Abstract
In recent years, studies of heavy metal air pollution have increasingly gone beyond determining total concentrations of individual toxic metals. Chemical fractionation of potentially toxic elements in airborne particles is becoming an important part of these studies. This review covers the articles that have been published over the last three decades. Attention was paid to the issue of atmospheric aerosol sampling, sample pretreatment, sequential extraction schemes and conditions of individual extractions. Geochemical forms of metals occurring in the air in urban areas were considered in detail. Based on the data sets from chemical fractionation of particulate matter samples by three sequential extraction procedures (SEPs)—Fernández Espinosa, BCR and Chester’s—the compilation of the chemical distribution patterns of As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb and Zn was prepared. The human health risk posed by these toxic and/or carcinogenic elements via inhalation of atmospheric particles was estimated for two categories of polluted urban areas: the commonly encountered pollution level and the high pollution level.
1. Introduction
Among the primary air pollutants (CO, CO2, CH4, VOCs, NO, N2O, NH3, H2S, SO2 and particulate matter) and the secondary air pollutants (NO2, HNO3, O3, H2SO4, sulfate, nitrate and organic aerosols), atmospheric particulate matter (APM) is of special interest to researchers due to its adverse effect on human health [1]. In urban and industrial areas, APM is a mixture of particles originating from natural sources (seas and oceans, deserts, soil, volcanes, vegetation and wildfires) and from anthropogenic sources (traffic, industrial activity, building and housing). In rural areas, APM additionally originates from biomass burning and farming activity [1]. The composition of APM is complex and it depends on local emission sources. Major urban-APM constituents are organic matter, sulfate, mineral dust, nitrate, ammonium, elemental carbon (including black carbon) and chloride [2]. Particularly important constituents of APM are trace elements, because many of them are toxic and carcinogenic, the so-called potentially toxic elements (PTEs) [3].
The goal of this paper is to review the present state of knowledge on the distribution of chemical forms of potentially toxic elements in airborne particles. Earlier publications focused on the methodology of chemical fractionation [4,5]. The results of operational studies of PTE speciation in urban air have not been the subject of review elaborations so far. Formally, this review covers the papers published in the last three decades. In fact, 60% of them were published in the last decade [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33] and 30% were published in the years 2001–2010 [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. The studies on operational speciation of PTEs dealt primarily with air quality in Europe (40%) [6,10,11,14,19,20,28] and Asia (40%) [7,8,9,12,13,15,16,17,21,22,23,29,30,31,32,33]. The other studies were conducted in the Americas [27,35,43,48,52,56,57] and Africa [18,24,25]. Thus, this review concerns urban areas of different population sizes with low or high industrial activity, with various agricultural influences and with dry and wet climates. In total, 112 separate laboratory data sets published in 70 articles during the last thirty years were compiled and analyzed in this study. The data reflect quite well the current state of affairs regarding the operational speciation of particulate-bound elements determined by sequential extraction methods. The focus was on chemical fractionation results, while the results of the speciation analysis, including valence speciation and physical speciation, were set aside to be discussed separately.
Chemical fractionation of PTEs in atmospheric particulate matter has attracted less interest than chemical fractionation of metals in soils or sediments because of the very real difficulties in measuring low concentrations in low-mass samples. For comparison, only in the last decade the Scopus database registered over 750 articles with the terms “chemical fractionation” AND “metal” AND “soil” in titles, abstracts or keywords, 500 articles with “chemical fractionation” AND “metal” AND “sediment” but only 42 articles with “chemical fractionation” AND “metal” AND “PM”.
The most frequent subject of these studies was a group of toxic metals: As, Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn (50–90% of the papers) [9,12,13,16,21,27,30,33,34,35]. The elements such as Al [15,18,20,26,32,33], Co [9,12,14,17,21,23,26,27,28,29,30], V [23,26,27,33,57,58,59,60], Ti [10,19,20,23,26,27,32,33], Sb [10,11,14,20,27,28,32], Mo [20,32,33,59,60,61], Se [11,14,28,47,55,56,62,63] and Sn [11,14,28,36,47,56,62] were studied less frequently (10–40%). The study of operational speciation of Ag [18,61], Hg [11,14], Pt [59,60,61], Rh [60], Sc [60], Tl [37,46], Te [61,62] and Bi [59,61] was performed sporadically. The scope of the research presented in a single publication ranged from a few to roughly a dozen elements, with an average of nine covered per paper. Only a few publications were focused on a single element: As [6,7,8] and Pb [64].
Among all PTEs, Cd, Cr, Cu, Fe, Mn, Ni, Pb, V and Zn are the metallic elements of the most immediate concern because of their detection frequency. Exposure to these metals has been associated with cardiovascular diseases, cancer and many other adverse health effects, and their specific effects within different age groups vary widely [3,9,65,66].
Taking the above into account, the particular aims of this work were formulated as follows: to develop average distribution patterns of chemical forms for ten PTEs (As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb and Zn) occurring in airborne particles worldwide; and to develop inhalation risk predictions for city dwellers breathing air that is moderately or extremely polluted with these elements. The complementary goals were the assessment of the uniformity of the terminology and the laboratory procedures used.
2. Terminology
Even a superficial reading of the publications leads to the conclusion that the International Union of Pure and Applied Chemistry (IUPAC) Recommendations from 2000 have not been fully implemented [67]. The terms: “speciation analysis”, to refer to the activity of identifying and measuring species, and “speciation”, meaning the distribution of an element among defined chemical species, are commonly used. However, dissemination of symmetrical definitions of fractionation still remains a rather distant goal. It is worth recalling that IUPAC Recommendations were made to restrict the use of the term fractionation to refer to analytical activities. A scan of 70 peer-reviewed articles on fractionation of PTEs in APMs published over the last 30 years reflects various approaches in this regard. The authors fractionated the elements taking into account both the chemical bond and the particle size. To specify the type of analytical activity, the term “chemical fractionation” was often used for fractionation by chemical bonding via sequential extraction procedures [8,10,11,12,13,14,15,34,35,36,37,38,63,68,69,70]. One may also encounter terms that are equivalent to the term “speciation analysis”, i.e., “fractionation analysis” [11,39] or “chemical fractionation analysis” [37]. The term “fractionation” was also used in the sense of the distribution of forms of an element [16,17,18,19,71]. However, for this purpose, the authors much more often used other terms: “speciation” or “chemical speciation” [6,10,18,20,21,22,34,39,40,58,69,71,72,73,74], “solid-state speciation” [41,75,76] and “operational speciation” or “operationally defined speciation” [19,42,77]. In this review paper the term “chemical fractionation” is used exclusively for analytical activities, whereas the distribution of chemical forms of a certain element is defined in this paper as its “operational speciation” or the “distribution pattern of its chemical fractions” (also geochemical forms and operationally defined fractions). The lack of a uniform terminology also relates to the research subject. In the set of publications reviewed, the research subject was defined as “heavy metal(s)” [13,16,17,23,43,71], “trace metal(s)” [24,25,34,41,44,75] and “trace element(s)” [10,11,14,26,27,45]. All three terms appeared in similar numbers. In the titles of those articles, the term “heavy metals” occurred more often than “trace metals” or “trace elements”, whilst in keywords it occurred twice as frequently as these other terms. The terms which were proposed to replace the criticized but commonly used term “heavy metals” [78,79] were rarely used: “potentially toxic metals” [13,17,19,20,68,69,80], “potentially toxic elements” [19,46,69], “potentially toxic trace elements” [11,63] and “potential hazardous elements” [70]. In our work, we wanted to respond positively to these terminological proposals and therefore all particulate-bound elements for which chemical fractionation data have been published are described as “potentially toxic elements” (PTEs).
3. Chemical Fractionation Procedures
In the reviewed articles, chemical fractionation of PTEs was performed on samples of different grain fractions of atmospheric aerosols: total suspended particulate fraction (TSP)—28%, PM10—26% and PM2.5—37% of the total number of studies. Occasionally, studies were also carried out on PM7 [43], PM1 [6,11,14,42] and PM0.6 [42] samples. The airborne particulate matter was collected on quartz fibre filter membranes (38%), PTFE filter membranes (29%), fibreglass filters (18%) and others (e.g., cotton paper filters, cellulose ester filters, polycarbonate membrane filters). Before sampling, the membrane filters were conditioned in a desiccator. Quartz filters were sometimes additionally pre-heated at 450–800 °C to eliminate organic contamination [13,22,23,34,50,61,69,74], while cellulose filters were occasionally soaked in ultra-pure HNO3 or HCl to reduce blank concentrations of metallic elements [32,72]. Field blank filters were collected to reduce gravimetric bias due to filter handling during and after sampling [30,80]. APM samples were collected at the height of 1–6 m [14,21,25,28,43,45,61,64,69,77], 12–20 m (roofs of buildings) [9,16,23,34,44,47,64,80] and in a few cases even at 30–35 m [76,81]. Low-volume and high-volume samplers were used exclusively, which, for the most frequently collected 24 h aerosol samples, was associated with the flow of several dozen or several thousand cubic meters of air. It is worth pointing out that some of the more general descriptions of sampling did not contain such detailed information.
Many sequential extraction methods have been reported, but the procedure developed by Fernández Espinosa [40] turned out to be the most widely used to evaluate the possible chemical associations of PTEs with different geochemical fractions of APM—27% of the surveyed papers (Figure 1).
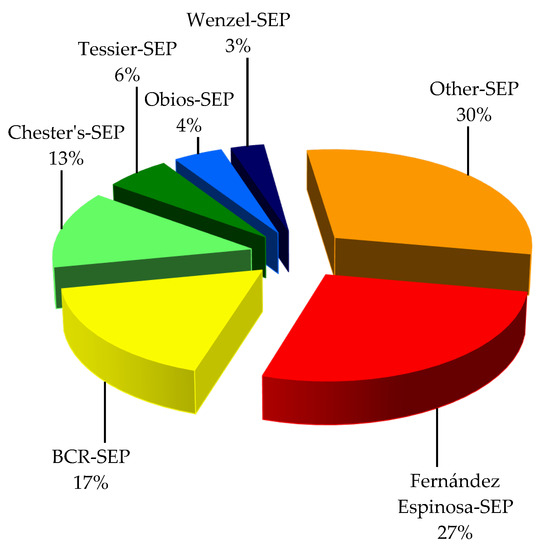
Figure 1.
Sequential extraction procedures used for chemical fractionation of potentially toxic elements in airborne particles.
The procedures that were used less often are the BCR procedure [82,83]—17% of the papers—and Chester’s procedure [75]—used in 13% of the papers. The remaining sequential extraction procedures were employed occasionally: the Tessier SEP [84]—6%, the Obios SEP [85]—4% and the Wenzel SEP [86]—3%.
Alternative sequential extraction procedures comprising two, three or four stages were proposed by authors to accomplish a specific research aim—30% of the papers in total. The above statistics do not mean that the SEPs that were used followed the precise methodologies outlined in the resource articles (Table 1).

Table 1.
Most widely used original procedures for chemical fractionation of PTEs in atmospheric particulate matter.
The Fernández Espinosa procedure in its original form was used only in 40% of the papers. In the remaining studies this procedure included a number of changes with a potentially different impact on chemical fractionation results. Ultrasound-assisted extraction at both RT [17] and at 70 °C [15] was used to reduce the time of extraction of the soluble and exchangeable fraction from 3 h to 30 min. It is worth mentioning the results of the studies carried out by Desboeufs et al. (2005), who demonstrated that 90% of the total dissolved content of individual metals is always released in the first 20 min of dissolution [87]. Modifications of the release of the carbonate and oxide fraction mainly consisted in reducing the extraction time from 5 h to 4 h [48], to 2 h [17,18,24] and to 20 min, but under ultrasound-assisted conditions [15]. In the same way Wu et al. (2021) accelerated the third step, from 90 min shaking at RT to 20 min shaking in an ultrasonic water bath also at RT [15]. The most serious modifications concerned the extraction of the residual fraction. The introduction of HF into the acid mixture, variously HNO3, HCl, HF [15,17,18,24], HNO3, HF, H2O2 [20] and HNO3, HF, HClO4 [48], resulted in an increase in the range of chemical fractionation from the pseudo-total content of PTEs to their total content.
By using microwave-assisted or ultrasonic assisted extraction, it was possible to accelerate digestion from 5 h to 20 min [15,18,24,48]. Microwave-assisted extraction was also used to digest APM samples in a typical mixture of acids (HNO3, HCl, HClO4) [29,30]. Conventional acid digestion of the residual fraction was carried out also under milder conditions e.g., at RT [10,11,28] and under more extreme conditions e.g., at 180 °C [34] than those proposed in the source procedure (95 °C). Chemical fractionation of atmospheric particle-bound metallic elements was carried out using two BCR procedures, according to the original BCR sequential extraction protocol [82] and to the modified BCR protocol [83]. Only in a few studies did the fractionation procedure accurately adhere to the developed protocols [71,73]. A reduction in the fractionation time was achieved using microwave-assisted extraction [58,63,74] or ultrasound-assisted extraction [39]. The largest range of modifications concerned the digestion of the residual fraction. Instead of the recommended aqua regia, other mixtures of concentrated acids were used, comprising HNO3, HClO4, HF [19], HNO3, HClO4, HCl [21], HNO3, H2O2 and HF [63] or only concentrated HNO3 [74]. The digestion process was carried out both under milder and under more drastic conditions than the recommended ones: e.g., RT for 30 min [31] or 90–160 °C for 16 h [19] or 190 °C for 24 h [72].
The described modifications of Chester’s procedure involved the addition of an extra stage (water extraction) to the three-stage SEP [12,35,49] and a change in the digestion mixture composition [12,35,49]. The release of the water-soluble fraction was carried out during a short (15 min) shaking at RT [35,49] or a longer extraction (60 min) assisted by ultrasound [12]. In order to ensure the complete dissolution of residual particles, concentrated HCl [49] or 30% H2O2 [12,35] were added to the mixture of HNO3 and HF acids. Acid digestion was performed conventionally as in the original procedure [41,50,51,75,76,81] or by using microwave-assisted extraction [12,35].
The Tessier procedure was employed relatively infrequently [9,25,45,52]. The introduced modifications consisted in determination of the water-soluble fraction of PTEs prior to the conventional procedure [45,52] and in the combined release of the carbonates fraction and the Fe/Mn oxides fraction by leaching with NH2OH∙HCl/25% AcOH solution at 96 °C for 6 h [25]. The Obios procedure was used only according to the original version [53,77,85]. In the studies on fraction distribution of arsenic in APM samples [7,8], the chemical fractionation was carried out according to the Wenzel procedure [86].
It is worth emphasizing that although the modifications of the original SEPs undoubtedly have some justification, in practice, each change to an operationally defined procedure makes it still more difficult to compare the results obtained in different laboratories for samples originating from distinct geographical areas or even from similar urban centers.
Metal and metalloid elements in sequential extracts and digests were determined using the following instrumental techniques: inductively coupled plasma optical emission spectrometry (ICP-OES or ICP-AES); inductively coupled plasma mass spectrometry (ICP-MS); atomic absorption spectrometry using either a flame or a flameless mode depending on concentration level (FAAS and GFAAS); energy dispersive X-ray fluorescence spectrometry (EDXRF); atomic fluorescence spectrometry (AFS); capillary electrophoresis (CE); or differential pulse anodic stripping voltammetry (DPASV). The three most commonly applied techniques were ICP-OES—32%, ICP-MS—30% and FAAS/GFAAS—19% of the papers (Figure 2).
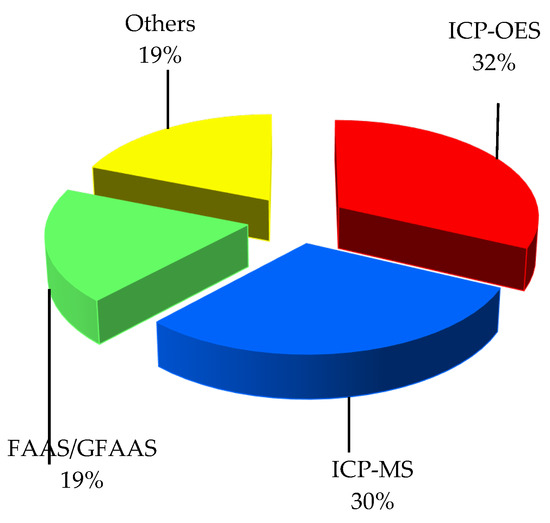
Figure 2.
Analytical techniques used for the determination of metallic and metalloid elements in digests and extract solutions of APM samples.
The other techniques were used in the quantitative elemental analysis by individual research teams, as follows: EDXRF [62], DPASV [45] and CE [39], with AFS used only for the determination of arsenic [7,8]. Since ICP-OES and ICP-MS do not yield the same reliable results for all investigated elements, multi-element analysis was performed in many speciation studies using both these techniques [13,16,19,20,31,37,71] or ICP-OES and GFAAS [41].
It is worth noting that the determined PTE concentrations were always corrected using their average blank concentrations (blank filter, reagents and vessels). Since standard reference materials regarding the chemical fraction dependent composition are not available, validations of chemical fractionation procedures were carried out using various other reference materials, as follows: VKI (QC loam soil A); PACS-2 (marine sediment) [49]; ERM-CZ120 (fine dust) and SRM 1648a (urban particulate matter) [11,14,26]; SRM 1648 (urban particulate matter) [47,48,55,56]; CRM BCR-701 (lake sediment) [19,25,26,48]; and SRM 1633b (coal fly ash) [27,56]. The metallic elements recovery of sequential extraction procedure was enhanced by comparing the sum of all the chemical fraction concentrations with their certified total concentrations. In turn, an internal check of the recovery of metallic elements by the SEP was performed by comparing the sum of all the chemical fractions with the total contents of these elements in examined samples [13,16,17,21,22,29,33,70,71]. It was considered that the SEP was reliable and repeatable if the recovery range was 85–115% for studied elements [13,16,17,22,29,33,70].
4. Operational Speciation of As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb and Zn
The distribution patterns of the 10 elements in airborne particles were developed for the three most frequently used procedures: the Fernández Espinosa SEP, the BCR SEPs and Chester’s SEP. All literature data regarding operational speciation used in this review are shown in Figures S1–S10. Individual data sets for a specific element and a given SEP were obtained in various laboratories and for various APM samples. Mean concentrations of most of these elements in the air were of the same order of magnitude in the groups defined by a given procedure of chemical fractionation. Statistical analysis of these literature data sets is shown in Table 2, Table 3 and Table 4. In general, the results (percentage of a chemical fraction) show high dispersion, since two thirds of the variation coefficient values are above 50%. It can be observed that 75% of the skewness coefficients are positive, and therefore the percentage fraction values are grouped around the values below the mean percentage (positively skewed distribution). The near zero skewness coefficient (0.0 ± 0.3) applies only to a small amount of data, and most often symmetry describes the percentage of the F(1) fraction, e.g., Cd, Co, Cu and Zn. The kurtosis does not show dominant tendencies; for each of the three data sets, the number of negative values is equivalent to the number of positive values with few but significantly high values of the kurtosis being positive. It is worth emphasizing that the kurtosis values for more than half of these data are in the range of ±1, which indicates a normal distribution of the data.

Table 2.
Descriptive statistics of literature data of operational speciation of As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb and Zn occurring in urban air particulate matter by Fernández Espinosa procedure. The element fraction content is given as a percentage of its total content [%].

Table 3.
Descriptive statistics of literature data of operational speciation of As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb and Zn occurring in urban air particulate matter by BCR procedure. The element fraction content is given as a percentage of its total content [%].

Table 4.
Descriptive statistics of literature data of operational speciation of As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb and Zn occurring in urban air particulate matter by Chester’s procedure. The element fraction content is given as a percentage of its total content [%].
The most commonly used Fernández Espinosa scheme enables the division of the mobilizable content of potentially toxic elements into four fractions: F(1)—water-soluble and exchangeable; F(2)—carbonate, oxides and reducible; F(3)—bound to organic matter, oxidizable and sulfidic; and F(4)—residual fraction. Chemical distribution patterns of the 10 elements in airborne particles were compiled from 11–23 (av. 18) sets of data obtained from various urban areas from around the world (Table 2).
A distinctive feature of the occurrence of PTEs in APM was the significant content of the water-soluble and exchangeable fraction. The highest percentage is achieved by Cd (42.5%), whereas Co (35.7%), Zn (32.3%), Mn (30.8%) and As (29.2%) are characterized by a slightly lower level of the mobile fraction. The percentage of the water-soluble fraction does not drop below 20% for Cu, Ni and Pb. Only in the case of iron and chromium, the water-soluble fraction binds a small percentage of these metallic elements: 6.5% and 14.6%, respectively. Low mobility of these elements is also confirmed by a high proportion of the residual fraction: 51.0% and 46.0%, respectively.
The compilation of chemical distribution patterns of heavy metals according to the BCR schemes (original and optimized) was developed on the basis of 8–20 (av. 15) data sets from chemical fractionation of particulate matter samples (Table 3). Using the BCR SEP, it is possible to divide the content of metallic elements into four operationally defined fractions: acid-soluble fraction, reducible fraction, oxidizable fraction and residual fraction. In the case of five elements (As, Cd, Cu, Pb and Zn), the dominant fraction was the most mobile fraction—the acid-soluble fraction. As 0.11 M AcOH is a more “aggressive” reagent than water, it was expected that the mean percentage of the acid-soluble fraction should not be lower than the mean percentage of the water-soluble fraction. In fact, only in the case of Co and Ni did the percentage of the acid-soluble fraction (BCR SEP) prove to be lower than the percentage of the water-soluble and exchangeable fraction (F-E SEP).
Operational speciation of the selected PTEs according to Chester’s scheme was developed on the basis of the published 7–15 (av. 12) data sets from chemical fractionation of urban-APM samples (Table 4). This chemical fractionation procedure enables the division of the content of PTEs into three fractions: loosely held fraction; carbonate and oxide fraction; and refractory and organic fraction. Although formally the fraction F(3) in this scheme can be treated as a sum of two fractions, F(3) and F(4), in the Fernández Espinosa and the BCR schemes, it should not be forgotten that the differences in the procedures of their release are significant (Table 1).
In spite of the fact that the results of the sequential extraction of environmental solid samples depend on the scheme used for their determination, an attempt has been made to compare the results of the chemical fractionation of PTEs in APM by using the Fernández Espinosa, BCR and Chester procedures. The evaluation focused on the percentages of individual chemical fractions. The similarity level turned out to be small. Irrespective of the procedure used, only Cd shows a high percentage of the F(1) fraction. A moderate proportion of this fraction (22.6–37.8%) can be found in the case of As, Cu and Mn, while the percentage of F(1)-Fe is definitely low: 6.5% (F-E SEP), 9.8% (BCR SEPs) and 6.1% (Chester’s SEP). The percentage values of F(2) make it impossible to conduct an analogous evaluation, whereas a similar proportion of the residual fraction concerns only Fe: 51.0% (F-E SEP), 47.9% (BCR SEPs) and 65.7% (Chester’s SEP).
The mean operational speciation of selected PTEs in the urban air worldwide is given in Figure 3.
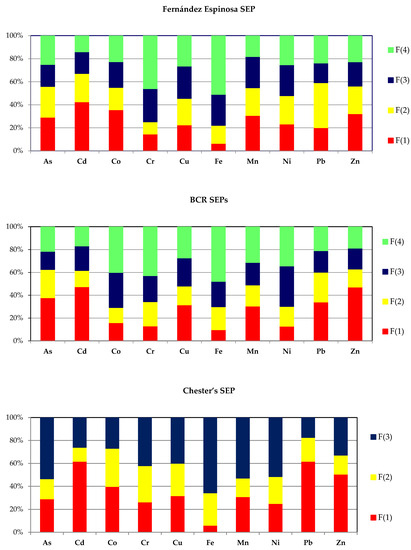
Figure 3.
Average operational speciation of selected PTEs in the urban air worldwide.
Operational speciation of individual elements is as follows:
Arsenic
The chemical distribution of As performed by F-E SEP is relatively balanced (Table 2). The percentages of fractions F(1)-As, F(2)-As and F(4)-As are quite similar (29.2%, 26.7% and 25.0%, respectively). Only the percentage of the organic, sulfidic and oxidizable fraction is lower—19.2%. The patterns of chemical distribution of As by the BCR SEPs (Table 3) and Chester’s SEP (Table 4) are different. The speciation studies using the BCR method have shown that As is present mainly in the acid-soluble fraction (37.8%), while the results obtained by means of Chester’s procedure show that As is mainly bound to the refractory and organic fractions (53.5%). The view that the important chemical species of As in urban APM are As2O3, As2S3, As2O5, As2S5 and organoarsenic compounds [48,51] is not divergent from these results.
Cadmium
Cd is one of the metallic elements for which operational speciation is almost independent of the applied fractionation procedure (F-E SEP, BCR SEPs or Chester’s SEP). Cd is mostly accumulated in the most mobile fraction F(1): 42.5%, 47.3% and 61.8%, respectively. The residual fraction bound only 14.0–16.9% of Cd (F-E SEP and BCR SEPs). In Chester’s scheme, an analogous fraction is not determined.
From among the cadmium species detected in aerosols (Cd, CdCl2, CdO, Cd(OH)2, CdS and mixed oxides with Cu and Zn) [16,51], the F(1)-Cd fraction should be linked primarily with the presence of CdCl2, although CdO and Cd(OH)2 may also be present.
Cobalt
Distribution of chemical forms of cobalt shows a high share of the water-soluble fraction (35.7%) and an even partition between the other three fractions (F-E SEP). The distribution pattern developed on the basis of the results of the chemical fractionation by Chester’s procedure is similar, while the operational speciation of Co based on the results of the chemical fractionation according to the BCR scheme is different in character. The residual fraction is dominant (40.0%), whereas mobile fractions F(1)-Co and F(2)-Co bind only 15.9% and 13.3% of cobalt, respectively.
Chromium
Chromium shows low mobility and is characterized by a large proportion in the residual fraction: 46.0% (F-E scheme), 42.8% (BCR-scheme) and 42.1% (Chester’s scheme), and a relatively low proportion in the F(1)-Cr fraction: 14.6% (F-E scheme), 13.0% (BCR scheme) and 26.4% (Chester’s scheme). The relatively high percentage of the F(1)-Cr fraction in the data set of Chester’s scheme can be easily explained by the use of an extractant with strong complexing properties of Cr(III)—1 M AcONH4.
Copper
Copper occurring in urban APM is characterized by an exceptionally uniform distribution among four chemical fractions regardless of the chemical fractionation procedure used: F-E SEP or BCR SEP. The even partition among three chemical fractions also refers to operational speciation by Chester’s SEP. Due to considerable interest in copper speciation in APM, there is relatively extensive literature on the identification of Cu species present in individual fractions. The water-soluble and exchangeable fraction is explained by the presence of CuCl2, CuSO4 and Cu(NO3)2 [16,22,27,45,72]. The organic, sulfidic and oxidizable fraction comprises sulfide species such as Cu2S, CuS, CuFeS2 and Cu5FeS4 [16,47,48]. The immobile residual fraction is formed by Cu bound to silicates [27,47] and magnetite with a significant Cu content emitted both by steel metallurgical industry and by vehicle traffic [19].
Iron
Iron is an element with the least mobility and its operational speciation is characterized by a significant diversity of distribution. The percentage of iron bound to individual chemical fractions increases, regardless of the chemical fractionation procedure used, in the following order: F(1) = 6.5% < F(2) = 15.7% < F(3) = 26.9% < F(4) = 51.0% (F-E SEP), F(1) = 9.8% < F(2) = 20.1% < F(3) = 22.3% < F(4) = 47.9% (BCR SEPs) and F(1) = 6.1% < F(2) = 28.2% < F(3) = 65.7% (Chester’s SEP). The water-soluble fraction of iron is linked with the presence of iron sulfate, nitrate and/or oxalate [16,48]. Possible compounds contained in the F(2)-Fe fraction are Fe2O3, FeO(OH) and FeCO3 [16,48], while magnetite, hematite and iron species in clay mineral can be present in the residual fraction [20,39,47,48].
Manganese
Chemical distribution patterns of Mn developed for three different data sets (F-E SEP, BCR SEPs and Chester’s SEP) are very similar. The percentage of F(1)-Mn is the same in each of the distribution patterns: 30.8%, 30.5% and 31.0% (Table 2, Table 3 and Table 4). The overall proportions of the refractory and oxidizable fractions are also similar: 45.2%, 51.0% and 52.9%. Mn may occur as MnCl2 and MnSO4 in the soluble and exchangeable fraction [16]. MnO2, MnCO3 [16] and MnFe2O4 [48] can be components of the reducible fraction. MnS is assigned to the organic matter and sulfide fraction whereas Mn present in silicates and aluminosilicates is included in the residual fraction [48].
Nickel
Ni, like Cu, is very evenly divided among the four fractions of the F-E scheme. The distribution pattern of Ni derived from a data set for the BCR scheme is different. Of the total Ni, 35.5% and 34.3% is accumulated in the oxidizable fraction and the residual fraction, respectively. Operational speciation of Ni developed on the basis of Chester’s scheme is very similar to the speciation of Ni by the Fernández Espinosa scheme. There is a view that the source of the water-soluble and exchangeable fraction is mainly NiSO4, which is derived from coal combustion, while the carbonate and reducible fraction of Ni is formed by NiO and/or more complex oxides [16,29,43].
Lead
Pb(II), similar to Cr(III), very readily forms acetate complexes under the extraction conditions of stage one of Chester’s scheme. Extraction with 0.11 M AcOH solution or only with water is considerably less efficient: 34.0% (BCR SEPs) and 20.1% (F-E SEP) compared with 61.8% (Chester’s SEP), meaning the lower the complexing power, the higher the percentage of Pb determined as the carbonate and oxide fraction: 39.0% (F-E SEP), 26.1% (BCR SEPs) and 20.8% (Chester’s SEP).
The exchangeable and water-soluble fraction may contain soluble lead halides (PbCl2, PbClBr, PbBr2 and PbBrCl·2NH4Cl) [16]. Less soluble Pb species, such as PbS, PbO, PbSO4, PbCrO4 and PbCO3, are considered to be components of the carbonate fraction or reducible fraction [16,20]. However, on the other hand, Funasaka et al. (2013) report that Pb0, PbSO4, PbSiO3, PbS and PbO2 are mainly extracted with the residual fraction [64].
Zinc
The chemical distribution pattern of Zn reflects the mobile nature of this element. Most Zn is found in the F(1)-Zn fraction, regardless of the fractionation procedure used to study its chemical distribution: 32.3% (F-E SEP), 47.1% (BCR SEPs) and 50.6% (Chester’s SEP). A considerably lower proportion of Zn is in the residual fractions (F-E SEP and BCR SEPs) or the refractory and organic fraction (Chester’s SEP): 22.8%, 18.7% and 32.8%, respectively.
The water-soluble fraction of Zn is mainly formed by ZnSO4 and ZnCl2 [16,19,27,47], whereas the carbonate fraction or the reducible fraction mostly comprises ZnO and carbonates e.g., CaCO3·ZnCO3 [16]. In the residual fraction, Zn may be bound with Fe and Mn oxides, aluminosilicates and silicates [20,27,47].
A detailed analysis of the reported results of the chemical fractionation of Zn and Cd leads to the conclusion that each of the three procedures (Fernández Espinosa SEP, BCR SEP and Chester’s SEP) offers equivalent possibilities for studying operational speciation. Comparable chemical distribution patterns were obtained for elements with similar chemical properties (the same subgroup of the periodic table—the zinc family) (Figure 4).
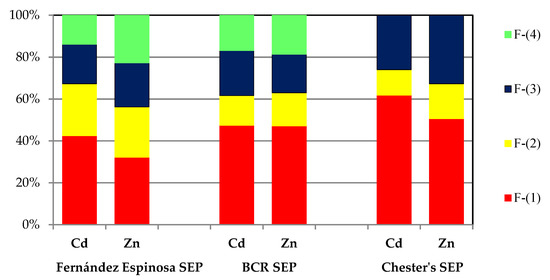
Figure 4.
Comparison of the average operational speciation of cadmium and zinc in atmospheric particulate matter.
5. Water-Soluble Fraction of Potentially Toxic Elements
It is assumed that the water-soluble fraction of PTEs in APM contains free metal ions and metal ions complexed with soluble organic substances [4]. An increase in ionic strength caused by the most rapidly dissolved metal species (chlorides, sulfates, nitrates, acetates, etc.) can also contribute to a release of exchangeable metal species [40]. Soluble PTEs are generated mainly from industrial sources (50–70%) [27]. The metals associated with the water-leachable fraction are considered mobile in the environment and bioaccessible to organisms, and therefore represent a potentially harmful component of atmospheric particles. The release of metal elements from atmospheric PM has an important impact also on aqueous atmospheric chemistry and the toxicity of wet deposition, especially in surface waters, because dissolved fractions of metal elements are directly accessible for the phytoplankton [88]. Water extraction is considered the simplest and most universal extraction procedure in toxicity testing by many authors [44]. Since the total or pseudo-total content of PTEs is usually also determined, the whole of the analytical process, regardless of whether the extractions were carried out sequentially or in parallel, allows for the establishment of the distribution of PTEs between the water-soluble fraction and the much less environmentally mobile fraction [47]. This approach to chemical fractionation is considered a good compromise between costs, analytical times and achievable information [54].
In this work, the water solubility data set of ten metallic and metalloid elements included the results of the water extraction of APM samples that were obtained in a two-step extraction procedure [6,27,28,32,44,46,47,59,60,61,64,80] or as the first step in recognized sequential extraction procedures, e.g., Fernández Espinosa SEP [40], modified Tessier SEP [52] and in other SEPs [45,49,55,62]. This data set also includes the results of extraction with 1 M MgCl2 and 1% NaCl solutions obtained by using the Tessier SEP and the Obios SEP [9,25,53,77,85]. The water-soluble proportion is expressed as the percentage of the total or pseudo-total concentration of those ten elements. The literature set of ordered pairs (concentration in air, percentage of the water-soluble fraction) has been split into two groups: “Commonly encountered pollution level” (CEPL) and “High pollution level” (HPL). The division was performed fairly arbitrarily, taking into consideration the values of PTE concentrations, air quality standards set by WHO and EC and an intention to build up a CEPL data set as large as possible, but without significant outliers. As a result, the CEPL set for individual elements has from 18 to 60 literature datapoints (average subset size of an element—34 values) while the HPL set has from four to 24 literature datapoints (average subset size of an element—11 values). The data set of CEPL is considered by us to be characteristic of an urban environment and thus the basic data set.
The statistical properties of this data set are provided in Table 5.

Table 5.
The mean concentrations and the percentage of the water-soluble fraction of the ten PTEs in the air in urban areas around the world.
Generally, low skewness values justify the use of an arithmetic mean as a measure of central tendency (only two skewness values are clearly greater than 1). The mean percentages of water-soluble fractions are in most cases close to their corresponding median values, while the mean values of concentrations of most PTEs are slightly higher than the corresponding median values (typical of a positively skewed normal distribution, which is characteristic of naturally occurring phenomena). The negative kurtosis values (platykurtic distribution) indicate the absence of outliers and a greater clustering of results around the mean. The exceptions are the large kurtosis coefficients for the concentrations of Co and Ni.
Even a cursory assessment of the collected literature data reveals considerable differences among concentrations of individual PTEs in the urban air. For metal concentrations classified as CEPL, Co and Cd have the lowest mean concentrations—1.2 ± 0.5 ng/m3 and 3.3 ± 0.5 ng/m3, respectively (Table 5). The mean concentration of Cd is below the EU target value (5 ng/m3) [89]. Slightly higher levels can be found for the concentrations of Cr (13 ± 2 ng/m3), Ni (16 ± 4 ng/m3) and As (19 ± 3 ng/m3) but the concentration of Ni is below the EU target value of 20 ng/m3, whereas the concentration of As exceeds the EU target value (6 ng/m3) threefold [89]. The mean concentration of Mn (38 ± 6 ng/m3) is considerably lower than the WHO guideline value of 150 ng/m3 [91]. The highest values are exhibited by the mean concentrations of Fe (4.6 × 102 ± 0.7 × 102 ng/m3), Pb (1.3 × 102 ± 0.2 × 102 ng/m3) and Zn (2.6 × 102 ± 0.4 × 102 ng/m3). It is worth pointing out that the mean concentration of Pb is well below the EU limit value (500 ng/m3) [90]. In urban areas classified by us as extremely polluted, mean concentrations of TMEs are one to two orders of magnitude higher. Against this background, air pollution with toxic trace metals in the EU area looks more favourable. In 2018, from among 700 monitoring stations located in 28 European countries, only a few reported concentrations of As, Cd, Pb and Ni above the target values, and 95–98% of the stations reported As, Cd, Pb and Ni concentrations below or equal to the lower assessment threshold (LAT): 2.4 ng/m3, 2 ng/m3, 250 ng/m3 and 10 ng/m3, respectively [91].
Despite such significant differences in concentrations of PTEs in the urban air, it was discovered that the proportion of the water-soluble fraction of these elements was not subject to large fluctuations (Figure 5).
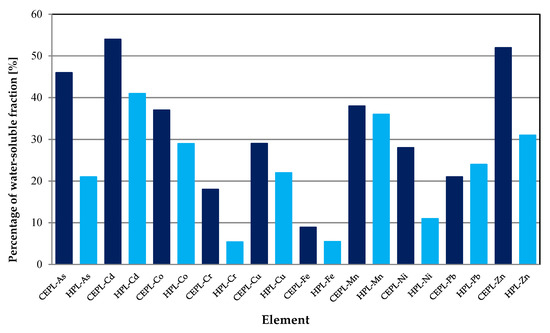
Figure 5.
Mean proportion of water-soluble fractions of PTEs in the urban air classified as follows: CEPL—commonly encountered pollution level; HPL—high pollution level.
Generally, PTEs reaching extremely high concentrations in the urban air are characterized by lower proportions in the most mobile fraction. Only the percentage of the water-soluble fraction of Mn and Pb remains at a stable level, irrespective of its concentration in air. The greatest reduction in water solubility was observed for Cr, for the CEPL—18% and for the HPL—5.5%. In the moderately polluted urban air, the order of water leachability of the PTEs was as follows: Cd ≈ Zn > As > Co ≈ Mn > Cu ≈ Ni > Pb > Cr > Fe. This was different from the order of the water leachabilities of these elements determined for highly polluted urban areas: Cd > Mn > Zn > Co > Pb > Cu ≈ As > Ni > Fe ≈ Cr. However, these differences are not large. For the data set belonging to the CEPL, Cd, Zn and As have the highest proportion in the water-soluble fraction: 54%, 52% and 46%, respectively. The percentage of the water-soluble fraction of Mn, Co, Cu and Ni is lower, but still relatively high (from 28% to 38%). Only Fe is characterized by a low proportion in the water-soluble fraction (8.9%). Water extractions are very often used to assess the bioaccessibility of PTEs. Thus, according to the classification proposed by Mbengue et al. (2015) [20], the analyzed PTEs can be divided into three groups depending on their potential bioaccessibility for CEPL:
- (1)
- not very bioaccessible (<20%): Fe and Cr;
- (2)
- moderately bioaccessible (20–40%): Pb, Ni, Cu, Co and Mn;
- (3)
- highly bioaccessible (40–60%): As, Zn and Cd.
For HPL, on the other hand, the PTEs can be divided as follows:
- (1)
- not very bioaccessible: Cr, Fe, and Ni;
- (2)
- moderately bioaccessible: As, Cu, Pb, Co, Zn, and Mn;
- (3)
- highly bioaccessible: Cd.
Due to dissimilar water solubilities, the water-soluble concentrations of elements may differ from the sequence of their concentrations in APM [32]. Hence, taking into account the concentration of the water-soluble fraction of these elements in the air, it is Zn, Pb, Mn and Cu that pose the highest potential risk of getting into human bodily fluids and then into the bloodstream. Notable aspects of the research on atmospheric operational speciation also include the effects of seasonal changes and size distribution of PM on PTE water-solubility. On the whole, a proportion of the water-soluble fraction of PTEs was higher in a fine fraction than in a coarse fraction of atmospheric particles [12,32,44,47]. The differences are not significant, although a few exceptions have been found, e.g., Ni (4×, Edinburgh, UK) [44] and Cu (10×, Pune, India) [12]. The study of seasonal variations in the water-soluble proportion usually includes four seasons; however, due to emissions from fossil fuel combustion, winter was often the season with the highest percentage of metals in the water-soluble fraction [6,15,32,33,48,61]. Due to a relatively small number of reports, the latter observations do not constitute a sufficient basis for broader generalizations.
6. Environmental Risk Assessment
In order to assess the risk in an environment polluted by PTEs, it is common to use a number of individual and complex indices, the most important being the following: geoaccumulation index (Igeo); enrichment factor (EF); bioavailability index (BI); individual and global contamination factor (ICF and GCF); risk assessment code (RAC); pollution load index (PLI); degree of contamination (Cdeg); and potential ecological risk (RI) [12,16,92]. Some indices are defined by the total metal concentration (e.g., Igeo, EF and PLI) and others by a concentration of a defined fraction of these metals (e.g., BI, RAC and ICF). Wider use of these indices beyond a specific research project necessitates the comparison of a set of indicators determined by various research teams. The QA/QC system commonly implemented in analytical laboratories ensures the collection of reliable data on total concentrations of trace elements in environmental samples. However, establishing the traceability of speciation analysis results using operational chemical fractionation procedures is still a challenge for environmental analysts [93].
As mentioned above, individual SEPs applied to a defined analytical task can yield different results. The differences result from a different approach to the kind and sequence of geochemical phases being dissolved and from different conditions of extraction of even the same geochemical phases. Thus, expecting good compatibility of environmental indices determined on the basis of data from multistage chemical fractionation is rather unrealistic. In the case of the procedures that are most frequently used for fractionation of PTEs in APM, a comparison of the results of the first stage of extraction is ineffective due to the different ranges of these extractions: the soluble and exchangeable fraction (Fernández Espinosa SEP), the exchangeable, water- and acid-soluble fraction (BCR SEP) and the loosely held fraction (Chester’s SEP) (Table 1). A similar remark applies to the second stage of these chemical fractionation schemes: the carbonate, oxide and reducible fraction (Fernández Espinosa SEP), the reducible fraction (BCR SEP) and the carbonate and oxide fraction (Chester’s SEP). The common element of these three data sets can be the combined fraction that is the sum of the extraction results of the first and second stages and therefore it can serve as a suitable tool for making comparisons. For a given SEP, the fraction defined in this way includes water-soluble and exchangeable PTEs as well as PTEs bound to carbonates and oxides. Jan et al. (2018) define this combined fraction as a non-resistant fraction of a metallic element that has become available to a human body and/or plants, and the remaining part of this element as its resistant fraction [12]. For elements that are potentially toxic to humans, the percentage of the mobile fractions F(1) and F(2) is of special interest because it determines their bioavailability [68]. The contents of the non-resistant fractions as a percentage of the mean total concentrations of PTEs are shown in Table 6.

Table 6.
Mean proportion of the non-resistant fraction of ten PTEs in urban particulate matter worldwide, and corresponding bioavailability indices and individual contamination factors.
All the data concern the results of the chemical fractionation of PTEs in APM samples from the group “Commonly encountered pollution level”. The most similar percentages of the non-resistant fraction were obtained for Mn, Zn, Fe and Cd; the differences between the largest and smallest values were 7.6%, 10.9%, 12.1% and 12.5%, respectively. An individual contamination factor for each of the ten PTEs was determined as a measure of the degree of metal contamination in the environment with respect to a retention time of these elements [94] and the bioavailability index that is used to evaluate the potential mobility and bioavailability of metals [34].
ICF was calculated as the ratio of the content of the non-resistant fraction to the resistant fraction of the metal. PTEs were categorized into four classes of contamination: low contamination (ICF < 1), moderate contamination (1 ≤ ICF < 3), considerable contamination (3 ≤ ICF < 6) and high contamination (ICF ≥ 6) [12]. Metals with a higher ICF have a lower retention time, higher environmental mobility and exert a higher risk to the local environment [16].
On the other hand, BI was calculated as the ratio of the content of the non-resistant fraction to the total content of the metal. BI values were divided into three categories: low bioavailability, when BI < 0.3; medium bioavailability, when 0.3 ≤ BI < 0.5; and high bioavailability, when BI ≥ 0.5 [16].
Regardless of the SEP used, the same class of contamination was determined for four PTEs (Cd, Fe, Ni and Zn), whereas the same bioavailability category was determined only for three PTEs (Cd, Pb and Zn). Generally, the indices calculated based on the results from extraction according to the Fernández Espinosa SEP and the BCR SEP showed better comparability than those from extractions according to the Chester SEP and the Fernández Espinosa SEP, or for the Chester SEP and the BCR SEP. The results of extractions by means of the Fernández Espinosa SEP and the BCR SEPs yielded the same class of contamination in the case of eight elements (As, Cd, Cr, Cu, Fe, Ni, Pb and Zn) out of the ten that were analyzed. The comparability of bioavailability was worse; the same category was determined for only six elements (As, Cd, Cu, Fe, Pb and Zn).
7. Health Risk Assessment
Metallic and metalloid elements bound in respirable APM can easily get deep into human lung tissues through breathing. The intake of metals into the human body through inhalation over a long period of time and their accumulation can cause serious health risks [34]. For this reason, many researchers have not only studied the contamination of air with PTEs but have also evaluated their impact on human health (slightly over 30% of the reviewed 70 articles). The human-health risk posed by the PTEs due to inhalation of atmospheric particles was most frequently estimated [10,12,13,14,17,24,33], although health risks from other routes of exposure (ingestion and dermal contact) have also been determined [9,15,18,25,29,31,70]. Since PM2.5 is a stronger risk factor than the coarse part of PM 10 [65], this grain fraction is the most commonly used for characterizing inhalation exposures to particulate matter [8,9,10,13,16,18,30]. The risk calculations less frequently took into account the contents of PTEs in the fractions PM10 [12,80], PM1 [14] or TSP [25,70]. Carcinogenic and non-carcinogenic risks were usually assessed [10,12,13,15,16,17,18,24,31,33,63,71], but there are some publications in which only the carcinogenic risk was calculated [29,34,80].
The human risk assessment was estimated on the basis of the human-health risk model developed by the US Environmental Protection Agency [95,96]. The collection of publications reviewed includes papers that estimated health risks according to the current recommended approach for inhaled contaminants (US EPA 2009, Part F) [8,10,14,16,17,18,33,71], and also according to a previous approach (USEPA 1989; Part A) [9,25,31,34,70,80]. It is worth noting that the earlier approach is still being applied, even in recent publications (2018–2021) [9,12,15,25,31]. This kind of approach is not only a formal matter, because in the case of similar concentrations of toxic elements in the air, risk indicators (Hazard Quotient, HQ and Hazard Index, HI) determined according to these models differ considerably. Lower indicator values reflecting smaller health hazards are obtained according to the previously recommended risk assessment model [15,16,17,24,25,70].
The health risk assessment was usually conducted only for adults [12,34,58,80] or for adults and children [8,10,16,25,71]. There are also some studies in which the risk was estimated separately for a few groups of children and/or adults. The children were classified into two groups (e.g., I: 1–6 years; II: 6–18 years) [24,29] or three groups: infants (0–1 year), toddlers (0.5–5 years) and children (5–19 years) [17], whereas adults were classified into two groups: males and females [9,17].
The human-health risk posed by particulate toxic elements via inhalation has been estimated on the basis of the following: their total [12,13] or pseudo-total concentration [9,29,30,80]; the concentration of mobile fractions (sum of all fractions except the residual fraction, regardless of the SEP used) [12,29,30]; the concentration of the bioaccessible fraction [10,15,16,17,18,24,33,34]; the concentration of the exchangeble fraction [25]; and the concentration of the water-soluble fraction [29,80].
Among all the chemical fractions of the analyzed elements, the water-soluble fraction is the most readily bioaccessible form [17,46] as it can easily transfer into the dissolved fraction and enter the bloodstream from lung fluids [22,58]. As it is this fraction that has the highest potential to cause adverse health effects [17,63,97], in our work, the carcinogenic and non-carcinogenic risk assessment was performed based on literature data regarding the water-soluble fractions of airborne PTEs. The reference point was the risk calculated for their corresponding total concentration of PTEs. A similar approach to health risk assessment can be found in studies by other authors in which the risk determined for mobile fractions or bioaccessible fractions of PTEs was compared with the risk estimated on the basis of the total concentration of PTEs in the air [12,29,30].
In our work, human-health risk was calculated using a risk assessment model recommended by US EPA (2009, Part F) [95]. The exposure concentration (ECi) of non-carcinogenic and carcinogenic metals was calculated using Equation (1):
where Ci is the mean concentration of a toxic element in the air [μg m−3]; ET—exposure time [h day−1], EF—exposure frequency [days year−1], ED—exposure duration [years] and ATn is the averaging time of exposure [h].
A residential scenario could consist of inhalation exposure for up to 24 h per day, up to 350 days per year for 6 to 30 years [95]. In the reviewed publications, the most commonly used parameters for adults were ED = 24 years [8,10,13,17,29,33,71] and ET = 24 h day−1 [8,10,13,16,17,18,29,30,33,71]. Lower values were used far less frequently e.g., ED = 20 years [10,30] and ET = 8 h day−1 [13]. A greater variation relates to the exposure frequency value. EF = 350 days per year was most commonly used in risk calculations [8,16,25,30,71], but there are publications in which EF has lower values: 250 days per year [10,13] or 180 days per year [17,29,33]. The exposure parameters used in our study are: ED = 24 years, ET = 24 h day−1 and EF = 350 days per year (Table 7).

Table 7.
Values of parameters used in the health risk assessment.
The average time of exposure for carcinogens was calculated as ATn = 70 years⋅365 days year−1⋅24 h day−1, while for non-carcinogens this was calculated as ATn = 24 years⋅365 days year−1⋅24 h day−1. The water-soluble fraction concentrations and total concentrations of toxic and carcinogenic elements calculated by us as the mean of literature data were used as Ci (Table 5). The data refer mostly to the PM2.5 fraction, e.g., for Pb: PM2.5 (42% of the papers), PM1 (5%), PM10 (25%) and TSP (28%).
The non-carcinogenic health risk for each toxic metal was evaluated by means of hazard quotient (HQ) using Equation (2):
where RfCi, is the inhalation reference concentration [mg m−3]. The overall potential non-carcinogenic risk posed by more than one toxic element was calculated as hazard index (HI) using Equation (3):
Cancer risk (CR) was calculated for each carcinogenic element using Equation (4):
The total cancer risk (TCR) posed by more than one carcinogenic element was calculated using Equation (5):
A carcinogenic risk value higher than the upper limit (1 × 10−4) suggests that the presence of carcinogens in the air results in a high probability of developing cancer through lifetime exposure, while values below the lower limit (1 × 10−6) indicate no significant cancer risk. The acceptable risk range is between 1 × 10−6 (1 in 1,000,000) and 1 × 10−4 (1 in 10,000) [96]. An HQ and/or HI below 1 indicates that there is no significant risk of non-carcinogenic effects. If HQ and/or HI equals or exceeds 1, non-carcinogenic effects might occur.
Non-carcinogenic effects were estimated only for As, Cd, Co, Cr, Ni and Mn, since for the rest of the analyzed elements reference concentrations for inhalation exposure were not evaluated [98]. Carcinogenic effects and CR values were calculated only for As, Cd, Co, Cr, Ni and Pb, for which IUR values were available.
Risk characterization was conducted both for the residents living in cities with low or moderate PTE pollution (CEPL) and those living in cities with air extremely highly polluted with PTEs (HPL). The estimated potential carcinogenic risks are given in Table 8.

Table 8.
Carcinogenic risk from metallic and metalloid elements via inhalation exposure to airborne particles.
The risks estimated on the basis of the water-soluble fraction of an individual element (As, Cd, Co, Cr, Ni and Pb) for residents of CEPL-cities were within the acceptable risk range (between 1 × 10−6 and 1 × 10−4) and ranged from 1.1 × 10−7 to 1.3 × 10−5. The TCR = 2.5 × 10−5 was also lower than the maximum acceptable limit of 1 × 10−4, indicating that the carcinogenic risk was acceptable and amounted to a negligible risk posed by As, Cd, Co, Cr, Ni and Pb. It is worth noting that the TCR calculated on the basis of the total concentrations of these elements (8.6 × 10−5) was also acceptable (Table 8). For residents of HPL-cities, the inhalation cancer risk based also on water-soluble fractions of individual elements was higher than the maximum acceptable limit only in the case of As (4.3 × 10−4) and Co (1.5 × 10−4), which may indicate potential carcinogenic risks from these two elements. However, the total cancer risk (TCR = 6.8 × 10−4) exceeded 1 × 10−4, suggesting a high probability of developing cancer through lifetime exposure.
The reference value calculated for total concentrations of PTEs was almost six times higher (TCR = 4 × 10−3). The estimated non-carcinogenic inhalation risks from PTEs are listed in Table 9.

Table 9.
Non-carcinogenic risk from metallic and metalloid elements via inhalation exposure to airborne particles.
The HQ values for Cd, Co, Cr, Ni and Mn for residents of CEPL-cities calculated even on the basis of the total concentration were lower than the safe level (HQ = 1) and ranged from 1.2 × 10−1 to 7.2 × 10−1, indicating no non-carcinogenic risks from each individual element. Only in the case of As was the hazard quotient slightly higher than unity (HQ = 1.2). The non-carcinogenic risk estimated on the basis of the concentrations of water-soluble fractions of these metals was, on average, three times lower. The total non-carcinogenic risk was acceptable (HI = 1.1) only for calculations based on concentrations of water-soluble fractions.
Much greater non-carcinogenic risks exist for residents of HPL-cities (Table 9). In this case, only Cr (HQ = 1.8 × 10−1) and Ni (HQ = 4.2 × 10−1) pose no threat. HQ values for As, Cd, Co and Mn exceed the acceptable risk level many times over. HQ for arsenic reached as much as 20. In the case of the water-soluble fraction, the non-carcinogenic health risk due to exposure to all analyzed PTEs was very high (HI = 34). An even higher HI = 1.4 × 102 was obtained when the non-carcinogenic risk was estimated on the basis of the total concentration.
Our calculation results demonstrate convincingly that health risks estimated on the basis of total concentrations of metals may result in an overestimation. A similar view has been presented by the authors of the articles reviewed, who suggest that taking into consideration the bioaccessible form rather than the total content of heavy metals in the air gives a more adequate health risk estimate [17,18,24,33]. It is also worth noting that the toxicological studies have suggested it is the soluble toxic trace element content of APM that is more directly linked to its harmful effects, rather than their total content [100,101].
8. Conclusions
- Operational speciation of over 40 potentially toxic particulate-bound elements occurring in urban air has been described in the scientific literature. The most common research subjects were As, Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn.
- Unification of terminology, the procedure for sampling atmospheric aerosols, sample pretreatment and, above all, a sequential extraction procedure is still a distant goal.
- Most operational speciation studies of potentially toxic elements were carried out using one of these three procedures: Fernández Espinosa SEP > BCR SEPs > Chester’s SEP. However, the declared application of a specific procedure did not always mean that the authors strictly followed its original protocols; both physical and chemical operating parameters were altered.
- The distribution patterns developed for 10 potentially toxic elements in urban air particulate matter for three chemical fractionation schemes should serve as useful benchmarks for future atmospheric speciation studies.
- A distinctive feature of operational speciation of particulate-bound metallic elements is the large proportion of the most mobile fraction. From among 10 elements (As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb and Zn) present in urban air, Cd and Zn have the highest proportion of this fraction, whilst As, Co, Cu, Mn, Ni and Pb have a moderate proportion, and only Fe and Cr have a relatively low proportion, of this fraction.
- Ecological risk indices calculated on the basis of the results obtained from chemical fractionation according to the Fernández Espinosa SEP, BCR SEP and Chester’s SEP are poorly comparable. The same class of contamination was determined only for Cd, Fe, Ni and Zn, whereas the same bioavailability category was determined only for Cd, Pb and Zn. Generally, the ecological risk indices calculated from the extraction results by Fernández Espinosa SEP and BCR SEP showed better comparability than those calculated according to Chester’s SEP and Fernández Espinosa SEP, or using Chester’s SEP and BCR SEP.
- The total cancer inhalation risk estimated on the basis of both total concentrations of As, Cd, Co, Cr, Ni and Pb and their water-soluble fractions for residents of cities with low or moderate pollution appeared to be lower than the maximum acceptable limit of 1 × 10−4. However, the total non-cancerogenic inhalation risk was acceptable only when the risk was assessed based on water-soluble fractions of As, Cd, Co, Cr and Mn. The total non-carcinogenic inhalation risk for residents of highly polluted cities exceeded the acceptable risk level by 1–2 orders of magnitude.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxics10030124/s1, Figure S1: Operational speciation of As in urban atmospheric particulate matter based on literature data; Figure S2: Operational speciation of Cd in urban atmospheric particulate matter based on literature data; Figure S3: Operational speciation of Co in urban atmospheric particulate matter based on literature data; Figure S4: Operational speciation of Cr in urban atmospheric particulate matter based on literature data; Figure S5: Operational speciation of Cu in urban atmospheric particulate matter based on literature data; Figure S6: Operational speciation of Fe in urban atmospheric particulate matter based on literature data; Figure S7: Operational speciation of Mn in urban atmospheric particulate matter based on literature data; Figure S8: Operational speciation of Ni in urban atmospheric particulate matter based on literature data; Figure S9: Operational speciation of Pb in urban atmospheric particulate matter based on literature data; Figure S10: Operational speciation of Zn in urban atmospheric particulate matter based on literature data.
Author Contributions
Conceptualization, R.Ś.; methodology, R.Ś. and M.T.; data collection, curation and analysis, R.Ś. and M.T.; original draft preparation, R.Ś. and M.T.; editing, R.Ś. and M.T.; supervision, R.Ś. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and supplementary material.
Acknowledgments
The work was carried out as part of the own research of the Kazimierz Pulaski University of Technology and Humanities in Radom, research work No 3522/182/P.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Calvo, A.I.; Alves, C.; Castro, A.; Pont, V.; Vicente, A.M.; Fraile, R. Research on aerosol sources and chemical composition: Past, current and emerging issues. Atmos. Res. 2013, 120–121, 1–28. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, W.; Yu, Y.; Hu, B.; Xin, J.; Sun, Y.; Wang, L.; Wang, G.; Bi, X.; Zhang, G.; et al. Characteristics of PM2.5 mass concentrations and chemical species in urban and background areas of China: Emerging results from the CARE-China network. Atmos. Chem. Phys. 2018, 18, 8849–8871. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Filgueiras, A.V.; Lavilla, I.; Bendicho, C. Chemical sequential extraction for metal partitioning in environmental solid samples. J. Environ. Monit. 2002, 4, 823–857. [Google Scholar] [CrossRef] [PubMed]
- Smichowski, P.; Polla, G.; Gómez, D. Metal fractionation of atmospheric aerosols via sequential chemical extraction: A review. Anal. Bioanal. Chem. 2005, 381, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Nocoń, K.; Rogula-Kozłowska, W. Speciation of arsenic: A case study of PM1 in Zabrze. SN Appl. Sci. 2019, 1, 450. [Google Scholar] [CrossRef]
- Xie, J.J.; Yuan, C.G.; Xie, J.; Shen, Y.W.; Zha, D.W.; Zhang, K.G.; Zhu, H.T. Fraction distribution of arsenic in different-sized atmospheric particulate matters. Environ. Sci. Pollut. Res. 2019, 26, 30826–30835. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.J.; Yuan, C.G.; Xie, J.; Niu, X.D.; Zhang, X.R.; Zhang, K.G.; Xu, P.Y.; Ma, X.Y.; Lv, X.B. Comparison of arsenic fractions and health risks in PM2.5 before and after coal-gas replacement. Environ. Pollut. 2020, 259, 113881. [Google Scholar] [CrossRef]
- Wang, K.; Wang, W.; Li, L.; Li, J.; Wei, L.; Chi, W.; Hong, L.; Zhao, Q.; Jiang, J. Seasonal concentration distribution of PM1.0 and PM2.5 and a risk assessment of bound trace metals in Harbin, China: Effect of the species distribution of heavy metals and heat supply. Sci. Rep. 2020, 10, 8160. [Google Scholar] [CrossRef] [PubMed]
- Juda-Rezler, K.; Zajusz-Zubek, E.; Reizer, M.; Maciejewska, K.; Kurek, E.; Bulska, E.; Klejnowski, K. Bioavailability of elements in atmospheric PM2.5 during winter episodes at Central Eastern European urban background site. Atmos. Environ. 2021, 245, 117993. [Google Scholar] [CrossRef]
- Zajusz-Zubek, E.; Kaczmarek, K.; Mainka, A. Trace elements speciation of submicron particulate matter (PM1) collected in the surroundings of power plants. Int. J. Environ. Res. Public Health 2015, 12, 13085–13103. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Roy, R.; Yadav, S.; Satsangi, P.G. Chemical fractionation and health risk assessment of particulate matter-bound metals in Pune, India. Environ. Geochem. Health 2018, 40, 255–270. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Wang, Q.G.; Qian, X.; Qian, Y.; Yang, M.; Li, F.; Lu, H.; Wang, C. Chemical fractionation of arsenic and heavy metals in fine particle matter and its implications for risk assessment: A case study in Nanjing, China. Atmos. Environ. 2015, 103, 339–346. [Google Scholar] [CrossRef]
- Zajusz-Zubek, E.; Radko, T.; Mainka, A. Fractionation of trace elements and human health risk of submicron particulate matter (PM1) collected in the surroundings of coking plants. Environ. Monit. Assess. 2017, 189, 389. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Liu, P.; He, X.; Xu, H.; Shen, Z. Bioavailability of heavy metals bounded to PM2.5 in Xi’an, China: Seasonal variation and health risk assessment. Environ. Sci. Pollut. Res. 2021, 28, 35844–35853. [Google Scholar] [CrossRef] [PubMed]
- Sah, D.; Verma, P.K.; Kandikonda, M.K.; Lakhani, A. Chemical fractionation, bioavailability, and health risks of heavy metals in fine particulate matter at a site in the Indo-Gangetic Plain, India. Environ. Sci. Pollut. Res. 2019, 26, 19749–19762. [Google Scholar] [CrossRef] [PubMed]
- Sah, D.; Verma, P.K.; Kumari, K.M.; Lakhani, A. Chemical fractionation of heavy metals in fine particulate matter and their health risk assessment through inhalation exposure pathway. Environ. Geochem. Health 2019, 41, 1445–1458. [Google Scholar] [CrossRef] [PubMed]
- Anake, W.U.; Benson, N.U.; Tenebe, I.T.; Emenike, P.C.; Ana, G.R.E.E.; Zhang, S. Chemical speciation and health risks of airborne heavy metals around an industrial community in Nigeria. Hum. Ecol. Risk Assess. 2020, 26, 242–254. [Google Scholar] [CrossRef]
- Sipos, P.; Choi, C.; May, Z. Combination of single and sequential chemical extractions to study the mobility and host phases of potentially toxic elements in airborne particulate matter. Chem. Erde-Geochem. 2016, 76, 481–489. [Google Scholar] [CrossRef]
- Mbengue, S.; Alleman, L.Y.; Flament, P. Bioaccessibility of trace elements in fine and ultrafine atmospheric particles in an industrial environment. Environ. Geochem. Health 2015, 37, 875–889. [Google Scholar] [CrossRef]
- Pandey, M.; Pandey, A.K.; Mishra, A.; Tripathi, B.D. Speciation of carcinogenic and non-carcinogenic metals in respirable suspended particulate matter (PM10) in Varanasi, India. Urban Clim. 2017, 19, 141–154. [Google Scholar] [CrossRef]
- Xie, J.J.; Yuan, C.G.; Xie, J.; Shen, Y.W.; He, K.Q.; Zhang, K.G. Speciation and bioaccessibility of heavy metals in PM2.5 in Baoding city, China. Environ. Pollut. 2019, 252, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, Y.; Wu, T.; Shen, Z.; Xu, H. Acid-extractable heavy metals in PM2.5 over Xi’an, China: Seasonal distribution and meteorological influence. Environ. Sci. Pollut. Res. 2019, 26, 34357–34367. [Google Scholar] [CrossRef] [PubMed]
- Anake, W.U.; Ana, G.R.E.E.; Williams, A.B.; Fred-Ahmadu, O.H.; Benson, N.U. Chemical speciation and health risk assessment of fine particulate bound trace metals emitted from Ota Industrial Estate, Nigeria. In Proceedings of the 3rd International Conference on Advances in Environment Research, IOP Conference Series: Earth and Environmental Science, Beijing, China, 23–25 May 2017; Volume 68, p. 012005. [Google Scholar]
- Olumayede, E.G.; Ediagbonya, T.F. Sequential extractions and toxicity potential of trace metals absorbed into airborne particles in an urban atmosphere of Southwestern Nigeria. Sci. World J. 2018, 2018, 6852165. [Google Scholar] [CrossRef]
- Conca, E.; Malandrino, M.; Giacomino, A.; Costa, E.; Ardini, F.; Inaudi, P.; Abollino, O. Optimization of a sequential extraction procedure for trace elements in Arctic PM10. Anal. Bioanal. Chem. 2020, 412, 7429–7440. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.A.; Sánchez, K.; Richter, P.; Pey, J.; Gramsch, E. Partitioning of the water soluble versus insoluble fraction of trace elements in the city of Santiago, Chile. Atmosfera 2018, 31, 373–387. [Google Scholar] [CrossRef]
- Zajusz-Zubek, E.; Mainka, A. Analysis of trace elements in the mobile form of respirable fraction PM2.5 collected in the surroundings of Power Plant. Eng. Prot. Environ. 2015, 18, 245–258. (In Polish) [Google Scholar]
- Sah, D.; Verma, P.K.; Kumari, K.M.; Lakhani, A. Chemical partitioning of fine particle-bound As, Cd, Cr, Ni, Co, Pb and assessment of associated cancer risk due to inhalation, ingestion and dermal exposure. Inhal. Toxicol. 2017, 29, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Rajouriya, K.; Rohra, H.; Taneja, A. Levels of fine particulate matter bound trace metals in air of glass industrial area; Firozabad. Pollution 2020, 6, 555–568. [Google Scholar]
- Huang, L.; Bai, Y.H.; Ma, R.Y.; Zhuo, Z.M.; Chen, L. Winter chemical partitioning of metals bound to atmospheric fine particles in Dongguan, China, and its health risk assessment. Environ. Sci. Pollut. Res. 2019, 26, 13664–13675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, H.; Wei, X.; Fu, Z. Preliminary assessment of size distribution of airborne metals and metalloids in the urban aerosols of Guiyang, southwest China. Atmos. Pollut. Res. 2015, 6, 635–643. [Google Scholar] [CrossRef]
- Li, H.; Wang, Q.; Shao, M.; Wang, J.; Wang, C.; Sun, Y.; Qian, X.; Wu, H.; Yang, M.; Li, F. Fractionation of airborne particulate-bound elements in haze-fog episode and associated health risks in a megacity of southeast China. Environ. Pollut. 2016, 208, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.D.; Dang, Z.; Huang, W.L.; Yang, C. Chemical speciation of fine particle bound trace metals. Int. J. Environ. Sci. Technol. 2009, 6, 337–346. [Google Scholar] [CrossRef]
- Richter, P.; Griño, P.; Ahumada, I.; Giordano, A. Total element concentration and chemical fractionation in airborne particulate matter from Santiago, Chile. Atmos. Environ. 2007, 41, 6729–6738. [Google Scholar] [CrossRef]
- Astolfi, M.L.; Canepari, S.; Carderelli, E.; Ghighi, S.; Marzo, M.L. Chemical fractionation of elements in airborne particulate matter: Primary results on PM10 and PM2.5 samples in the Lazio region (central Italy). Ann. Chim. 2006, 96, 183–194. [Google Scholar] [CrossRef]
- Canepari, S.; Perrino, C.; Olivieri, F.; Astolfi, M.L. Characterisation of the traffic sources of PM through size-segregated sampling, sequential leaching and ICP analysis. Atmos. Environ. 2008, 42, 8161–8175. [Google Scholar] [CrossRef]
- Canepari, S.; Pietrodangelo, A.; Perrino, C.; Astolfi, M.L.; Marzo, M.L. Enhancement of source traceability of atmospheric PM by elemental chemical fractionation. Atmos. Environ. 2009, 43, 4754–4765. [Google Scholar] [CrossRef]
- Dabek-Zlotorzynska, E.; Kelly, M.; Chen, H.; Chakrabarti, C.L. Evaluation of capillary electrophoresis combined with a BCR sequential extraction for determining distribution of Fe, Zn, Cu, Mn, and Cd in airborne particulate matter. Anal. Chim. Acta 2003, 498, 175–187. [Google Scholar] [CrossRef]
- Fernández Espinosa, A.J.; Rodriguez, M.T.; Barragán de la Rosa, F.J.; Sánchez, J.C.J. A chemical speciation of trace metals for fine urban particles. Atmos. Environ. 2002, 36, 773–780. [Google Scholar] [CrossRef]
- Koçak, M.; Kubilay, N.; Herut, B.; Nimmo, M. Trace metal solid state speciation in aerosols of the Northern Levantine Basin, East Mediterranean. J. Atmos. Chem. 2007, 56, 239–257. [Google Scholar] [CrossRef]
- Fernández-Espinosa, A.J.; Ternero-Rodriguez, M. Study of traffic pollution by metals in Seville (Spain) by physical and chemical speciation methods. Anal. Bioanal. Chem. 2004, 379, 684–699. [Google Scholar] [CrossRef] [PubMed]
- Voutsa, D.; Samara, C. Labile and bioaccessible fractions of heavy metals in the airborne particulate matter from urban and industrial areas. Atmos. Environ. 2002, 36, 3583–3590. [Google Scholar] [CrossRef]
- Heal, M.R.; Hibbs, L.R.; Agius, R.M.; Beverland, I.J. Total and water-soluble trace metal content of urban background PM10, PM2.5 and black smoke in Edinburgh, U.K. Atmos. Environ. 2005, 39, 1417–1430. [Google Scholar] [CrossRef]
- Al-Masri, M.S.; Al-Kharfan, K.; Al-Shamali, K. Speciation of Pb, Cu and Zn determined by sequential extraction for identification of air pollution sources in Syria. Atmos. Environ. 2006, 40, 753–761. [Google Scholar] [CrossRef]
- Turšič, J.; Radić, H.; Kovačević, M.; Veber, M. Determination of selected trace elements in airborne aerosols particles using different sample preparation. Arch. Ind. Hyg. Toxicol. 2008, 59, 111–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dos Santos, M.; Gómez, D.; Dawidowski, L.; Gautier, E.; Smichowski, P. Determination of water-soluble and insoluble compounds in size classified airborne particulate matter. Microchem. J. 2009, 91, 133–139. [Google Scholar] [CrossRef]
- Fujiwara, F.; Dos Santos, M.; Marrero, J.; Polla, G.; Gómez, D.; Dawidowski, L.; Smichowski, P. Fractionation of eleven elements by chemical bonding from airborne particulate matter collected in an industrial city of Argentina. J. Environ. Monit. 2006, 8, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Pöykiö, R.; Perämäki, P.; Välimäki, I.; Kuokkanen, T. Estimation of environmental mobility of heavy metals using a sequential leaching of particulate material emitted from an opencast chrome mine complex. Anal. Bioanal. Chem. 2002, 373, 190–194. [Google Scholar] [CrossRef]
- Wu, Y.F.; Liu, C.Q.; Tu, C.L. Atmospheric deposition of metals in TSP of Guiyang, PR China. Bull. Environ. Contam. Toxicol. 2008, 80, 465–468. [Google Scholar] [CrossRef]
- Bikkes, M.; Polyák, K.; Hlavay, J. Fractionation of elements by particle size and chemical bonding from aerosols followed by ETAAS determination. J. Anal. At. Spectrom. 2001, 16, 74–81. [Google Scholar] [CrossRef]
- Preciado, H.F.; Li, L.Y. Evaluation of metal loadings and bioavailability in air, water and soil along two highways of British Columbia, Canada. Water Air Soil Pollut. 2006, 172, 81–108. [Google Scholar] [CrossRef]
- Jeong, G.H.; Lee, J.Y.; Moon, J.Y.; Lee, S.I. Chemical speciation of trace metals in airborne particles at an industrialized site. J. Environ. Sci. 2006, 15, 503–511. [Google Scholar]
- Canepari, S.; Astolfi, M.L.; Moretti, S.; Curini, R. Comparison of extracting solutions for elemental fractionation in airborne particulate matter. Talanta 2010, 82, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Canepari, S.; Cardarelli, E.; Perrino, C.; Catrambone, M.; Pietrodangelo, A.; Strincone, M. Two-stage chemical fractionation method for the analysis of elements and non-volatile inorganic ions in PM10 samples: Application to ambient samples collected in Rome (Italy). Atmos. Environ. 2006, 40, 7908–7923. [Google Scholar] [CrossRef]
- Niu, J.; Rasmussen, P.E.; Hassan, N.M.; Vincent, R. Concentration distribution and bioaccessibility of trace elements in nano and fine urban airborne particulate matter: Influence of particle size. Water Air Soil Pollut. 2010, 213, 211–225. [Google Scholar] [CrossRef]
- Celo, V.; Mahmoud, M.; Yassine, M.M.; Dabek-Zlotorzynska, E. Insights into elemental composition and sources of fine and coarse particulate matter in dense traffic areas in Toronto and Vancouver, Canada. Toxics 2021, 9, 264. [Google Scholar] [CrossRef] [PubMed]
- Betha, R.; Pradani, M.; Lestari, P.; Joshi, U.M.; Reid, J.S.; Balasubramanian, R. Chemical speciation of trace metals emitted from Indonesian peat fires for health risk assessment. Atmos. Res. 2013, 122, 571–578. [Google Scholar] [CrossRef]
- Szigeti, T.; Mihucz, V.G.; Óvári, M.; Baysal, A.; Atılgan, S.; Akman, S.; Záray, G. Chemical characterization of PM2.5 fractions of urban aerosol collected in Budapest and Istanbul. Microchem. J. 2013, 107, 86–94. [Google Scholar] [CrossRef]
- Cancio, J.L.; Sánchez, A.D.; Alemán, P.S. Metallic species in ambient air particles of Canary Islands. Soluble fraction in total suspended matter. Afinidad 2013, 70, 34–42. [Google Scholar]
- Muránszky, G.; Óvári, M.; Virág, I.; Csiba, P.; Dobai, R.; Záray, G. Chemical characterization of PM10 fractions of urban aerosol. Microchem. J. 2011, 98, 1–10. [Google Scholar] [CrossRef]
- Rogula-Kozłowska, W.; Błaszczak, B.; Szopa, S.; Klejnowski, K.; Sówka, I.; Zawoździak, A.; Jabłońska, M.; Mathews, B. PM2.5 in the central part of Upper Silesia, Poland: Concentrations, elemental composition, and mobility of components. Environ. Monit. Assess. 2013, 185, 581–601. [Google Scholar] [CrossRef]
- Betha, R.; Behera, S.N.; Balasubramanian, R. 2013 Southheast Asian smoke haze: Fractionation of particulate-bound elements and associated health risk. Environ. Sci. Technol. 2014, 48, 4327–4335. [Google Scholar] [CrossRef] [PubMed]
- Funasaka, K.; Tojo, T.; Kaneco, S.; Takaoka, M. Different chemical properties of lead in atmospheric particles from urban roadside and residential areas. Atmos. Pollut. Res. 2013, 4, 362–369. [Google Scholar] [CrossRef][Green Version]
- Zhao, Z.; Jing, Y.; Luo, X.S.; Li, H.; Tang, M. Overview and research progresses in chemical speciation and in vitro bioaccessibility analyses of airborne particulate trace metals. Curr. Pollut. Rep. 2021, 7, 540–548. [Google Scholar] [CrossRef]
- Mishra, A.; Pervez, S.; Candeias, C.; Verma, M.; Bano, S.; Dugga, P.; Verma, S.R.; Tamrakar, A.; Shafi, S.; Pervez, Y.F.; et al. Bioaccessiblity features of particulate bound toxic elements: Review of extraction approaches, concentrations and health risks. J. Indian Chem. Soc. 2021, 98, 100212. [Google Scholar] [CrossRef]
- Templeton, D.M.; Ariese, F.; Cornelis, R.; Danielsson, L.-G.; Muntau, H.; van Leeuwen, H.P.; Lobinski, R. Guidelines for terms related to chemical speciation and fractionation of elements. Definitions, structural aspects, and methodological approaches (IUPAC Recommendations 2000). Pure Appl. Chem. 2000, 72, 1453–1470. [Google Scholar] [CrossRef]
- Schleicher, N.; Norra, S.; Chai, F.; Chen, Y.; Wang, S.; Cen, K.; Yu, Y.; Stüben, D. Mobility of trace metals in urban atmospheric particulate matter from Beijing, China. In Urban Environment, Proceedings of the 10th Urban Environment Symposium; Springer: Berlin/Heidelberg, Germany, 2012; pp. 191–200. [Google Scholar]
- Schleicher, N.J.; Norra, S.; Chai, F.; Chen, Y.; Wang, S.; Cen, K.; Yu, Y.; Stüben, D. Temporal variability of trace metal mobility of urban particulate matter from Beijinge. A contribution to health impact assessments of aerosols. Atmos. Environ. 2011, 45, 7248–7265. [Google Scholar] [CrossRef]
- Li, X.; Feng, L.; Huang, C.; Yan, X.; Zhang, X. Potential hazardous elements (PHEs) in atmospheric particulate matter (APM) in the south of Xi’an during the dust episodes of 2001–2012 (NW China): Chemical fractionation, ecological and health risk assessment. Environ. Earth Sci. 2014, 71, 4115–4126. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Wu, J.; Lian, H.; Chen, Y. Fractionation and health risks of atmospheric particle-bound As and heavy metals in summer and winter. Sci. Total Environ. 2014, 493, 487–494. [Google Scholar] [CrossRef]
- Shao, L.; Xiao, H.; Wu, D. Speciation of heavy metals in airborne particles, road dust, and soils along expressways in China. Chin. J. Geochem. 2013, 32, 420–429. [Google Scholar] [CrossRef]
- Gao, J.; Tian, H.; Cheng, K.; Lu, L.; Wang, Y.; Wu, Y.; Zhu, C.; Liu, K.; Zhou, J.; Liu, X.; et al. Seasonal and spatial variation of trace elements in multi-size airborne particulate matters of Beijing, China: Mass concentration, enrichment characteristics, source apportionment, chemical speciation and bioavailability. Atmos. Environ. 2014, 99, 257–265. [Google Scholar] [CrossRef]
- Zhai, Y.; Liu, X.; Chen, H.; Xu, B.; Zhu, L.; Li, C.; Zeng, G. Source identification and potential ecological risk assessment of heavy metals in PM2.5 from Changsha. Sci. Total Environ. 2014, 493, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Chester, R.; Lin, F.J.; Murphy, K.J.T. A three stage sequential leaching scheme for the characterisation of the sources and environmental mobility of trace metals in the marine aerosol. Environ. Technol. Lett. 1989, 10, 887–900. [Google Scholar] [CrossRef]
- Hlavay, J.; Polyák, K.; Bódog, I.; Molnár, Á.; Mészáros, E. Distribution of trace elements in filter-collected aerosol samples. Fresenius J. Anal. Chem. 1996, 354, 227–232. [Google Scholar] [CrossRef]
- Fernández, A.J.; Ternero, M.; Barragán, F.J.; Jiménez, J.C. An approach to characterization of sources of urban airborne particles through heavy metal speciation. Chemosphere-Glob. Chang. Sci. 2000, 2, 123–136. [Google Scholar] [CrossRef]
- Pourret, O.; Bollinger, J.-C. “Heavy Metals”—What to do now: To use or not to use? Sci. Total Environ. 2018, 610–611, 419–420. [Google Scholar] [CrossRef]
- Pourret, O.; Hursthouse, A. It’s time to replace the term “heavy metals” with “potentially toxic elements” when reporting environmental research. Int. J. Environ. Res. Public Health 2019, 16, 4446. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Satsangi, P.G. Characterization of particulate matter and its related metal toxicity in an urban location in South West India. Environ. Monit. Assess. 2013, 185, 7365–7379. [Google Scholar] [CrossRef] [PubMed]
- Hlavay, J.; Polyak, K.; Molnar, A.; Meszaros, E. Determination of the distribution of elements as a function of particle size in aerosol samples by sequential leaching. Analyst 1998, 123, 859–863. [Google Scholar] [CrossRef]
- Ure, A.M.; Quevauviller, P.; Muntau, H.; Griepink, B. Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. Int. J. Environ. Anal. Chem. 1993, 51, 135–151. [Google Scholar] [CrossRef]
- Rauret, G.; López-Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–850. [Google Scholar] [CrossRef]
- Obiols, J.; Devesa, R.; Sol, A. Speciation of heavy metals in suspended particulates in urban air. Toxicol. Environ. Chem. 1986, 13, 121–128. [Google Scholar] [CrossRef]
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D.C. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]
- Desboeufs, K.V.; Sofikitis, A.; Losno, R.; Colin, J.L.; Ausset, P. Dissolution and solubility of trace metals from natural and anthropogenic aerosol particulate matter. Chemosphere 2005, 58, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Chester, R.; Nimmo, M.; Preston, M.R. The trace metal chemistry of atmospheric dry deposition samples collected at Cap Ferrat: A coastal site in the Western Mediterranean. Mar. Chem. 1999, 68, 15–30. [Google Scholar] [CrossRef]
- Directive 2004/107/EC of the European Parliament and of the Council of 15 December 2004 Relating to Arsenic, Cadmium, Mercury, Nickel and Polycyclic aromatic Hydrocarbons in Ambient Air. Official Journal of the European Union L 23/3. 26 January 2005. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2005:023:0003:0016:EN:PDF (accessed on 22 September 2021).
- Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on Ambient Air Quality and Cleaner Air for Europe. Official Journal of the European Union L 152/1. 11 June 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008L0050&from=en (accessed on 25 September 2021).
- European Environment Agency. Air Quality in Europe—2020 Report; EEA Report No 09/2020; European Environment Agency: København, Danmark, September 2020. [Google Scholar]
- Kowalska, J.B.; Mazurek, R.; Gąsiorek, M.; Zaleski, T. Pollution indices as useful tools for the comprehensive evaluation of the degree of soil contamination—A review. Environ. Geochem. Health 2018, 40, 2395–2420. [Google Scholar] [CrossRef] [PubMed]
- Quevauviller, P. Operationally defined extraction procedures for soil and sediment analysis I. Standardization. Trends Anal. Chem. 1998, 17, 289–297. [Google Scholar] [CrossRef]
- Naji, A.; Ismail, A.; Ismail, A.R. Chemical speciation and contamination assessment of Zn and Cd by sequential extraction in surface sediment of Klang River, Malaysia. Microchem. J. 2010, 95, 285–292. [Google Scholar] [CrossRef]
- US EPA. Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part F, Supplemental Guidance for Inhalation Risk Assessment); Office of Superfund Remediation and Technology Innovation Environmental Protection Agency: Washington, DC, USA, 2009.
- US EPA. Risk Assessment Guidance for Superfund, Vol. I, Human Health Evaluation Manual (Part A); EPA/540/1-89/002; Office of Emergency and Remedial Response: Washington, DC, USA, 1989; p. 20450.
- Mukhtar, A.; Limbeck, A. Recent developments in assessment of bio–accessible trace metal fractions in airborne particulate matter: A review. Anal. Chim. Acta 2013, 774, 11–25. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Recommended Default Exposure Factors; US Environmental Protection Agency: Washington, DC, USA, February 2014.
- US EPA. Regional Screening Levels (RSLs)—Generic Tables. 2021. Available online: http://www.epa.gov/region9/superfund/prg/ (accessed on 14 July 2021).
- Costa, D.L.; Dreher, K.L. Bioavailable transition metals in particulate matter mediate cardiopulmonary injury in healthy and compromised animal models. Environ. Health Perspect. 1997, 105, 1053–1060. [Google Scholar] [PubMed]
- Adamson, I.Y.R.; Prieditis, H.; Hedgecock, C.; Vincent, R. Zinc is the toxic factor in the lung response to an atmospheric particulate sample. Toxicol. Appl. Pharmacol. 2000, 166, 111–119. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).