Growth Hormones in Broad Bean (Vicia faba L.) and Radish (Raphanus raphanistrum subsp. sativus L.) Are Associated with Accumulated Concentrations of Perfluoroalkyl Substances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Sample Pre-Treatment and Extraction
2.3. Statistical Analyses
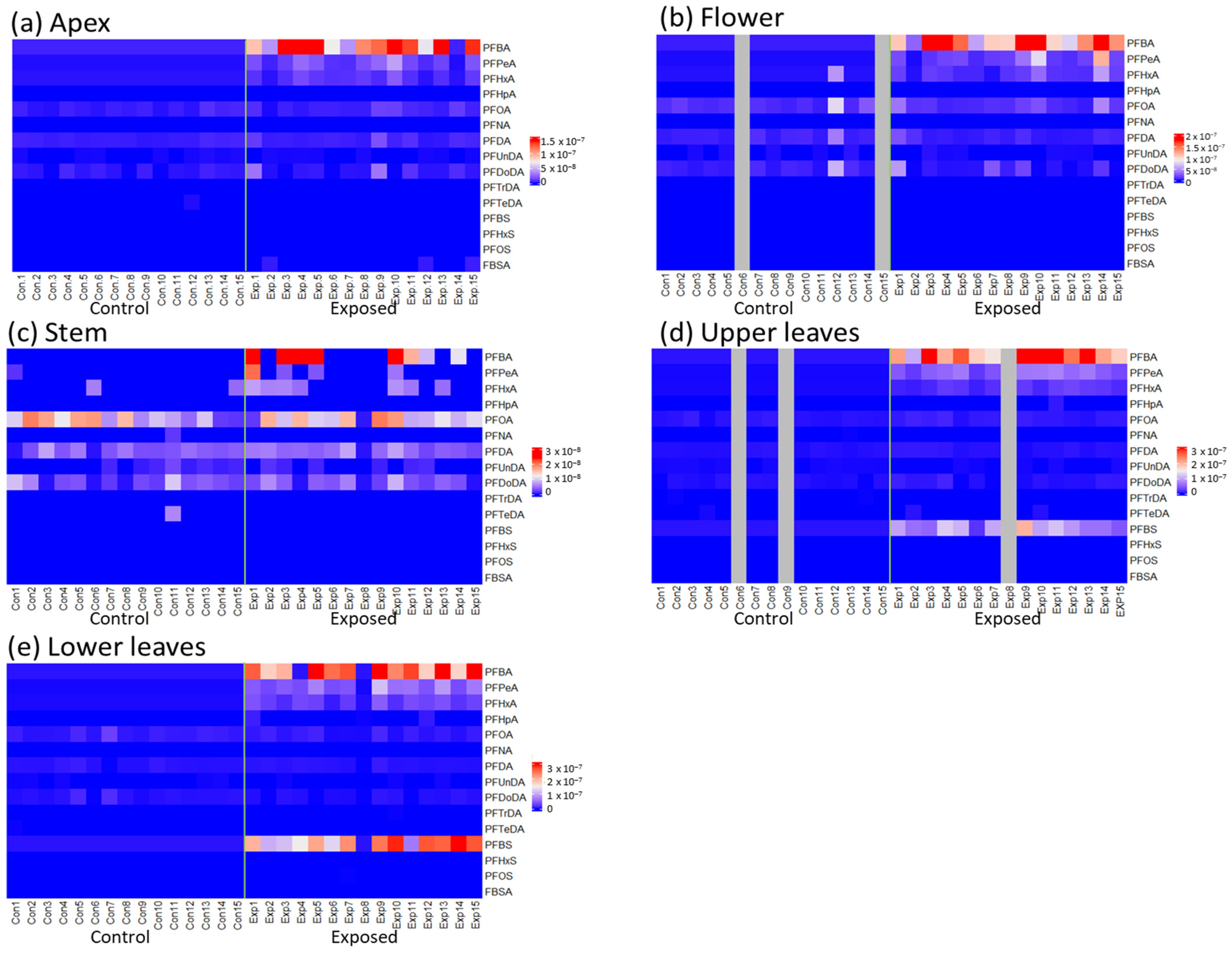
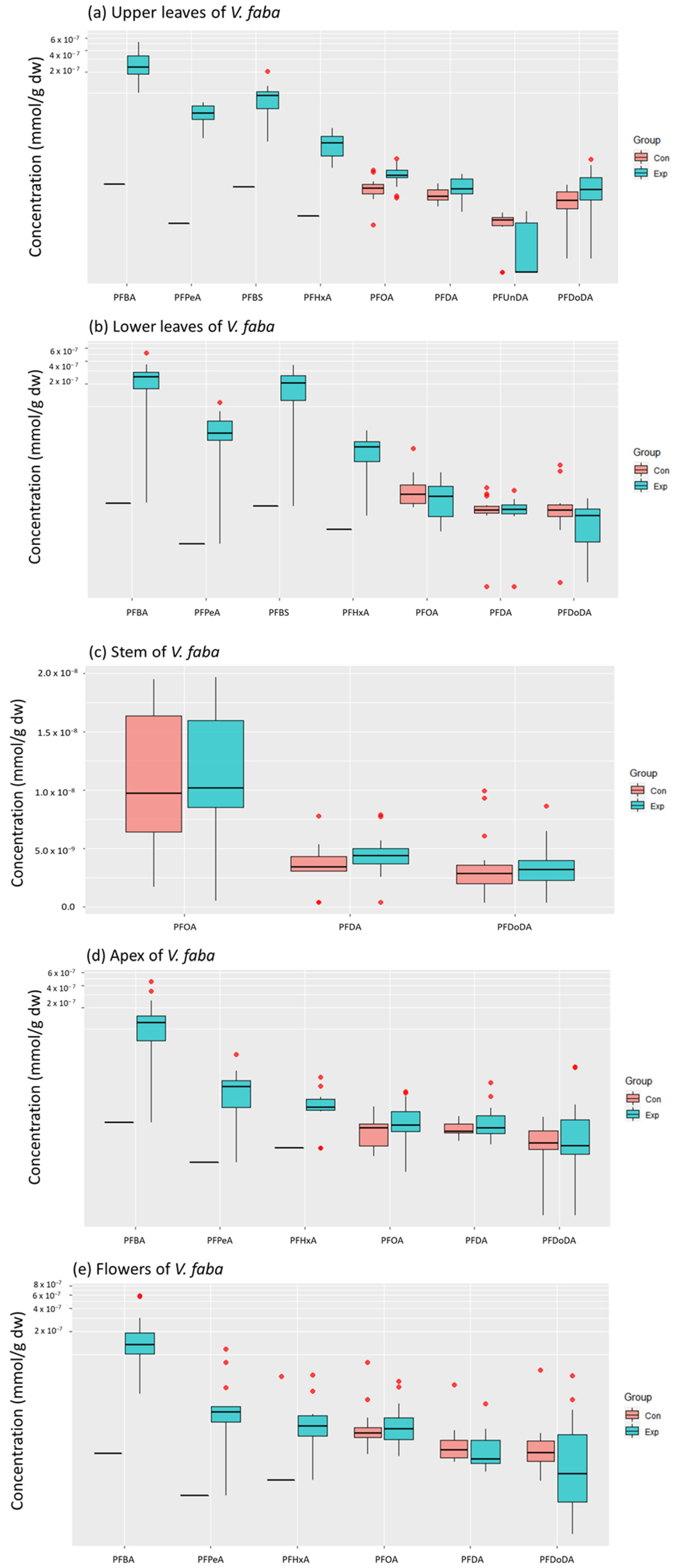
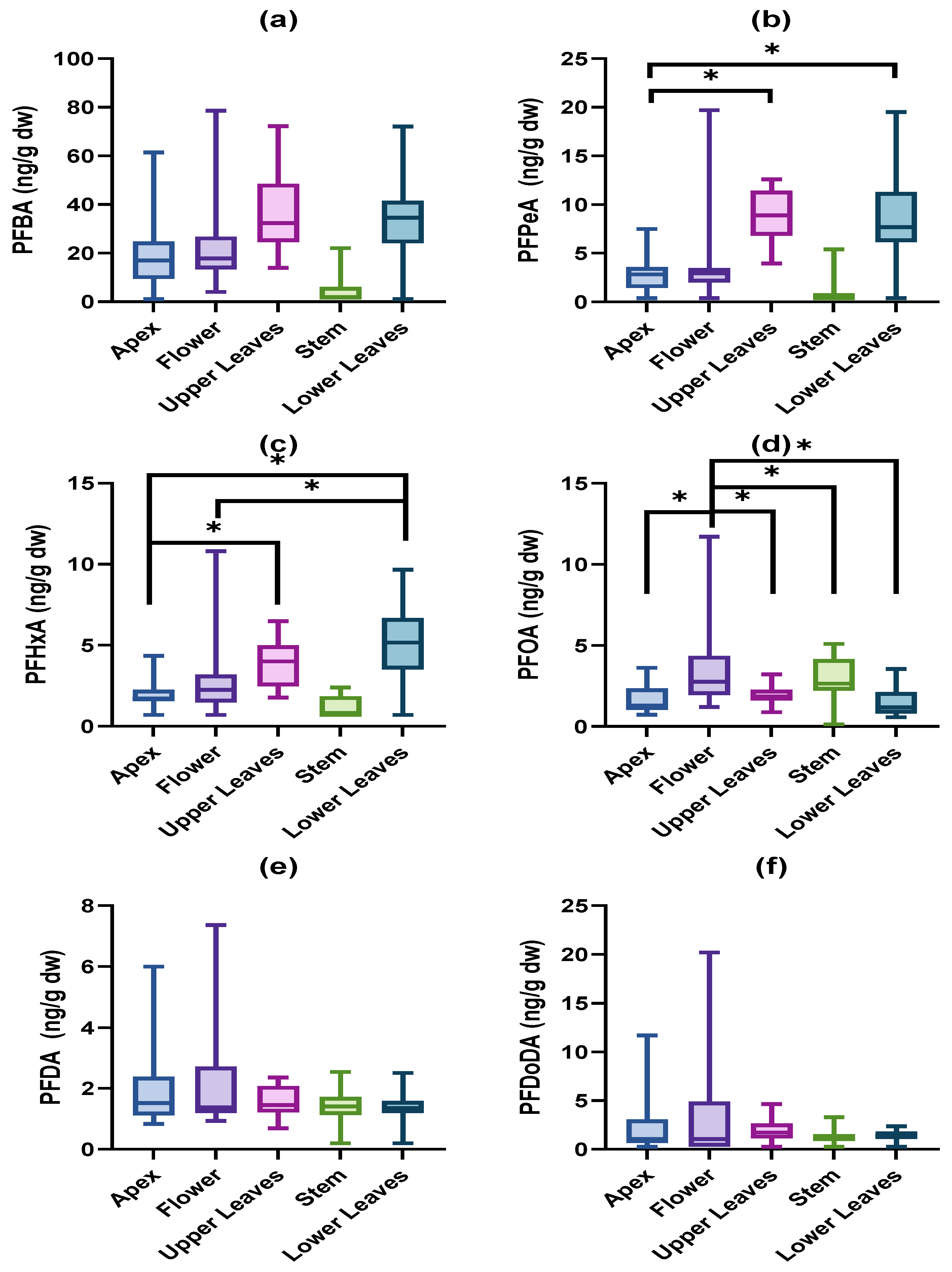
3. Results
3.1. Accumulated PFAS Concentrations and Profiles
3.2. Growth Hormone Levels and Plant Growth
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Astrup Jensen, A.; Kannan, K.; Mabury, S.; van Leeuwen, S.P.J. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef] [PubMed]
- Cousins, I.T.; Johansson, J.H.; Salter, M.E.; Sha, B.; Scheringer, M. Outside the safe operating space of a new planetary boundary for per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Technol. 2022, 56, 11172–11179. [Google Scholar] [CrossRef] [PubMed]
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci. Total Environ. 2022, 809, 151003. [Google Scholar] [CrossRef] [PubMed]
- Groffen, T.; Prinsen, E.; Devos Stoffels, O.-A.; Maas, L.; Vincke, P.; Lasters, R.; Eens, M.; Bervoets, L. PFAS accumulation in several terrestrial plant and invertebrate species reveals species-specific differences. Environ. Sci. Pollut. Res. 2022, 30, 23820–23835. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Ankley, G.T.; Cureton, P.; Hoke, R.A.; Houde, M.; Kumar, A.; Kurias, J.; Lanno, R.; McCarthy, C.; Newsted, J.; Salice, C.J.; et al. Assessing the ecological risks of per- and polyfluoroalkyl substances: Current state-of-the science and a proposed path forward. Environ. Toxicol. Chem. 2021, 40, 564–605. [Google Scholar] [CrossRef]
- Groffen, T.; Eens, M.; Bervoets, L. Do concentrations of perfluoroalkylated acids (PFAAs) in isopods reflect concentrations in soil and songbirds? A study using a distance gradient from a fluorochemical plant. Sci. Total Environ. 2019, 657, 111–123. [Google Scholar] [CrossRef]
- Brendel, S.; Fetter, É.; Staude, C.; Vierke, L.; Biegel-Engler, A. Short-chain perfluoroalkyl acids: Environmental concerns and a regulatory strategy under REACH. Environ. Sci. Eur. 2018, 30, 9. [Google Scholar] [CrossRef]
- Blaine, A.C.; Rich, C.D.; Sedlacko, E.M.; Hundal, L.S.; Kumar, K.; Lau, C.; Mills, M.A.; Harris, K.M.; Higgins, C.P. Perfluoroalkyl acid distribution in various plant compartments of edible crops grown in biosolids-amended soils. Environ. Sci. Technol. 2014, 48, 7858–7865. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, Y.; Song, X.; Jones, K.; Sweetman, A.J.; Johnson, A.C.; Zhang, M.; Lu, X.; Su, C. Multiple crop bioaccumulation and human exposure of perfluoroalkyl substances around a mega fluorochemical industrial park, China: Implication for planting optimization and food safety. Environ. Int. 2019, 127, 671–684. [Google Scholar] [CrossRef]
- Wen, B.; Wu, Y.; Zhang, H.; Liu, Y.; Hu, X.; Huang, H.; Zhang, S. The roles of protein and lipid in the accumulation and distribution of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in plants grown in biosolids-amended soils. Environ. Pollut. 2016, 216, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Ghisi, R.; Vamerali, T.; Manzetti, S. Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: A review. Environ. Res. 2019, 169, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, J.; Li, P. Exposure routes, bioaccumulation and toxic effects of per- and polyfluoroalkyl substances (PFASs) on plants: A critical review. Environ. Int. 2022, 158, 106891. [Google Scholar] [CrossRef]
- Gray, W.M. Hormonal regulation of plant growth and development. PLoS Biol. 2004, 2, e311. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Irving, H.R. Developing a model of plant hormone interactions. Plant Signal. Behav. 2011, 6, 494–500. [Google Scholar] [CrossRef]
- Powley, C.R.; George, S.W.; Ryan, T.W.; Buck, R.C. Matrix effect-free analytical methods for determination of perfluorinated carboxylic acids in environmental matrixes. Anal. Chem. 2005, 77, 6353–6358. [Google Scholar] [CrossRef]
- Bervoets, L.; Voets, J.; Chu, S.G.; Covaci, A.; Schepens, P.; Blust, R. Comparison of accumulation of micropollutants between indigenous and transplanted zebra mussels (Dreissena polymorpha). Environ. Toxicol. Chem. 2004, 23, 1973–1983. [Google Scholar] [CrossRef]
- Adu, O.; Ma, X.; Sharma, V.K. Bioavailability, phytotoxicity and plant uptake of per- and polyfluoroalkyl substances (PFAS): A review. J. Hazard. Mater. 2023, 447, 130805. [Google Scholar] [CrossRef]
- Kavusi, E.; Shahi Khalaf Ansar, B.; Ebrahimi, S.; Sharma, R.; Shideh Ghoreishi, S.; Nobaharan, K.; Abdoli, S.; Dehghanian, Z.; Asgari Lajayer, B.; Senapathi, V.; et al. Critical review on phytoremediation of polyfluoroalkyl substances from environmental matrices: Need for global concern. Environ. Res. 2022, 217, 114844. [Google Scholar] [CrossRef]
- Costello, M.C.S.; Lee, L.S. Sources, fate and plant uptake in agricultural systems of per- and polyfluoroalkyl substances. Curr. Pollut. Rep. 2020, 1–21. [Google Scholar] [CrossRef]
- Wang, T.T.; Ying, G.G.; He, L.Y.; Liu, Y.S.; Zhao, J.L. Uptake mechanism, subcellular distribution, and uptake process of perfluorooctanoic acid and perfluorooctane sulfonic acid by wetland plant Alisma orientale. Sci. Total Environ. 2020, 733, 139383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, H.; Wang, Q.; Chen, H.; Yao, Y.; Zhao, Z.; Alder, A.C. Uptake mechanisms of perfluoroalkyl acids with different carbon chain lengths (C2-C8) by wheat (Triticum aestivum L.). Sci. Total Environ. 2019, 654, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.E.; LeFevre, G.H.; Timofte, A.E.; Hussain, F.A.; Sattely, E.S.; Luthy, R.G. Competing mechanisms for perfluoroalkyl acid accumulation in plants revealed using an Arabidopsis model system. Environ. Toxicol. Chem. 2016, 35, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- White, P. Long-distance transport in the xylem and phloem. In Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier: Oxford, UK, 2012; pp. 49–70. [Google Scholar]
- Bhhatarai, B.; Gramatica, P. Prediction of aqueous solubility, vapor pressure and critical micelle concentration for aquatic partitioning of perfluorinated chemicals. Environ. Sci. Technol 2011, 45, 8120–8128. [Google Scholar] [CrossRef] [PubMed]
- Krippner, J.; Brunn, H.; Falk, S.; Georgii, S.; Schubert, S.; Stahl, T. Effects of chain length and pH on the uptake and distribution of perfluoroalkyl substances in maize (Zea mays). Chemosphere 2014, 94, 85–90. [Google Scholar] [CrossRef]
- Barberon, M. The endodermis as a checkpoint for nutrients. New Phytol. 2017, 213, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Doblas, V.G.; Geldner, N.; Barberon, M. The endodermis, a tightly controlled barrier for nutrients. Curr. Opin. Plant Biol. 2017, 39, 136–143. [Google Scholar] [CrossRef]
- Enstone, D.E.; Peterson, C.A.; Ma, F. Root endodermis and exodermis: Structure, function, and responses to the environment. J. Plant Growth Regul. 2002, 21, 335–351. [Google Scholar] [CrossRef]
- Calvo-Polanco, M.; Ribeyre, Z.; Dauzat, M.; Reyt, G.; Hidalgo-Shrestha, C.; Diehl, P.; Frenger, M.; Simonneau, T.; Muller, B.; Salt, D.E.; et al. Physiological roles of Casparian strips and suberin in the transport of water and solutes. New Phytol. 2021, 232, 2295–2307. [Google Scholar] [CrossRef]
- Guelfo, J.L.; Higgins, C.P. Subsurface transport potential of perfluoroalkyl acids at aqueous film-forming foam (AFFF)-impacted sites. Environ. Sci. Technol. 2013, 47, 4164–4171. [Google Scholar] [CrossRef]
- Blaine, A.C.; Rich, C.D.; Hundal, L.S.; Lau, C.; Mills, M.A.; Harris, K.M.; Higgins, C.P. Uptake of perfluoroalkyl acids into edible crops via land applied biosolids: Field and greenhouse studies. Environ. Sci. Technol. 2013, 47, 14062–14069. [Google Scholar] [CrossRef] [PubMed]
- Felizeter, S.; McLachlan, M.S.; de Voogt, P. Uptake of perfluorinated alkyl acids by hydroponically grown lettuce (Lactuca sativa). Environ. Sci. Technol. 2012, 46, 11735–11743. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Li, L.; Zhang, H.; Ma, Y.; Shang, X.-Q.; Zhang, S. Field study on the uptake and translocation of perfluoroalkyl acids (PFAAs) by wheat (Triticum aestivum L.) grown in biosolids-amended soils. Environ. Pollut. 2014, 184, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Oyang, X.; Xie, X.; Li, Z.; Yang, H.; Xi, J.; Guo, Y.; Tian, X.; Liu, B.; Li, J.; et al. Phytotoxicity induced by perfluorooctanoic acid and perfluorooctane sulfonate via metabolomics. J. Hazard. Mater. 2019, 389, 121852. [Google Scholar] [CrossRef]
- Gupta, R.; Chakrabarty, S.K. Gibberellic acid in plant: Still a mystery unresolved. Plant Signal. Behav. 2013, 8, e25504. [Google Scholar] [CrossRef]
- Achard, P.; Cheng, H.; De Grauwe, L.; Decat, J.; Schoutteten, H.; Moritz, T.; Van Der Straeten, D.; Peng, J.; Harberd, N.P. Integration of plant responses to environmentally activated phytohormonal signals. Science 2006, 311, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, N.; Sun, T.-P.; Gubler, F. Gibberellin signaling: Biosynthesis, catabolism, and response pathways. Plant Cell 2002, 14, S61–S80. [Google Scholar] [CrossRef] [PubMed]
- Musgrave, A.; Jackson, M.B.; Ling, E. Callitriche stem elongation is controlled by ethylene and gibberellin. Nat. New Biol. 1972, 238, 93–96. [Google Scholar] [CrossRef]
- Zhang, N.; Xie, Y.-D.; Guo, H.-J.; Zhao, L.-S.; Xiong, H.-C.; Gu, J.-Y.; Li, J.-H.; Kong, F.-Q.; Sui, L.; Zhao, Z.-W.; et al. Gibberellins regulate the stem elongation rate without affecting the mature plant height of a quick development mutant of winter wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2016, 107, 228–236. [Google Scholar] [CrossRef]
- Amir, R. Current understanding of the factors regulating methionine content in vegetative tissues of higher plants. Amino Acids 2010, 39, 917–931. [Google Scholar] [CrossRef]
- Ravanel, S.; Gakière, B.; Job, D.; Douce, R. The specific features of methionine biosynthesis and metabolism in plants. Proc. Natl. Acad. Sci. USA 1998, 95, 7805–7812. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene role in plant growth, development and senescence: Interaction with other phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.-F.; Wei, J.-Y.; Chen, H.-W.; Liu, Y.-Y.; Lu, H.-Y.; Chou, J.-Y. Indole-3-acetic acid: A widespread physiological code in interactions of fungi with other organisms. Plant Signal. Behav. 2015, 10, e1048052. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Groffen, T.; Lasters, R.; Lemière, F.; Willems, T.; Eens, M.; Bervoets, L.; Prinsen, E. Development and validation of an extraction method for the analysis of perfluoroalkyl substances (PFAS) in environmental and biotic matrices. J. Chromatogr. B 2019, 1116, 30–37. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groffen, T.; Kuijper, N.; Oden, S.; Willems, T.; Bervoets, L.; Prinsen, E. Growth Hormones in Broad Bean (Vicia faba L.) and Radish (Raphanus raphanistrum subsp. sativus L.) Are Associated with Accumulated Concentrations of Perfluoroalkyl Substances. Toxics 2023, 11, 922. https://doi.org/10.3390/toxics11110922
Groffen T, Kuijper N, Oden S, Willems T, Bervoets L, Prinsen E. Growth Hormones in Broad Bean (Vicia faba L.) and Radish (Raphanus raphanistrum subsp. sativus L.) Are Associated with Accumulated Concentrations of Perfluoroalkyl Substances. Toxics. 2023; 11(11):922. https://doi.org/10.3390/toxics11110922
Chicago/Turabian StyleGroffen, Thimo, Niels Kuijper, Sevgi Oden, Tim Willems, Lieven Bervoets, and Els Prinsen. 2023. "Growth Hormones in Broad Bean (Vicia faba L.) and Radish (Raphanus raphanistrum subsp. sativus L.) Are Associated with Accumulated Concentrations of Perfluoroalkyl Substances" Toxics 11, no. 11: 922. https://doi.org/10.3390/toxics11110922
APA StyleGroffen, T., Kuijper, N., Oden, S., Willems, T., Bervoets, L., & Prinsen, E. (2023). Growth Hormones in Broad Bean (Vicia faba L.) and Radish (Raphanus raphanistrum subsp. sativus L.) Are Associated with Accumulated Concentrations of Perfluoroalkyl Substances. Toxics, 11(11), 922. https://doi.org/10.3390/toxics11110922





