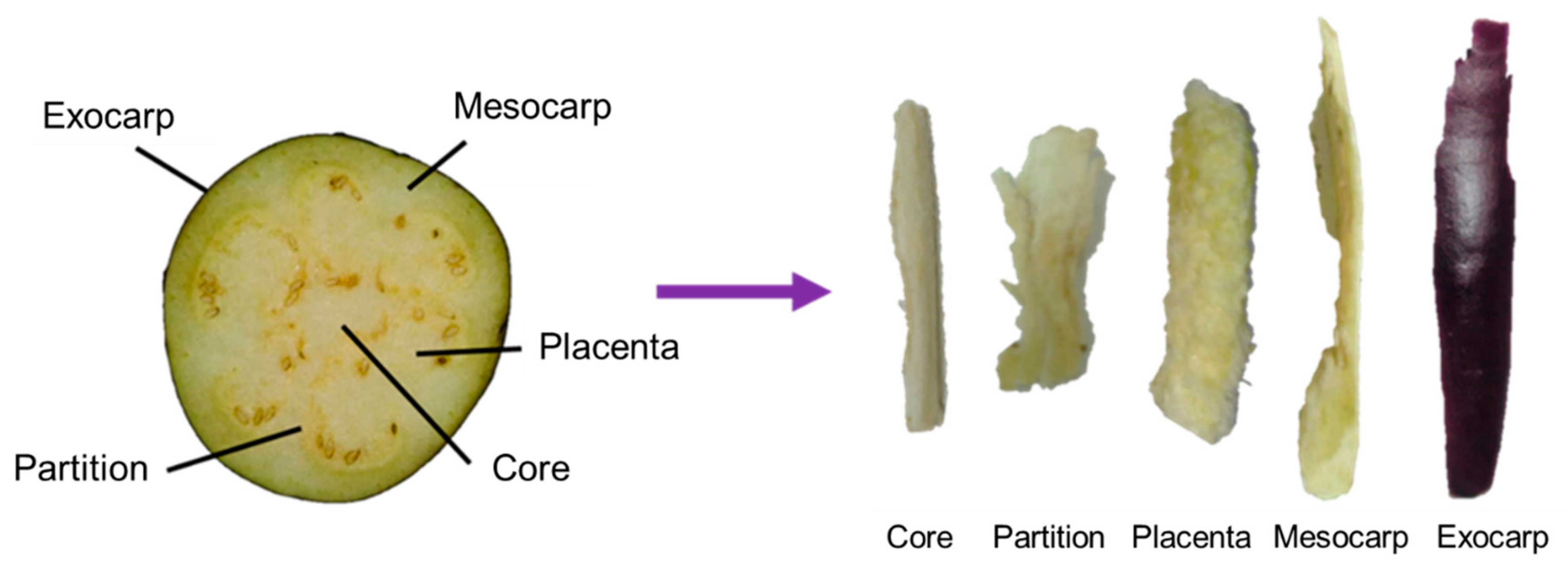
3.3. Quantification of ACh and Choline in Six Parts of Eggplant Fruit (Tosataka)
3.4. Quantification of ACh and Choline in the Base, Center, and Top Parts of Eggplant (Tosataka)
3.5. Influence of Heat Treatment on ACh Content in Eggplant (Tosataka)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. FAOSTAT. Statistics Division. Forestry Production and Trade. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 20 August 2020).
- Bhaduri, P.N. Inter-relationship of non-tuberiferous species of Solanum with some consideration on the origin of brinjal (Solanum melongena L.). Indian J. Genet. Plant Breed. 1951, 11, 75–82. [Google Scholar]
- Khan, R. Solanum melongena and its ancestral forms. In Solanaceae I. The Biology and Taxonomy of the Solanaceae; Hawkes, J.G., Lester, R.N., Skelding, A.D., Eds.; Academic Press: London, UK, 1979; pp. 629–636. [Google Scholar]
- Khan, R. Solanum melongena and the problem of its origin and phylogenetic affinities. J. Indian Bot. Soc. 1979, 58, 99–109. [Google Scholar]
- Gleddie, S.; Keller, W.; Setterfield, G. Eggplant. In Handbook of Plant Cell Culture, Techniques for Propagation and Breeding; Evans, D.A., Sharp, W.R., Eds.; MacMillan: New York, NY, USA, 1986; Volume 3, pp. 500–511. [Google Scholar]
- Horiuchi, Y.; Kimura, R.; Kato, N.; Fujii, T.; Seki, M.; Endo, T.; Kato, T.; Kawashima, K. Evolutional study on acetylcholine expression. Life Sci. 2003, 72, 1745–1756. [Google Scholar] [CrossRef]
- Yamada, T.; Fujii, T.; Kanai, T.; Amo, T.; Imanaka, T.; Nishimasu, H.; Wakagi, T.; Shoun, H.; Kamekura, M.; Kamagata, Y.; et al. Expression of acetylcholine (ACh) and ACh-synthesizing activity in Archaea. Life Sci. 2005, 77, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Matsumoto, K.; Koyama, M.; Tian, S.; Watanabe, M.; Takahashi, A.; Miyatake, K.; Nakamura, K. Antihypertensive effects of orally administered eggplant (Solanum melongena) rich in acetylcholine on spontaneously hypertensive rats. Food Chem. 2019, 276, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Suzuki, M.; Takahashi, R.; Yamaguchi, S.; Tsubaki, K.; Fujita, T.; Nishihira, J.; Nakamura, K. Daily ingestion of eggplant powder improves blood pressure and psychological state in stressed individuals: A randomized placebo-controlled study. Nutrition 2019, 11, 2797. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yamaguchi, S.; Koyama, M.; Tian, S.; Ino, A.; Miyatake, K.; Nakamura, K. LC–MS/MS analysis of choline compounds in Japanese-cultivated vegetables and fruits. Foods 2020, 9, 1029. [Google Scholar] [CrossRef] [PubMed]
- Diet, Nutrition and The Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; WHO Technical Report Series, No. 916; World Health Organization: Geneva, Switzerland, 2003.
- Nakamura, K. Choline Ester-Containing Composition for Oral Ingestion. U.S. Patent WO/2018/070545, 19 April 2018. [Google Scholar]
- Ota, Y. Physiological and genetical studies on the pungency of Capsicum, IV. Secretory organs, receptacles and distribution of capsaicin in the Capsicum fruit. Jpn. J. Breed. 1962, 12, 179–183. [Google Scholar] [CrossRef][Green Version]
- Lo Scalzo, R.; Fibiani, M.; Francese, G.; D’Alessandro, A.; Rotino, G.L.; Conte, P.; Mennella, G. Cooking influence on physico-chemical fruit characteristics of eggplant (Solanum melongena L.). Food Chem. 2016, 194, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Arkoub-Djermoune, L.; Boulekbache-Makhlouf, L.; Zeghichi-Hamri, S.; Bellili, S.; Boukhalfa, F.; Madani, K. Influence of the thermal processing on the physicochemical properties and the antioxidant activity of a Solanaceae vegetable: Eggplant. J. Food Qual. 2016, 39, 181–191. [Google Scholar] [CrossRef]
- Ramírez-Anaya, J.P.; Samaniego-Sánchez, C.; Castañeda-Saucedo, M.C.; Villalón-Mir, M.; López-García de la Serrana, H. Phenols and the antioxidant capacity of Mediterranean vegetables prepared with extra virgin olive oil using different domestic cooking techniques. Food Chem. 2015, 188, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Conte, A.; Cattivelli, A.; Tagliazucchi, D. Domestic cooking methods affect the stability and bioaccessibility of dark purple eggplant (Solanum melongena) phenolic compounds. Food Chem. 2021, 341, 128298. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; Koelle, G.B. Electron microscope localization of acetylcholinesterase and butyrylcholinesterase in the superior cervical ganglion of the cat. I. Normal Ganglion. J. Cell Biol. 1978, 78, 785–809. [Google Scholar]
- Dunat, Y.; Israel, M. The release of acetylcholine. Sci. Am. 1985, 252, 40–48. [Google Scholar]
- Israel, M.; Manaranehe, R. The release of acetylcholine: From the cellular towards a molecular mechanism. Biol. Cell. 1985, 55, 1–14. [Google Scholar] [CrossRef] [PubMed]


| Crop | Part | ACh (mg/100 g FW) |
|---|---|---|
| Eggplant (Senryo No. 2) | Leaf | 2.5 × 10−1 ± 1.5 × 10−1 |
| Root | 4.6 × 10−3 ± 2.1 × 10−3 | |
| Bud | 5.2 × 10−1 ± 3.7 × 10−1 | |
| Calyx | 8.2 × 10−1 ± 1.4 × 10−2 | |
| Ovary (0-week fruit) | 1.2 × 10−2 ± 3.1 × 10−3 | |
| Fruit (1 week after flowering) | 2.5 × 10−1 ± 7.7 × 10−3 | |
| Fruit (2 weeks after flowering) | 6.3 × 10−1 ± 1.8 × 10−1 | |
| Fruit (1.5 months after flowering) | 4.8 ± 1.2 | |
| Tomato (Home Momotaro) | Leaf | ND |
| Root | ND | |
| Flower | ND | |
| Fruit (2 weeks after flowering) | 4.2 × 10−3 ± 5.6 × 10−4 | |
| Fruit (2 months after flowering) | ND |
| Part | ACh (mg/100 g FW) | Choline (mg/100 g FW) |
|---|---|---|
| Exocarp | 7.5 ± 2.0 × 10−1 a | 3.6 ± 2.1 × 10−1 a |
| Mesocarp | 6.6 ± 2.7 × 10−1 ab | 1.3 ± 2.4 × 10−1 b |
| Partition | 6.0 ± 5.4 × 10−1 b | 2.8 ± 7.2 × 10−1 ac |
| Outer placenta | 6.0 ± 2.9 × 10−1 b | 2.9 ± 4.4 × 10−1 ac |
| Inner placenta | 6.5 ± 1.9 × 10−1 ab | 1.9 ± 1.8 × 10−1 bc |
| Core | 6.6 ± 2.6 × 10−1 ab | 2.4 ± 5.1 × 10−1 abc |
| Part | ACh (mg/100 g FW) | Choline (mg/100 g FW) |
|---|---|---|
| Fruit base | 7.0 ± 3.7 × 10−1 | 2.4 ± 2.2 × 10−1 |
| Fruit center | 6.7 ± 5.0 × 10−1 | 2.4 ± 1.7 × 10−1 |
| Fruit top | 6.2 ± 4.4 × 10−1 | 2.3 ± 2.7 × 10−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Yamaguchi, S.; Suzuki, A.; Wagu, N.; Koyama, M.; Takahashi, A.; Takada, R.; Miyatake, K.; Nakamura, K. Investigation of the Distribution and Content of Acetylcholine, a Novel Functional Compound in Eggplant. Foods 2021, 10, 81. https://doi.org/10.3390/foods10010081
Wang W, Yamaguchi S, Suzuki A, Wagu N, Koyama M, Takahashi A, Takada R, Miyatake K, Nakamura K. Investigation of the Distribution and Content of Acetylcholine, a Novel Functional Compound in Eggplant. Foods. 2021; 10(1):81. https://doi.org/10.3390/foods10010081
Chicago/Turabian StyleWang, Wenhao, Shohei Yamaguchi, Ayako Suzuki, Naomi Wagu, Masahiro Koyama, Akihiko Takahashi, Risa Takada, Koji Miyatake, and Kozo Nakamura. 2021. "Investigation of the Distribution and Content of Acetylcholine, a Novel Functional Compound in Eggplant" Foods 10, no. 1: 81. https://doi.org/10.3390/foods10010081
APA StyleWang, W., Yamaguchi, S., Suzuki, A., Wagu, N., Koyama, M., Takahashi, A., Takada, R., Miyatake, K., & Nakamura, K. (2021). Investigation of the Distribution and Content of Acetylcholine, a Novel Functional Compound in Eggplant. Foods, 10(1), 81. https://doi.org/10.3390/foods10010081







