Quantification and Chemical Characterization of Plastic Additives and Small Microplastics (<100 μm) in Highway Road Dust
Abstract
:1. Introduction
2. Materials and Methods
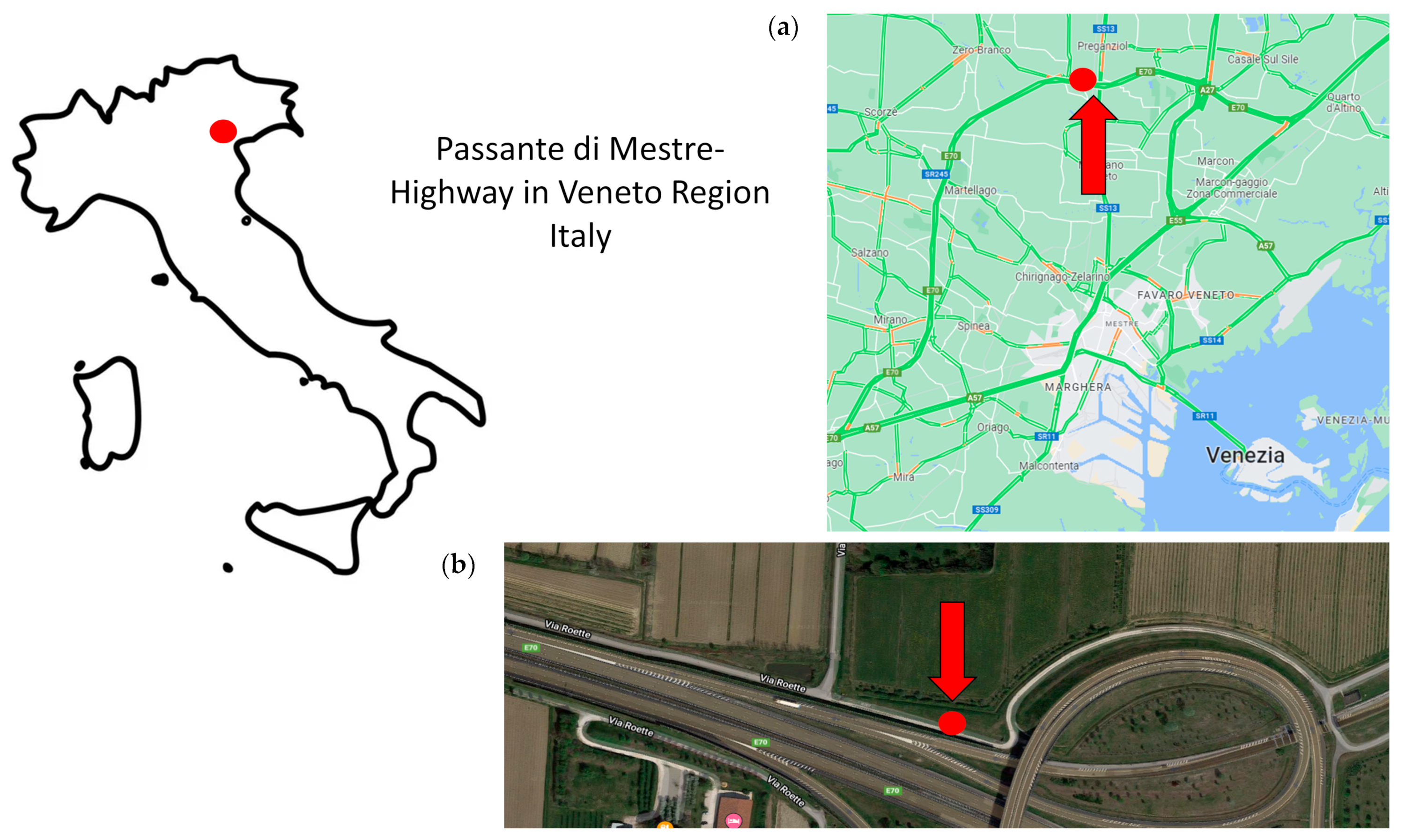
2.1. Sampling
2.2. Reagents
2.3. QA/QC
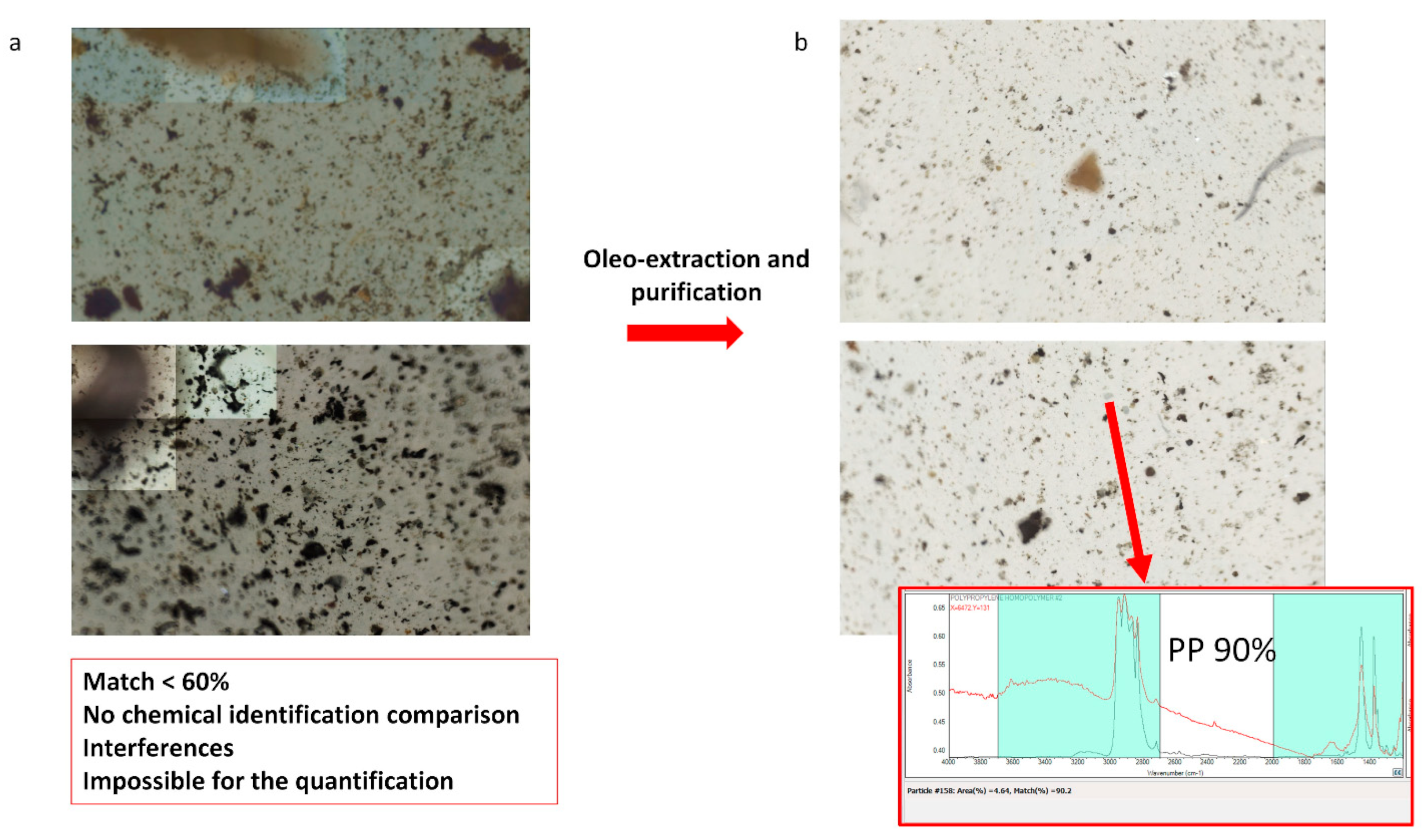
2.4. Oleoextraction, Purification, and Filtration of RD Samples
2.5. Quantification and Chemical Identification of SMPs and APFs Using Micro-FTIR
2.6. Statistical Analyses
3. Results and Discussion
3.1. Optimization of the Pretreatment (Oleoextraction, Filtration, and Purification)
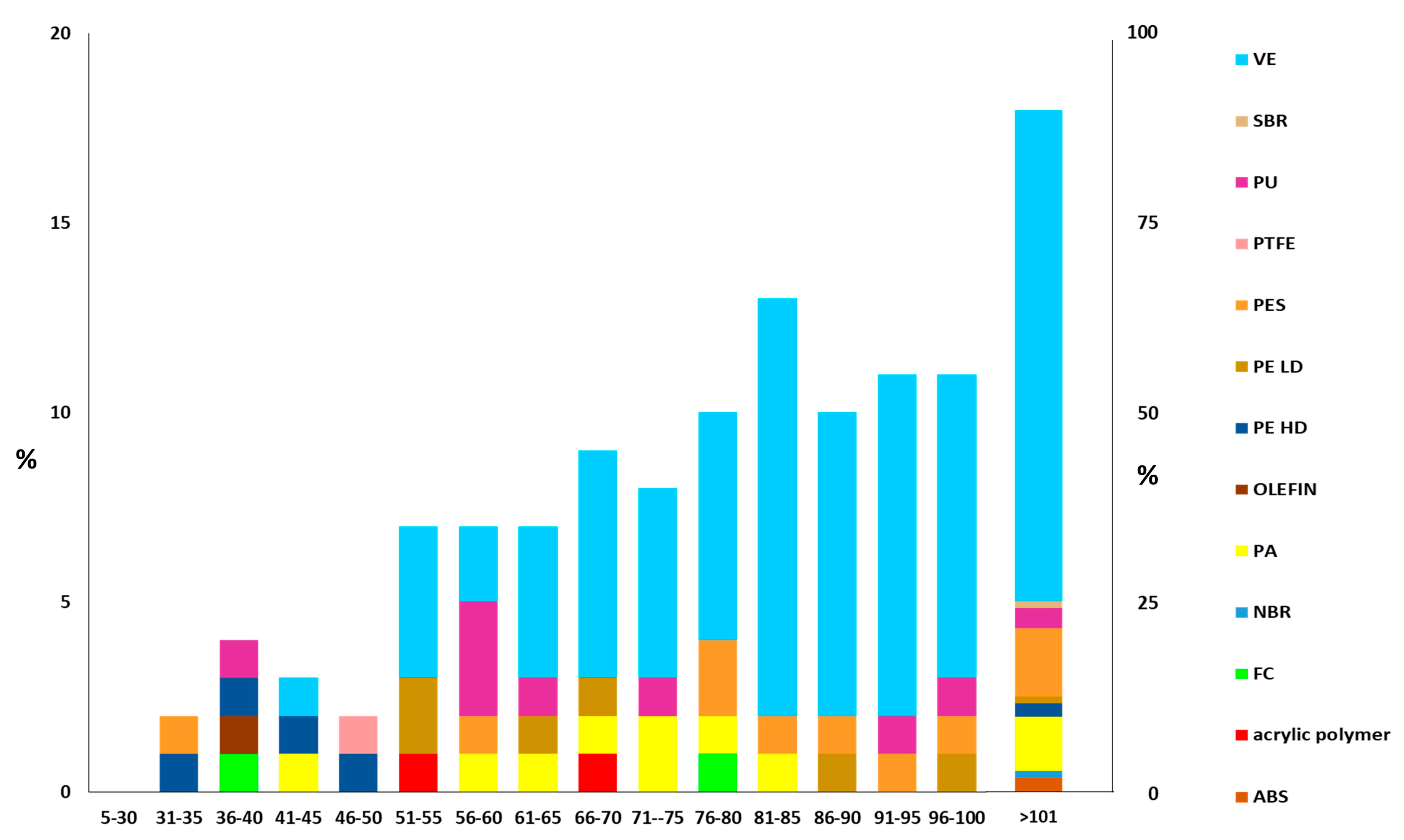
3.2. Quantification and Simultaneous Identification of SMPs in HWRD Samples
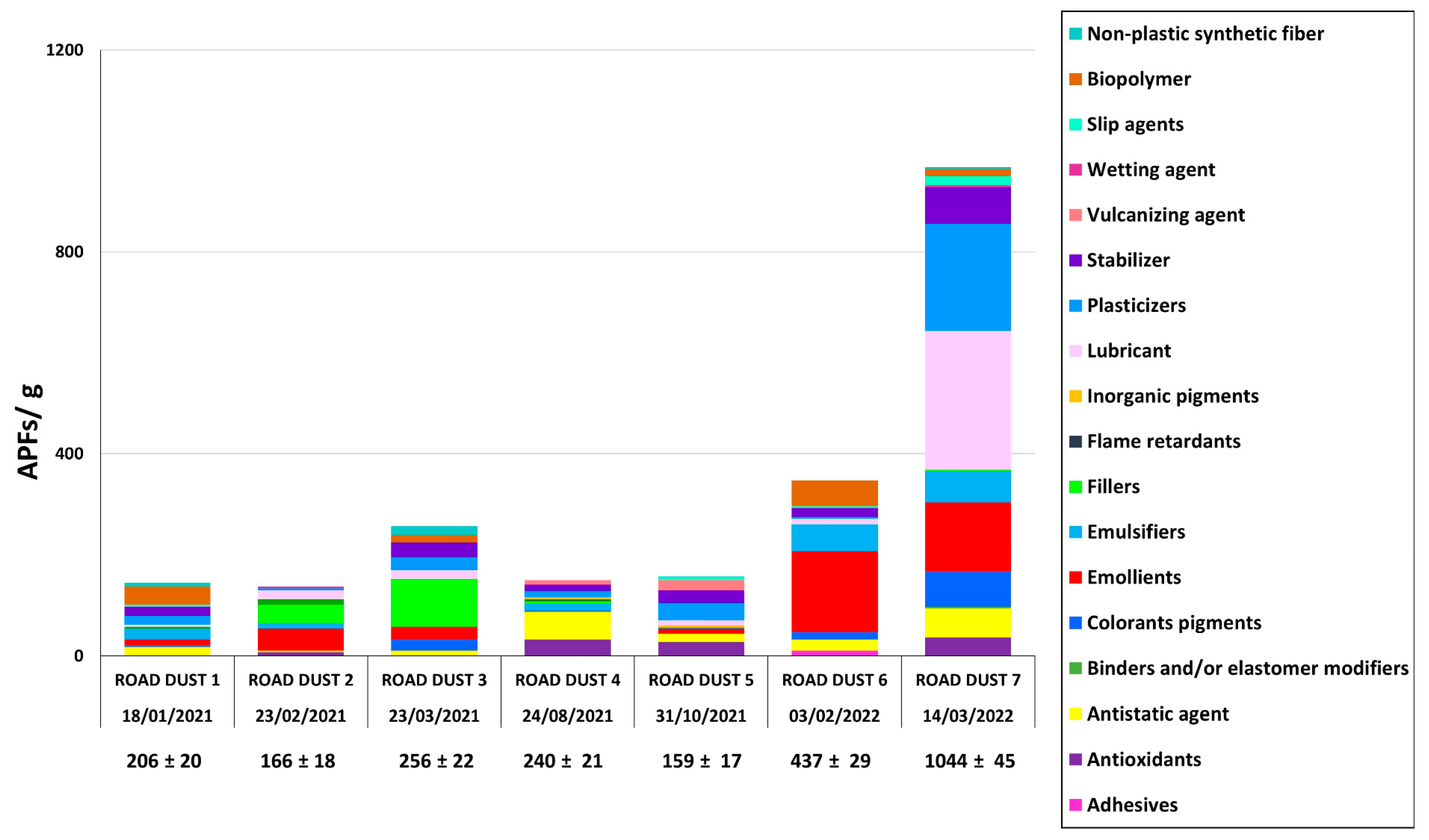
3.3. Quantification and Identification of APFs in HWRD Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hwang, H.; Fiala, M.J.; Wade, T.L.; Park, D. Review of pollutants in urban road dust: Part II. Organic contaminants from vehicles and road management. Int. J. Urban Sci. 2019, 23, 445–463. [Google Scholar] [CrossRef]
- ACEA. Vehichles in Use in Europe. Report. 2022. Available online: https://www.acea.auto/publication/report-vehicles-in-use-europe-2022/ (accessed on 10 August 2023).
- European Court Report Roads EU ECA Special Report Pursuant to Article 287 4 TFEU, 2020, Second Subparagraph. Available online: https://www.eca.europa.eu/Lists/ECADocuments/SR20_09/SR_Road_network_EN (accessed on 10 August 2023).
- European Environment Agency. pm10, European Air Quality Data for 2021, (Interpolated Data). 2023. Available online: https://airindex.eea.europa.eu/Map/AQI/Viewer/ (accessed on 6 September 2023).
- Gondwal, T.K.; Mandal, P. Review on Classification, Sources and Management of Road Dust and Determination of Uncertainty Associated with Measurement of Particle Size of Road Dust. MAPAN 2021, 36, 909–924. [Google Scholar] [CrossRef]
- Monira, S.; Bhuiyan, M.A.; Haque, N.; Shah, K.; Roychand, R.; Hai, F.I.; Pramanik, B.K. Understanding the fate and control of road dust-associated microplastics in stormwater. Process Saf. Environ. Prot. 2021, 152, 47–57. [Google Scholar] [CrossRef]
- Wei, L.; Yue, Q.; Chen, G.; Wang, J. Microplastics in rainwater/stormwater environments: Influencing factors, sources, transport, fate, and removal techniques. TrAC Trends Anal. Chem. 2023, 165, 117147. [Google Scholar] [CrossRef]
- Österlund, H.; Blecken, G.; Lange, K.; Marsalek, J.; Gopinath, K.; Viklander, M. Microplastics in urban catchments: Review of sources, pathways, and entry into stormwater. Scie Tot. Environ. 2023, 858, 159781. [Google Scholar] [CrossRef]
- Hwang, H.; Fiala, M.; Dongjoo, P.; Wade, T.L. Review of pollutants in urban RD and stormwater runoff: Part 1. Heavy metals released from vehicles. Int. J. Urban Sci. 2016, 20, 334–360. [Google Scholar] [CrossRef]
- Roychand, R.; Pramanik, B.K. Identification of micro-plastics in Australian RD. J. Environ. Chem. Eng. 2020, 8, 103647. [Google Scholar] [CrossRef]
- Vogelsang, C.; Lusher, A.; Dadkhah, M.; Sundvor, I.; Umar, M.; Ranneklev, S.; Pettersen, D.; Eidsvoll, M.S. Microplastics in RD-Characteristics, Pathways and Measures Environmental Science; NIVA-Rapport; Norwegian Institute for Water Research: Oslo, Norway, 2018; Volume 7526. [Google Scholar]
- Kitahara, K.I.; Nakata, H. Plastic additives as tracers of microplastic sources in Japanese road dust. Sci. Tot. Environ. 2020, 736, 139694. [Google Scholar] [CrossRef]
- Bhatt, V.; Chauhan, J.S. Microplastic in freshwater ecosystem: Bioaccumulation, trophic transfer, and biomagnification. Environ. Scie Pollut. Res. 2023, 30, 9389–9400. [Google Scholar] [CrossRef]
- Iannilli, V.; Pasquali, V.; Setini, A.; Corami, F. First evidence of microplastics ingestion in benthic amphipods from Svalbard. Environ. Res. 2019, 179, 108811. [Google Scholar] [CrossRef]
- Ma, M.; Wu, Z.; An, L.; Xu, Q.; Wang, H.; Zhang, Y.; Kang, Y. Microplastics transferring from abiotic to biotic in aquatic ecosystem: A mini review. Scie Tot. Environ. 2023, 893, 164686. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, J.P.; Chamkha, M.; Ksibi, M.; Sayadi, S. Effects of microplastics’ physical and chemical properties on aquatic organisms: State-of-the-art and future research trends. TrAC Trends Anal. Chem. 2023, 166, 117192. [Google Scholar] [CrossRef]
- ECHA Plastic Additives Initiative. 2018. Available online: https://echa.europa.eu/plastic-additives-initiative (accessed on 15 January 2020).
- Beiras, R.; Verdejo, E.; Campoy-Lopez, P.; Vidal-Linan, L. Aquatic toxicity of chemically defined microplastics can be explained by functional additives. J. Hazard. Mat. 2021, 406, 124338. [Google Scholar] [CrossRef] [PubMed]
- Corami, F.; Rosso, B.; Iannilli, V.; Ciadamidaro, S.; Bravo, B.; Barbante, C. Occurrence and characterization of small microplastics (< 100 μm), additives, and plasticizers in Larvae of simuliidae. Toxics 2022, 10, 383. [Google Scholar] [CrossRef]
- Fauser, P.; Vorkamp, K.; Strand, J. Residual additives in marine microplastics and their risk assessment–A critical review. Mar. Pollut. Bullet. 2022, 177, 113467. [Google Scholar] [CrossRef]
- Rauert, C.; Rødland, E.S.; Okoffo, E.D.; Reid, M.J.; Meland, S.; Thomas, K.V. Challenges with quantifying tire road wear particles: Recognizing the need for further refinement of the ISO technical specification. Environ. Sci. Technol. Lett. 2021, 8, 231–236. [Google Scholar] [CrossRef]
- Fred-Ahmadu, O.H.; Bhagwat, G.; Oluyoye, I.; Benson, N.U.; Ayejuyo, O.O.; Palanisami, T. Interaction of chemical contaminants with microplastics: Principles and perspectives. Scie Tot. Environ. 2020, 706, 135978. [Google Scholar] [CrossRef]
- Gunaalan, K.; Fabbri, E.; Capolupo, M. The hidden threat of plastic leachates: A critical review on their impacts on aquatic organisms. Water Res. 2020, 184, 116170. [Google Scholar] [CrossRef]
- Sendra, M.; Pereiro, P.; Figueras, A.; Novoa, B. An integrative toxicogenomic analysis of plastic additives. J. Hazard. Mater. 2021, 409, 124975. [Google Scholar] [CrossRef]
- Capolupo, M.; Sørensen, L.; Jayasena, K.D.R.; Booth, A.M.; Fabbri, E. Chemical composition and ecotoxicity of plastic and car tire rubber leachates to aquatic organisms. Water Res. 2020, 169, 115270. [Google Scholar] [CrossRef]
- Page, T.S.; Almeda, R.; Koski, M.; Bournaka, E.; Nielsen, T.G. Toxicity of tyre wear particle leachates to marine phytoplankton. Aquat. Toxicol. 2022, 252, 106299. [Google Scholar] [CrossRef] [PubMed]
- Rauert, C.; Charlton, N.; Okoffo, E.D.; Stanton, R.S.; Agua, A.R.; Pirrung, M.C.; Thomas, K.V. Concentrations of tire additive chemicals and tire road wear particles in an Australian urban tributary. Environ. Sci. Technol. 2022, 56, 2421–2431. [Google Scholar] [CrossRef] [PubMed]
- Do, A.T.N.; Ha, Y.; Kwon, J.H. Leaching of microplastic-associated additives in aquatic environments: A critical review. Environ, Pollut. 2020, 305, 119258. [Google Scholar] [CrossRef] [PubMed]
- Zha, F.; Shang, M.; Ouyang, Z.; Guo, X. The aging behaviors and release of microplastics: A review. Gondwana Res. 2022, 108, 60–71. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, B.; Wang, H. Analytical methods for microplastics in the environment: A review. Environ. Chem. Lett. 2023, 21, 383–401. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Al-Azzawi, M.S.M.; Kefer, S.; Weißer, J.; Reichel, J.; Schwaller, C.; Glas, K.; Knoop, O.; Drewes, J.E. Validation of sample preparation methods for microplastic analysis in wastewater matrices—Reproducibility and standardization. Water 2020, 12, 2445. [Google Scholar] [CrossRef]
- Corami, F.; Rosso, B.; Morabito, E.; Rensi, V.; Gambaro, A.; Barbante, C. Small microplastics (<100 μm), plasticizers and additives in seawater and sediments: Oleoextraction, purification, quantification, and polymer characterization using Micro-FTIR. Sci. Tot. Environ. 2021, 797, 148937. [Google Scholar] [CrossRef]
- Debraj, D.; Lavanya, M. Microplastics everywhere: A review on existing methods of extraction. Scie Tot. Environ. 2023, 893, 164878. [Google Scholar] [CrossRef]
- Yang, C.; Niu, S.; Xia, Y.; Wu, J. Microplastics in urban road dust: Sampling, analysis, characterization, pollution level, and influencing factors. TrAC Trends Anal. Chem. 2023, 168, 117348. [Google Scholar] [CrossRef]
- ISO Standards are Internationally Agreed by Experts. Available online: https://www.iso.org/standards.html (accessed on 10 October 2017).
- Corami, F.; Rosso, B.; Bravo, B.; Gambaro, A.; Barbante, C. A novel method for purification, quantitative analysis and characterization of microplastic fibers using Micro-FTIR. Chemosphere 2019, 238, 124564. [Google Scholar] [CrossRef] [PubMed]
- Corami, F.; Rosso, B.; Roman, M.; Picone, M.; Gambaro, A.; Barbante, C. Evidence of small microplastics (<100 μm) ingestion by Pacific oysters (Crassostrea gigas): A novel method of extraction, purification, and analysis using Micro-FTIR. Mar. Pollut. Bullet. 2020, 160, 111606. [Google Scholar] [CrossRef]
- ARPA Veneto. Available online: https://www.arpa.veneto.it/dati-ambientali/bollettini/meteo (accessed on 2 June 2022).
- Su, L.; Nan, B.; Craig, N.J.; Pettigrove, V. Temporal and spatial variations of microplastics in roadside dust from rural and urban Victoria, Australia: Implications for diffuse pollution. Chemosphere 2020, 252, 126567. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Park, S.; Lee, B.; Kim, I.; Kim, S. Concentration of Microplastics in Road Dust as a Function of the Drying Period—A Case Study in G City, Korea. Sustainability 2022, 14, 3006. [Google Scholar] [CrossRef]
- Myszka, R.; Enfrin, M.; Giustozzi, F. Microplastics in road dust: A practical guide for identification and characterisation. Chemosphere 2023, 315, 137757. [Google Scholar] [CrossRef]
- Narmadha, V.V.; Jose, J.; Patil, S.; Farooqui, M.O.; Srimuruganandam, B.S.; Saravanadevi, K.; Krishnamurthi, K. Assessment of Microplastics in Roadside Suspended Dust from Urban and Rural Environment of Nagpur, India. Int. J. Environ. Res. 2020, 14, 629–640. [Google Scholar] [CrossRef]
- Patchaiyappan, A.; Dowarah, K.; Zaki Ahmed, S.; Prabakaran, M.; Jayakumar, S.; Thirunavukkarasu, C.; Devipriya, S.P. Prevalence and characteristics of microplastics present in the street dust collected from Chennai metropolitan city, India. Chemosphere 2020, 269, 128757. [Google Scholar] [CrossRef]
- Mani, T.; Frehland, S.; Kalberer, A.; Burkhardt-Holm, P. Using castor oil to separate microplastics from four different environmental matrices. Anal. Methods 2019, 11, 1788–1794. [Google Scholar] [CrossRef]
- Rosso, B.; Corami, F.; Vezzaro, L.; Biondi, S.; Bravo, B.; Barbante, C.; Gambaro, A. Quantification and characterization of additives, plasticizers, and small microplastics (5–100 μm) in highway stormwater runoff. J. Environ. Manage. 2022, 15, 116348. [Google Scholar] [CrossRef]
- Rosso, B.; Corami, F.; Barbante, C.; Gambaro, A. Quantification and identification of airborne small microplastics (<100 μm) and other microlitter components in atmospheric aerosol via a novel elutriation and oleoextraction method. Environ. Pollut 2023, 318, 120889. [Google Scholar] [CrossRef]
- Scopetani, C.; Chelazzi, D.; Mikola, J.; Leiniö, V.; Heikkinen, R.; Cincinelli, A.; Pellinen, J. Olive oil-based method for the extraction, quantification and identification of microplastics in soil and compost samples. Scie Tot. Environ. 2020, 733, 139338. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.N.; Carré, F.; Hoarau-Belkhiri, A.; Joris, A.; Leonards, P.E.; Lamoree, M.H. Innovations in analytical methods to assess the occurrence of microplastics in soil. J. Environ. Chem. 2022, 10, 107421. [Google Scholar] [CrossRef]
- Opher, T.; Friedler, E. Factors Affecting Highway Runoff Quality. Urban Water J. 2010, 7, 155–172. [Google Scholar] [CrossRef]
- Shruti, V.C.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Kutralam-Muniasamy, G. Current trends and analytical methods for evaluation of microplastics in stormwater. Trends Environ. Anal. Chem. 2021, 30, e00123. [Google Scholar] [CrossRef]
- Pitt, R.E.; Williamson, D.; Voorhees, J.; Clark, S. Review of historical street dust and dirt accumulation and washoff data. J. Water Manag. Model. 2005, R223-12. [Google Scholar] [CrossRef]
- Rienda, I.C.; Alves, C.A. Road dust resuspension: A review. Atmos. Res. 2021, 261, 105740. [Google Scholar] [CrossRef]
- Gaylarde, C.C.; Neto, J.A.B.; da Fonseca, E.M. Paint fragments as polluting microplastics: A brief review. Mar. Pollut. Bull. 2021, 162, 111847. [Google Scholar] [CrossRef]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Delshab, H.; Soltani, N.; Sorooshian, A. Investigation of microrubbers, microplastics and heavy metals in street dust: A study in Bushehr city, Iran. Environ. Earth Sci. 2017, 76, 798. [Google Scholar] [CrossRef]
- Yukioka, S.; Tanaka, S.; Nabetani, Y.; Suzuki, Y.; Ushijima, T.; Fujii, S.; Takada, H.; Tran, Q.V.; Singh, S. Occurrence and characteristics of microplastics in surface road dust in Kusatsu (Japan), Da Nang (Vietnam), and Kathmandu (Nepal). Environ. Pollut. 2020, 256, 113447. [Google Scholar] [CrossRef]
- Wang, T.; Niu, S.; Wu, J.; Yu, J. Seasonal and daily occurrence of microplastic pollution in urban road dust. J. Clean. Prod. 2022, 380, 135025. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- PCC Group report Plastic Additives. 2018. Available online: https://www.products.pcc.eu/wp-content/uploads/export-category-page/5d4d7590ca39b/PCC-Group_Polymer-Additives_EN.pdf (accessed on 10 August 2023).
- Fatima, N.; Holmgren, A.; Marklund, P.; Minami, I.; Larsson, R. Degradation mechanism of automatic transmission fluid by water as a contaminant. Proceedings of the Institution of Mechanical Engineers. Part J J. Eng. Tribol. 2015, 229, 74–85. [Google Scholar] [CrossRef]
- Godwin, A.D. Plasticizers. In Applied Plastics Engineering Handbook, 2nd ed.; Kut, M., Ed.; William Andrew Imprint of Elsevier: Norwich, NY, USA, 2017; pp. 533–553. [Google Scholar]
- Pub Chem 2023, National Library of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound (accessed on 10 October 2022).
- He, M.; Li, Y.; Qiao, B.; Ma, X.; Song, J.; Wang, M. Effect of dicumyl peroxide and phenolic resin as a mixed curing system on the mechanical properties and morphology of TPVs based on HDPE/ground tire rubber. Polym. Compos. 2015, 36, 1907–1916. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Zedler, Ł.; Formela, K. Processing, performance properties, and storage stability of ground tire rubber modified by dicumyl peroxide and ethylene-vinyl acetate copolymers. Polymers 2021, 13, 4014. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.; Akil, H.M. The effects of dynamic vulcanization by dicumyl peroxide (DCP) and N, Nm-phenylene bismaleimide (HVA-2) on the properties of polypropylene (PP)/ethylene-propylene diene terpolymer (EPDM)/natural rubber (NR) blends. Polym. Plast Technol. Eng. 2005, 44, 1217–1234. [Google Scholar] [CrossRef]

| SMPs | Abbreviations |
| Acrylic polymer | Acrylic polymer |
| Acrylonitrile butadiene styrene | ABS |
| Ethylene methyl acrylate | EMA |
| Ethylene-vinyl acetate | EVA |
| Ethylene vinyl alcohol | EVOH |
| MODACRYLIC | MODACRYLIC |
| Nylon | PA |
| Olefin | OLEFIN |
| Polyarylamide | PARA |
| Polyethylene HD | PE HD |
| Polyethylene low density | PE LD |
| Polyester | PES |
| Polyolefin | PO |
| Polyoxymethylene | POM |
| Poly(p-phenylene oxide) | PPE |
| Polyphthalamide | PPA |
| Polypropylene | PP |
| Polystyrene | PS |
| Polytetrafluoroethylene | PTFE |
| Polyurethane | PU |
| Styrene butadiene rubber | SBR |
| Vinyl ester | VE |
| APFs | Abbreviations |
| (N-(2-ethoxy phenyl)-N-(2-ethyl phenyl)-ethanediamide) | 2E2ANI |
| 1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one | 1,7,7-TMBC-2,2,1-H |
| 2-(2-phenylpropan-2-ylperoxy)propan-2-ylbenzene (dicumyl peroxide) | DCP |
| 2-methoxyethyl stearate | MS |
| 50% active glycerol monostearate | AGM |
| 5-methyl-1h-benzotriazole | 5-MBTR |
| Adipic 1,3-butylene glycol polymer | ABGP |
| Antiozonant e-9604® | Antiozonant E-9604® |
| Bis(2-hydroxyethyl dimerate | BHD |
| Butyl (Z)-octadec-9-enoate | BO |
| Butyl benzyl sebacate | BB |
| Butyl epoxy stearate | BE |
| Butyl ricinoleate | BR |
| Butyl vinyl ether | NBVE |
| Calcium pelargonate | CP |
| Calcium stearate | CS |
| Calcium sulfonate | CS |
| Calcium zinc molybdate | CZM |
| Chloroalkyl phosphate ester | CPE |
| Cocoamidopropyl betaine | CAPB |
| Cyanox STDP antioxidant | CYANOX STDP Antioxidant |
| Dibasic lead phosphite | DLP |
| Dicapryl maleate | DOM |
| Dicetyl maleate | DM |
| Diester of 3-dodecylthio propionic acid and tetraethylene glycol, hydrated amorphous silica | 3-DTPA-DE/TEG |
| Dihydrogen phosphate;2-hydroxyethyl-dimethyl-[3-(octadecanoylamino)propyl]azanium | 2-HE2M-3-OAPAP |
| Dimethyl sebacate | DMS |
| Dimodan lsk | |
| Dioctyl adipate | DA |
| Dipropylene glycol dinonanoate | DGD |
| Ethylene glycol monooleate | EGM |
| Fatty acid ester | FAE |
| Glutaric acid-based monomeric ester | GAM |
| Glyceryl monoricinoleate | GMR/SSL |
| Glyceryl trioleate | GT |
| Ground calcium carbonate #1 | GCC |
| Isooctyl stearate | IS |
| Methoxyethyl acetyl ricinoleate | MAR |
| Methyl octadecadienoate (methyl (9z,12z)-octadeca-9,12-dienoate-metyl linoleate) | Mlo |
| Methyl oleate | MO |
| Methyl ricinoleate | MLO |
| Methyl tallowate | MT |
| Microcrystalline wax | MCW |
| Montan ester wax mixture | MEW |
| Naugatuck antioxidant 451® | Naugatuck Antioxidant 451® |
| Neopentyl glycol | NG |
| Octadecanoic acid, calcium salt | OC SALT |
| Octyl dipropionate | OD |
| Organo quaterarny antistatic agent | QAC |
| Pentaerythritol monoricinoleate | PM |
| Peptizer 566 | Peptizer 566 |
| Phthalocyanine | Pc |
| Plasthall p-670 (polyester adipate) | PEA |
| Polymeric epoxy plasticizer | PEP |
| Propylen glycol dilaurate | PGDL |
| Quaternary ammonium compound | QAC |
| Rayon | RAYON |
| Resinall cp-25 | Resinall CP-25 |
| sebacic acid polymer with a polyol and vegetable oil | SAP |
| Sodium salt of alkyls sulfonic acid in polyolefin | SSAS in PO |
| Sorbitan isostearate esters | SIE |
| Sorbitan laurate | SL |
| Sorbitan monostearate | SL |
| Sorbitan trioleate | STO |
| Tetrahydrofurfuryl oleate | TO |
| Triglycerol diisostearate | TD |
| Unsaturated distilled monoglyceride | UDM |
| Vanox sn-1 | Vanox SN-1 |
| WB7 (titanium dioxide) | TiO2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosso, B.; Bravo, B.; Gregoris, E.; Barbante, C.; Gambaro, A.; Corami, F. Quantification and Chemical Characterization of Plastic Additives and Small Microplastics (<100 μm) in Highway Road Dust. Toxics 2023, 11, 936. https://doi.org/10.3390/toxics11110936
Rosso B, Bravo B, Gregoris E, Barbante C, Gambaro A, Corami F. Quantification and Chemical Characterization of Plastic Additives and Small Microplastics (<100 μm) in Highway Road Dust. Toxics. 2023; 11(11):936. https://doi.org/10.3390/toxics11110936
Chicago/Turabian StyleRosso, Beatrice, Barbara Bravo, Elena Gregoris, Carlo Barbante, Andrea Gambaro, and Fabiana Corami. 2023. "Quantification and Chemical Characterization of Plastic Additives and Small Microplastics (<100 μm) in Highway Road Dust" Toxics 11, no. 11: 936. https://doi.org/10.3390/toxics11110936
APA StyleRosso, B., Bravo, B., Gregoris, E., Barbante, C., Gambaro, A., & Corami, F. (2023). Quantification and Chemical Characterization of Plastic Additives and Small Microplastics (<100 μm) in Highway Road Dust. Toxics, 11(11), 936. https://doi.org/10.3390/toxics11110936







