Toxicity of the Pesticides Imidacloprid, Difenoconazole and Glyphosate Alone and in Binary and Ternary Mixtures to Winter Honey Bees: Effects on Survival and Antioxidative Defenses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Honey Bees
2.3. Exposure to Pesticides
2.4. Analysis of Physiological Life History Traits
2.5. Lipid Peroxidation
2.6. Quantification of Protein Carbonylation
2.7. Statistical Analysis
2.8. Mode of Interaction between Pesticides
3. Results
3.1. Chronic Toxicity of Pesticides Alone or in Combination
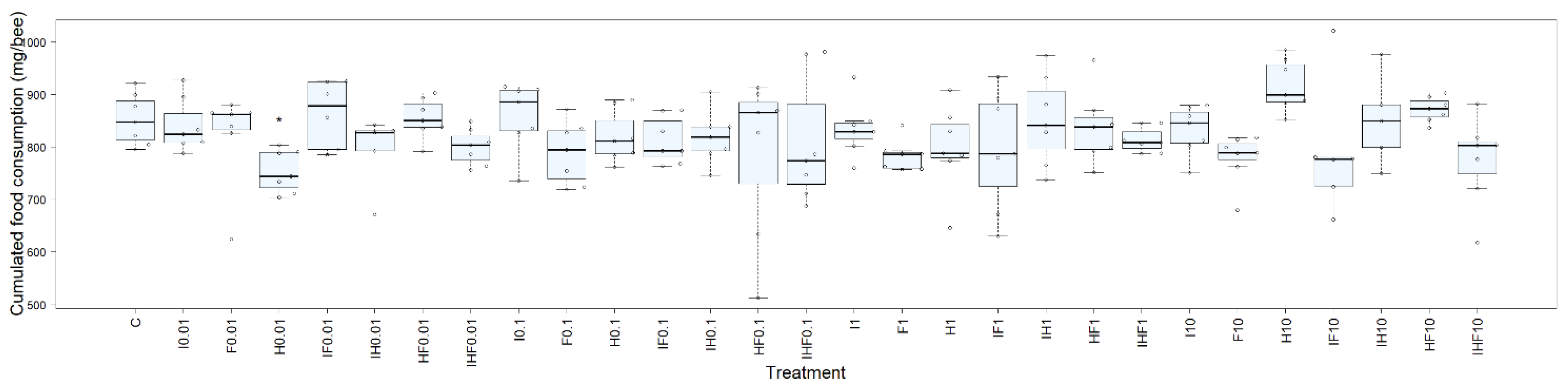
3.2. Effects of Pesticides on Feeding Behavior
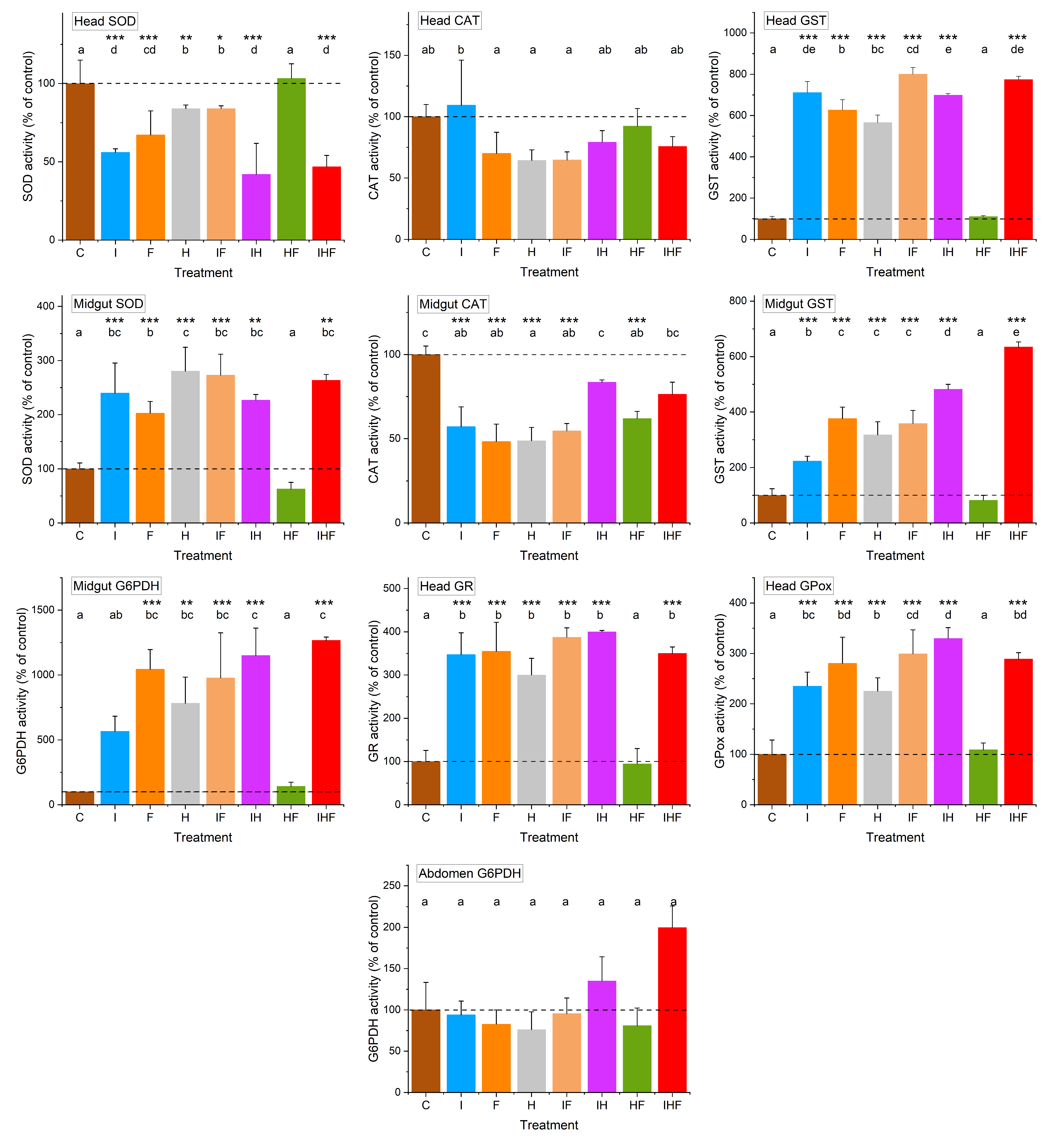
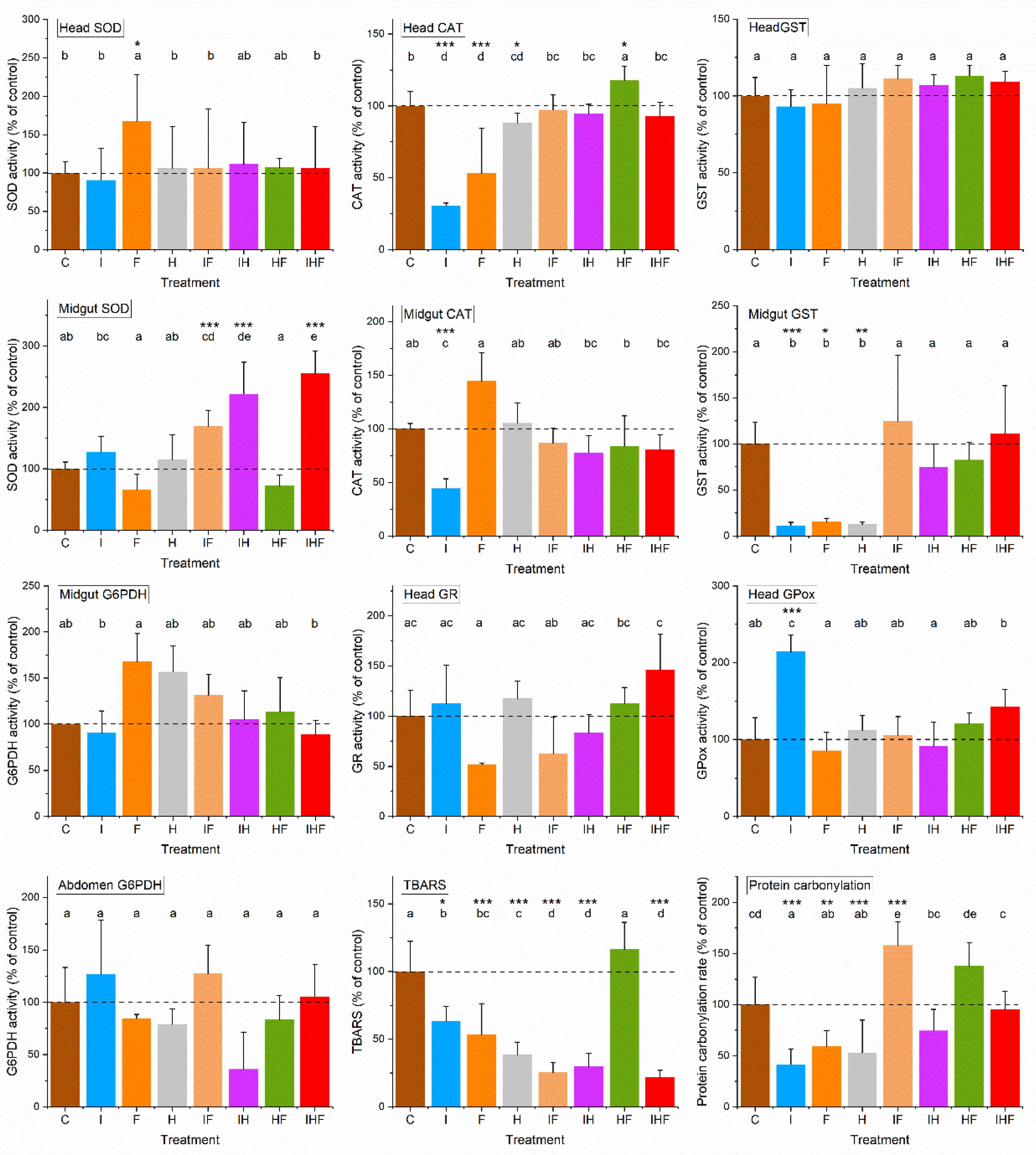
3.3. Variations in Physiological Life History Traits by Pesticides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Hung, K.-L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. B Boil. Sci. 2018, 285, 20172140. [Google Scholar] [CrossRef] [Green Version]
- Breeze, T.D.; Vaissière, B.E.; Bommarco, R.; Petanidou, T.; Seraphides, N.; Kozák, L.; Scheper, J.; Biesmeijer, J.C.; Kleijn, D.; Gyldenkaerne, S.; et al. Agricultural Policies Exacerbate Honeybee Pollination Service Supply-Demand Mismatches Across Europe. PLoS ONE 2014, 9, e82996. [Google Scholar] [CrossRef] [Green Version]
- Gallai, N.; Salles, J.-M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Neumann, P.; Carreck, N. Honey bee colony losses. J. Apic. Res. 2010, 49, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Vanengelsdorp, D.; Meixner, M.D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010, 103, S80–S95. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAOSTAT. ProdSTAT Database. 2018. Available online: http://www.fao.org/faostat/en/#data/QA/visualize (accessed on 21 September 2020).
- Moritz, R.F.A.; de Miranda, J.; Fries, I.; Le Conte, Y.; Neumann, P.J.; Paxton, R. Research strategies to improve honeybee health in Europe. Apidologie 2010, 41, 227–242. [Google Scholar] [CrossRef] [Green Version]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Beekman, M.; Ratnieks, F.L.W. Long-range foraging by the honey-bee, Apis mellifera L. Funct. Ecol. 2000, 14, 490–496. [Google Scholar] [CrossRef] [Green Version]
- Chauzat, M.-P.; Martel, A.-C.; Cougoule, N.; Porta, P.; Lachaize, J.; Zeggane, S.; Aubert, M.; Carpentier, P.; Faucon, J.-P. An assessment of honeybee colony matrices, Apis mellifera (Hymenoptera: Apidae) to monitor pesticide presence in continental France. Environ. Toxicol. Chem. 2011, 30, 103–111. [Google Scholar] [CrossRef]
- Mullin, C.A.; Frazier, M.; Frazier, J.L.; Ashcraft, S.; Simonds, R.; Vanengelsdorp, D.; Pettis, J.S. High Levels of Miticides and Agrochemicals in North American Apiaries: Implications for Honey Bee Health. PLoS ONE 2010, 5, e9754. [Google Scholar] [CrossRef] [Green Version]
- Rubio, F.; Guo, E.; Kamp, L. Survey of Glyphosate Residues in Honey, Corn and Soy Products. J. Environ. Anal. Toxicol. 2014, 5. [Google Scholar] [CrossRef]
- Lambert, O.; Piroux, M.; Puyo, S.; Thorin, C.; L’Hostis, M.; Wiest, L.; Buleté, A.; Delbac, F.; Pouliquen, H. Widespread Occurrence of Chemical Residues in Beehive Matrices from Apiaries Located in Different Landscapes of Western France. PLoS ONE 2013, 8, e67007. [Google Scholar] [CrossRef]
- Bridi, R.; Larena, A.; Pizarro, P.N.; Giordano, A.; Montenegro, G. LC-MS/MS analysis of neonicotinoid insecticides: Residue findings in chilean honeys. Cienc. Agrotec. 2018, 42, 51–57. [Google Scholar] [CrossRef]
- Villalba, A.; Maggi, M.; Ondarza, P.M.; Szawarski, N.; Miglioranza, K.S.B. Influence of land use on chlorpyrifos and persistent organic pollutant levels in honey bees, bee bread and honey: Beehive exposure assessment. Sci. Total Environ. 2020, 713, 136554. [Google Scholar] [CrossRef]
- Belzunces, L.P.; Tchamitchian, S.; Brunet, J.-L. Neural effects of insecticides in the honey bee. Apidologie 2012, 43, 348–370. [Google Scholar] [CrossRef] [Green Version]
- Meikle, W.G.; Adamczyk, J.J.; Weiss, M.; Ross, J.; Werle, C.; Beren, E. Sublethal concentrations of clothianidin affect honey bee colony behavior and interact with landscapes to affect colony growth. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wintermantel, D.; Odoux, J.-F.; Decourtye, A.; Henry, M.; Allier, F.; Bretagnolle, V. Neonicotinoid-induced mortality risk for bees foraging on oilseed rape nectar persists despite EU moratorium. Sci. Total Environ. 2019, 704, 135400. [Google Scholar] [CrossRef]
- Catae, A.F.; Roat, T.C.; Pratavieira, M.; Menegasso, A.R.D.S.; Palma, M.S.; Malaspina, O. Exposure to a sublethal concentration of imidacloprid and the side effects on target and nontarget organs of Apis mellifera (Hymenoptera, Apidae). Ecotoxicology 2018, 27, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-J.; Qiao, N.-H.; Diao, Q.-Y.; Jing, Z.; Vukanti, R.; Dai, P.-L.; Ge, Y. Thiacloprid exposure perturbs the gut microbiota and reduces the survival status in honeybees. J. Hazard. Mater. 2020, 389, 121818. [Google Scholar] [CrossRef]
- Colin, M.-E.; Belzunces, L.P. Evidence of synergy between prochloraz and deltamethrin in Apis mellifera L.: A convenient biological approach. Pestic. Sci. 1992, 36, 115–119. [Google Scholar] [CrossRef]
- Meled, M.; Thrasyvoulou, A.; Belzunces, L.P. Seasonal variations in susceptibility of Apis melliferato the synergistic action of prochloraz and deltamethrin. Environ. Toxicol. Chem. 1998, 17, 2517–2520. [Google Scholar] [CrossRef]
- Chalvet-Monfray, K.; Sabatier, P.; Belzunces, L.P.; Colin, M.E.; Fléché, C. Synergy between deltamethrin and prochloraz in bees: Modeling approach. Environ. Toxicol. Chem. 1996, 15, 525–534. [Google Scholar] [CrossRef]
- Gill, R.J.; Ramos-Rodriguez, O.; Raine, N.E. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 2012, 491, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Tosi, S.; Nieh, J.C. Lethal and sublethal synergistic effects of a new systemic pesticide, flupyradifurone (Sivanto®), on honeybees. Proc. R. Soc. B Boil. Sci. 2019, 286, 20190433. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Zhu, Y.C.; Li, W.H. Interaction patterns and combined toxic effects of acetamiprid in combination with seven pesticides on honey bee (Apis mellifera L.). Ecotoxicol. Environ. Saf. 2020, 190, 110100. [Google Scholar] [CrossRef]
- Pilling, E.D.; Jepson, P.C. Synergism between EBI fungicides and a pyrethroid insecticide in the honeybee (Apis mellifera). Pestic. Sci. 1993, 39, 293–297. [Google Scholar] [CrossRef]
- Papaefthimiou, C.; Theophilidis, G. The Cardiotoxic Action of the Pyrethroid Insecticide Deltamethrin, the Azole Fungicide Prochloraz, and Their Synergy on the Semi-Isolated Heart of the Bee Apis mellifera macedonica. Pestic. Biochem. Physiol. 2001, 69, 77–91. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Shi, T.; Xu, S.; Lu, B.; Qin, H.; Yu, L. Synergistic toxicity and physiological impact of thiamethoxam alone or in binary mixtures with three commonly used insecticides on honeybee. Apidologie 2019, 51, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Schmuck, R.; Stadler, T.; Schmidt, H.-W. Field relevance of a synergistic effect observed in the laboratory between an EBI fungicide and a chloronicotinyl insecticide in the honeybee (Apis mellifera L., Hymenoptera). Pest Manag. Sci. 2003, 59, 279–286. [Google Scholar] [CrossRef]
- Hydrology, C.F.E.; Spurgeon, D.; Hesketh, H.; Lahive, E.; Svendsen, C.; Baas, J.; Robinson, A.; Horton, A.; Heard, M. Chronic oral lethal and sub-lethal toxicities of different binary mixtures of pesticides and contaminants in bees (Apis mellifera, Osmia bicornis and Bombus terrestris). EFSA Support. Publ. 2016, 13, 1076E. [Google Scholar] [CrossRef] [Green Version]
- Iwasa, T.; Motoyama, N.; Ambrose, J.T.; Roe, R.M. Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop Prot. 2004, 23, 371–378. [Google Scholar] [CrossRef]
- Han, W.; Yang, Y.; Gao, J.; Zhao, D.; Ren, C.; Wang, S.; Zhao, S.; Zhong, Y. Chronic toxicity and biochemical response of Apis cerana cerana (Hymenoptera: Apidae) exposed to acetamiprid and propiconazole alone or combined. Ecotoxicology 2019, 28, 399–411. [Google Scholar] [CrossRef]
- Lopez, S.H.; Lozano, A.; Sosa, A.; Hernando, M.D.; Fernández-Alba, A.R. Screening of pesticide residues in honeybee wax comb by LC-ESI-MS/MS. A pilot study. Chemosphere 2016, 163, 44–53. [Google Scholar] [CrossRef]
- Piechowicz, B.; Grodzicki, P.; Podbielska, M.; Tyrka, N.; Śliwa, M. Transfer of Active Ingredients from Plant Protection Products to a Honeybee (Apis mellifera F.) Hive from Winter Oilseed Rape Crops Protected with Conventional Methods. Pol. J. Environ. Stud. 2018, 27, 1219–1228. [Google Scholar] [CrossRef]
- Škerl, M.I.S.; Bolta, V.; Česnik, H.B.; Gregorc, A. Residues of Pesticides in Honeybee (Apis mellifera carnica) Bee Bread and in Pollen Loads from Treated Apple Orchards. Bull. Environ. Contam. Toxicol. 2009, 83, 374–377. [Google Scholar] [CrossRef]
- Almasri, H.; Tavares, D.A.; Pioz, M.; Sené, D.; Tchamitchian, S.; Cousin, M.; Brunet, J.-L.; Belzunces, L.P. Mixtures of an insecticide, a fungicide and a herbicide induce high toxicities and systemic physiological disturbances in winter Apis mellifera honey bees. Ecotoxicol. Environ. Saf. 2020, 203, 111013. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants—An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Neonicotinoid Insecticide Toxicology: Mechanisms of Selective Action. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Li, J.-Z.; Guo, B.-Y.; Wang, H.-L. Determination and Safety Evaluation of Difenoconazole Residues in Apples and Soils. Bull. Environ. Contam. Toxicol. 2010, 85, 427–431. [Google Scholar] [CrossRef] [Green Version]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef] [PubMed]
- FAO. Pesticides use, pesticides trade and pesticides indicators. In Global, Regional and Country Trends: 1990-FAOSTAT Analytical Brief Series No. 29; Botts, F., Ed.; FAO: Rome, Italy, 2021. [Google Scholar]
- Abdallah, O.I.; Hanafi, A.; Ghani, S.B.A.; Ghisoni, S.; Lucini, L. Pesticides contamination in Egyptian honey samples. J. Consum. Prot. Food Saf. 2017, 12, 317–327. [Google Scholar] [CrossRef]
- Johnson, R.M.; Ellis, M.D.; Mullin, C.A.; Frazier, M. Pesticides and honey bee toxicity—USA. Apidologie 2010, 41, 312–331. [Google Scholar] [CrossRef] [Green Version]
- Juan-Borrás, M.; Domenech, E.; Escriche, I. Mixture-risk-assessment of pesticide residues in retail polyfloral honey. Food Control 2016, 67, 127–134. [Google Scholar] [CrossRef]
- Karise, R.; Raimets, R.; Bartkevics, V.; Pugajeva, I.; Pihlik, P.; Keres, I.; Williams, I.H.; Viinalass, H.; Mänd, M. Are pesticide residues in honey related to oilseed rape treatments? Chemosphere 2017, 188, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Piechowicz, B.; Woś, I.; Podbielska, M.; Grodzicki, P. The transfer of active ingredients of insecticides and fungicides from an orchard to beehives. J. Environ. Sci. Health Part B-Pestic. Contam. Agric. Wastes 2017, 53, 18–24. [Google Scholar] [CrossRef]
- Wiest, L.; Buleté, A.; Giroud, B.; Fratta, C.; Amic, S.; Lambert, O.; Pouliquen, H.; Arnaudguilhem, C. Multi-residue analysis of 80 environmental contaminants in honeys, honeybees and pollens by one extraction procedure followed by liquid and gas chromatography coupled with mass spectrometric detection. J. Chromatogr. A 2011, 1218, 5743–5756. [Google Scholar] [CrossRef]
- Paradis, D.; Bérail, G.; Bonmatin, J.-M.; Belzunces, L.P. Sensitive analytical methods for 22 relevant insecticides of 3 chemical families in honey by GC-MS/MS and LC-MS/MS. Anal. Bioanal. Chem. 2014, 406, 621–633. [Google Scholar] [CrossRef]
- Almasri, H.; Tavares, D.A.; Diogon, M.; Pioz, M.; Alamil, M.; Sené, D.; Tchamitchian, S.; Cousin, M.; Brunet, J.-L.; Belzunces, L.P. Physiological effects of the interaction between Nosema ceranae and sequential and overlapping exposure to glyphosate and difenoconazole in the honey bee Apis mellifera. Ecotoxicol. Environ. Saf. 2021, 217, 112258. [Google Scholar] [CrossRef]
- Belzunces, L.P.; Theveniau, M.; Masson, P.; Bounias, M. Membrane acetylcholinesterase from Apis mellifera head solubilized by phosphatidylinositol-specific phospholipase C interacts with an anti-CRD antibody. Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 1990, 95, 609–612. [Google Scholar] [CrossRef]
- Badiou-Bénéteau, A.; Carvalho, S.M.; Brunet, J.-L.; Carvalho, G.A.; Buleté, A.; Giroud, B.; Belzunces, L.P. Development of biomarkers of exposure to xenobiotics in the honey bee Apis mellifera: Application to the systemic insecticide thiamethoxam. Ecotoxicol. Environ. Saf. 2012, 82, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases—First enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Dussaubat, C.; Brunet, J.-L.; Higes, M.; Colbourne, J.K.; Lopez, J.; Choi, J.H.; Martin-Hernández, R.; Botías, C.; Cousin, M.; McDonnell, C.; et al. Gut Pathology and Responses to the Microsporidium Nosema ceranae in the Honey Bee Apis mellifera. PLoS ONE 2012, 7, e37017. [Google Scholar] [CrossRef] [Green Version]
- Beers, R.F., Jr.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Armstrong, D.; Browne, R. The Analysis of Free Radicals, Lipid Peroxides, Antioxidant Enzymes and Compounds Related to Oxidative Stress as Applied to the Clinical Chemistry Laboratory. In Free Radicals in Diagnostic Medicine; Springer: New York, NY, USA, 1994; pp. 43–58. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Requena, J.R.; Chao, C.-C.; Levine, R.L.; Stadtman, E.R. Glutamic and aminoadipic semialdehydes are the main carbonyl products of metal-catalyzed oxidation of proteins. Proc. Natl. Acad. Sci. USA 2000, 98, 69–74. [Google Scholar] [CrossRef]
- Piggott, J.J.; Townsend, C.R.; Matthaei, C.D. Reconceptualizing synergism and antagonism among multiple stressors. Ecol. Evol. 2015, 5, 1538–1547. [Google Scholar] [CrossRef]
- Wegener, J.; Ruhnke, H.; Milchreit, K.; Kleebaum, K.; Franke, M.; Mispagel, S.; Bischoff, G.; Kamp, G.; Bienefeld, K. Secondary biomarkers of insecticide-induced stress of honey bee colonies and their relevance for overwintering strength. Ecotoxicol. Environ. Saf. 2016, 132, 379–389. [Google Scholar] [CrossRef]
- Vázquez, D.E.; Ilina, N.; Pagano, E.A.; Zavala, J.A.; Farina, W.M. Glyphosate affects the larval development of honey bees depending on the susceptibility of colonies. PLoS ONE 2018, 13, e0205074. [Google Scholar] [CrossRef] [Green Version]
- Boily, M.; Sarrasin, B.; DeBlois, C.; Aras, P.; Chagnon, M. Acetylcholinesterase in honey bees (Apis mellifera) exposed to neonicotinoids, atrazine and glyphosate: Laboratory and field experiments. Environ. Sci. Pollut. Res. 2013, 20, 5603–5614. [Google Scholar] [CrossRef]
- Baines, D.; Wilton, E.; Pawluk, A.; De Gorter, M.; Chomistek, N. Neonicotinoids act like endocrine disrupting chemicals in newly-emerged bees and winter bees. Sci. Rep. 2017, 7, 10979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagarde, F.; Beausoleil, C.; Belcher, S.M.; Belzunces, L.P.; Emond, C.; Guerbet, M.; Rousselle, C. Non-monotonic dose-response relationships and endocrine disruptors: A qualitative method of assessment. Environ. Health Glob. Access Sci. Source 2015, 14, 13. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.M.; Dahlgren, L.; Siegfried, B.D.; Ellis, M.D. Acaricide, Fungicide and Drug Interactions in Honey Bees (Apis mellifera). PLoS ONE 2013, 8, e54092. [Google Scholar] [CrossRef] [Green Version]
- Mao, W.; Rupasinghe, S.G.; Johnson, R.M.; Zangerl, A.R.; Schuler, M.A.; Berenbaum, M.R. Quercetin-metabolizing CYP6AS enzymes of the pollinator Apis mellifera (Hymenoptera: Apidae). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 154, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Schuler, M.A.; Berenbaum, M.R. CYP9Q-mediated detoxification of acaricides in the honey bee (Apis mellifera). Proc. Natl. Acad. Sci. USA 2011, 108, 12657–12662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laignelet, L.; Narbonne, J.-F.; Lhuguenot, J.-C.; Riviere, J.-L. Induction and inhibition of rat liver cytochrome(s) P-450 by an imidazole fungicide (prochloraz). Toxicology 1989, 59, 271–284. [Google Scholar] [CrossRef]
- Snégaroff, J.; Bach, J. Effects of the fungicide prochloraz on xenobiotic metabolism in rainbow trout: Inhibition in vitro and time course of induction in vivo. Xenobiotica 1989, 19, 255–267. [Google Scholar] [CrossRef]
- Rivière, J.-L. Prochloraz, a potent inducer of the microsomal cytochrome P-450 system. Pestic. Biochem. Physiol. 1983, 19, 44–52. [Google Scholar] [CrossRef]
- Tozzi, A. 1—A Brief History of the Development of Piperonyl Butoxide as an Insecticide Synergist. In Piperonyl Butoxide; Jones, D.G., Ed.; Academic Press: London, UK, 1999; pp. 1–5. [Google Scholar]
- Willoughby, L.; Batterham, P.; Daborn, P.J. Piperonyl butoxide induces the expression of cytochrome P450 and glutathioneS-transferase genes inDrosophila melanogaster. Pest Manag. Sci. 2007, 63, 803–808. [Google Scholar] [CrossRef]
- Hodgson, E.; Levi, P.E. Interactions of Piperonyl Butoxide with Cytochrome P450. In Piperonyl Butoxide; Elsevier: Cambridge, UK, 1999; p. 41. [Google Scholar] [CrossRef]
- Suchail, S.; De Sousa, G.; Rahmani, R.; Belzunces, L.P. In vivo distribution and metabolisation of14C-imidacloprid in different compartments of Apis mellifera L. Pest Manag. Sci. 2004, 60, 1056–1062. [Google Scholar] [CrossRef]
- Suchail, S.; Guez, D.; Belzunces, L.P. Discrepancy between acute and chronic toxicity induced by imidacloprid and its metabolites in Apis mellifera. Environ. Toxicol. Chem. 2001, 20, 2482–2486. [Google Scholar] [CrossRef] [PubMed]
- Blot, N.; Veillat, L.; Rouzé, R.; Delatte, H. Glyphosate, but not its metabolite AMPA, alters the honeybee gut microbiota. PLoS ONE 2019, 14, e0215466. [Google Scholar] [CrossRef]
- Gauthier, M.; Aras, P.; Paquin, J.; Boily, M. Chronic exposure to imidacloprid or thiamethoxam neonicotinoid causes oxidative damages and alters carotenoid-retinoid levels in caged honey bees (Apis mellifera). Sci. Rep. 2018, 8, 16274. [Google Scholar] [CrossRef] [PubMed]
- Kessler, S.C.; Tiedeken, E.J.; Simcock, K.L.; Derveau, S.; Mitchell, J.; Softley, S.; Radcliffe, A.; Stout, J.; Wright, G.A. Bees prefer foods containing neonicotinoid pesticides. Nature 2015, 521, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; Yao, J.; Adamczyk, J.; Luttrell, R. Feeding toxicity and impact of imidacloprid formulation and mixtures with six representative pesticides at residue concentrations on honey bee physiology (Apis mellifera). PLoS ONE 2017, 12, e0178421. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.-H.; Wu, W.-Y.; Berenbaum, M.R. Behavioral responses of honey bees (Apis mellifera) to natural and synthetic xenobiotics in food. Sci. Rep. 2017, 7, 15924. [Google Scholar] [CrossRef]
- Almasri, H.; Tavares, D.A.; Tchamitchian, S.; Pélissier, M.; Sené, D.; Cousin, M.; Brunet, J.-L.; Belzunces, L.P. Toxicological status changes the susceptibility of the honey bee Apis mellifera to a single fungicidal spray application. Environ. Sci. Pollut. Res. 2021, 28, 42807–42820. [Google Scholar] [CrossRef]
- Dupré-Crochet, S.; Erard, M.; Nüβe, O. ROS production in phagocytes: Why, when, and where? J. Leukoc. Biol. 2013, 94, 657–670. [Google Scholar] [CrossRef]
- Finkel, T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003, 15, 247–254. [Google Scholar] [CrossRef]
- Barouki, R. Ageing free radicals and cellular stress. Med. Sci. 2006, 22, 266–272. [Google Scholar] [CrossRef] [Green Version]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohen, R.; Nyska, A. Invited Review: Oxidation of Biological Systems: Oxidative Stress Phenomena, Antioxidants, Redox Reactions, and Methods for Their Quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H. Strategies of antioxidant defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar] [CrossRef]
- Corona, M.; Robinson, G.E. Genes of the antioxidant system of the honey bee: Annotation and phylogeny. Insect Mol. Biol. 2006, 15, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Nwani, C.D.; Lakra, W.S.; Nagpure, N.S.; Kumar, R.; Kushwaha, B.; Srivastava, S.K. Toxicity of the Herbicide Atrazine: Effects on Lipid Peroxidation and Activities of Antioxidant Enzymes in the Freshwater Fish Channa Punctatus (Bloch). Int. J. Environ. Res. Public Health 2010, 7, 3298–3312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lushchak, O.V.; Kubrak, O.I.; Storey, J.M.; Storey, K.B.; Lushchak, V.I. Low toxic herbicide Roundup induces mild oxidative stress in goldfish tissues. Chemosphere 2009, 76, 932–937. [Google Scholar] [CrossRef]
- De Aguiar, L.M.; Figueira, F.H.; Gottschalk, M.; da Rosa, C.E. Glyphosate-based herbicide exposure causes antioxidant defence responses in the fruit fly Drosophila melanogaster. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 185–186, 94–101. [Google Scholar] [CrossRef]
- Puértolas, L.; Damásio, J.; Barata, C.; Soares, A.M.V.M.; Prat, N. Evaluation of side-effects of glyphosate mediated control of giant reed (Arundo donax) on the structure and function of a nearby Mediterranean river ecosystem. Environ. Res. 2010, 110, 556–564. [Google Scholar] [CrossRef]
- Jasper, R.; Locatelli, G.O.; Pilati, C.; Locatelli, C. Evaluation of biochemical, hematological and oxidative parameters in mice exposed to the herbicide glyphosate-Roundup®. Interdiscip. Toxicol. 2012, 5, 133–140. [Google Scholar] [CrossRef]
- Guilherme, S.; Gaivao, I.; Santos, M.A.; Pacheco, M. European eel (Anguilla anguilla) genotoxic and pro-oxidant responses following short-term exposure to Roundup(R)-a glyphosate-based herbicide. Mutagenesis 2010, 25, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Ordoñez, E.; Uría-Galicia, E.; Ruiz-Picos, R.A.; Duran, A.G.S.; Trejo, Y.H.; Sedeño-Díaz, J.E.; López-López, E. Effect of Yerbimat Herbicide on Lipid Peroxidation, Catalase Activity, and Histological Damage in Gills and Liver of the Freshwater Fish Goodea Atripinnis. Arch. Environ. Contam. Toxicol. 2011, 61, 443–452. [Google Scholar] [CrossRef]
- Malev, O.; Klobučar, R.S.; Fabbretti, E.; Trebše, P. Comparative toxicity of imidacloprid and its transformation product 6-chloronicotinic acid to non-target aquatic organisms: Microalgae Desmodesmus subspicatus and amphipod Gammarus fossarum. Pestic. Biochem. Physiol. 2012, 104, 178–186. [Google Scholar] [CrossRef]
- Contardo-Jara, V.; Klingelmann, E.; Wiegand, C. Bioaccumulation of glyphosate and its formulation Roundup Ultra in Lumbriculus variegatus and its effects on biotransformation and antioxidant enzymes. Environ. Pollut. 2009, 157, 57–63. [Google Scholar] [CrossRef]
- Helmer, S.H.; Kerbaol, A.; Aras, P.; Jumarie, C.; Boily, M. Effects of realistic doses of atrazine, metolachlor, and glyphosate on lipid peroxidation and diet-derived antioxidants in caged honey bees (Apis mellifera). Environ. Sci. Pollut. Res. 2015, 22, 8010–8021. [Google Scholar] [CrossRef]
- Jumarie, C.; Aras, P.; Boily, M. Mixtures of herbicides and metals affect the redox system of honey bees. Chemosphere 2017, 168, 163–170. [Google Scholar] [CrossRef]
- Gregorc, A.; Alburaki, M.; Rinderer, N.; Sampson, B.; Knight, P.R.; Karim, S.; Adamczyk, J. Effects of coumaphos and imidacloprid on honey bee (Hymenoptera: Apidae) lifespan and antioxidant gene regulations in laboratory experiments. Sci. Rep. 2018, 8, 15003. [Google Scholar] [CrossRef]
- Vázquez, D.E.; Latorre-Estivalis, J.M.; Ons, S.; Farina, W.M. Chronic exposure to glyphosate induces transcriptional changes in honey bee larva: A toxicogenomic study. Environ. Pollut. 2020, 261, 114148. [Google Scholar] [CrossRef] [PubMed]
- Kairo, G.; Poquet, Y.; Haji, H.; Tchamitchian, S.; Cousin, M.; Bonnet, M.; Pelissier, M.; Kretzschmar, A.; Belzunces, L.P.; Brunet, J.-L. Assessment of the toxic effect of pesticides on honey bee drone fertility using laboratory and semifield approaches: A case study of fipronil. Environ. Toxicol. Chem. 2017, 36, 2345–2351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Li, M.; He, J.; Zhao, X.; Chaimanee, V.; Huang, W.-F.; Nie, H.; Zhao, Y.; Su, S. Differential physiological effects of neonicotinoid insecticides on honey bees: A comparison between Apis mellifera and Apis cerana. Pestic. Biochem. Physiol. 2017, 140, 1–8. [Google Scholar] [CrossRef]
- Carvalho, S.M.; Belzunces, L.P.; Carvalho, G.A.; Brunet, J.-L.; Badiou-Beneteau, A. Enzymatic biomarkers as tools to assess environmental quality: A case study of exposure of the honeybee Apis mellifera to insecticides. Environ. Toxicol. Chem. 2013, 32, 2117–2124. [Google Scholar] [CrossRef]
- Renzi, M.T.; Amichot, M.; Pauron, D.; Tchamitchian, S.; Brunet, J.-L.; Kretzschmar, A.; Maini, S.; Belzunces, L.P. Chronic toxicity and physiological changes induced in the honey bee by the exposure to fipronil and Bacillus thuringiensis spores alone or combined. Ecotoxicol. Environ. Saf. 2016, 127, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Dicus, M.; Ho, N.D.; Boroujerdi-Rad, L.; Sindhu, R.K. Oxidative stress and dysregulation of superoxide dismutase and NADPH oxidase in renal insufficiency. Kidney Int. 2003, 63, 179–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michiels, C.; Raes, M.; Toussaint, O.; Remacle, J. Importance of SE-glutathione peroxidase, catalase, and CU/ZN-SOD for cell survival against oxidative stress. Free Radic. Biol. Med. 1994, 17, 235–248. [Google Scholar] [CrossRef]
- Du Rand, E.E.; Smit, S.; Beukes, M.; Apostolides, Z.; Pirk, C.W.W.; Nicolson, S. Detoxification mechanisms of honey bees (Apis mellifera) resulting in tolerance of dietary nicotine. Sci. Rep. 2015, 5, 11779. [Google Scholar] [CrossRef] [Green Version]
- Berenbaum, M.R.; Johnson, R.M. Xenobiotic detoxification pathways in honey bees. Curr. Opin. Insect Sci. 2015, 10, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Suchail, S.; Debrauwer, L.; Belzunces, L.P. Metabolism of imidacloprid in Apis mellifera. Pest Manag. Sci. 2003, 60, 291–296. [Google Scholar] [CrossRef]
- Pisa, L.W.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Downs, C.A.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; McField, M.; et al. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. 2015, 22, 68–102. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.-Y.; Luo, Q.-H.; Hou, C.-S.; Wang, Q.; Dai, P.; Gao, J.; Liu, Y.-J.; Diao, Q.-Y. Sublethal effects of imidacloprid on targeting muscle and ribosomal protein related genes in the honey bee Apis mellifera L. Sci. Rep. 2017, 7, 15943. [Google Scholar] [CrossRef] [PubMed]
- Colin, T.; Plath, J.A.; Klein, S.; Vine, P.; Devaud, J.-M.; Lihoreau, M.; Meikle, W.G.; Barron, A.B. The miticide thymol in combination with trace levels of the neonicotinoid imidacloprid reduces visual learning performance in honey bees (Apis mellifera). Apidologie 2020, 51, 499–509. [Google Scholar] [CrossRef]
- Llewellyn, H.J.; Hare-Harris, A.; Hranitz, J.M.; Surmacz, C.A.; Surmacz, C. Sublethal Doses of the Neonicotinoid Imidacloprid Alters mRNA Expression in Cellular Stress Pathways in Honey Bees. Integr. Comp. Biol. 2020, 60, E367. [Google Scholar]
- Gooley, Z.C.; Gooley, A.C.; Reeve, J.D. Neonicotinoid-contaminated diet causes behavior changes in forager honey bees (Apis mellifera) that may reduce colony survival during late fall. J. Apic. Res. 2021, 60, 726–733. [Google Scholar] [CrossRef]
- Balbuena, M.S.; Tison, L.; Hahn, M.-L.; Greggers, U.; Menzel, R.; Farina, W.M. Effects of sublethal doses of glyphosate on honeybee navigation. J. Exp. Biol. 2015, 218, 2799–2805. [Google Scholar] [CrossRef] [Green Version]
- Faita, M.R.; Oliveira, E.D.; Alves, V.V.; Orth, A.I.; Nodari, R.O. Changes in hypopharyngeal glands of nurse bees (Apis mellifera) induced by pollen-containing sublethal doses of the herbicide Roundup(R). Chemosphere. 2018, 211, 566–572. [Google Scholar] [CrossRef]
- Castelli, L.; Balbuena, S.; Branchiccela, B.; Zunino, P.; Liberti, J.; Engel, P.; Antúnez, K. Impact of Chronic Exposure to Sublethal Doses of Glyphosate on Honey Bee Immunity, Gut Microbiota and Infection by Pathogens. Microorganisms 2021, 9, 845. [Google Scholar] [CrossRef]
- Delkash-Roudsari, S.; Chicas-Mosier, A.M.; Goldansaz, S.H.; Talebi-Jahromi, K.; Ashouri, A.; Abramson, C.I. Assessment of lethal and sublethal effects of imidacloprid, ethion, and glyphosate on aversive conditioning, motility, and lifespan in honey bees (Apis mellifera L.). Ecotoxicol. Environ. Saf. 2020, 204, 111108. [Google Scholar] [CrossRef]
- Motta, E.V.S.; Mak, M.; De Jong, T.K.; Powell, J.E.; O’Donnell, A.; Suhr, K.J.; Riddington, I.M.; Moran, N.A. Oral or Topical Exposure to Glyphosate in Herbicide Formulation Impacts the Gut Microbiota and Survival Rates of Honey Bees. Appl. Environ. Microbiol. 2020, 86, e01150-20. [Google Scholar] [CrossRef]
- Vázquez, D.E.; Balbuena, M.S.; Chaves, F.; Gora, J.; Menzel, R.; Farina, W.M. Sleep in honey bees is affected by the herbicide glyphosate. Sci. Rep. 2020, 10, 10516. [Google Scholar] [CrossRef]
- Ihara, M.; Matsuda, K. Neonicotinoids: Molecular mechanisms of action, insights into resistance and impact on pollinators. Curr. Opin. Insect Sci. 2018, 30, 86–92. [Google Scholar] [CrossRef]
- Monk, B.C.; Sagatova, A.A.; Hosseini, P.; Ruma, Y.N.; Wilson, R.K.; Keniya, M.V. Fungal Lanosterol 14α-demethylase: A target for next-generation antifungal design. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2020, 1868, 140206. [Google Scholar] [CrossRef]
- Iverson, A.; Hale, C.; Richardson, L.; Miller, O.; McArt, S. Synergistic effects of three sterol biosynthesis inhibiting fungicides on the toxicity of a pyrethroid and neonicotinoid insecticide to bumble bees. Apidologie 2019, 50, 733–744. [Google Scholar] [CrossRef]
- Syromyatnikov, M.Y.; Kokina, A.V.; Lopatin, A.V.; Starkov, A.A.; Popov, V.N. Evaluation of the toxicity of fungicides to flight muscle mitochondria of bumblebee (Bombus terrestris L.). Pestic. Biochem. Physiol. 2017, 135, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Vandame, R.; Belzunces, L.P. Joint actions of deltamethrin and azole fungicides on honey bee thermoregulation. Neurosci. Lett. 1998, 251, 57–60. [Google Scholar] [CrossRef]
- European Food Safety, A. Review of the existing maximum residue levels for glyphosate according to Article 12 of Regulation (EC) No 396/2005–revised version to take into account omitted data. EFSA J. 2019, 17, e05862. [Google Scholar] [CrossRef] [PubMed]
- Mañas, F.; Peralta, L.; Raviolo, J.; Ovando, H.G.; Weyers, A.; Ugnia, L.; Cid, M.G.; Larripa, I.; Gorla, N. Genotoxicity of AMPA, the environmental metabolite of glyphosate, assessed by the Comet assay and cytogenetic tests. Ecotoxicol. Environ. Saf. 2009, 72, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Ostiguy, N.; Drummond, F.A.; Aronstein, K.; Eitzer, B.; Ellis, J.D.; Spivak, M.; Sheppard, W.S. Honey Bee Exposure to Pesticides: A Four-Year Nationwide Study. Insects 2019, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Gray, A.; Brodschneider, R.; Adjlane, N.; Ballis, A.; Brusbardis, V.; Charrière, J.-D.; Chlebo, R.; Coffey, M.F.; Cornelissen, B.; Da Costa, C.A.; et al. Loss rates of honey bee colonies during winter 2017/18 in 36 countries participating in the COLOSS survey, including effects of forage sources. J. Apicult. Res. 2019, 58, 479–485. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, E.; Almasri, H.; Paris, L.; Diogon, M.; Pioz, M.; Cousin, M.; Sené, D.; Tchamitchian, S.; Tavares, D.A.; Delbac, F.; et al. Toxicity of the Pesticides Imidacloprid, Difenoconazole and Glyphosate Alone and in Binary and Ternary Mixtures to Winter Honey Bees: Effects on Survival and Antioxidative Defenses. Toxics 2022, 10, 104. https://doi.org/10.3390/toxics10030104
Pal E, Almasri H, Paris L, Diogon M, Pioz M, Cousin M, Sené D, Tchamitchian S, Tavares DA, Delbac F, et al. Toxicity of the Pesticides Imidacloprid, Difenoconazole and Glyphosate Alone and in Binary and Ternary Mixtures to Winter Honey Bees: Effects on Survival and Antioxidative Defenses. Toxics. 2022; 10(3):104. https://doi.org/10.3390/toxics10030104
Chicago/Turabian StylePal, Elisa, Hanine Almasri, Laurianne Paris, Marie Diogon, Maryline Pioz, Marianne Cousin, Déborah Sené, Sylvie Tchamitchian, Daiana Antonia Tavares, Frédéric Delbac, and et al. 2022. "Toxicity of the Pesticides Imidacloprid, Difenoconazole and Glyphosate Alone and in Binary and Ternary Mixtures to Winter Honey Bees: Effects on Survival and Antioxidative Defenses" Toxics 10, no. 3: 104. https://doi.org/10.3390/toxics10030104
APA StylePal, E., Almasri, H., Paris, L., Diogon, M., Pioz, M., Cousin, M., Sené, D., Tchamitchian, S., Tavares, D. A., Delbac, F., Blot, N., Brunet, J.-L., & Belzunces, L. P. (2022). Toxicity of the Pesticides Imidacloprid, Difenoconazole and Glyphosate Alone and in Binary and Ternary Mixtures to Winter Honey Bees: Effects on Survival and Antioxidative Defenses. Toxics, 10(3), 104. https://doi.org/10.3390/toxics10030104







