Removal of Pb from Contaminated Kaolin by Pulsed Electrochemical Treatment Coupled with a Permeable Reactive Barrier: Tuning Removal Efficiency and Energy Consumption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
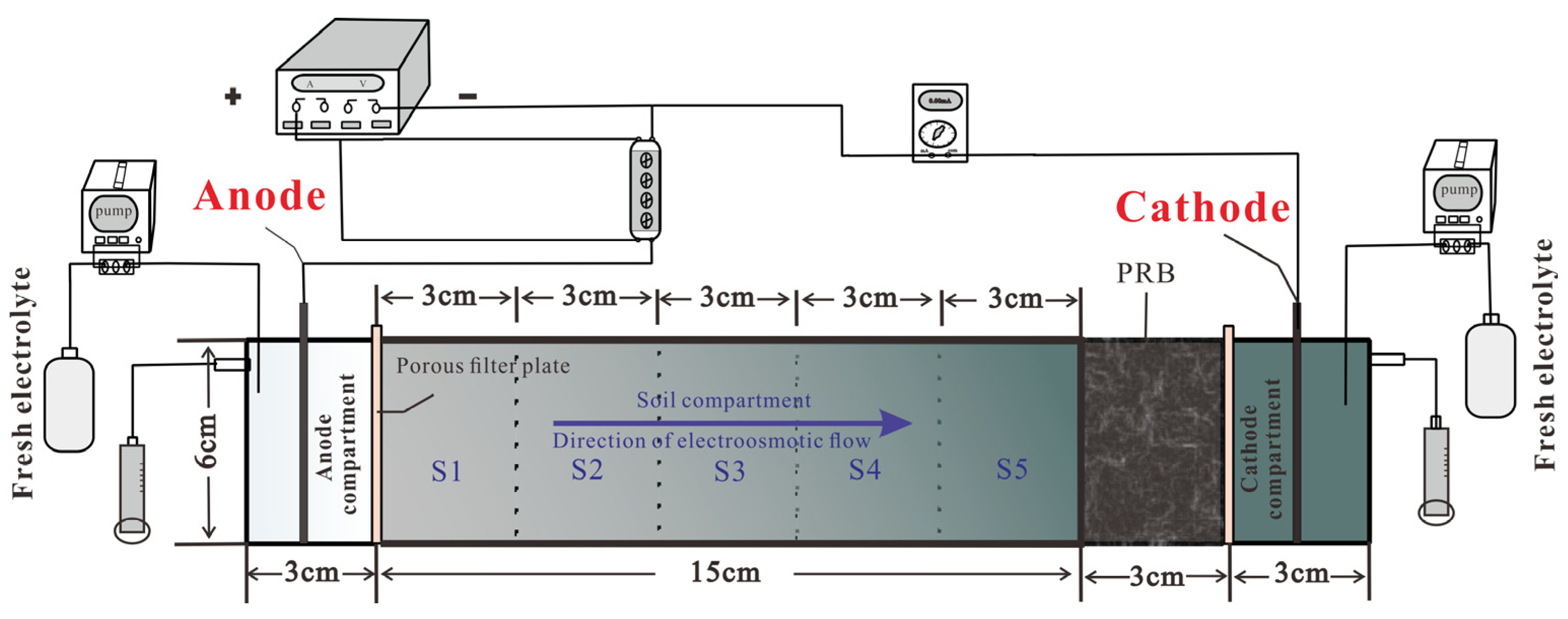
2.2. Experimental Setup
2.3. Experimental Procedure
2.4. Instrumental Analysis
3. Results
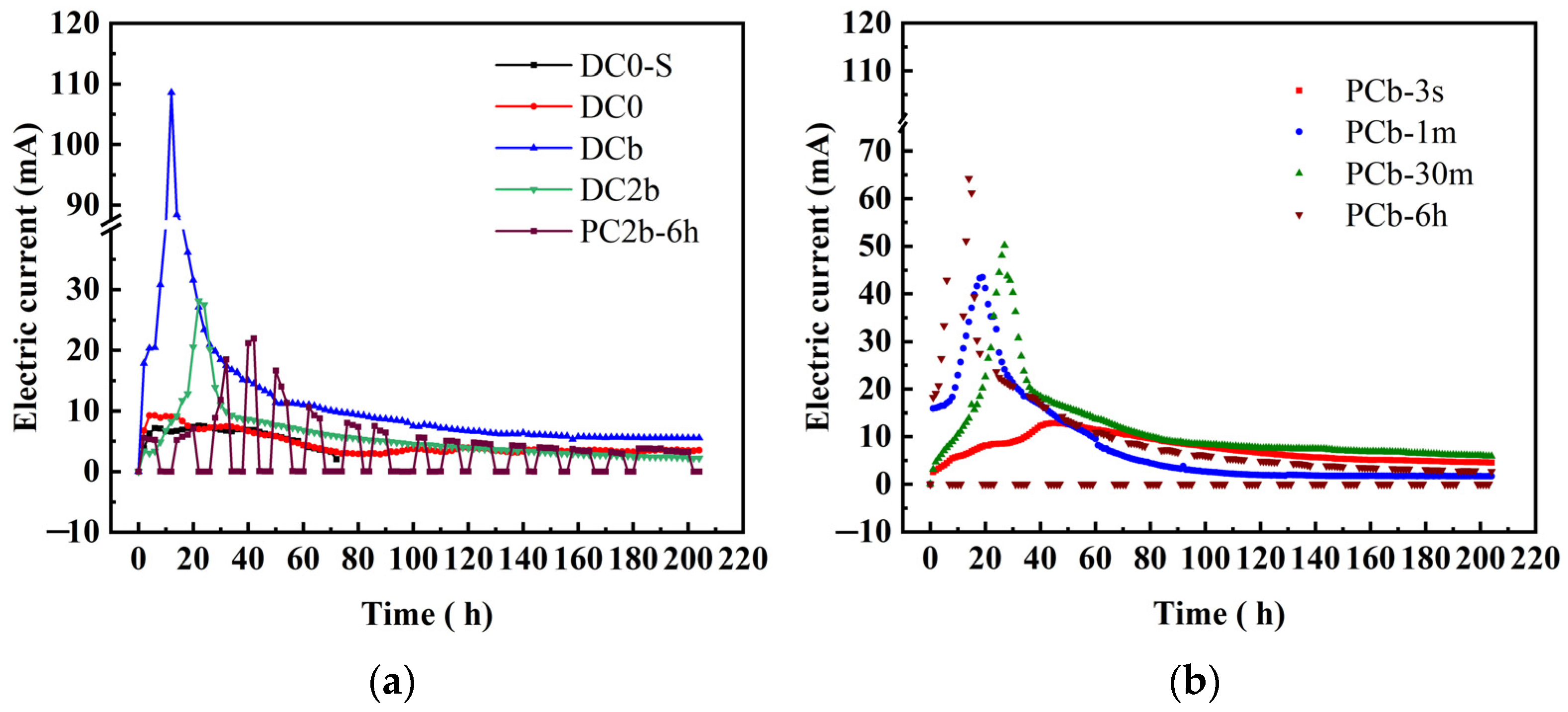
3.1. Electrical Current
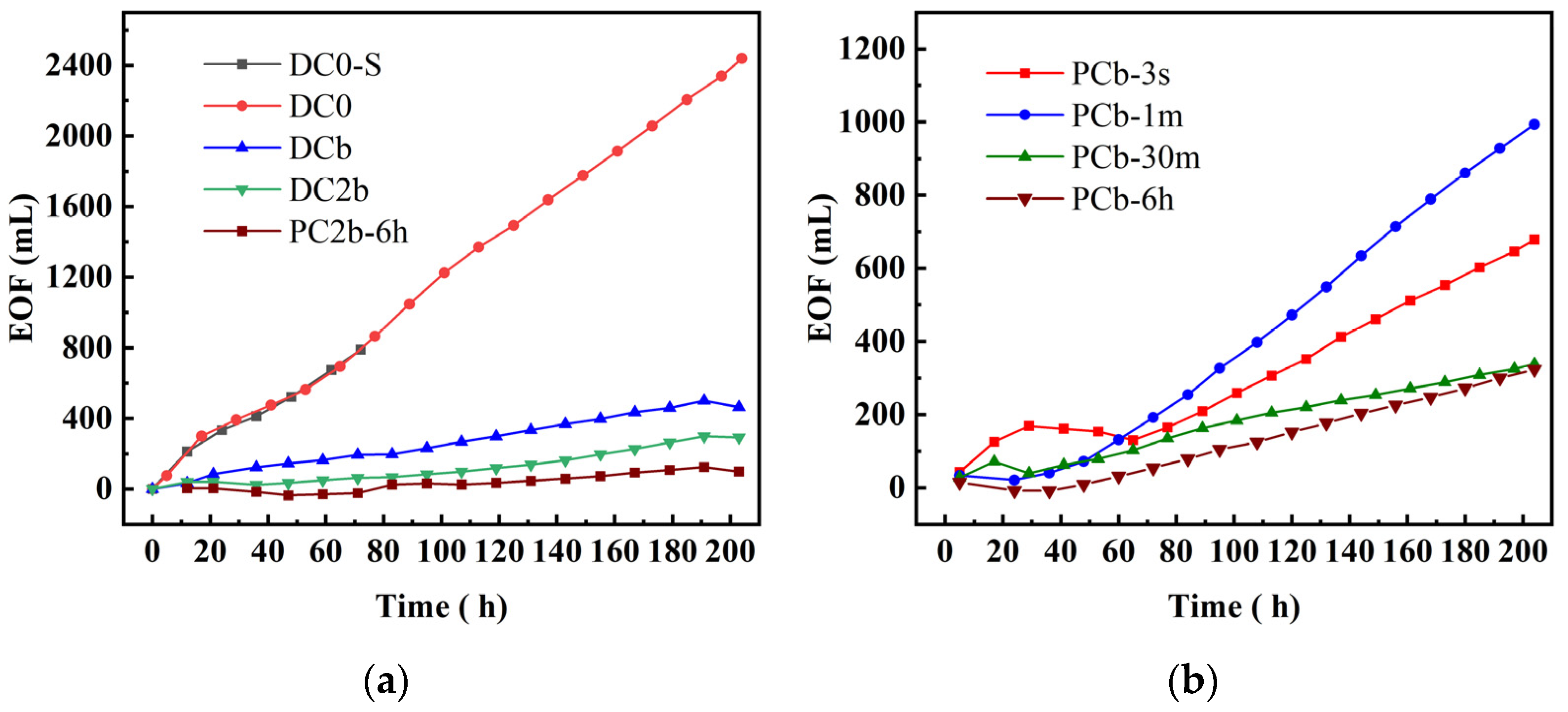
3.2. Electroosmotic Flow
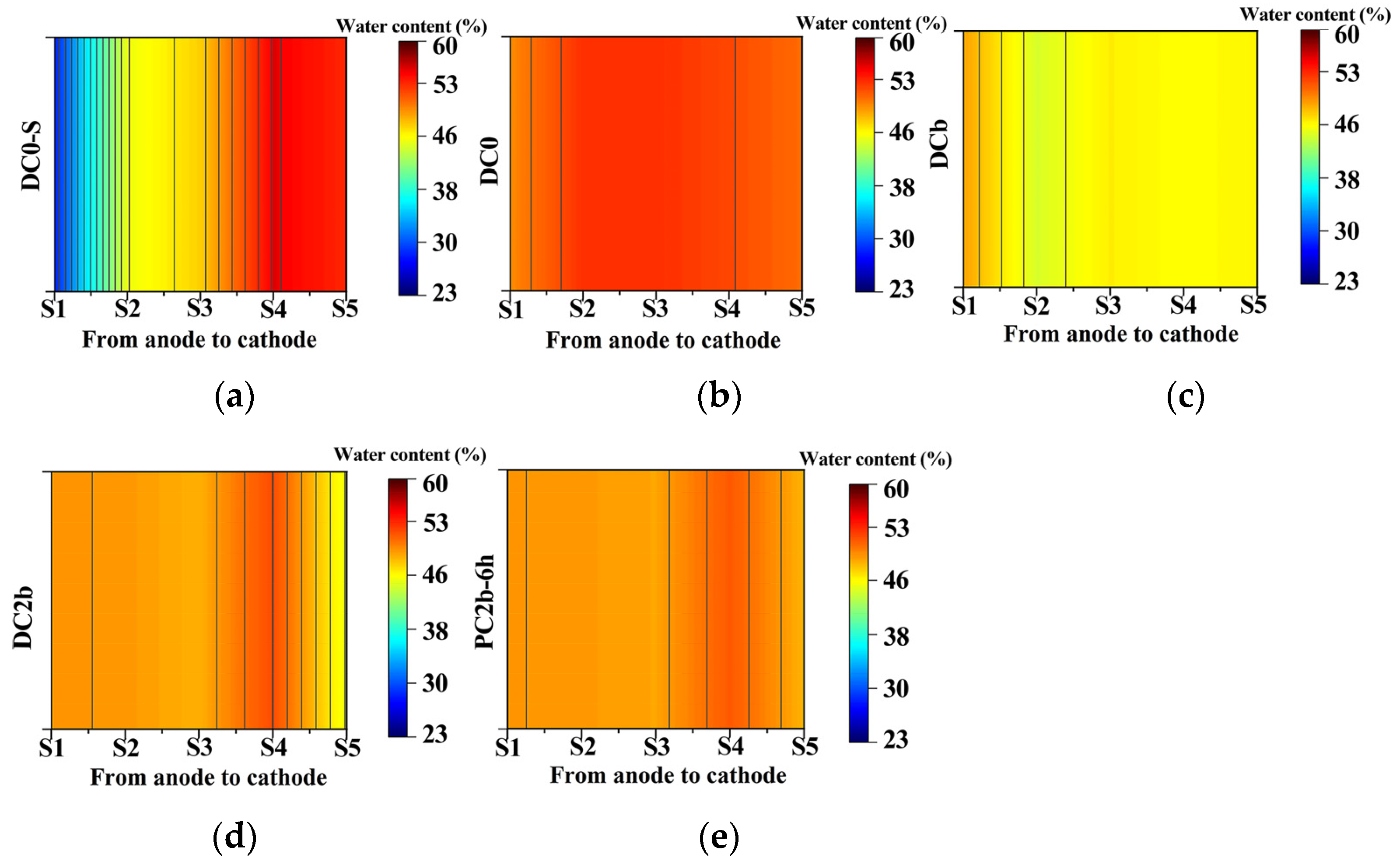
3.3. Moisture Content of Treated Soil
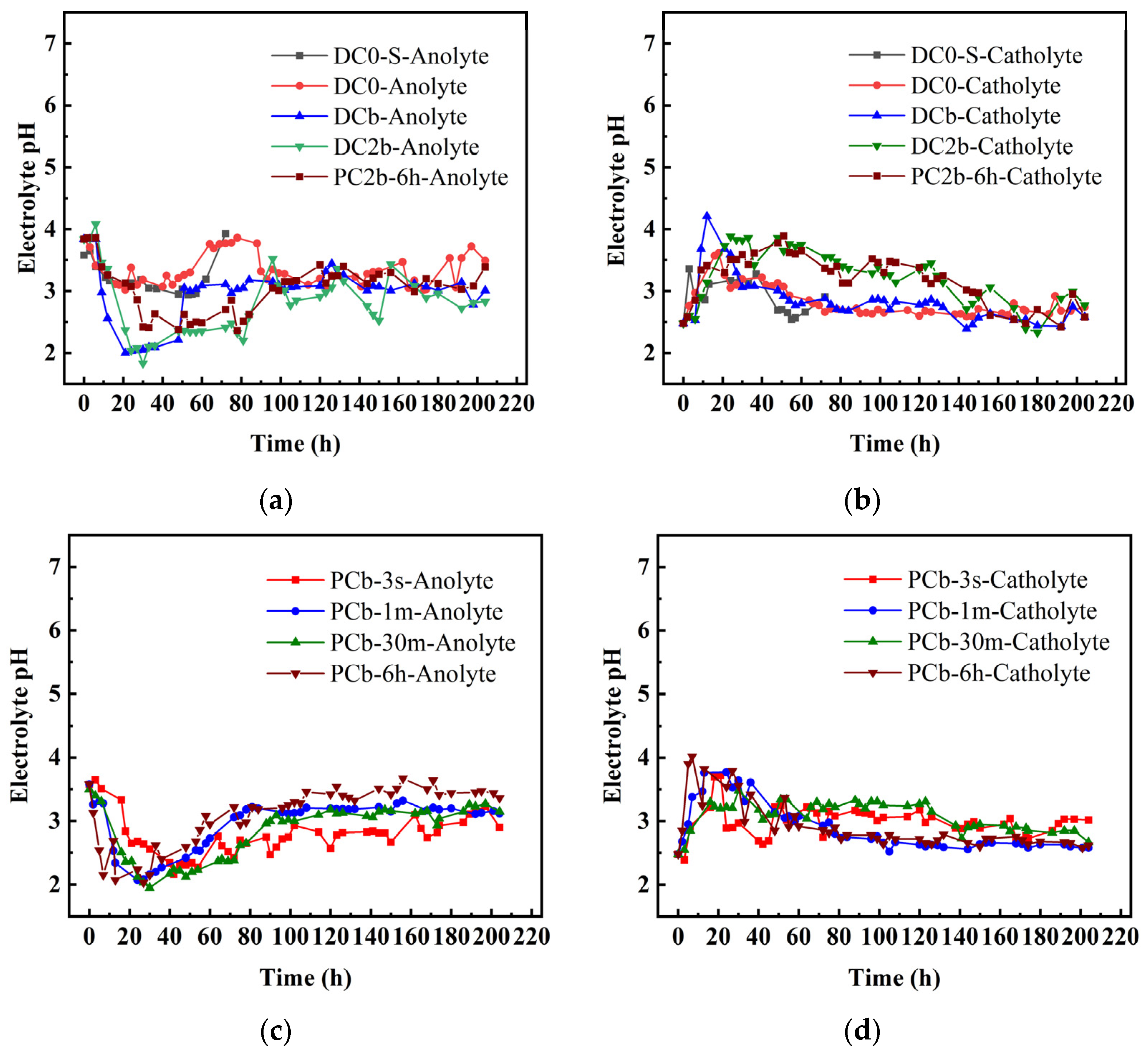
3.4. Electrolyte pH
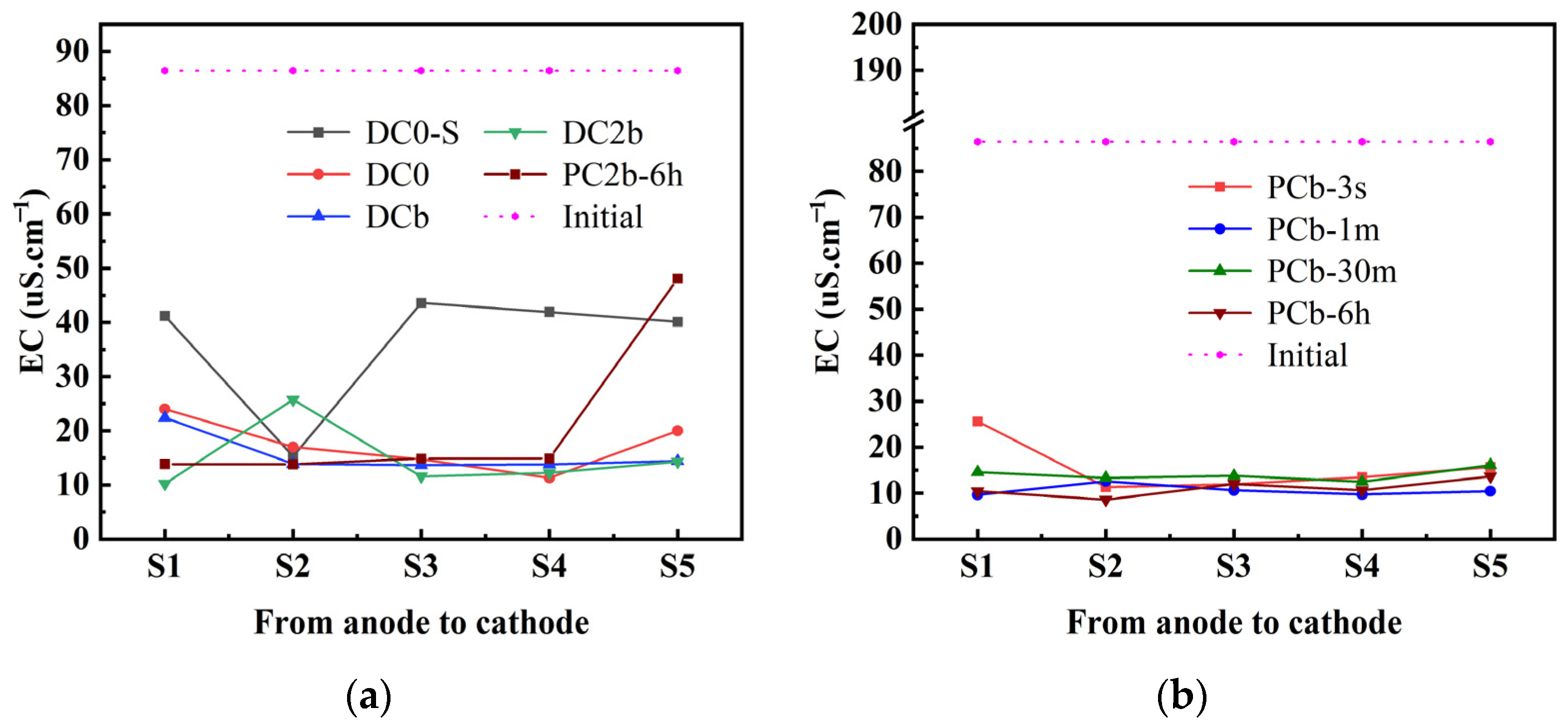
3.5. Soil pH and Soil Conductivity
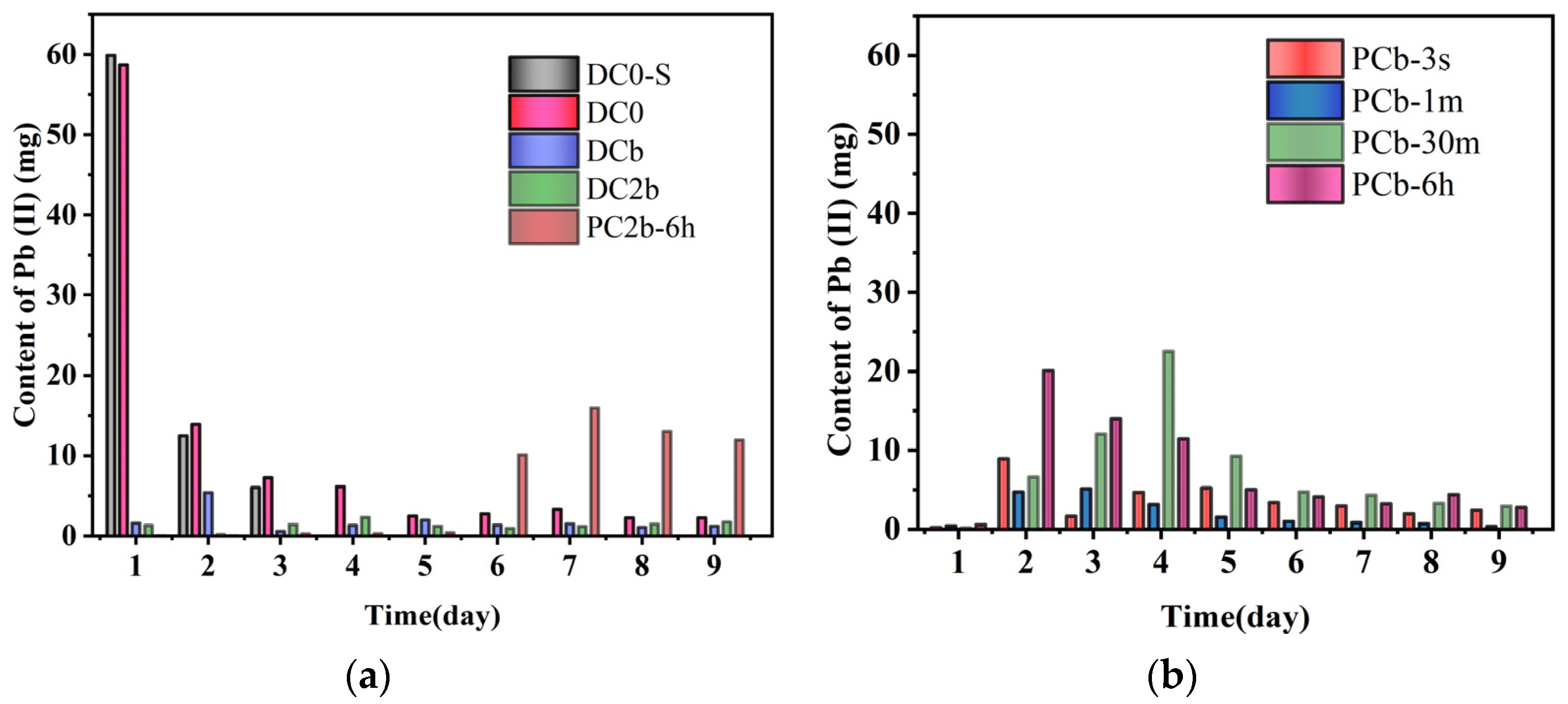
3.6. Residual Content of Pb in Collected Catholyte
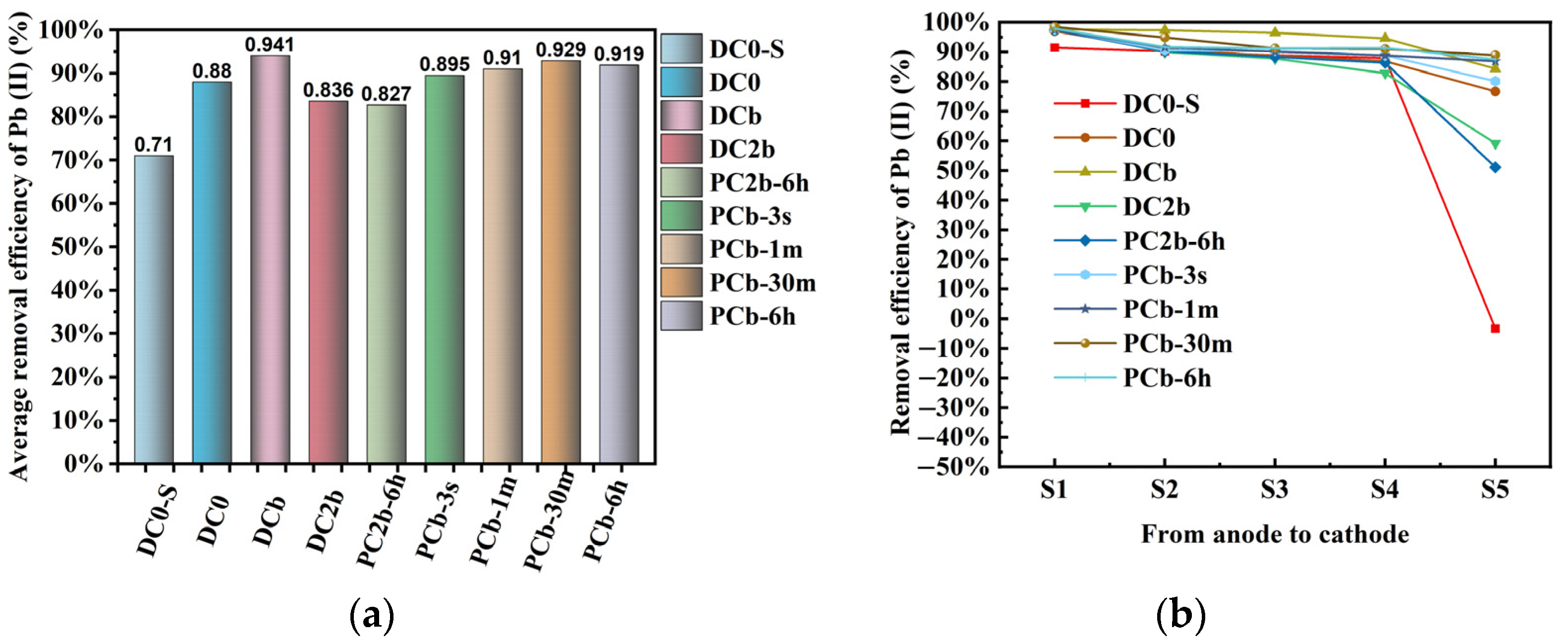
3.7. Removal Efficiency of Pb
3.8. Electrical Energy Consumption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amrate, S.; Akretche, D.E.; Innocent, C.; Seta, P. Removal of Pb from a calcareous soil during EDTA-enhanced electrokinetic extraction. Sci. Total Environ. 2005, 349, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, Z.; Liu, C.; Dong, Y. Technologies for removing heavy metal from contaminated soils on farmland: A review. Chemosphere 2022, 305, 135457. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Cheng, W.; Wen, S.; Kang, N. Revealing underlying mechanisms affecting electrokinetic remediation of an artificially Cu- and Pb-contaminated loess using the external regulatory system with adsorbent. Front. Mater. 2022, 9, 967871. [Google Scholar] [CrossRef]
- Taneja, S.; Karaca, O.; Haritash, A.K. Treatment of Pb-contaminated soil by electrokinetics: Enhancements by varying voltage, chelant, and electrode material. J. Geochem. Explor. 2023, 250, 107240. [Google Scholar] [CrossRef]
- Shareef, Z.N.; Yatiya, S.; Sulaiman, M.M. Enhanced of electro-kinetic technology for removal of lead ions from silty clay contaminated soil using EDTA and multi anode electrode. S. Afr. J. Chem. Eng. 2023, 45, 120–126. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Cheng, F.; Guo, P.; Guo, S. Enhancement of electrokinetic-bioremediation by ryegrass: Sustainability of electrokinetic effect and improvement of n-hexadecane degradation. Environ. Res. 2020, 188, 109717. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Jo, S.-U.; Yoo, J.-C.; Baek, K. Ex situ pilot scale electrokinetic restoration of saline soil using pulsed current. Sep. Purif. Technol. 2013, 120, 282–288. [Google Scholar] [CrossRef]
- Acar, Y.B.; Gale, R.J.; Alshawabkeh, A.N.; Marks, R.E.; Puppala, S.; Bricka, M.; Parker, R. Electrokinetic remediation: Basics and technology status. J. Hazard. Mater. 1995, 40, 117–137. [Google Scholar] [CrossRef]
- Bessaim, M.M.; Missoum, H.; Bendani, K.; Laredj, N.; Bekkouche, M.S. Sodic-saline soil remediation by electrochemical treatment under uncontrolled pH conditions. Arab. J. Geosci. 2020, 13, 199. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jo, S.-U.; Choi, J.-H.; Yang, J.-S.; Baek, K. Hexagonal two dimensional electrokinetic systems for restoration of saline agricultural lands: A pilot study. Chem. Eng. J. 2012, 198–199, 110–121. [Google Scholar] [CrossRef]
- Altin, A.; Degirmenci, M. Lead (II) removal from natural soils by enhanced electrokinetic remediation. Sci. Total. Environ. 2005, 337, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yu, C.; Mao, Y.; Zong, Y.; Zhang, B.; Chu, H.; Wu, D. Can flow-electrode capacitive deionization become a new in-situ soil remediation technology for heavy metal removal? J. Hazard. Mater. 2021, 402, 123568. [Google Scholar] [CrossRef] [PubMed]
- Niinae, M.; Nishigaki, K.; Aoki, K. Removal of Lead from Contaminated Soils with Chelating Agents. Mater. Trans. 2008, 49, 2377–2382. [Google Scholar] [CrossRef]
- Ryu, B.G.; Park, G.-Y.; Yang, J.-W.; Baek, K. Electrolyte conditioning for electrokinetic remediation of As, Cu, and Pb-contaminated soil. Sep. Purif. Technol. 2011, 79, 170–176. [Google Scholar] [CrossRef]
- Benamar, A.; Ammami, M.; Song, Y.; Portet-Koltalo, F. Scale-up of electrokinetic process for dredged sediments remediation. Electrochim. Acta 2020, 352, 136488. [Google Scholar] [CrossRef]
- Chen, R.; Zhou, L.; Wang, W.; Cui, D.; Hao, D.; Guo, J. Enhanced Electrokinetic Remediation of Copper-Contaminated Soil by Combining Steel Slag and a Permeable Reactive Barrier. Appl. Sci. 2022, 12, 7981. [Google Scholar] [CrossRef]
- Wang, J.; Gao, W.; Zhu, J.; Yang, Y.; Niu, Y. Remediation of Cd and Cu Contaminated Agricultural Soils near Oilfields by Biochar Combined with Sodium Humate-Wood Vinegar. Agronomy 2023, 13, 1009. [Google Scholar] [CrossRef]
- Zhu, Z.; Kong, Y.; Yang, H.; Tian, Y.; Zhou, X.; Zhu, Y.; Fang, Z.; Zhang, L.; Tang, S.; Fan, Y. Effects of Pretreatment and Polarization Shielding on EK-PRB of Fe/Mn/C-LDH for Remediation of Arsenic Contaminated Soils. Nanomaterials 2023, 13, 325. [Google Scholar] [CrossRef]
- Silva, K.N.; Henrique, J.M.; Vilar, V.J.; A Martínez-Huitle, C.; dos Santos, E.V. Cork-based permeable reactive barriers coupled to electrokinetic processes for interrupting pollutants reaching groundwater: A case study of lead-contaminated soil. J. Chem. Technol. Biotechnol. 2022, 97, 2861–2870. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, M.; Chen, L.; Gong, Z.; Hu, J.; Ma, D. Electrokinetic remediation for the removal of heavy metals in soil: Limitations, solutions and prospection. Sci. Total. Environ. 2023, 903, 165970. [Google Scholar] [CrossRef]
- Yuan, L.; Xu, X.; Li, H.; Wang, Q.; Wang, N.; Yu, H. The influence of macroelements on energy consumption during periodic power electrokinetic remediation of heavy metals contaminated black soil. Electrochim. Acta 2017, 235, 604–612. [Google Scholar] [CrossRef]
- Usman, A.K.; Mu’azu, N.D.; Lukman, S.; Essa, M.; Bukhari, A.A.; Al-Malack, M.H. Removal of Lead and Copper from Contaminated Mixed Clay Soils Using Pulsed Electrokinetics. Soil Sediment Contam. Int. J. 2020, 29, 465–480. [Google Scholar] [CrossRef]
- Ryu, B.-G.; Yang, J.-S.; Kim, D.-H.; Baek, K. Pulsed electrokinetic removal of Cd and Zn from fine-grained soil. J. Appl. Electrchem. 2020, 40, 1039–1047. [Google Scholar] [CrossRef]
- Sun, T.R.; Ottosen, L.M. Effects of pulse current on energy consumption and removal of heavy metals during electrodialytic soil remediation. Electrochim. Acta 2012, 86, 28–35. [Google Scholar] [CrossRef]
- Song, P.; Xu, D.; Yue, J.; Ma, Y.; Dong, S.; Feng, J. Recent advances in soil remediation technology for heavy metal contaminated sites: A critical review. Sci. Total. Environ. 2022, 838, 156417. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hu, A.; Wang, F.; Zhang, P.; Zhao, Z.; Zhao, Y.; Liu, X. Effective remediation of cadmium and zinc co-contaminated soil by electrokinetic-permeable reactive barrier with a pretreatment of complexing agent and microorganism. Chem. Eng. J. 2021, 407, 126923. [Google Scholar] [CrossRef]
- Vizcaíno, R.L.; Yustres, A.; Asensio, L.; Saez, C.; Cañizares, P.; Rodrigo, M.; Navarro, V. Enhanced electrokinetic remediation of polluted soils by anolyte pH conditioning. Chemosphere 2018, 199, 477–485. [Google Scholar] [CrossRef]
- Zhang, T.; Zou, H.; Ji, M.; Li, X.; Li, L.; Tang, T. Enhanced electrokinetic remediation of lead-contaminated soil by complexing agents and approaching anodes. Environ. Sci. Pollut. Res. 2013, 21, 3126–3133. [Google Scholar] [CrossRef]
- Pei, L.; Zhang, X.; Yuan, Z. Remediation of Cu Contaminated Soil by Fe78Si9B13AP Permeability Reaction Barrier Combined with Electrokinetic Method. J. Renew. Mater. 2023, 11, 2969–2983. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, S.; Liu, F.; Zhou, D. Pulse-enhanced electrokinetic remediation of fluorine-contaminated soil. Korean J. Chem. Eng. 2014, 31, 2008–2013. [Google Scholar] [CrossRef]
- Wang, H.X.; Teng, H.W.; Wang, X.Y.; Xu, J.L.; Sheng, L.X. Physicochemical modification of corn straw biochar to improve performance and its application of constructed wetland substrate to treat city tail water. J. Environ. Manag. 2022, 310, 114758. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xu, J.; Lv, S.; Liu, Z.; Liu, W. Removal of cadmium in contaminated kaolin by new-style electrokinetic remediation using array electrodes coupled with permeable reactive barrier. Sep. Purif. Technol. 2020, 239, 116544. [Google Scholar] [CrossRef]
- Wang, Q.; Shaheen, S.M.; Jiang, Y.; Li, R.; Slaný, M.; Abdelrahman, H.; Kwon, E.; Bolan, N.; Rinklebe, J.; Zhang, Z. Fe/Mn- and P-modified drinking water treatment residuals reduced Cu and Pb phytoavailability and uptake in a mining soil. J. Hazard. Mater. 2021, 403, 123628. [Google Scholar] [CrossRef]
- Wang, Q.; Slaný, M.; Gu, X.; Miao, Z.; Du, W.; Zhang, J.; Gang, C. Lubricity and Rheological Properties of Highly Dispersed Graphite in Clay-Water-Based Drilling Fluids. Materials 2022, 15, 1083. [Google Scholar] [CrossRef]
- Mu’azu, N.D.; Usman, A.; Jarrah, N.; Alagha, O. Pulsed Electrokinetic Removal of Chromium, Mercury and Cadmium from Contaminated Mixed Clay Soils. Soil Sediment Contam. Int. J. 2016, 25, 757–775. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Z.; Li, X.; Xu, J. Remediation of lead (II)-contaminated soil using electrokinetics assisted by permeable reactive barrier with different filling materials. J. Hazard. Mater. 2021, 408, 124885. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuang, Y.-F.; Xiao, F.; Liu, Z. Mechanism for reverse electroosmotic flow and its impact on electrokinetic remediation of lead-contaminated kaolin. Acta Geotech. 2023, 18, 124885. [Google Scholar] [CrossRef]
- Beyrami, H. Effect of different treatments on electrokinetic remediation of Zn, Pb and Cd from a contaminated calcareous soil. Chin. J. Chem. Eng. 2021, 38, 255–265. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Xu, L.-H.; Zhuang, Y.-F. Effect of electrolyte, potential gradient and treatment time on remediation of hexavalent chromium contaminated soil by electrokinetic remediation and adsorption. Environ. Earth Sci. 2023, 82, 40. [Google Scholar] [CrossRef]
- Xie, N.; Chen, Z.; Wang, H.; You, C. Activated carbon coupled with citric acid in enhancing the remediation of Pb-Contaminated soil by electrokinetic method. J. Clean. Prod. 2021, 308, 127433. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, H.-P.; Zhu, L. Remediation of soil contaminated with organic compounds by nanoscale zero-valent iron: A review. Sci. Total Environ. 2021, 760, 143413. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M.J.; Moayedi, H.; Sadeghi, M.M.; Hajiannia, A. A review of combinations of electrokinetic applications. Environ. Geochem. Health 2016, 38, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D.J.; Madden, T.R. Induced polarization, a study of its causes. Geophysics 1959, 24, 790–816. [Google Scholar] [CrossRef]
- Cameselle, C.; Reddy, K.R. Development and enhancement of electro-osmotic flow for the removal of contaminants from soils(Conference Paper). Electrochim. Acta 2012, 86, 10–22. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.E.; Neretnieks, I. Removal of CU(II) and CR(III) from naturally contaminated loam by electromigration. J. Environ. Sci. Health-Toxic/Hazard. Subst. Environ. Eng. 1997, 32, 1293–1308. [Google Scholar] [CrossRef]
- Qiao, R.; Aluru, N.R. Charge Inversion and Flow Reversal in a Nanochannel Electro-osmotic Flow. Phys. Rev. Lett. 2004, 92, 198301. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total. Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; He, X.; Liu, Y. Optimization analysis and mechanism exploration on the removal of cadmium from contaminated soil by electrokinetic remediation. Sep. Purif. Technol. 2020, 250, 117180. [Google Scholar] [CrossRef]
- Gao, J.; Luo, Q.; Zhang, C.; Li, B.; Meng, L. Enhanced electrokinetic removal of cadmium from sludge using a coupled catholyte circulation system with multilayer of anion exchange resin. Chem. Eng. J. 2013, 234, 1–8. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Zhang, J.; Liu, J. Study on Electrokinetic Remediation of Pb-contaminated Saturated Sand. Int. J. Electrochem. Sci. 2020, 15, 1486–1497. [Google Scholar] [CrossRef]
- Zhou, D.-M.; Deng, C.-F.; Cang, L. Electrokinetic remediation of a Cu contaminated red soil by conditioning catholyte pH with different enhancing chemical reagents. Chemosphere 2004, 56, 265–273. [Google Scholar] [CrossRef]
- Xiao, J.; Pang, Z.; Zhou, S.; Chu, L.; Rong, L.; Liu, Y.; Li, J.; Tian, L. The mechanism of acid-washed zero-valent iron/activated carbon as permeable reactive barrier enhanced electrokinetic remediation of uranium-contaminated soil. Sep. Purif. Technol. 2020, 244, 116667. [Google Scholar] [CrossRef]
- Suzuki, T.; Kawai, K.; Moribe, M.; Niinae, M. Recovery of Cr as Cr(III) from Cr(VI)-contaminated kaolinite clay by electrokineics coupled with a permeable reactive barrier. J. Hazard. Mater. 2014, 278, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Guo, S.; Li, S.; Zhang, L.; Wang, S. Comparison of approaching and fixed anodes for avoiding the ‘focusing’ effect during electrokinetic remediation of chromium-contaminated soil. Chem. Eng. J. 2012, 203, 231–238. [Google Scholar] [CrossRef]
- Rojo, A.; Hansen, H.K.; Monárdez, O. Electrokinetic remediation of mine tailings by applying a pulsed variable electric field. Miner. Eng. 2014, 55, 52–56. [Google Scholar] [CrossRef]
- Mena, E.; Villaseñor, J.; Rodrigo, M.A.; Cañizares, P. Electrokinetic remediation of soil polluted with insoluble organics using biological permeable reactive barriers: Effect of periodic polarity reversal and voltage gradient. Chem. Eng. J. 2016, 299, 30–36. [Google Scholar] [CrossRef]
- Rezaee, M.; Asadollahfardi, G.; Gomez-Lahoz, C.; Villen-Guzman, M.; Paz-Garcia, J.M. Modeling of electrokinetic remediation of Cd- and Pb-contaminated kaolinite. J. Hazard. Mater. 2018, 366, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; He, G.; Shi, W.; Zou, M.; Liu, C. The polarization characteristics of lithium-ion batteries under cyclic charge and discharge. J. Solid State Electrochem. 2019, 23, 1887–1902. [Google Scholar] [CrossRef]
- Shen, Z.; Chen, X.; Jia, J.; Qu, L.; Wang, W. Comparison of electrokinetic soil remediation methods using one fixed anode and approaching anodes. Environ. Pollut. 2007, 150, 193–199. [Google Scholar] [CrossRef]
- Xu, J.; Liu, C.; Hsu, P.-C.; Zhao, J.; Wu, T.; Tang, J.; Liu, K.; Cui, Y. Remediation of heavy metal contaminated soil by asymmetrical alternating current electrochemistry. Nat. Commun. 2019, 10, 2440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Jin, C.; Sun, Z.; Huang, G.; She, Z. Assessment of Acid Enhancement Schemes for Electrokinetic Remediation of Cd/Pb Contaminated Soil. Water Air Soil Poll. 2016, 227, 217. [Google Scholar] [CrossRef]
- Kim, S.; Moon, S.-H.; Kim, K.-W. Removal of Heavy Metals from Soils using Enhanced Electrokinetic Soil Processing. Water Air Soil Poll. 2001, 125, 259–272. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Q.; Jiang, S.; Du, Z.; Zhang, X.; Chen, H.; Cao, W.; Nghiem, L.-D.; Ngo, H.-H. Enhancement of lead removal from soil by in-situ release of dissolved organic matters from biochar in electrokinetic remediation. J. Clean. Prod. 2022, 361, 132294. [Google Scholar] [CrossRef]


| The Properties of Kaolin | Value |
|---|---|
| pH | 5.77 ± 0.21 |
| EC (μs/cm) | 86.45 ± 25.74 |
| SiO2 (%) | 54.42 ± 0.11 |
| Al2O3 (%) | 42.68 ± 0.09 |
| TiO2 (%) | 1.77 ± 0.01 |
| Fe2O3 (%) | 0.58 ± 0.009 |
| CaO (%) | 0.23 ± 0.007 |
| K2O (%) | 0.13 ± 0.005 |
| P2O5 (%) | 0.08 ± 0.002 |
| MgO (%) | 0.06 ± 0.001 |
| SrO (%) | 0.03 ± 0.001 |
| Cr2O3 (%) | 0.02 ± 0.001 |
| The Properties of Biochar | Value |
|---|---|
| Organic carbon content (%) | 42.21 ± 0.21 |
| Total nitrogen content (%) | 8.34 ± 0.05 |
| Total phosphorus content (%) | 2.31 ± 0.01 |
| Total potassium content (%) | 16.12 ± 0.09 |
| Ash content (%) | 7.23 ± 0.03 |
| Others (%) | 23.79 ± 0.11 |
| pH | 9.46 ± 0.05 |
| No. | PRB | Power Type | Power On/Off Interval Periods | Voltage Gradient (V/cm) | Treatment Time (h) |
|---|---|---|---|---|---|
| DC0-S | no PRB | DC power | Constant voltage | 4 | 72 |
| DC0 | no PRB | DC power | Constant voltage | 4 | 204 |
| DCb | biochar | DC power | Constant voltage | 4 | 204 |
| DC2b | biochar | DC power | Constant voltage | 2 | 204 |
| PCb-3 s | biochar | PC power | 3 s/3 s | 4 | 204 |
| PCb-1 m | biochar | PC power | 1 min /1 min | 4 | 204 |
| PCb-30 m | biochar | PC power | 30 min/30 min | 4 | 204 |
| PCb-6 h | biochar | PC power | 6 h/6 h | 4 | 204 |
| PC2b-6 h | biochar | PC power | 6 h/6 h | 2 | 204 |
| Exp. | S1 (%) | S2 (%) | S3 (%) | S4 (%) | S5 (%) | Average Removal (%) | Energy Consumption (kWh/m3) |
|---|---|---|---|---|---|---|---|
| DC0-S | 91.5 | 90.2 | 88.9 | 87.9 | −3.4 | 71 | 99.6 |
| DC0 | 97 | 90.9 | 88.6 | 86.9 | 76.6 | 88 | 213.9 |
| DCb | 97.6 | 97.4 | 96.5 | 94.6 | 84.4 | 94.1 | 563.2 |
| DC2b | 98.1 | 89.9 | 87.8 | 82.8 | 59.2 | 83.6 | 133.5 |
| PCb-3 s | 97.6 | 90.6 | 90.4 | 88.8 | 80.2 | 89.5 | 177.6 |
| PCb-1 m | 97.4 | 91.6 | 90.1 | 88.8 | 86.9 | 91 | 197.9 |
| PCb-30 m | 98.4 | 94.9 | 91.3 | 91.1 | 88.9 | 92.9 | 271.6 |
| PCb-6 h | 97.9 | 91.7 | 91.3 | 91.4 | 87.5 | 91.9 | 296 |
| PC2b-6 h | 97.4 | 90.2 | 88.3 | 86.4 | 51.1 | 82.7 | 68.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zang, L.; Zhao, Y.; Wei, Q.; Han, J. Removal of Pb from Contaminated Kaolin by Pulsed Electrochemical Treatment Coupled with a Permeable Reactive Barrier: Tuning Removal Efficiency and Energy Consumption. Toxics 2023, 11, 961. https://doi.org/10.3390/toxics11120961
Zhang Y, Zang L, Zhao Y, Wei Q, Han J. Removal of Pb from Contaminated Kaolin by Pulsed Electrochemical Treatment Coupled with a Permeable Reactive Barrier: Tuning Removal Efficiency and Energy Consumption. Toxics. 2023; 11(12):961. https://doi.org/10.3390/toxics11120961
Chicago/Turabian StyleZhang, Yinyin, Libin Zang, Yuyan Zhao, Qiaoqiao Wei, and Jiangtao Han. 2023. "Removal of Pb from Contaminated Kaolin by Pulsed Electrochemical Treatment Coupled with a Permeable Reactive Barrier: Tuning Removal Efficiency and Energy Consumption" Toxics 11, no. 12: 961. https://doi.org/10.3390/toxics11120961
APA StyleZhang, Y., Zang, L., Zhao, Y., Wei, Q., & Han, J. (2023). Removal of Pb from Contaminated Kaolin by Pulsed Electrochemical Treatment Coupled with a Permeable Reactive Barrier: Tuning Removal Efficiency and Energy Consumption. Toxics, 11(12), 961. https://doi.org/10.3390/toxics11120961









