Environmentally Toxic Solid Nanoparticles in Noradrenergic and Dopaminergic Nuclei and Cerebellum of Metropolitan Mexico City Children and Young Adults with Neural Quadruple Misfolded Protein Pathologies and High Exposures to Nano Particulate Matter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area Air Quality
2.2. Study Design and Brain Samples
2.3. Transmission Electron Microscopy (TEM), High Resolution Scanning and Transmission Electron Microscopy (HRSTEM) and Energy-Dispersive X-ray Spectrometry (EDX) Studies
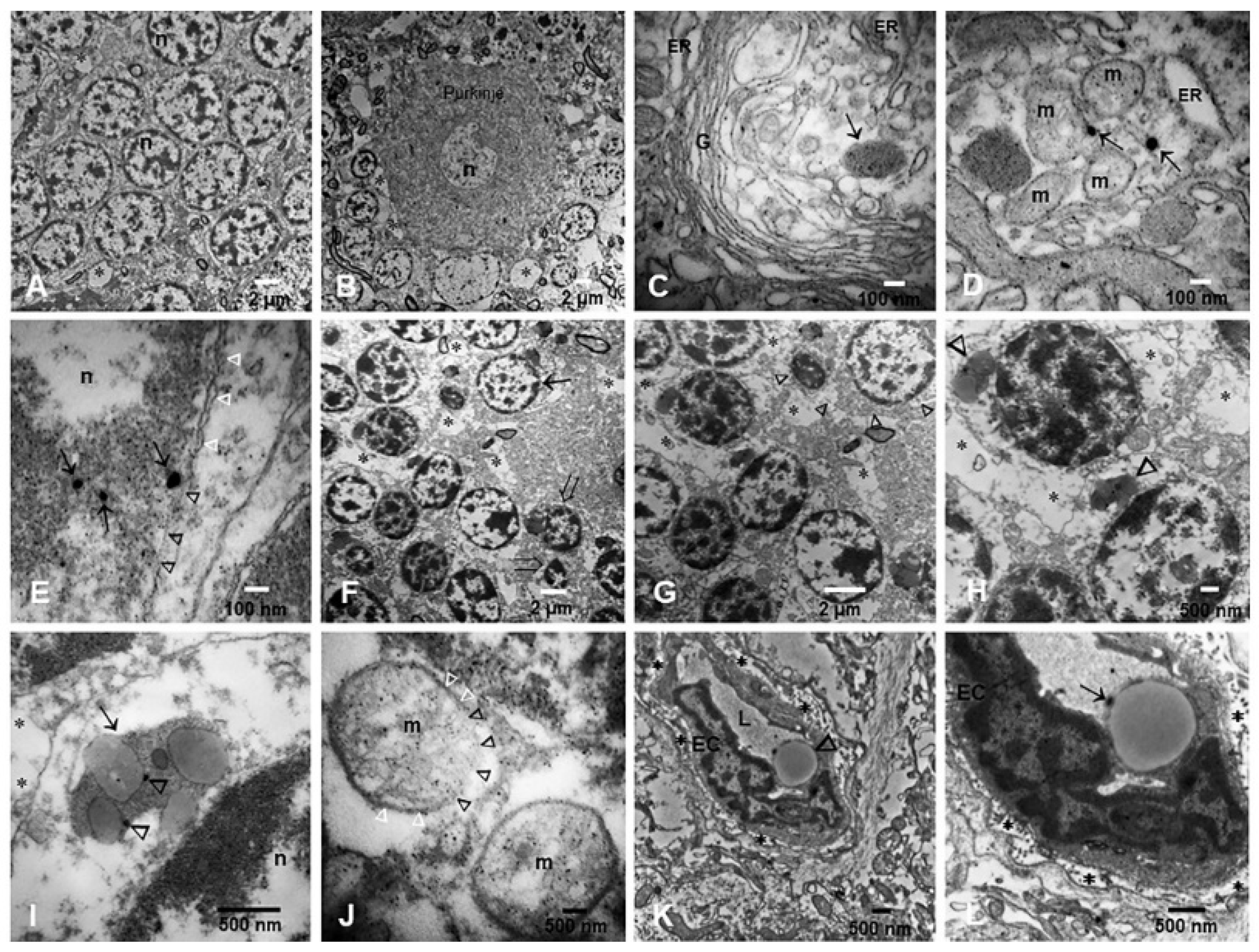
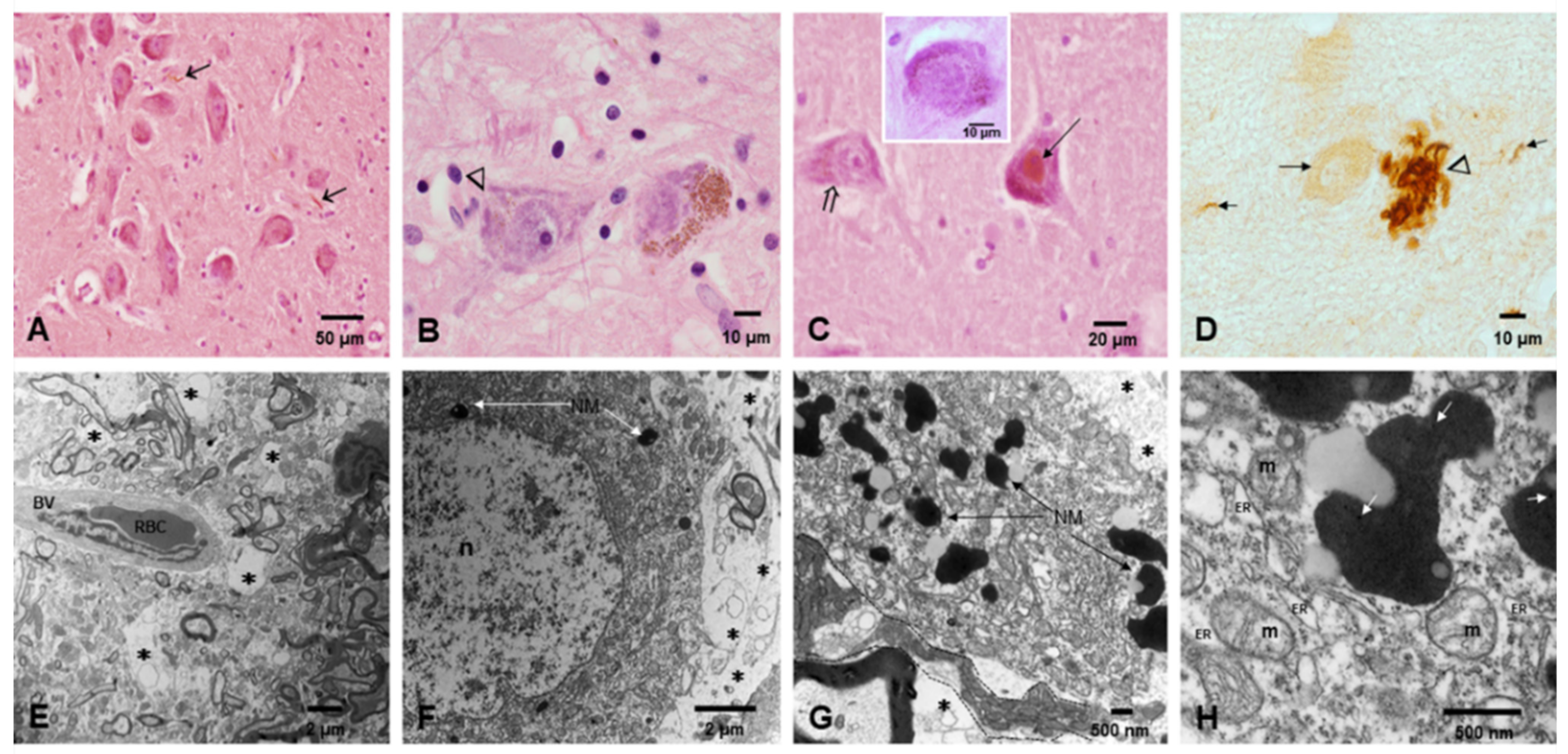
3. Results
3.1. Air Pollution
3.2. Light and Electron Microscopy in Cerebellum, Substantia Nigrae and Locus Coeruleus
3.3. EDX
4. Discussion
5. Concluding Remarks
- The portals of entry and the specific characteristics (composition and size) of the NPs are critical in defining which cells and organelles are affected. The SN, LC and the cerebellum are early NP targets and p-τ is the most common aberrant protein ID in young urbanites [12].
- Strikingly, we have identified in SNpc and LC neural cells and macrophages in situ NPs containing Fe, Ti, W and Hg associated with extensive NVU, mitochondrial and nuclear damage. The elongated Ti-rich NPs are similar to the ones described by our group in neuroenteric neurons, strongly supporting the gastrointestinal portal of entry is at work in young urbanites [12].
- Iron-rich NPs in SN, LC and cerebellar children’s tissues potentially represent a severe risk; they can generate heat under an alternating magnetic field and/or magnetic field gradients, making possible particle displacement/rotation and localized heating through microwave absorption [106,107,108,109,110]. Children are extensively exposed to low frequency electric and magnetic fields (EMFs) of various frequencies and wireless networks Wi-Fi involving at least one Wi-Fi antenna using a 2.4 GHz band [147,148,149]. High-voltage power lines, transformer buildings, domestic appliances e.g., hair dryers, electric shavers, induction cookers plus compact fluorescent lamps, inductive charging systems for electric cars and security or anti-theft devices ought to be included for possible future risk analysis as clearly stated by Gajsek et al. in their Electromagnetic Field (EMF) Exposure Assessment in Europe [148].
- The presence of HgNPs in neural cells is extremely worrisome, and the accumulation of Hg and Fe NPs in macrophage-like cells associated with LC and SNpc neurons is striking and heightens the possibility of neuronal damage by activated macrophages capable of accumulating NPs, likely through scavenger receptor-mediated endocytosis and lysosomal internalization as seen experimentally upon the administration of iron oxide nanoparticles [150].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johannson, K.A.; Balmes, J.R.; Collard, H.R. Air pollution exposure: A novel environmental risk factor for interstitial lung disease? Chest 2015, 147, 1161–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corea, F.; Silvestrelli, G.; Baccarelli, A.; Giua, A.; Previdi, P.; Siliprandi, G.; Murgia, N. Airborne Pollutants and Lacunar Stroke: A Case Cross-Over Analysis on Stroke Unit Admissions. Neurol. Int. 2012, 4, 44–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newby, D.E.; Mannucci, P.M.; Tell, G.S.; Baccarelli, A.; Brook, R.D.; Donaldson, K.; Forastiere, F.; Franchini, M.; Franco, O.; Graham, I.; et al. Expert position paper on air pollution and cardiovascular disease. Eur. Heart J. 2015, 36, 83–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, C.R.; Lin, Y.T.; Hwang, B. Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: A population-based cohort study in Taiwan. J. Alzheimers Dis. 2015, 44, 573–584. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Kwong, J.C.; Copes, R.; Tu, K.; Villeneuve, P.; van Donkelaar, A.; Hystad, P.; Martin, R.V.; Murray, B.; Jessiman, B.; et al. Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: A population-based cohort study. Lancet 2017, 389, 718–726. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Ayala, A. Air pollution, ultrafine particles, and your brain—Are combustion nanoparticle emissions and engineered nanoparticles causing preventable fatal neurodegenerative diseases and common neuropsychiatric outcomes? Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Pérez-Calatayud, A.; González-Maciel, A.; Reynoso-Robles, R.; Silva-Pereyra, H.G.; Ramos-Morales, A.; Torres-Jardón, R.; Soberanes-Cerino, C.D.J.; Carrillo-Esper, R.; Briones-Garduño, J.C.; et al. Environmental Nanoparticles Reach Human Fetal Brains. Biomedicines 2022, 10, 410. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Reed, W.; Maronpot, R.R.; Henríquez-Roldán, C.; Delgado-Chavez, R.; Calderón-Garcidueñas, A.; Dragustinovis, I.; Franco-Lira, M.; Aragón-Flores, M.; Solt, A.C.; et al. Brain Inflammation and Alzheimer’s-Like Pathology in Individuals Exposed to Severe Air Pollution. Toxicol. Pathol. 2004, 32, 650–658. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Reynoso-Robles, R.; Pérez-Guillé, B.; Mukherjee, P.S.; Gónzalez-Maciel, A. Combustion derived nanoparticles, the neuroenteric system, cervical vagus, hyperphosphorilated alpha synuclein and tau in young Mexico City residents. Environ. Res. 2017, 159, 186–201. [Google Scholar] [CrossRef] [Green Version]
- Calderón-Garcidueñas, L.; Gónzalez-Maciel, A.; Reynoso-Robles, R.; Delgado-Chávez, R.; Mukherjee, P.S.; Kulesza, R.J.; Torres-Jardón, R.; Ávila-Ramírez, J.; Villarreal-Ríos, R. Hallmarks of Alzheimer disease are evolving relentlessly in Metropolitan Mexico City infants, children and young adults. APOE4 carriers have higher suicide risk and higher odds of reaching NFT stage V at ≤40 years of age. Environ. Res. 2018, 164, 475–487. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Herrera-Soto, A.; Jury, N.; Maher, B.A.; González-Maciel, A.; Reynoso-Robles, R.; Ruiz-Rudolph, P.; van Zundert, B.; Varela-Nallar, L. Reduced repressive epigenetic marks, increased DNA damage and Alzheimer’s disease hallmarks in the brain of humans and mice exposed to particulate urban air pollution. Environ. Res. 2020, 183, 109226. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; González-Maciel, A.; Reynoso-Robles, R.; Hammond, J.; Kulesza, R.; Lachmann, I.; Torres-Jardón, R.; Mukherjee, P.S.; Maher, B.A. Quadruple abnormal protein aggregates in brainstem pathology and exogenous metal-rich magnetic nanoparticles (and engineered Ti-rich nanorods). The substantia nigrae is a very early target in young urbanites and the gastrointestinal tract a key brainstem portal. Environ. Res. 2020, 191, 110139. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L. Parkinson disease and air pollution: Does what we breathe matter? Nat. Rev. Neurol. 2021, 17, 467–468. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Franco-Lira, M.; Mora-Tiscareño, A.; Medina-Cortina, H.; Torres-Jardón, R.; Kavanaugh, M. Early Alzheimer’s and Parkinson’s Disease Pathology in Urban Children: Friend versus Foe Responses—It Is Time to Face the Evidence. BioMed Res. Int. 2013, 2013, 161687. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; González-González, L.O.; Kulesza, R.J.; Fech, T.M.; Pérez-Guillé, G.; Luna, M.A.J.-B.; Soriano-Rosales, R.E.; Solorio, E.; Miramontes-Higuera, J.D.J.; Chew, A.G.-M.; et al. Exposures to fine particulate matter (PM2.5) and ozone above USA standards are associated with auditory brainstem dysmorphology and abnormal auditory brainstem evoked potentials in healthy young dogs. Environ. Res. 2017, 158, 324–332. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; González-Maciel, A.; Reynoso-Robles, R.; Kulesza, R.J.; Mukherjee, P.S.; Torres-Jardón, R.; Rönkkö, T.; Doty, R.L. Alzheimer’s disease and alpha-synuclein pathology in the olfactory bulbs of infants, children, teens and adults ≤40 years in Metropolitan Mexico City. APOE4 carriers at higher risk of suicide accelerate their olfactory bulb pathology. Environ. Res. 2018, 166, 348–362. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; D’Angiulli, A.; Kulesza, R.J.; Torres-Jardón, R.; Osnaya, N.; Romero, L.; Keefe, S.; Herritt, L.; Brooks, D.M.; Avila-Ramirez, J.; et al. Air pollution is associated with brainstem auditory nuclei pathology and delayed brainstem auditory evoked potentials. Int. J. Dev. Neurosci. 2011, 29, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Calderón-Garcidueñas, L.; Torres-Solorio, A.K.; Kulesza, R.J.; Torres-Jardón, R.; González-González, L.O.; García-Arreola, B.; Chávez-Franco, D.A.; Luévano-Castro, S.C.; Hernández-Castillo, A.; Carlos-Hernández, E.; et al. Gait and balance disturbances are common in young urbanites and associated with cognitive impairment. Air pollution and the historical development of Alzheimer’s disease in the young. Environ. Res. 2020, 191, 110087. [Google Scholar] [CrossRef]
- Venkatraman, A.; Edlow, B.L.; Immordino-Yang, M.H. The Brainstem in Emotion: A Review. Front. Neuroanat. 2017, 11, 15. [Google Scholar] [CrossRef]
- Zhang, Y.; Larcher, K.M.-H.; Misic, B.; Dagher, A. Anatomical and functional organization of the human substantia nigra and its connections. eLife 2017, 6, e26653. [Google Scholar] [CrossRef]
- Zelena, D.; Menant, O.; Andersson, F.; Chaillou, E. Periaqueductal gray and emotions: The complexity of the problem and the light at the end of the tunnel, the magnetic resonance imaging. Endocr. Regul. 2018, 52, 222–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamaszek, M.; D’Agata, F.; Ferrucci, R.; Habas, C.; Keulen, S.; Kirkby, K.C.; Leggio, M.; Mariën, P.; Molinari, M.; Moulton, E.; et al. Consensus Paper: Cerebellum and Emotion. Cerebellum 2017, 16, 552–576. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.K.; Park, H.H.; Saw, N.L.; Singhal, K.; Ogawa, G.; Leib, R.D.; Shamloo, M. Age-related neuroinflammation and pathology in the locus coeruleus and hippocampus: Beta-adrenergic antagonists exacerbate impairment of learning and memory in aged mice. Neurobiol. Aging 2021, 106, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Sergio, T.D.O.; Lei, K.; Kwok, C.; Ghotra, S.; Wegner, S.A.; Walsh, M.; Waal, J.; Darevsky, D.; Hopf, F.W. The role of anterior insula–brainstem projections and alpha-1 noradrenergic receptors for compulsion-like and alcohol-only drinking. Neuropsychopharmacology 2021, 46, 1918–1926. [Google Scholar] [CrossRef]
- Bolton, C.J.; Tam, J.W. Differential Involvement of the Locus Coeruleus in Early- and Late-Onset Alzheimer’s Disease: A Potential Mechanism of Clinical Differences? J. Geriatr. Psychiatry Neurol. 2021. [Google Scholar] [CrossRef]
- Prasuhn, J.; Prasuhn, M.; Fellbrich, A.; Strautz, R.; Lemmer, F.; Dreischmeier, S.; Kasten, M.; Münte, T.F.; Hanssen, H.; Heldmann, M.; et al. Association of Locus Coeruleus and Substantia Nigra Pathology with Cognitive and Motor Functions in Patients with Parkinson Disease. Neurology 2021, 97, e1007–e1016. [Google Scholar] [CrossRef]
- Langley, J.; Hussain, M.S.; Huddleston, D.E.; Bennett, I.J.; Hu, X.P. Impact of Locus Coeruleus and Its Projections on Memory and Aging. Brain Connect. 2021. [Google Scholar] [CrossRef]
- Fischer, N.M.; Hinkle, J.T.; Perepezko, K.; Bakker, C.C.; Morris, M.; Broen, M.P.; Butala, A.; Dawson, T.M.; Leentjens, A.F.; Mari, Z.; et al. Brainstem Pathologies Correlate with Depression and Psychosis in Parkinson’s Disease. Am. J. Geriatr. Psychiatry 2021, 29, 958–968. [Google Scholar] [CrossRef]
- Lamotte, G.; Shouman, K.; Benarroch, E.E. Stress and central autonomic network. Auton. Neurosci. 2021, 235, 102870. [Google Scholar] [CrossRef]
- Grella, S.L.; Gomes, S.M.; Lackie, R.E.; Renda, B.; Marrone, D.F. Norepinephrine as a spatial memory reset signal. Behav. Pharmacol. 2021, 32, 531–548. [Google Scholar] [CrossRef]
- Llorca-Torralba, M.; Camarena-Delgado, C.; Suárez-Pereira, I.; Bravo, L.; Mariscal, P.; Garcia-Partida, J.A.; López-Martín, C.; Wei, H.; Pertovaara, A.; Mico, J.A.; et al. Pain and depression comorbidity causes asymmetric plasticity in the locus coeruleus neurons. Brain 2021, 2021, awab239. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain 2015, 138 Pt 10, 2814–2833. [Google Scholar] [CrossRef]
- Arnsten, A.F.T.; Datta, D.; Del Tredici, K.; Braak, H. Hypothesis: Tau pathology is an initiating factor in sporadic Alzheimer’s disease. Alzheimer’s Dement. 2021, 17, 115–124. [Google Scholar] [CrossRef]
- Harley, C.W.; Walling, S.G.; Yuan, Q.; Martin, G.M. The ‘a, b, c’s of pretangle tau and their relation to aging and the risk of Alzheimer’s Disease. Semin. Cell Dev. Biol. 2021, 116, 125–134. [Google Scholar] [CrossRef]
- Tilley, B.S.; Patel, S.R.; Goldfinger, M.H.; Pearce, R.K.; Gentleman, S.M. Locus Coeruleus Pathology Indicates a Continuum of Lewy Body Dementia. J. Park. Dis. 2021, 11, 1641–1650. [Google Scholar] [CrossRef]
- Pamphlett, R.; Bishop, D.P. Mercury is present in neurons and oligodendrocytes in regions of the brain affected by Parkinson’s disease and co-localises with Lewy bodies. PLoS ONE 2022, 17, e0262464. [Google Scholar] [CrossRef]
- Pamphlett, R.; Bishop, D.P.; Jew, S.K.; Doble, P. Age-related accumulation of toxic metals in the human locus ceruleus. PLoS ONE 2018, 13, e0203627. [Google Scholar] [CrossRef]
- Stopschinski, B.E.; Del Tredici, K.; Estill-Terpack, S.-J.; Ghebremdehin, E.; Yu, F.F.; Braak, H.; Diamond, M.I. Anatomic survey of seeding in Alzheimer’s disease brains reveals unexpected patterns. Acta Neuropathol. Commun. 2021, 9, 164. [Google Scholar] [CrossRef]
- Molina, L.T.; Velasco, E.; Retama, A.; Zavala, M. Experience from Integrated Air Quality Management in the Mexico City Metropolitan Area and Singapore. Atmosphere 2019, 10, 512. [Google Scholar] [CrossRef] [Green Version]
- Zavala, M.; Brune, W.H.; Velasco, E.; Retama, A.; Cruz-Alavez, L.A.; Molina, L.T. Changes in ozone production and VOC reactivity in the atmosphere of the Mexico City Metropolitan Area. Atmos. Environ. 2020, 238, 117747. [Google Scholar] [CrossRef]
- Molina, L.T.; Madronich, S.; Gaffney, J.S.; Apel, E.; de Foy, B.; Fast, J.; Ferrare, R.; Herndon, S.; Jimenez, J.L.; Lamb, B.; et al. An overview of the MILAGRO 2006 Campaign: Mexico City emissions and their transport and transformation. Atmos. Chem. Phys. 2010, 10, 8697–8760. [Google Scholar] [CrossRef] [Green Version]
- Torres-Jardón, R. Políticas públicas y su efecto en la calidad del aire de la Zona Metropolitana de la Ciudad de México. In Transversalidad de la Política del Aire en México; Núñez, G.S.S., Ed.; Instituto de Investigaciones Dr. José María Luis Mora: México City, Mexico, 2018; pp. 43–74. [Google Scholar]
- SEDEMA. Calidad del aire en la Ciudad de México, informe 2017. In Dirección General de Gestión de la Calidad del Aire, Dirección de Monitoreo Atmosférico; Secretaría del Medio Ambiente de la Ciudad de México: México City, Mexico, 2018. [Google Scholar]
- Velasco, E.; Retama, A. Ozone’s threat hits back Mexico city. Sustain. Cities Soc. 2017, 31, 260–263. [Google Scholar] [CrossRef]
- SEDEMA. Secretaría del Medio Ambiente de la Ciudad de México. 2021. Available online: http://www.aire.cdmx.gob.mx/default.php (accessed on 10 September 2021).
- Just, A.C.; Wright, R.O.; Schwartz, J.; Coull, B.A.; Baccarelli, A.A.; Tellez-Rojo, M.M.; Moody, E.; Wang, Y.; Lyapustin, A.; Kloog, I. Using High-Resolution Satellite Aerosol Optical Depth to Estimate Daily PM2.5 Geographical Distribution in Mexico City. Environ. Sci. Technol. 2015, 49, 8576–8584. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, F.; Alvarez-Ospina, H.; Retama, A.; López-Medina, A.; Castro, T.; Salcedo, D. Seasonal changes in the PM1 chemical composition north of Mexico City. Atmósfera 2017, 30, 243–258. [Google Scholar] [CrossRef] [Green Version]
- Retama, A.; Baumgardner, D.; Raga, G.B.; McMeeking, G.R.; Walker, J.W. Seasonal and diurnal trends in black carbon properties and co-pollutants in Mexico City. Atmos. Chem. Phys. 2015, 15, 9693–9709. [Google Scholar] [CrossRef] [Green Version]
- Peralta, O.; Ortínez-Alvarez, A.; Basaldud, R.; Santiago, N.; Alvarez-Ospina, H.; de la Cruz, K.; Barrera, V.; de la Luz Espinosa, M.; Saavedra, I.; Castro, T.; et al. Atmospheric black carbon concentrations in Mexico. Atmos. Res. 2019, 230, 104626. [Google Scholar] [CrossRef]
- Dzepina, K.; Arey, J.; Marr, L.C.; Worsnop, D.R.; Salcedo, D.; Zhang, Q.; Onasch, T.B.; Molina, L.T.; Molina, M.J.; Jimenez, J.L. Detection of particle-phase polycyclic aromatic hydrocarbons in Mexico City using an aerosol mass spectrometer. Int. J. Mass Spectrom. 2007, 263, 152–170. [Google Scholar] [CrossRef]
- Ladino, L.A.; Raga, G.B.; Baumgardner, D. On particle-bound polycyclic aromatic hydrocarbons (PPAH) and links to gaseous emissions in Mexico city. Atmos. Environ. 2018, 194, 31–40. [Google Scholar] [CrossRef]
- Amador-Muñoz, O.; Martínez-Domínguez, Y.; Gómez-Arroyo, S.; Peralta, O. Current situation of polycyclic aromatic hydrocarbons (PAH) in PM2.5 in a receptor site in Mexico City and estimation of carcinogenic PAH by combining non-real-time and real-time measurement techniques. Sci. Total Environ. 2020, 703, 134526. [Google Scholar] [CrossRef]
- Dunn, M.J.; Jimenez, J.L.; Baumgardner, D.; Castro, T.; McMurry, P.H.; Smith, J.N. Measurements of Mexico City nanoparticle size distributions: Observations of new particle formation and growth. Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef] [Green Version]
- Caudillo, L.; Salcedo, D.; Peralta, O.; Castro, T.; Alvarez-Ospina, H. Nanoparticle size distributions in Mexico city. Atmos. Pollut. Res. 2020, 11, 78–84. [Google Scholar] [CrossRef]
- Velasco, E.; Retama, A.; Segovia, E.; Ramos, R. Particle exposure and inhaled dose while commuting by public transport in Mexico City. Atmos. Environ. 2019, 219, 117044. [Google Scholar] [CrossRef]
- Aiken, A.C.; Salcedo, D.; Cubison, M.J.; Huffman, J.A.; DeCarlo, P.F.; Ulbrich, I.M.; Docherty, K.S.; Sueper, D.; Kimmel, J.R.; Worsnop, D.R.; et al. Mexico City aerosol analysis during MILAGRO using high resolution aerosol mass spectrometry at the urban supersite (T0)—Part 1: Fine particle composition and organic source apportionment. Atmos. Chem. Phys. 2009, 9, 6633–6653. [Google Scholar] [CrossRef] [Green Version]
- Stone, E.A.; Hedman, C.J.; Zhou, J.; Mieritz, M.; Schauer, J.J. Insights into the nature of secondary organic aerosol in Mexico City during the MILAGRO experiment 2006. Atmos. Environ. 2010, 44, 312–319. [Google Scholar] [CrossRef]
- Querol, X.; Pey, J.; Minguillón, M.C.; Pérez, N.; Alastuey, A.; Viana, M.; Moreno, T.; Bernabé, R.M.; Blanco, S.; Cárdenas, B.; et al. PM speciation and sources in Mexico during the MILAGRO-2006 Campaign. Atmos. Chem. Phys. 2008, 8, 111–128. [Google Scholar] [CrossRef] [Green Version]
- Morton-Bermea, O.; Garza-Galindo, R.; Hernández-Álvarez, E.; Amador-Munoz, O.; Garcia-Arreola, M.E.; Ordoñez-Godínez, S.L.; Beramendi-Orosco, L.E.; Santos-Medina, G.L.; Miranda, J.; Pérez, I.A.R. Recognition of the importance of geogenic sources in the content of metals in PM2.5 collected in the Mexico City Metropolitan Area. Environ. Monit. Assess. 2018, 190, 83. [Google Scholar] [CrossRef]
- Hernández-López, A.E.; Miranda Martín del Campo, J.; Álvarez, V.M.; Valle-Hernández, B.L.; Mejía-Ponce, L.V.; Pineda-Santamaría, J.C.; Reynoso-Cruces, S.; Mendoza-Flores, J.A.; Rozanes-Valenzuela, D. A study of PM2.5 elemental composition in southwest Mexico City and development of receptor models with positive matrix factorization. Rev. Int. Contam. Ambie. 2021, 37, 67–88. [Google Scholar] [CrossRef]
- Morton-Bermea, O.; Garza-Galindo, R.; Hernández-Álvarez, E.; Ordoñez-Godínez, S.L.; Amador-Muñoz, O.; Beramendi-Orosco, L.; Miranda, J.; Rosas-Pérez, I. Atmospheric PM2.5 Mercury in the Metropolitan Area of Mexico City. Bull. Environ. Contam. Toxicol. 2018, 100, 588–592. [Google Scholar] [CrossRef]
- Mugica-Alvarez, V.; Figueroa-Lara, J.D.J.; Romo, M.A.R.; Sepúlveda-Sánchez, J.; López-Moreno, T. Concentrations and properties of airborne particles in the Mexico City subway system. Atmos. Environ. 2012, 49, 284–293. [Google Scholar] [CrossRef]
- Baumgardner, D.; Raga, G.B.; Kok, G.; Ogren, J.; Rosas, I.; Baez, A.; Novakov, T. On the evolution of aerosol properties at a mountain site above Mexico City. J. Geophys. Res. Earth Surf. 2000, 105, 22243–22253. [Google Scholar] [CrossRef] [Green Version]
- Kleinman, L.I.; Springston, S.R.; Wang, J.; Daum, P.H.; Lee, Y.-N.; Nunnermacker, L.J.; Senum, G.I.; Weinstein-Lloyd, J.; Alexander, M.L.; Hubbe, J.; et al. The time evolution of aerosol size distribution over the Mexico City plateau. Atmos. Chem. Phys. 2009, 9, 4261–4278. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.-S.; Ryu, M.H.; Carlsten, C. Ultrafine particles: Unique physicochemical properties relevant to health and disease. Exp. Mol. Med. 2020, 52, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Morawska, L.; Birmili, W.; Paasonen, P.; Hu, M.; Kulmala, M.; Harrison, R.M.; Norford, L.; Britter, R. Ultrafine particles in cities. Environ. Int. 2014, 66, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, V.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Contribution of transition metals in the reactive oxygen species activity of PM emissions from retrofitted heavy-duty vehicles. Atmos. Environ. 2010, 44, 5165–5173. [Google Scholar] [CrossRef]
- Sanderson, P.; Su, S.; Chang, I.; Delgado-Saborit, J.M.; Kepaptsoglou, D.; Weber, R.; Harrison, R.M. Characterisation of iron-rich atmospheric submicrometre particles in the roadside environment. Atmos. Environ. 2016, 140, 167–175. [Google Scholar] [CrossRef]
- Adachi, K.; Buseck, P.R. Hosted and Free-Floating Metal-Bearing Atmospheric Nanoparticles in Mexico City. Environ. Sci. Technol. 2010, 44, 2299–2304. [Google Scholar] [CrossRef] [PubMed]
- Moffet, R.C.; de Foy, B.; Molina, L.T.; Molina, M.J.; Prather, K.A. Measurement of ambient aerosols in northern Mexico City by single particle mass spectrometry. Atmos. Chem. Phys. 2008, 8, 4499–4516. [Google Scholar] [CrossRef] [Green Version]
- Gonet, T.; Maher, B.A. Airborne, Vehicle-Derived Fe-Bearing Nanoparticles in the Urban Environment: A Review. Environ. Sci. Technol. 2019, 53, 9970–9991. [Google Scholar] [CrossRef]
- De Jesus, A.L.; Rahman, M.M.; Mazaheri, M.; Thompson, H.; Knibbs, L.D.; Jeong, C.; Evans, G.; Nei, W.; Ding, A.; Qiao, L.; et al. Ultrafine particles and PM2.5 in the air of cities around the world: Are they representative of each other? Environ. Int. 2019, 129, 118–135. [Google Scholar] [CrossRef]
- Jeong, C.-H.; Evans, G.J.; Hopke, P.K.; Chalupa, D.J.; Utell, M. Influence of Atmospheric Dispersion and New Particle Formation Events on Ambient Particle Number Concentration in Rochester, United States, and Toronto, Canada. J. Air Waste Manag. Assoc. 2006, 56, 431–443. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, S.; Van Dingenen, R.; Putaud, J.-P.; Dell’Acqua, A.; Pey, J.; Querol, X.; Alastuey, A.; Chenery, S.; Ho, K.-F.; Harrison, R.; et al. A study on the relationship between mass concentrations, chemistry and number size distribution of urban fine aerosols in Milan, Barcelona and London. Atmos. Chem. Phys. 2007, 7, 2217–2232. [Google Scholar] [CrossRef] [Green Version]
- Bravo, A.H.; Urone, P. The Altitude: A Fundamental Parameter in the Use of Air Quality Standards. J. Air Pollut. Control Assoc. 1981, 31, 264–265. [Google Scholar] [CrossRef]
- Schaeffer, S.; Iadecola, C. Revisiting the neurovascular unit. Nat. Neurosci. 2021, 24, 1198–1209. [Google Scholar] [CrossRef]
- Coccini, T.; Pignatti, P.; Spinillo, A.; De Simone, U. Developmental Neurotoxicity Screening for Nanoparticles Using Neuron-Like Cells of Human Umbilical Cord Mesenchymal Stem Cells: Example with Magnetite Nanoparticles. Nanomaterials 2020, 10, 1607. [Google Scholar] [CrossRef]
- Mehrbeheshti, N.; Esmaili, Z.; Ahmadi, M.; Moosavi, M. A dose response effect of oral aluminum nanoparticle on novel object recognition memory, hippocampal caspase-3 and MAPKs signaling in mice. Behav. Brain Res. 2022, 417, 113615. [Google Scholar] [CrossRef]
- Mortensen, N.P.; Pathmasiri, W.; Snyder, R.W.; Caffaro, M.M.; Watson, S.L.; Patel, P.R.; Beeravalli, L.; Prattipati, S.; Aravamudhan, S.; Sumner, S.J.; et al. Oral administration of TiO2 nanoparticles 21 ± 5 nm during early life impacts cardiac and neurobehavioral performance and metabolite profile in an age- and sex-related manner. Part. Fibre Toxicol. 2022, 19, 3. [Google Scholar] [CrossRef]
- Korzhevskii, D.E.; Kirik, O.V.; Guselnikova, V.V.; Tsyba, D.L.; Fedorova, E.A.; Grigorev, I.P. Changes in cytoplasmic and extracellular neuromelanin in human substantia nigra with normal aging. Eur. J. Histochem. 2021, 65, 3283. [Google Scholar] [CrossRef]
- Panja, P.; Jana, N.R. Arginine-Terminated Nanoparticles of <10 nm Size for Direct Membrane Penetration and Protein Delivery for Straight Access to Cytosol and Nucleus. J. Phys. Chem. Lett. 2020, 11, 2363–2368. [Google Scholar] [CrossRef]
- Gutiérrez, L.; De La Cueva, L.; Moros, M.; Mazarío, E.; De Bernardo, S.; De La Fuente, J.M.; Morales, M.D.P.; Salas, G. Aggregation effects on the magnetic properties of iron oxide colloids. Nanotechnology 2019, 30, 112001. [Google Scholar] [CrossRef] [Green Version]
- Bridges, C.C.; Zalups, R.K. Transport of Inorganic Mercury and Methylmercury in Target Tissues and Organs. J. Toxicol. Environ. Health Part B 2010, 13, 385–410. [Google Scholar] [CrossRef]
- Raj, K.; Kaur, P.; Gupta, G.; Singh, S. Metals associated neurodegeneration in Parkinson’s disease: Insight to physiological, pathological mechanisms and management. Neurosci. Lett. 2021, 753, 135873. [Google Scholar] [CrossRef]
- Cernichiari, E.; Brewer, R.; Myers, G.J.; Marsh, D.O.; Lapham, L.W.; Cox, C.; Shamlaye, C.F.; Berlin, M.; Davidson, P.W.; Clarkson, T.W. Monitoring methylmercury during pregnancy: Maternal hair predicts fetal brain exposure. NeuroToxicology 1995, 16, 705–710. [Google Scholar]
- Beckers, F.; Rinklebe, J. Cycling of mercury in the environment: Sources, fate, and human health implications: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 693–794. [Google Scholar] [CrossRef]
- Gustin, M.S.; Bank, M.S.; Bishop, K.; Bowman, K.; Branfireun, B.; Chételat, J.; Eckley, C.S.; Hammerschmidt, C.R.; Lamborg, C.; Lyman, S.; et al. Mercury biogeochemical cycling: A synthesis of recent scientific advances. Sci. Total Environ. 2020, 737, 139619. [Google Scholar] [CrossRef]
- Andreoli, V.; Sprovieri, F. Genetic Aspects of Susceptibility to Mercury Toxicity: An Overview. Int. J. Environ. Res. Public Health 2017, 14, 93. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Shimohata, T. Vascular Dysfunction Induced by Mercury Exposure. Int. J. Mol. Sci. 2019, 20, 2435. [Google Scholar] [CrossRef] [Green Version]
- Grant, M.; VanderSchee, C.; Chou, H.; Bolt, A.; Epure, L.; Kuter, D.; Antoniou, J.; Bohle, S.; Mann, K.; Mwale, F. Tungsten accumulates in the intervertebral disc and vertebrae stimulating disc degeneration and upregulating markers of inflammation and pain. Eur. Cells Mater. 2021, 41, 517–530. [Google Scholar] [CrossRef]
- Chinde, S.; Grover, P. Toxicological assessment of nano and micron-sized tungsten oxide after 28 days repeated oral administration to Wistar rats. Mutat. Res. Toxicol. Environ. Mutagen. 2017, 819, 1–13. [Google Scholar] [CrossRef]
- Javdani, N.; Rahpeyma, S.S.; Ghasemi, Y.; Raheb, J. Effect of superparamagnetic nanoparticles coated with various electric charges on α-synuclein and β-amyloid proteins fibrillation process. Int. J. Nanomed. 2019, 14, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, S.; Nikkhah, M. TiO2 Nanoparticles as Potential Promoting Agents of Fibrillation of α-Synuclein, a Parkinson’s Disease-Related Protein. Iran. J. Biotechnol. 2017, 15, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Rahgozar, S.; Zarrabi, A. Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation. BMC Neurosci. 2017, 18, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, H.; Wu, J. Silica nanoparticles induce alpha-synuclein induction and aggregation in PC12-cells. Chem. Biol. Interact. 2016, 258, 197–204. [Google Scholar] [CrossRef]
- Wu, J.; Xie, H. Effects of titanium dioxide nanoparticles on α-synuclein aggregation and the ubiquitin-proteasome system in dopaminergic neurons. Artif. Cells Nanomed. Biotechnol. 2016, 44, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Gan, M.; Yen, S.-H.; Dickson, D.W. Nanoparticles with Affinity for α-Synuclein Sequester α-Synuclein to Form Toxic Aggregates in Neurons with Endolysosomal Impairment. Front. Mol. Neurosci. 2021, 14, 234. [Google Scholar] [CrossRef] [PubMed]
- Gilan, S.S.T.; Rayat, D.Y.; Mustafa, T.A.; Aziz, F.M.; Shahpasand, K.; Akhtari, K.; Salihi, A.; Abou-Zied, O.K.; Falahati, M. α-synuclein interaction with zero-valent iron nanoparticles accelerates structural rearrangement into amyloid-susceptible structure with increased cytotoxic tendency. Int. J. Nanomed. 2019, 14, 4637–4648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad-Beigi, H.; Zanganeh, M.; Scavenius, C.; Eskandari, H.; Farzadfard, A.; Shojaosadati, S.A.; Enghild, J.J.; Otzen, D.E.; Buell, A.K.; Sutherland, D.S. A Protein Corona Modulates Interactions of α-Synuclein with Nanoparticles and Alters the Rates of the Microscopic Steps of Amyloid Formation. ACS Nano 2022, 16, 1102–1118. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.A.; Lin, W.; Woods, W.S.; George, J.M.; Murphy, C.J. α-Synuclein’s Adsorption, Conformation, and Orientation on Cationic Gold Nanoparticle Surfaces Seeds Global Conformation Change. J. Phys. Chem. B 2014, 118, 3559–3571. [Google Scholar] [CrossRef]
- González-Fernández, C.; Baños, F.D.; Esteban, M.; Cuesta, A. Functionalized Nanoplastics (NPs) Increase the Toxicity of Metals in Fish Cell Lines. Int. J. Mol. Sci. 2021, 22, 7141. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. Neuropathological Staging of Brain Pathology in Sporadic Parkinson’s disease: Separating the Wheat from the Chaff. J. Park. Dis. 2017, 7, S71–S85. [Google Scholar] [CrossRef] [Green Version]
- Karanth, S.; Nelson, P.T.; Katsumata, Y.; Kryscio, R.J.; Schmitt, F.A.; Fardo, D.W.; Cykowski, M.D.; Jicha, G.A.; Van Eldik, L.J.; Abner, E.L. Prevalence and Clinical Phenotype of Quadruple Misfolded Proteins in Older Adults. JAMA Neurol. 2020, 77, 1299–1307. [Google Scholar] [CrossRef]
- Tsugita, M.; Morimoto, N.; Nakayama, M. SiO2 and TiO2 nanoparticles synergistically trigger macrophage inflammatory responses. Part. Fibre Toxicol. 2017, 14, 11. [Google Scholar] [CrossRef] [Green Version]
- Semerád, J.; Moeder, M.; Filip, J.; Pivokonský, M.; Filipová, A.; Cajthaml, T. Oxidative stress in microbes after exposure to iron nanoparticles: Analysis of aldehydes as oxidative damage products of lipids and proteins. Environ. Sci. Pollut. Res. Int. 2019, 26, 33670–33682. [Google Scholar] [CrossRef]
- Vereda, F.; De Vicente, J.; Hidalgo-Álvarez, R. Physical Properties of Elongated Magnetic Particles: Magnetization and Friction Coefficient Anisotropies. ChemPhysChem 2009, 10, 1165–1179. [Google Scholar] [CrossRef]
- Abu-Bakr, A.F.; Zubarev, A.Y. On the theory of magnetic hyperthermia: Clusterization of nanoparticles. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2020, 378, 20190251. [Google Scholar] [CrossRef] [Green Version]
- Ovejero, J.G.; Spizzo, F.; Morales, M.P.; Del Bianco, L. Mixing iron oxide nanoparticles with different shape and size for tunable magneto-heating performance. Nanoscale 2021, 13, 5714–5729. [Google Scholar] [CrossRef]
- Sola-Leyva, A.; Jabalera, Y.; Chico-Lozano, M.A.; Carrasco-Jiménez, M.P.; Iglesias, G.R.; Jimenez-Lopez, C. Reactive oxygen species (ROS) production in HepG2 cancer cell line through the application of localized alternating magnetic field. J. Mater. Chem. B 2020, 8, 7667–7676. [Google Scholar] [CrossRef]
- Pacakova, B.; Kubickova, S.; Salas, G.; Mantlikova, A.R.; Marciello, M.; Morales, M.P.; Niznansky, D.; Vejpravova, J. The internal structure of magnetic nanoparticles determines the magnetic response. Nanoscale 2017, 9, 5129–5140. [Google Scholar] [CrossRef]
- Takakusaki, K. Neurophysiology of gait: From the spinal cord to the frontal lobe. Mov. Disord. 2013, 28, 1483–1491. [Google Scholar] [CrossRef]
- Deval, A.; Bayot, M.; Defebvre, L.; Dujardin, K. Cortical Oscillations during Gait: Wouldn’t Walking be so Automatic? Brain Sci 2020, 10, 90. [Google Scholar] [CrossRef] [Green Version]
- Bove, C.; Travagli, R.A. Neurophysiology of the brain stem in Parkinson’s disease. J. Neurophysiol. 2019, 121, 1856–1864. [Google Scholar] [CrossRef]
- Cassidy, C.M.; Therriault, J.; Pascoal, T.A.; Cheung, V.; Savard, M.; Tuominen, L.; Chamoun, M.; McCall, A.; Celebi, S.; Lussier, F.; et al. Association of locus coeruleus integrity with Braak stage and neuropsychiatric symptom severity in Alzheimer’s disease. Neuropsychopharmacology 2022, 47, 1128–1136. [Google Scholar] [CrossRef]
- Klann, E.M.; Dissanayake, U.; Gurrala, A.; Farrer, M.; Shukla, A.W.; Ramirez-Zamora, A.; Mai, V.; Vedam-Mai, V. The Gut–Brain Axis and Its Relation to Parkinson’s Disease: A Review. Front. Aging Neurosci. 2022, 13. [Google Scholar] [CrossRef]
- Mishra, A.K.; Dixit, A. Dopaminergic Axons: Key Recitalists in Parkinson’s Disease. Neurochem. Res. 2021, 47, 234–248. [Google Scholar] [CrossRef]
- Iarkov, A.; Mendoza, C.; Echeverria, V. Cholinergic Receptor Modulation as a Target for Preventing Dementia in Parkinson’s Disease. Front. Neurosci. 2021, 15, 1209. [Google Scholar] [CrossRef]
- Panicker, N.; Ge, P.; Dawson, V.L.; Dawson, T.M. The cell biology of Parkinson’s disease. J. Cell Biol. 2021, 220, e202012095. [Google Scholar] [CrossRef]
- Hansen, N. Locus Coeruleus Malfunction Is Linked to Psychopathology in Prodromal Dementia with Lewy Bodies. Front. Aging Neurosci. 2021, 13, 78. [Google Scholar] [CrossRef]
- Minakaki, G.; Krainc, D.; Burbulla, L.F. The Convergence of Alpha-Synuclein, Mitochondrial, and Lysosomal Pathways in Vulnerability of Midbrain Dopaminergic Neurons in Parkinson’s Disease. Front. Cell Dev. Biol. 2020, 8, 1465. [Google Scholar] [CrossRef]
- Paredes-Rodriguez, E.; Vegas-Suárez, S.; Morera-Herreras, T.; De Deurwaerdère, P.; Miguelez, C. The Noradrenergic System in Parkinson’s Disease. Front. Pharmacol. 2020, 11, 435. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Landin-Romero, R.; Kumfor, F.; Irish, M.; Hodges, J.R.; Piguet, O. Cerebellar structural connectivity and contributions to cognition in frontotemporal dementias. Cortex 2020, 129, 57–67. [Google Scholar] [CrossRef]
- Pierce, J.E.; Péron, J. The basal ganglia and the cerebellum in human emotion. Soc. Cogn. Affect. Neurosci. 2020, 15, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Stommel, E.W.; Rajkumar, R.P.; Mukherjee, P.S.; Ayala, A. Particulate Air Pollution and Risk of Neuropsychiatric Outcomes. What We Breathe, Swallow, and Put on Our Skin Matters. Int. J. Environ. Res. Public Health 2021, 18, 11568. [Google Scholar] [CrossRef]
- Van der Heijden, M.E.; Sillitoe, R.V. Interactions Between Purkinje Cells and Granule Cells Coordinate the Development of Functional Cerebellar Circuits. Neuroscience 2020, 462, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Hernández-Luna, J.; Mukherjee, P.S.; Styner, M.; Chávez-Franco, D.A.; Luévano-Castro, S.C.; Crespo-Cortés, C.N.; Stommel, E.W.; Torres-Jardón, R. Hemispheric Cortical, Cerebellar and Caudate Atrophy Associated to Cognitive Impairment in Metropolitan Mexico City Young Adults Exposed to Fine Particulate Matter Air Pollution. Toxics 2022, 10, 156. [Google Scholar] [CrossRef]
- Fens, M.H.; Van Wijk, R.; Andringa, G.; Van Rooijen, K.L.; Dijstelbloem, H.M.; Rasmussen, J.T.; De Vooght, K.M.; Schiffelers, R.; Gaillard, C.A.; Van Solinge, W.W. A role for activated endothelial cells in red blood cell clearance: Implications for vasopathology. Haematologica 2012, 97, 500–508. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Wang, C.; Liu, Z. Red Blood Cells as Smart Delivery Systems. Bioconjug. Chem. 2018, 29, 852–860. [Google Scholar] [CrossRef]
- Föller, M.; Lang, F. Ion Transport in Eryptosis, the Suicidal Death of Erythrocytes. Front. Cell Dev. Biol. 2020, 8, 597. [Google Scholar] [CrossRef]
- Vanneste, J.; Bosch, L.V.D. The Role of Nucleocytoplasmic Transport Defects in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2021, 22, 12175. [Google Scholar] [CrossRef]
- Odeh, H.M.; Fare, C.M.; Shorter, J. Nuclear-Import Receptors Counter Deleterious Phase Transitions in Neurodegenerative Disease. J. Mol. Biol. 2022, 434, 167220. [Google Scholar] [CrossRef]
- Amore, G.; Spoto, G.; Ieni, A.; Vetri, L.; Quatrosi, G.; Di Rosa, G.; Nicotera, A.G. A Focus on the Cerebellum: From Embryogenesis to an Age-Related Clinical Perspective. Front. Syst. Neurosci. 2021, 15, 646052. [Google Scholar] [CrossRef]
- Kang, S.; Jun, S.; Baek, S.J.; Park, H.; Yamamoto, Y.; Tanaka-Yamamoto, K. Recent Advances in the Understanding of Specific Efferent Pathways Emerging from the Cerebellum. Front. Neuroanat. 2021, 15, 75994815. [Google Scholar] [CrossRef]
- Gatti, D.; Rinaldi, L.; Ferreri, L.; Vecchi, T. The Human Cerebellum as a Hub of the Predictive Brain. Brain Sci. 2021, 11, 1492. [Google Scholar] [CrossRef]
- Van Overwalle, F.; Baeken, C.; Campanella, S.; Crunelle, C.L.; Heleven, E.; Kornreich, C.; Leggio, M.; Noël, X.; Vanderhasselt, M.-A.; Baetens, K. The Role of the Posterior Cerebellum in Dysfunctional Social Sequencing. Cerebellum 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Clark, S.V.; Semmel, E.S.; Aleksonis, H.A.; Steinberg, S.N.; King, T.Z. Cerebellar-Subcortical-Cortical Systems as Modulators of Cognitive Functions. Neuropsychol. Rev. 2021, 31, 422–446. [Google Scholar] [CrossRef]
- Flace, P.; Livrea, P.; Basile, G.A.; Galletta, D.; Bizzoca, A.; Gennarini, G.; Bertino, S.; Branca, J.J.V.; Gulisano, M.; Bianconi, S.; et al. The Cerebellar Dopaminergic System. Front. Syst. Neurosci. 2021, 15, 50. [Google Scholar] [CrossRef]
- Haghshomar, M.; Shobeiri, P.; Seyedi, S.A.; Abbasi-Feijani, F.; Poopak, A.; Sotoudeh, H.; Kamali, A. Cerebellar Microstructural Abnormalities in Parkinson’s Disease: A Systematic Review of Diffusion Tensor Imaging Studies. Cerebellum 2022, 1–27. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Momodu, O.I. The Developing Cerebellum as a Target for Toxic Substances: Protective Role of Antioxidants. Cerebellum 2021, 20, 614–630. [Google Scholar] [CrossRef]
- Camuso, S.; La Rosa, P.; Fiorenza, M.T.; Canterini, S. Pleiotropic effects of BDNF on the cerebellum and hippocampus: Implications for neurodevelopmental disorders. Neurobiol. Dis. 2022, 163, 105606. [Google Scholar] [CrossRef]
- Koga, S.; Sekiya, H.; Kondru, N.; Ross, O.A.; Dickson, D.W. Neuropathology and molecular diagnosis of Synucleinopathies. Mol. Neurodegener. 2021, 16, 83. [Google Scholar] [CrossRef]
- Lawn, T.; Ffytche, D. Cerebellar involvement in hallucinations may transcend clinical conditions and perceptual modalities. Cortex 2021, 143, 290–294. [Google Scholar] [CrossRef]
- Devita, M.; Alberti, F.; Fagnani, M.; Fabio, M.; Ara, E.; Sergi, G.; Mapelli, D.; Coin, A. Novel insights into the relationship between cerebellum and dementia: A narrative review as a toolkit for clinicians. Ageing Res. Rev. 2021, 70, 101389. [Google Scholar] [CrossRef]
- Chen, Y.; Kumfor, F.; Landin-Romero, R.; Irish, M.; Piguet, O. The Cerebellum in Frontotemporal Dementia: A Meta-Analysis of Neuroimaging Studies. Neuropsychol. Rev. 2019, 29, 450–464. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Rajkumar, R.; Stommel, E.; Kulesza, R.; Mansour, Y.; Rico-Villanueva, A.; Flores-Vázquez, J.; Brito-Aguilar, R.; Ramírez-Sánchez, S.; García-Alonso, G.; et al. Brainstem Quadruple Aberrant Hyperphosphorylated Tau, Beta-Amyloid, Alpha-Synuclein and TDP-43 Pathology, Stress and Sleep Behavior Disorders. Int. J. Environ. Res. Public Health 2021, 18, 6689. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhu, J.C. A Narrative Review of Cerebellar Malfunctions and Sleep Disturbances. Front. Neurosci. 2021, 15, 590619. [Google Scholar] [CrossRef] [PubMed]
- Pall, M.L. Wi-Fi is an important threat to human health. Environ. Res. 2018, 164, 405–416. [Google Scholar] [CrossRef]
- Gajšek, P.; Ravazzani, P.; Grellier, J.; Samaras, T.; Bakos, J.; Thuróczy, G. Review of Studies Concerning Electromagnetic Field (EMF) Exposure Assessment in Europe: Low Frequency Fields (50 Hz–100 kHz). Int. J. Environ. Res. Public Health 2016, 13, 875. [Google Scholar] [CrossRef] [Green Version]
- Belpomme, D.; Carlo, G.; Irigaray, P.; Carpenter, D.; Hardell, L.; Kundi, M.; Belyaev, I.; Havas, M.; Adlkofer, F.; Heuser, G.; et al. The Critical Importance of Molecular Biomarkers and Imaging in the Study of Electrohypersensitivity. A Scientific Consensus International Report. Int. J. Mol. Sci. 2021, 22, 7321. [Google Scholar] [CrossRef]
- Feng, Q.; Xu, X.; Wei, C.; Li, Y.; Wang, M.; Lv, C.; Wu, J.; Dai, Y.; Han, Y.; Lesniak, M.S.; et al. The Dynamic Interactions between Nanoparticles and Macrophages Impact Their Fate in Brain Tumors. Small 2021, 17, 2103600. [Google Scholar] [CrossRef]
- Beckwith, T.; Cecil, K.; Altaye, M.; Severs, R.; Wolfe, C.; Percy, Z.; Maloney, T.; Yolton, K.; Lemasters, G.; Brunst, K.; et al. Reduced gray matter volume and cortical thickness associated with traffic-related air pollution in a longitudinally studied pediatric cohort. PLoS ONE 2020, 15, e0228092. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Torres-Jardón, R.; Franco-Lira, M.; Kulesza, R.; González-Maciel, A.; Reynoso-Robles, R.; Brito-Aguilar, R.; García-Arreola, B.; Revueltas-Ficachi, P.; Barrera-Velázquez, J.A.; et al. Environmental Nanoparticles, SARS-CoV-2 Brain Involvement, and Potential Acceleration of Alzheimer’s and Parkinson’s Diseases in Young Urbanites Exposed to Air Pollution. J. Alzheimer’s Dis. 2020, 78, 479–503. [Google Scholar] [CrossRef]






| Anatomical Area | Number of Samples | Pτ Positive IHC | α Syn + IHC | % Positive Cases Pτ/α Syn | Average Age IHC + Cases | TEM/EDX Selected Samples |
|---|---|---|---|---|---|---|
| Substantia nigrae (SN) | 184 | 100 | 42 | 54.3%/22.8% | 25.8 ± 9.3 years Pτ 26.7 ± 8.1 years α S | 34+ and 28− for Pτ and/or α Syn |
| Locus coeruleus (LC) | 180 | 82 | 44 | 44.5%/24.4% | 26.7 ± 8.5 years Pτ 27.9 ± 8.2 years α S | 34+ and 23− for Pτ/α Syn |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Garcidueñas, L.; González-Maciel, A.; Reynoso-Robles, R.; Silva-Pereyra, H.G.; Torres-Jardón, R.; Brito-Aguilar, R.; Ayala, A.; Stommel, E.W.; Delgado-Chávez, R. Environmentally Toxic Solid Nanoparticles in Noradrenergic and Dopaminergic Nuclei and Cerebellum of Metropolitan Mexico City Children and Young Adults with Neural Quadruple Misfolded Protein Pathologies and High Exposures to Nano Particulate Matter. Toxics 2022, 10, 164. https://doi.org/10.3390/toxics10040164
Calderón-Garcidueñas L, González-Maciel A, Reynoso-Robles R, Silva-Pereyra HG, Torres-Jardón R, Brito-Aguilar R, Ayala A, Stommel EW, Delgado-Chávez R. Environmentally Toxic Solid Nanoparticles in Noradrenergic and Dopaminergic Nuclei and Cerebellum of Metropolitan Mexico City Children and Young Adults with Neural Quadruple Misfolded Protein Pathologies and High Exposures to Nano Particulate Matter. Toxics. 2022; 10(4):164. https://doi.org/10.3390/toxics10040164
Chicago/Turabian StyleCalderón-Garcidueñas, Lilian, Angélica González-Maciel, Rafael Reynoso-Robles, Héctor G. Silva-Pereyra, Ricardo Torres-Jardón, Rafael Brito-Aguilar, Alberto Ayala, Elijah W. Stommel, and Ricardo Delgado-Chávez. 2022. "Environmentally Toxic Solid Nanoparticles in Noradrenergic and Dopaminergic Nuclei and Cerebellum of Metropolitan Mexico City Children and Young Adults with Neural Quadruple Misfolded Protein Pathologies and High Exposures to Nano Particulate Matter" Toxics 10, no. 4: 164. https://doi.org/10.3390/toxics10040164
APA StyleCalderón-Garcidueñas, L., González-Maciel, A., Reynoso-Robles, R., Silva-Pereyra, H. G., Torres-Jardón, R., Brito-Aguilar, R., Ayala, A., Stommel, E. W., & Delgado-Chávez, R. (2022). Environmentally Toxic Solid Nanoparticles in Noradrenergic and Dopaminergic Nuclei and Cerebellum of Metropolitan Mexico City Children and Young Adults with Neural Quadruple Misfolded Protein Pathologies and High Exposures to Nano Particulate Matter. Toxics, 10(4), 164. https://doi.org/10.3390/toxics10040164






