Physicochemical and Functional Properties of Active Fish Gelatin-Based Edible Films Added with Aloe Vera Gel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Aloe Vera Inner Gel
2.3. Preparation of Edible Films
2.4. Characterization of Aloe Vera Inner Gel
2.4.1. Carbohydrate Composition Study
2.4.2. Structural Characterization by ATR-FTIR
2.4.3. Thermal Characterization
2.4.4. Total Phenolic Content
2.4.5. Antioxidant Activity by DPPH, ABTS and FRAP Methods
2.5. Characterization of Edible Films
2.5.1. Thickness, Light Transmittance and Transparency Values
2.5.2. Morphological Characterization
2.5.3. Structural Characterization
2.5.4. Thermal Characterization
2.5.5. Mechanical Properties
2.5.6. Barrier Properties
2.5.7. Antimicrobial Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Aloe Vera Inner Gel
3.1.1. Carbohydrate and Polysaccharide Analysis
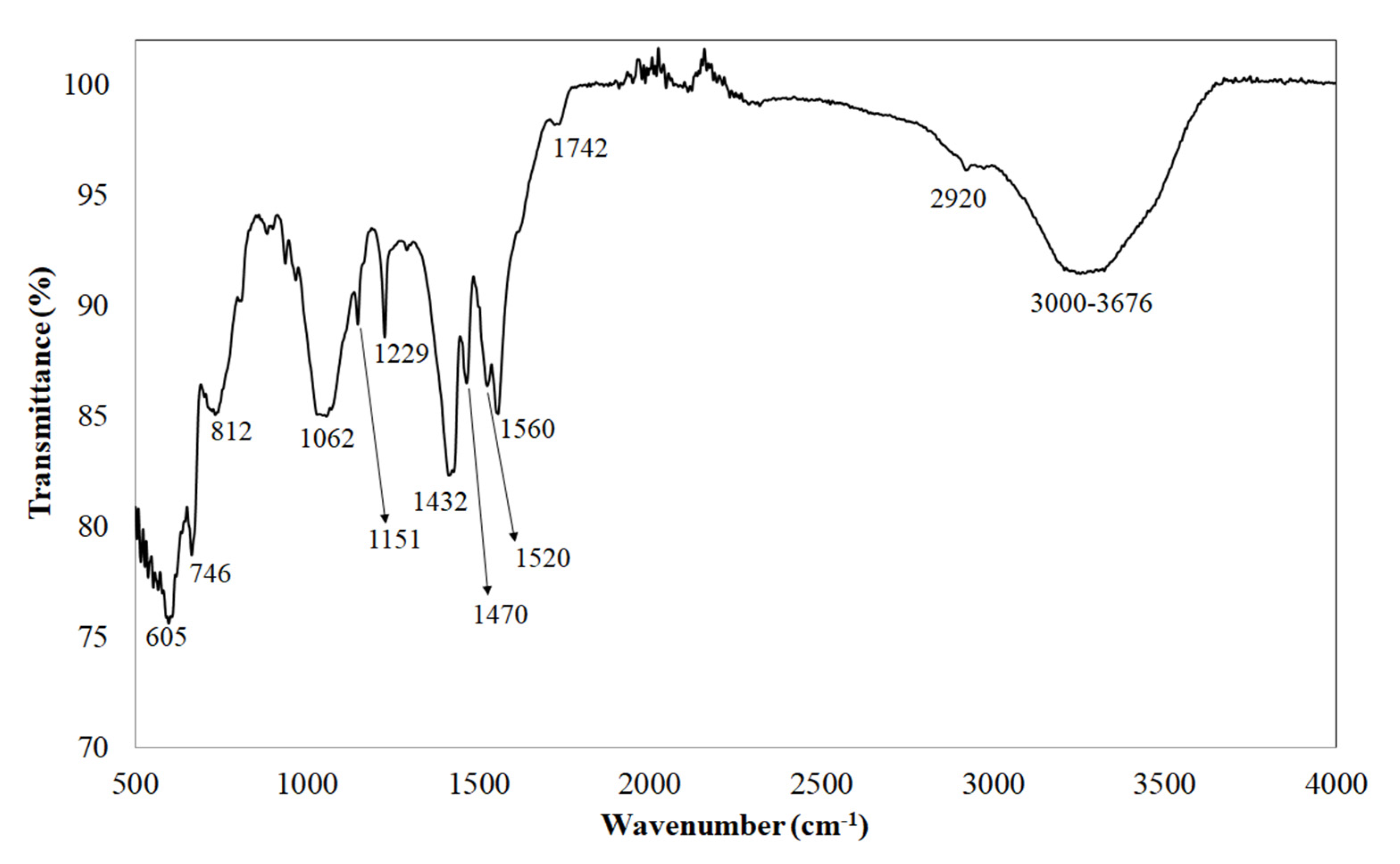
3.1.2. Structural Study by ATR-FTIR
3.1.3. Thermal Characterization
3.1.4. TPC and Antioxidant Activity
3.2. Characterization of Edible Films
3.2.1. Visual Appearance, Thickness and Transparency
3.2.2. Morphological Characterization by SEM
3.2.3. Structural Characterization by ATR-FTIR
3.2.4. Thermal Characterization
3.2.5. Mechanical and Barrier Properties
3.2.6. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mukama, M.; Ambaw, A.; Opara, U.L. Advances in design and performance evaluation of fresh fruit ventilated distribution packaging: A review. Food Packag. Shelf Life 2020, 24, 100472. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Alburquerque, N.; Valverde, J.M.; Guillén, F.; Castillo, S.; Valero, D.; Serrano, M. Postharvest sweet cherry quality and safety maintenance by Aloe vera treatment: A new edible coating. Postharvest Biol. Technol. 2006, 39, 93–100. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). SAVE FOOD: Global Initiative on Food Loss and Waste Reduction. 2017. Available online: http://www.fao.org/3/a-i4068e.pdf (accessed on 2 February 2020).
- Sahraee, S.; Milani, J.M.; Regenstein, J.M.; Kafil, H.S. Protection of foods against oxidative deterioration using edible films and coatings: A review. Food Biosci. 2019, 32, 100451. [Google Scholar] [CrossRef]
- Kchaou, H.; Benbettaieb, N.; Jridi, M.; Nasri, M.; Debeaufort, F. Influence of Maillard reaction and temperature on functional, structure and bioactive properties of fish gelatin films. Food Hydrocoll. 2019, 97, 105196. [Google Scholar] [CrossRef]
- Moula Ali, A.M.; Prodpran, T.; Benjakul, S. Effect of squalene as a glycerol substitute on morphological and barrier properties of golden carp (Probarbus jullieni) skin gelatin film. Food Hydrocoll. 2019, 97, 105201. [Google Scholar] [CrossRef]
- Valdés, A.; Garcia-Serna, E.; Martínez-Abad, A.; Vilaplana, F.; Jimenez, A.; Garrigós, M.C. Gelatin-based antimicrobial films incorporating pomegranate (Punica granatum L.) seed juice by-product. Molecules 2020, 25, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoque, M.S.; Benjakul, S.; Prodpran, T. Properties of film from cuttlefish (Sepia pharaonis) skin gelatin incorporated with cinnamon, clove and star anise extracts. Food Hydrocoll. 2011, 25, 1085–1097. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils. Food Chem. 2012, 134, 1571–1579. [Google Scholar] [CrossRef]
- Valdés, A.; Mellinas, A.C.; Ramos, M.; Burgos, N.; Jiménez, A.; Garrigós, M.C. Use of herbs, spices and their bioactive compounds in active food packaging. RSC Adv. 2015, 5, 40324–40335. [Google Scholar] [CrossRef] [Green Version]
- Parven, A.; Sarker, R.; Megharaj, M.; Meftaul, I. Prolonging the shelf life of Papaya (Carica papaya L.) using Aloe vera gel at ambient temperature. Sci. Hortic. 2020, 265, 109228. [Google Scholar] [CrossRef]
- Kaveh, H.; Vatandoost, S. Possible use of organic compounds on shelf life and quality properties of peeled pomegranate. Food Sci. Nut. 2020, 8, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Radha, M.H.; Laxmipriya, N.P. Evaluation of biological properties and clinical effectiveness of Aloe vera: A systematic review. J. Tradit. Complement. Med. 2015, 5, 21–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hęś, M.; Dziedzic, K.; Górecka, D.; Jędrusek-Golińska, A.; Gujska, E. Aloe vera (L.) Webb.: Natural Sources of Antioxidants—A Review. Plant Foods Hum. Nutr. 2019, 74, 255–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, A.; Dutta Gupta, S.; Ghosh, S. Isolation and characterization of potent bioactive fraction with antioxidant and UV absorbing activity from Aloe barbadensis Miller gel. J. Plant Biochem. Biotechnol. 2013, 22, 483–487. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Rastegar, S. Preservation of mango fruit with guar-based edible coatings enriched with Spirulina platensis and Aloe vera extract during storage at ambient temperature. Sci. Hortic. 2020, 265, 109258. [Google Scholar] [CrossRef]
- Masood, S.; Randhawa, M.A.; Ahmad, W.; Butt, M.S.; Asghar, M.; Jabbar, S. Quality of tomatoes as influenced by bio-chemicals and controlled atmosphere during storage. Proc. Pakistan Acad. Sci. 2019, 55, 39–48, ISSN 2518-4261 (print), ISSN 2518-427X (online). [Google Scholar]
- Sogvar, O.B.; Koushesh Saba, M.; Emamifar, A. Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Vieira, J.M.; Flores-López, M.L.; de Rodríguez, D.J.; Sousa, M.C.; Vicente, A.A.; Martins, J.T. Effect of chitosan—Aloe vera coating on postharvest quality of blueberry (Vaccinium corymbosum) fruit. Postharvest Biol. Technol. 2016, 116, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.; Tojeira, A.; Vaz, D.C.; Mendes, A.; Bártolo, P. Preparation and characterization of films based on alginate and aloe vera. Int. J. Polym. Anal. Charact. 2011, 16, 449–464. [Google Scholar] [CrossRef]
- Sui Chin, S.; Han Lyn, F.; Nur Hanani, Z.A. Effect of Aloe vera (Aloe barbadensis Miller) gel on the physical and functional properties of fish gelatin films as active packaging. Food Packag. Shelf Life 2017, 12, 128–134. [Google Scholar] [CrossRef]
- Mohsen, R.; Elham, F.; Hamid-Reza, A.; Sedigheh, A. Effect of Gelatin-Based Edible Coatings Incorporated with Aloe vera and Black and Green Tea Extracts on the Shelf Life of Fresh-Cut Oranges. J. Food Qual. 2017, 1, 9764650. [Google Scholar] [CrossRef] [Green Version]
- Aloe Vera Las Coronas, Córdoba, Spain. Available online: https://www.youtube.com/watch?v=rHVjetB6xdg (accessed on 1 July 2020).
- Assefa, G.Y.; Admassu, S.E. Antimicrobial Activity, Physicochemical and Mechanical Properties of Aloe (Aloe debrana) Based Packaging Films. Br. J. Appl. Sci. Technol. 2013, 3, 1257–1275. [Google Scholar] [CrossRef]
- Pettolino, F.A.; Walsh, C.; Fincher, G.B.; Bacic, A. Determining the polysaccharide composition of plant cell walls. Nat. Protoc. 2012, 7, 1590–1607. [Google Scholar] [CrossRef] [PubMed]
- Carpita, N.C.; Shea, E.M. Linkage Structure of Carbohydrates by Gas Chromatography–Mass Spectrometry (GC–MS) of Partially Methylated Alditol Acetates. In Analysis of Carbohydrates by GLC and MS; Biermann, M.G.D.C.J., Ed.; CRC Press, Inc.: Boca Raton, FL, USA, 1989; pp. 157–216. [Google Scholar]
- Lucini, L.; Pellizzoni, M.; Pellegrino, R.; Molinari, G.P.; Colla, G. Phytochemical constituents and in vitro radical scavenging activity of different Aloe species. Food Chem. 2015, 170, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Ramful, D.; Tarnus, E.; Aruoma, O.I.; Bourdon, E.; Bahorun, T. Polyphenol composition, vitamin C content and antioxidant capacity of Mauritian citrus fruit pulps. Food Res. Int. 2011, 44, 2088–2099. [Google Scholar] [CrossRef]
- Saini, D.K.; Saini, M.R. Evaluation of radioprotective efficacy and possible mechanism of action of Aloe gel. Environ. Toxicol. Pharmacol. 2011, 31, 427–435. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Guerrero, P.; Nur Hanani, Z.A.; Kerry, J.P.; de la Caba, K. Characterization of soy protein-based films prepared with acids and oils by compression. J. Food Eng. 2011, 107, 41–49. [Google Scholar] [CrossRef]
- Valdés, A.; Ramos, M.; Sanahuja, A.; Garrigós, M.C. Characterization and degradation characteristics of poly(ε-caprolactone)-based composites reinforced with almond skin residues. Polym. Degrad. Stab. 2014, 108, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Bio-based composite edible films containing Origanum vulgare L. essential oil. Ind. Crop. Prod. 2015, 67, 403–413. [Google Scholar] [CrossRef]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P. Properties and characteristics of nanocomposite films from tilapia skin gelatin incorporated with ethanolic extract from coconut husk. J. Food Sci. Technol. 2015, 52, 7669–7682. [Google Scholar] [CrossRef] [PubMed]
- Tham, W.L.; Poh, B.T.; Mohd Ishak, Z.A.; Chow, W.S. Transparent poly(lactic acid)/halloysite nanotube nanocomposites with improved oxygen barrier and antioxidant properties. J. Therm. Anal. Calorim. 2016, 126, 1331–1337. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Grossmann, M.V.E.; Yamashita, F.; Pineda, E.A.G. Antimicrobial, Mechanical, and Barrier Properties of Cassava Starch—Chitosan Films Incorporated with Oregano Essential oil. J. Agric. Food Chem. 2009, 57, 7499–7504. [Google Scholar] [CrossRef] [PubMed]
- Foyle, T.; Jennings, L.; Mulcahy, P. Compositional analysis of lignocellulosic materials: Evaluation of methods used for sugar analysis of waste paper and straw. Bioresour. Technol. 2007, 98, 3026–3036. [Google Scholar] [CrossRef] [PubMed]
- Campestrini, L.H.; Silveira, J.L.M.; Duarte, M.E.R.; Koop, H.S.; Noseda, M.D. NMR and rheological study of Aloe barbadensis partially acetylated glucomannan. Carbohydr. Polym. 2013, 94, 511–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manna, S.; McAnalley, B.H. Determination of the position of the O-acetyl group in a β-(1 → 4)-mannan (acemannan) from Aloe barbardensis Miller. Carbohydr. Res. 1993, 241, 317–319. [Google Scholar] [CrossRef]
- Chinchilla, N.; Carrera, C.; Durán, A.G.; Macías, M.; Torres, A.; Macías, F.A. Aloe barbadensis: How a miraculous plant becomes reality. Phytochem. Rev. 2013, 12, 581–602. [Google Scholar] [CrossRef]
- Femenia, A.; Sánchez, E.S.; Simal, S.; Rosselló, C. Compositional features of polysaccharides from Aloe vera (Aloe barbadensis Miller) plant tissues. Carbohydr. Polym. 1999, 39, 109–117. [Google Scholar] [CrossRef]
- Ni, Y.; Turner, D.; Yates, K.M.; Tizard, I. Isolation and characterization of structural components of Aloe vera L. leaf pulp. Int. Immunopharmacol. 2004, 4, 1745–1755. [Google Scholar] [CrossRef]
- Grindlay, D.; Reynolds, T. The Aloe vera phenomenon: A review of the properties and modern uses of the leaf parenchyma gel. J. Ethnopharmacol. 1986, 16, 117–151. [Google Scholar] [CrossRef]
- Salinas, C.; Handford, M.; Pauly, M.; Dupree, P.; Cardemil, L. Structural modifications of fructans in aloe barbadensis miller (Aloe vera) grown under water stress. PLoS ONE 2016, 11, e0159819. [Google Scholar] [CrossRef] [Green Version]
- Drabczyk, A.; Kudlacik-Kramarczyk, S.; Glab, M.; Kedzierska, M.; Jaromin, A.; Mierzwinski, D.; Tyliszczak, B. Physicochemical investigations of chitosan-based hydrogels containing Aloe Vera designed for biomedical use. Materials 2020, 13, 3073. [Google Scholar] [CrossRef] [PubMed]
- Jithendra, P.; Rajam, A.M.; Kalaivani, T.; Mandal, A.B.; Rose, C. Preparation and characterization of aloe vera blended Collagen-Chitosan composite scaffold for tissue engineering applications. ACS Appl. Mater. Interfaces 2013, 5, 7291–7298. [Google Scholar] [CrossRef] [PubMed]
- Kiran, P.; Rao, P.S. Rheological and structural characterization of prepared aqueous Aloe vera dispersions. Food Res. Int. 2014, 62, 1029–1037. [Google Scholar] [CrossRef]
- Nejatzadeh-Barandozi, F.; Enferadi, S.T. FT-IR study of the polysaccharides isolated from the skin juice, gel juice, and flower of Aloe vera tissues affected by fertilizer treatment. Org. Med. Chem. Lett. 2012, 2, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.-D.; Nie, S.-P.; Yin, J.-Y.; Que, Z.-Q.; Zhang, L.-J.; Huang, X.-J. Polysaccharide from leaf skin of Aloe barbadensis Miller: Part I. Extraction, fractionation, physicochemical properties and structural characterization. Food Hydrocoll. 2017, 73, 176–183. [Google Scholar] [CrossRef]
- Nema, J.; Shrivastava, S.; Mitra, N.G. Physicochemical study of acemannan polysaccharide in Aloe species under the influence of soil reaction (pH) and moisture application. Afr. J. Pure Appl. Chem. 2012, 6, 132–136. [Google Scholar] [CrossRef] [Green Version]
- Alvarado-González, J.S.; Chanona-Pérez, J.; Welti-Chanes, J.; Calderón-Domínguez, G.; Arzate-Vázquez, I.; Pacheco-Alcalá, S.U.; Garibay-Febles, V.; Gutiérrez-López, G.F. Optical, microstructural, functional and nanomechanical properties of aloe vera gel/gellan gum edible films. Rev. Mex. Ing. Quim. 2012, 11, 193–210. [Google Scholar]
- Godinho, J.F.; Berti, F.V.; Müller, D.; Rambo, C.R.; Porto, L.M. Incorporation of Aloe vera extracts into nanocellulose during biosynthesis. Cellulose 2016, 23, 545–555. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Benjakul, S.; Prodpran, T. Influence of palm oil and glycerol on properties of fish skin gelatin-based films. J. Food Sci. Technol. 2016, 53, 2715–2724. [Google Scholar] [CrossRef] [Green Version]
- Arfat, Y.A.; Ahmed, J.; Hiremath, N.; Auras, R.; Joseph, A. Thermo-mechanical, rheological, structural and antimicrobial properties of bionanocomposite films based on fish skin gelatin and silver-copper nanoparticles. Food Hydrocoll. 2017, 62, 191–202. [Google Scholar] [CrossRef]
- Evranos, B.; Aycan, D.; Alemdar, N. Production of ciprofloxacin loaded chitosan/gelatin/bone ash wound dressing with improved mechanical properties. Carbohydr. Polym. 2019, 222, 115007. [Google Scholar] [CrossRef]
- Jeya Shakila, R.; Jeevithan, E.; Varatharajakumar, A.; Jeyasekaran, G.; Sukumar, D. Comparison of the properties of multi-composite fish gelatin films with that of mammalian gelatin films. Food Chem. 2012, 135, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
- Gullon, B.; Pintado, M.E.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Assessment of polyphenolic profile and antibacterial activity of pomegranate peel (Punica granatum) flour obtained from co-product of juice extraction. Food Control 2016, 59, 94–98. [Google Scholar] [CrossRef]
- Saritha, V.; Anilakumar, K.R.; Khanum, F. Antioxidant and antibacterial activity of Aloe vera gel extracts. Int. J. Pharm. Biol. Arch. 2010, 1, 376–384. [Google Scholar]
- Pugh, N.; Ross, S.A.; ElSohly, M.A.; Pasco, D.S. Characterization of aloeride, a new high-molecular-weight polysaccharide from Aloe vera with potent immunostimulatory activity. J. Agric. Food Chem. 2001, 49, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Mendy, T.; Misran, A.; Mashmuf, T.; Ismall, S. Antifungal properties of Aloe vera through in vitro and in vivo screening against postharvest pathogens of papaya fruit. Sci. Hortic. 2019, 257, 108767. [Google Scholar] [CrossRef]






| Linkage | Relative Abundance (%mol) |
|---|---|
| t-Araf | 0.50 ± 0.08 |
| 5-Araf | 0.09 ± 0.03 |
| Total Ara | 0.59 ± 0.12 |
| t-Xylp | 0.09 ± 0.01 |
| 2-Xylp/4-Xylp * | 1.01 ± 0.15 |
| 2,4-Xylp | 0.17 ± 0.05 |
| Total Xyl | 1.26 ± 0.22 |
| t-Glcp | 1.00 ± 0.24 |
| 2-Glcp | 0.23 ± 0.08 |
| 3-Glcp | 0.13 ± 0.02 |
| 4-Glcp | 10.80 ± 0.50 |
| 4,6-Glcp | 0.29 ± 0.03 |
| Total Glc | 12.45 ± 0.87 |
| t-Manp | 0.64 ± 0.10 |
| 2-Manp | 0.20 ± 0.02 |
| 4-Manp | 82.16 ± 0.90 |
| 3,4-Manp | 0.08 ± 0.03 |
| 2,4-Manp | 0.20 ± 0.09 |
| 4,6-Manp | 0.28 ± 0.05 |
| Total Man | 83.56 ± 1.17 |
| t-Galp | 0.47 ± 0.08 |
| 3-Galp | 0.75 ± 0.12 |
| 6-Galp | 0.26 ± 0.03 |
| 3,6-Galp | 1.06 ± 0.34 |
| Total Gal | 2.54 ± 0.58 |
| t-GalpA | 0.03 ± 0.01 |
| 4-GalpA | 0.26 ± 0.15 |
| Total GalA | 0.29 ± 0.60 |
| t-GlcpA | 0.01 ± 0.01 |
| 4-GlcpA | 0.02 ± 0.01 |
| Total GlcA | 0.03 ± 0.02 |
| Wavenumber (cm−1) | Transmittance (%) | ||||
|---|---|---|---|---|---|
| FG | FG/AV1 | FG/AV4 | FG | FG/AV1 | FG/AV4 |
| 3281 ± 12 a | 3273 ± 9 a | 3283 ± 1 a | 79 ± 11 a | 78 ± 9 a | 55 ± 2 b |
| 2932 ± 2 a | 2932 ± 1 a | 2932 ± 1 a | 88 ± 7 a | 86 ± 5 a,b | 73 ± 1 b |
| 1630 ± 1 a | 1629 ± 1 a | 1631 ± 1 a | 67 ± 17 a | 62 ± 14 a,b | 31 ± 2 b |
| 1546 ± 1 a | 1542 ± 5 a | 1545 ± 1 a | 70 ± 15 a | 66 ± 12 a | 36 ± 2 b |
| 1237 ± 1 a | 1236 ± 1 a | 1237 ± 1 a | 76 ± 12 a | 72 ± 10 a,b | 49 ± 1 b |
| 1034 ± 1 a | 1033 ± 1 a | 1034 ± 1 a | 72 ± 15 a | 67 ± 11 a,b | 41 ± 1 b |
| 894 ± 7 a | 898 ± 3 a | 913 ± 2 b | 92 ± 1 a | 78 ± 2 b | 71 ± 1 c |
| 817 ± 6 a | 829 ± 2 b | 835 ± 4 b | 90 ± 2 a | 77 ± 1 b | 72 ± 1 c |
| Thermal Parameter | FG | FG/AV1 | FG/AV4 | |
|---|---|---|---|---|
| DSC parameter | ΔHd (J g−1) | 26 ± 2 a | 27 ± 4 a | 15 ± 4 b |
| Td (°C) | 80 ± 4 a | 83 ± 1 a | 71 ± 5 a | |
| Tg (°C) | 22 ± 6 a | 34 ± 5 b | 38 ± 2 b | |
| TGA parameter | Volatiles loss (%) | 8 ± 1 a | 9± 1 a | 9 ± 2 a |
| Tmax1 (°C) | 81 ± 2 a | 85 ± 1 a | 83 ± 3 a | |
| Tmax2 (°C) | 259 ± 1 a | 259 ± 2 a | 263 ± 5 a | |
| Tmax3 (°C) | 330 ± 3 a | 329 ± 2 a | 328 ± 2 a | |
| Residual weight (700 °C, %) | 15 ± 6 a | 21 ± 1 a | 21 ± 1 a |
| Property | FG | FG/AV1 | FG/AV4 |
|---|---|---|---|
| Elastic modulus (MPa) | 1150 ± 130 a | 1100 ± 200 a | 1400 ± 300 a |
| Elongation at break (%) | 9 ± 3 a | 6 ± 3 a | 8 ± 3 a |
| Tensile strength (MPa) | 36 ± 2 a | 40 ± 10 a | 38 ± 8 a |
| OTR.e (cm3 mm m−2 day) | 0.41 ± 0.08 a | 0.45 ± 0.03 a | 0.45 ± 0.08 a |
| WVP × 10−11 (kg m Pa−1 s−1 m−2) | 4.2 ± 1.2 a | 4.0 ± 0.4 a | 4.8 ± 0.6 a |
| Solubility (%) | 31 ± 1 a | 34 ± 2 a | 44 ± 1 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, J.T.; García, A.V.; Martínez-Abad, A.; Vilaplana, F.; Jiménez, A.; Garrigós, M.C. Physicochemical and Functional Properties of Active Fish Gelatin-Based Edible Films Added with Aloe Vera Gel. Foods 2020, 9, 1248. https://doi.org/10.3390/foods9091248
Sánchez JT, García AV, Martínez-Abad A, Vilaplana F, Jiménez A, Garrigós MC. Physicochemical and Functional Properties of Active Fish Gelatin-Based Edible Films Added with Aloe Vera Gel. Foods. 2020; 9(9):1248. https://doi.org/10.3390/foods9091248
Chicago/Turabian StyleSánchez, Jorge Trujillo, Arantzazu Valdés García, Antonio Martínez-Abad, Francisco Vilaplana, Alfonso Jiménez, and María Carmen Garrigós. 2020. "Physicochemical and Functional Properties of Active Fish Gelatin-Based Edible Films Added with Aloe Vera Gel" Foods 9, no. 9: 1248. https://doi.org/10.3390/foods9091248
APA StyleSánchez, J. T., García, A. V., Martínez-Abad, A., Vilaplana, F., Jiménez, A., & Garrigós, M. C. (2020). Physicochemical and Functional Properties of Active Fish Gelatin-Based Edible Films Added with Aloe Vera Gel. Foods, 9(9), 1248. https://doi.org/10.3390/foods9091248