Recent Advances in Black Phosphorous-Based Photocatalysts for Degradation of Emerging Contaminants
Abstract
:1. Introduction
2. Preparation of Bulk BP
2.1. High-Temperature and High-Pressure Method
2.2. Mercury Catalysis and Liquid Bismuth Recrystallization Methods
2.3. Ball Milling Method
2.4. CVT Method
3. Preparation of BPNS
3.1. Mechanical Exfoliation Method
3.2. Liquid-Phase Exfoliation Method
3.3. Electrochemical Electrode Stripping Method
3.4. Chemical Vapor Deposition Method
3.5. Pulsed Laser Method
4. Photocatalytic Degradation of ECs Using BP-Based Materials
4.1. Heterojunctions
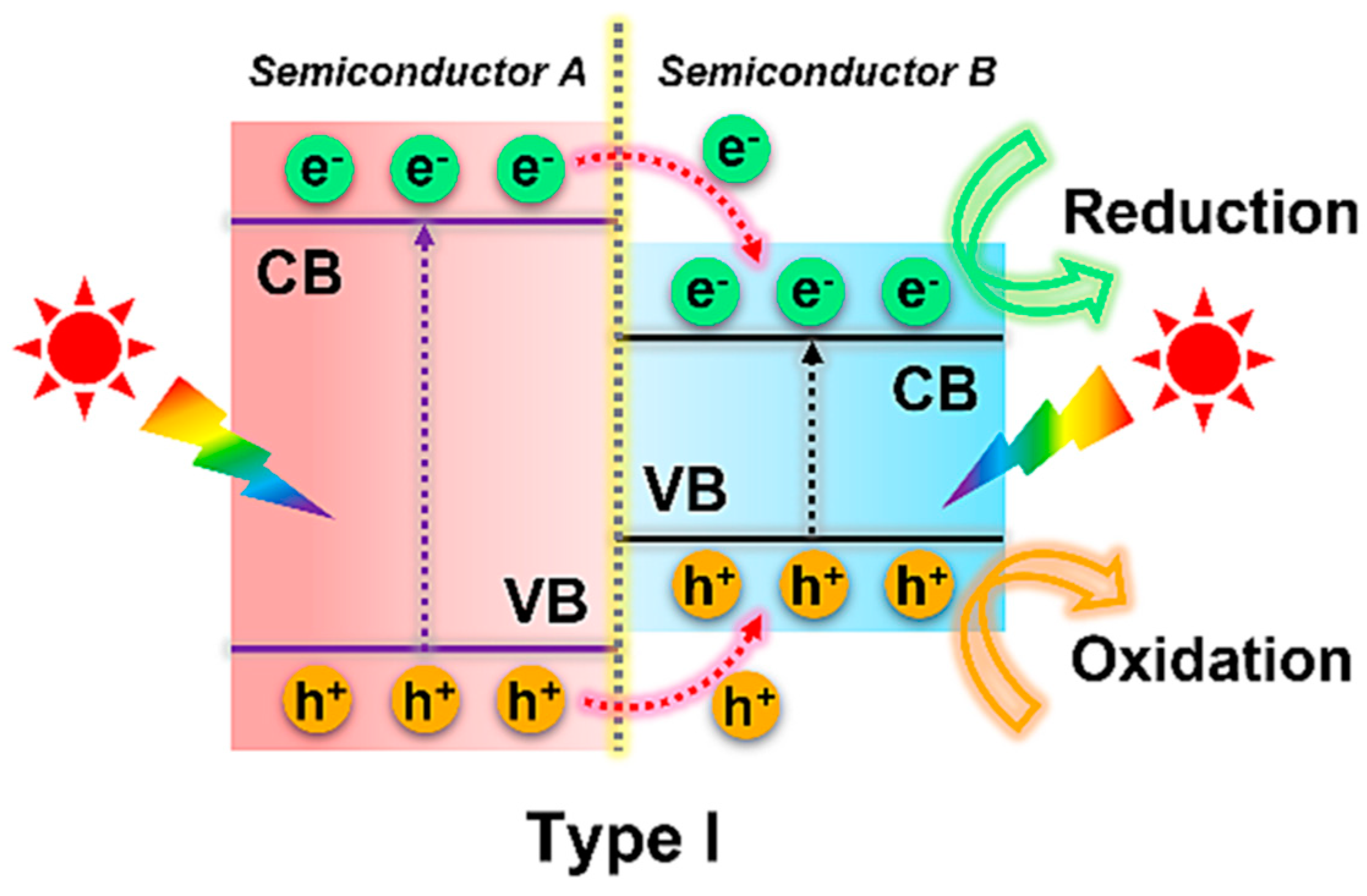
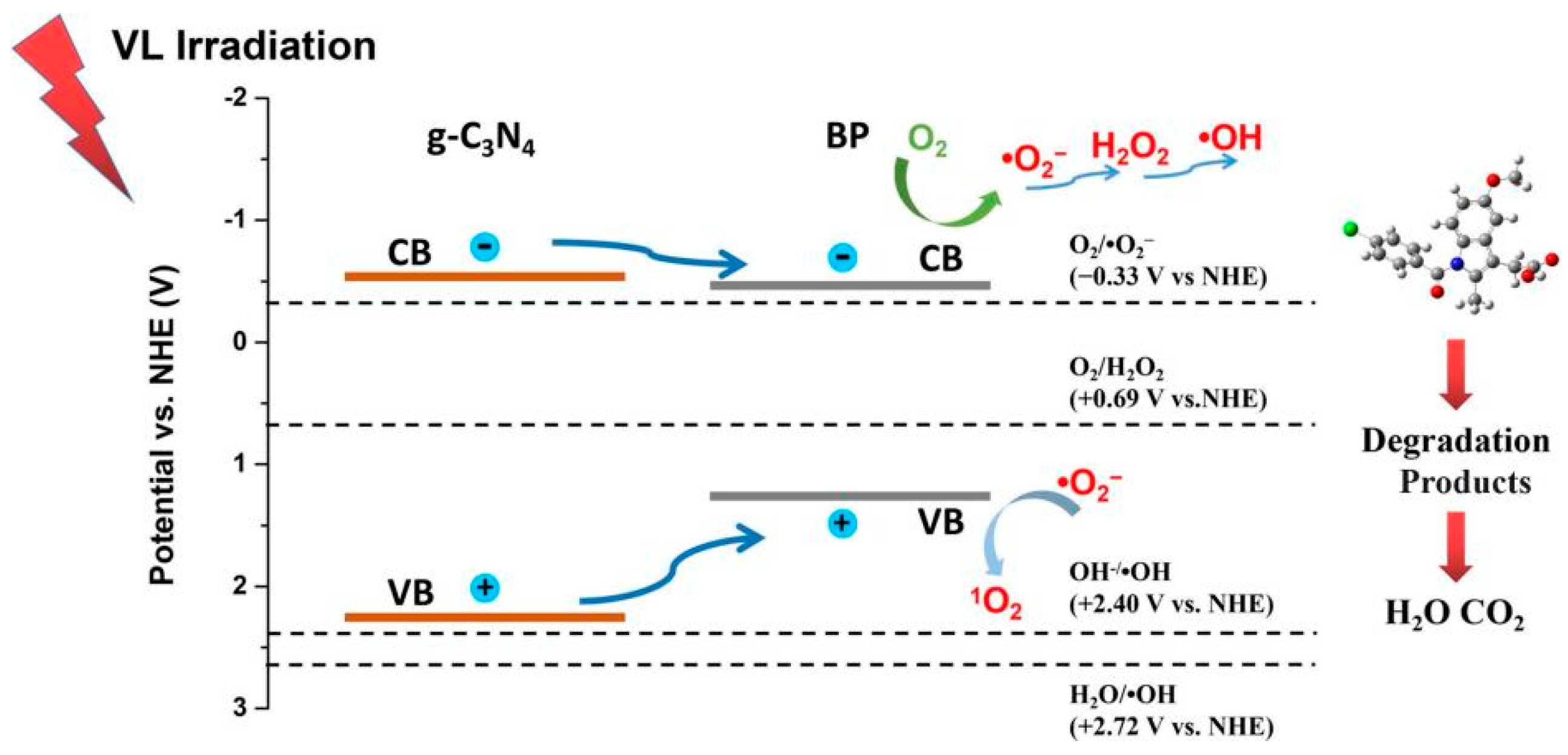
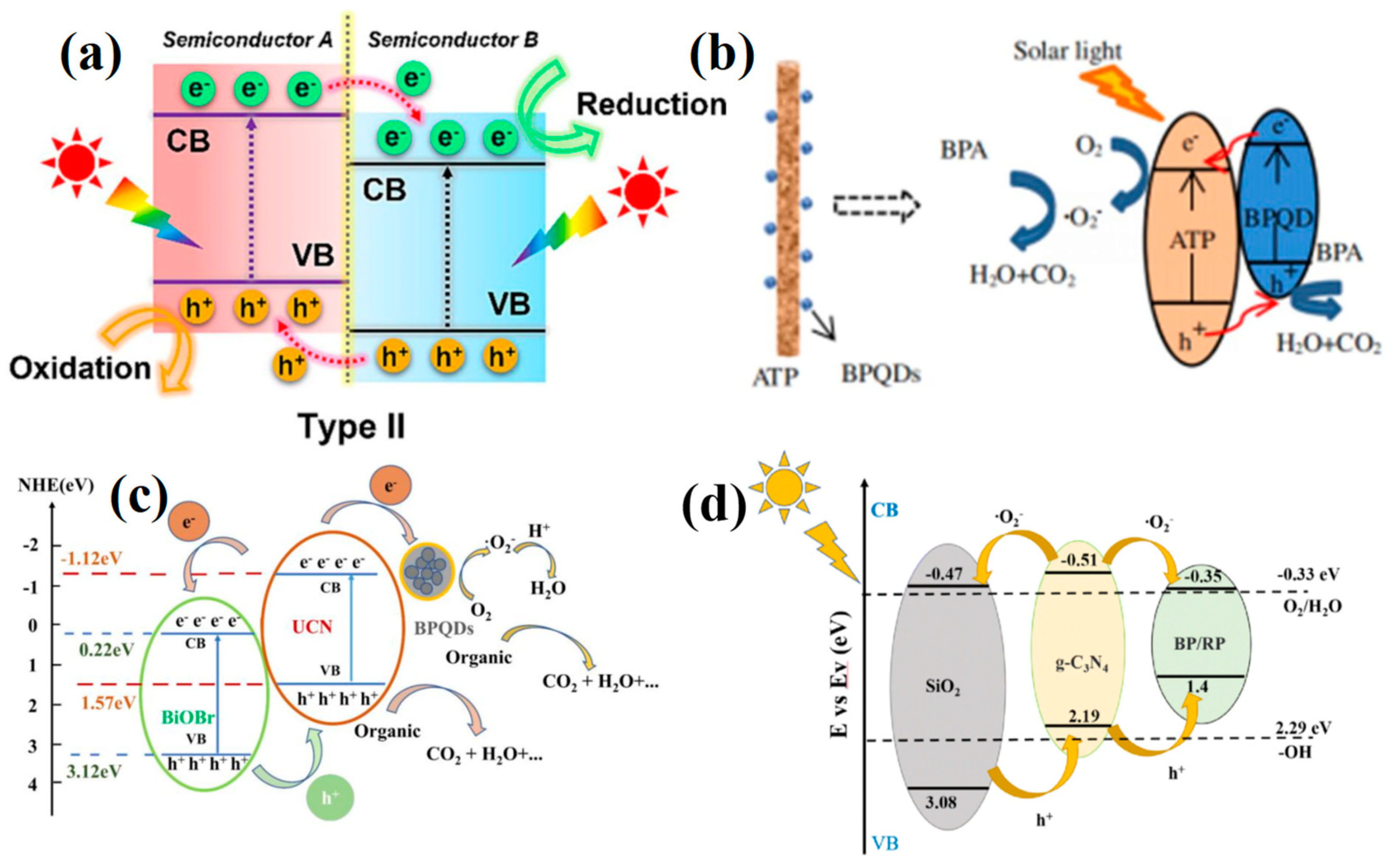
4.1.1. Type I Heterojunction
4.1.2. Type II Heterojunction
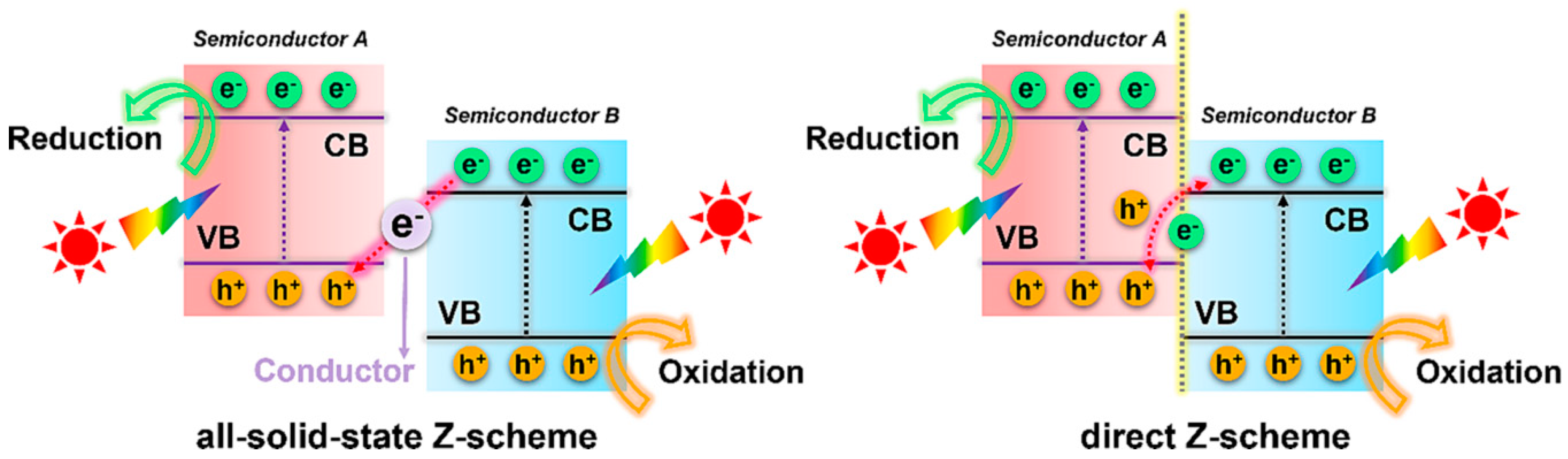
4.1.3. Z-Scheme Heterojunction
4.1.4. S-Scheme Heterojunction
4.2. Hybrids and Doped
5. Conclusions and Perspectives
- (1)
- To date, a diverse array of 2D nanomaterials, including GR, inorganic hexagonal boron nitride (h-BN), transition metal dichalcogenides (TMDs), and MXenes, as well as g-C3N4 and covalent organic frameworks (COFs), have been developed as catalysts for a broad spectrum of applications. Beyond the inherent advantages of 2D materials, BPNS exhibited a narrow band gap in the visible region, layer-dependent optical properties, high carrier mobility, and abundant lone-pairs for metal ion anchoring, rendering it a valuable candidate in catalytic fields. For instance, compared with BP, GR has found applications in various fields, including electrical and optical devices, owing to its exceptional carrier mobility, remarkable thermal conductivity, and optical transparency. Nevertheless, its intrinsic zero bandgap property disqualifies it as a proficient photocatalyst since it cannot be photoexcited to generate charge carriers. Conversely, TMDs, another extensively explored 2D crystal, exhibit tunable bandgap energies but suffer from low charge mobility, thereby limiting their suitability as ideal photocatalysts. Nevertheless, BP confronted challenges of instability in ambient environments due to chemical degradation, constituting the primary impediment to its prospective utilization in electronic devices, photocatalysis, and other scientific domains. Furthermore, the large-scale production of few-layer stable BP imposed additional constraints on its applications. Although the Earth’s crust contains abundant phosphorus, the production cost of stable few-layer BP is heightened due to the more stringent conditions required for its preparation compared to other 2D materials. Therefore, the exploration of large-scale production of stable, scalable, and cost-effective few-layer BP is particularly crucial.
- (2)
- The integration of machine learning stands as a promising avenue for guiding the production of high-activity BP-based catalysts for ECs degradation. Currently, machine learning has emerged as a prominent and efficacious research methodology within the realm of photocatalysis. It enables the targeted prediction and selection of photocatalysts possessing requisite properties from extensive, pre-established databases. These encompass critical parameters such as the catalysts’ band structures, work functions, and interfacial interactions of composites, as well as the energy fluctuations associated with surface redox reactions.
- (3)
- Researchers can extend the application of BP-based materials to a wider range of photocatalytic reactions. While the application of BP-based photocatalysts has primarily focused on water splitting for H2 generation, there has been limited research on their use in the photocatalytic degradation of ECs. Therefore, it is essential to rationally design BP-based photocatalysts and apply them in the field of photocatalytic degradation of ECs. Investigating the corresponding photocatalytic mechanisms is equally imperative. What is more, the mineralization efficiency of BP-based photocatalysts still requires further enhancement. Previous studies have demonstrated a high photocatalytic degradation efficiency for ECs. However, TOC experiments indicated a certain reduction in mineralization efficiency compared to degradation efficiency. Therefore, further research is needed to investigate the mineralization efficiency of BP-based photocatalytic materials for ECs.
- (4)
- To gain deeper insights into the photocatalytic mechanism, it is imperative to employ advanced characterization techniques and essential theoretical calculations. In addition to conventional methods such as electron spin resonance and experiments for capturing active species, a comprehensive understanding of the catalytic reaction processes in BP-based materials can be achieved through various in situ characterizations including XPS, Fourier-transform infrared (FT-IR), and Raman spectroscopy. These techniques can provide detailed insights into the photocatalytic mechanism. Furthermore, femtosecond time-resolved transient absorption spectroscopy and photoirradiated Kelvin probe measurements are invaluable tools for directly examining the transfer processes of photoinduced charge carriers. Additionally, rational density functional theory (DFT) computations, involving the determination of the lowest-energy structure and local density of states (LDOS), enable a theoretical exploration of the enhanced photocatalytic mechanisms exhibited by BP-based photocatalysts at molecular and atomic levels.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jéquier, E.; Constant, F. Water as an essential nutrient: The physiological basis of hydration. Eur. J. Clin. Nutr. 2010, 64, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, S.M. Water: An Essential But Overlooked Nutrient. J. Am. Diet. Assoc. 1999, 99, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Rathi, B.S.; Kumar, P.S.; Show, P.-L. A review on effective removal of emerging contaminants from aquatic systems: Current trends and scope for further research. J. Hazard. Mater. 2021, 409, 124413. [Google Scholar] [CrossRef]
- Li, W.; Shi, Y.; Gao, L.; Liu, J.; Cai, Y. Occurrence, fate and risk assessment of parabens and their chlorinated derivatives in an advanced wastewater treatment plant. J. Hazard. Mater. 2015, 300, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Tchobanoglous, G.; Angelakis, A.N. Technologies for wastewater treatment appropriate for reuse: Potential for applications in Greece. Water Sci. Technol. 1996, 33, 15–24. [Google Scholar] [CrossRef]
- Guo, J.; Tu, K.; Chou, L.; Zhang, Y.; Wei, S.; Zhang, X.; Yu, H.; Shi, W. Deep mining of reported emerging contaminants in China’s surface water in the past decade: Exposure, ecological effects and risk assessment. Water Res. 2023, 243, 120318. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Luo, J.; Li, C.; Chen, J.; Zhu, N. Antiviral drugs in wastewater are on the rise as emerging contaminants: A comprehensive review of spatiotemporal characteristics, removal technologies and environmental risks. J. Hazard. Mater. 2023, 457, 131694. [Google Scholar] [CrossRef] [PubMed]
- Montes, R.; Méndez, S.; Cobas, J.; Carro, N.; Neuparth, T.; Alves, N.; Santos, M.M.; Quintana, J.B.; Rodil, R. Occurrence of persistent and mobile chemicals and other contaminants of emerging concern in Spanish and Portuguese wastewater treatment plants, transnational river basins and coastal water. Sci. Total Environ. 2023, 885, 163737. [Google Scholar] [CrossRef]
- Lemay, A.C.; Sontarp, E.J.; Martinez, D.; Maruri, P.; Mohammed, R.; Neapole, R.; Wiese, M.; Willemsen, J.A.R.; Bourg, I.C. Molecular Dynamics Simulation Prediction of the Partitioning Constants (KH, Kiw, Kia) of 82 Legacy and Emerging Organic Contaminants at the Water–Air Interface. Environ. Sci. Technol. 2023, 57, 6296–6308. [Google Scholar] [CrossRef]
- Zhou, Y.; He, J.; Li, X.; Lu, J.; Zhou, Y. Efficient removal of roxarsone and emerging organic contaminants by a solar light-driven in-situ Fenton system. Chem. Eng. J. 2021, 435, 132434. [Google Scholar] [CrossRef]
- Zhou, Y.; Ren, X.; Tsui, T.-H.; Barcelo, D.; Wang, Q.; Zhang, Z.; Ding, Y. Microplastics as an underestimated emerging contaminant in solid organic waste and their biological products: Occurrence, fate and ecological risks. J. Hazard. Mater. 2023, 445, 130596. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-W.; Park, M.; Wu, S.; Lopez, I.J.; Ji, W.; Scheideler, J.; Snyder, S.A. Strategies for selecting indicator compounds to assess attenuation of emerging contaminants during UV advanced oxidation processes. Water Res. 2019, 166, 115030. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.; Espana, V.A.A.; Liu, Y.; Jit, J. Emerging contaminants in the environment: Risk-based analysis for better management. Chemosphere 2016, 154, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Dsikowitzky, L.; Schwarzbauer, J. Industrial organic contaminants: Identification, toxicity and fate in the environment. Environ. Chem. Lett. 2014, 12, 371–386. [Google Scholar] [CrossRef]
- Wilkinson, J.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Occurrence, fate and transformation of emerging contaminants in water: An overarching review of the field. Environ. Pollut. 2017, 231, 954–970. [Google Scholar] [CrossRef] [PubMed]
- Ávila, C.; Bayona, J.M.; Martín, I.; Salas, J.J.; García, J. Emerging organic contaminant removal in a full-scale hybrid constructed wetland system for wastewater treatment and reuse. Ecol. Eng. 2015, 80, 108–116. [Google Scholar] [CrossRef]
- Omar, T.; Aris, A.Z.; Yusoff, F.M.; Mustafa, S. Occurrence and level of emerging organic contaminant in fish and mollusk from Klang River estuary, Malaysia and assessment on human health risk. Environ. Pollut. 2019, 248, 763–773. [Google Scholar] [CrossRef]
- Bolong, N.; Ismail, A.F.; Salim, M.R.; Matsuura, T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 2009, 239, 229–246. [Google Scholar] [CrossRef]
- Pereira, L.C.; de Souza, A.O.; Bernardes, M.F.F.; Pazin, M.; Tasso, M.J.; Pereira, P.H.; Dorta, D.J. A perspective on the potential risks of emerging contaminants to human and environmental health. Environ. Sci. Pollut. Res. 2015, 22, 13800–13823. [Google Scholar] [CrossRef]
- Martínez, C.; Canle, M.; Fernández, M.; Santaballa, J.; Faria, J. Aqueous degradation of diclofenac by heterogeneous photocatalysis using nanostructured materials. Appl. Catal. B Environ. 2011, 107, 110–118. [Google Scholar] [CrossRef]
- Valencia, S.; Cataño, F.; Rios, L.; Restrepo, G.; Marín, J. A new kinetic model for heterogeneous photocatalysis with titanium dioxide: Case of non-specific adsorption considering back reaction. Appl. Catal. B Environ. 2011, 104, 300–304. [Google Scholar] [CrossRef]
- Bretos, I.; Jiménez, R.; Pérez-Mezcua, D.; Salazar, N.; Ricote, J.; Calzada, M.L. Low-Temperature Liquid Precursors of Crystalline Metal Oxides Assisted by Heterogeneous Photocatalysis. Adv. Mater. 2015, 27, 2608–2613. [Google Scholar] [CrossRef] [PubMed]
- Júnior, O.G.; Neto, W.B.; Machado, A.E.; Daniel, D.; Trovó, A.G. Optimization of fipronil degradation by heterogeneous photocatalysis: Identification of transformation products and toxicity assessment. Water Res. 2017, 110, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Younis, S.A.; Kim, K.-H. Heterogeneous Photocatalysis Scalability for Environmental Remediation: Opportunities and Challenges. Catalysts 2020, 10, 1109. [Google Scholar] [CrossRef]
- Yu, L.; Wang, C.; Chen, F.; Zhang, J.; Ruan, Y.; Xu, J. Investigating the synergistic effects in tourmaline/TiO2-based heterogeneous photocatalysis: Underlying mechanism insights. J. Mol. Catal. A Chem. 2016, 411, 1–8. [Google Scholar] [CrossRef]
- Low, J.; Cao, S.; Yu, J.; Wageh, S. Two-dimensional layered composite photocatalysts. Chem. Commun. 2014, 50, 10768–10777. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Tian, N.; Zhang, Y.; Huang, H. Facet-selective charge separation in two-dimensional bismuth-based photocatalysts. Catal. Sci. Technol. 2021, 11, 3659–3675. [Google Scholar] [CrossRef]
- Sridharan, K.; Shenoy, S.; Kumar, S.G.; Terashima, C.; Fujishima, A.; Pitchaimuthu, S. Advanced Two-Dimensional Heterojunction Photocatalysts of Stoichiometric and Non-Stoichiometric Bismuth Oxyhalides with Graphitic Carbon Nitride for Sustainable Energy and Environmental Applications. Catalysts 2021, 11, 426. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Li, Z.; Smeu, M.; Rives, A.; Maraval, V.; Chauvin, R.; Ratner, M.A.; Borguet, E. Towards graphyne molecular electronics. Nat. Commun. 2015, 6, 6321. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; Chen, S.; Pan, Q.; Yu, Z.; Jia, X.; He, L.; Li, C.; Zhao, Y. Preparation of a Large Amount of Ultrathin Graphdiyne. Chem. Eur. J. 2022, 28, e202200442. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cao, C.-B.; Zhu, H.-S. Graphitic carbon nitride thin films deposited by electrodeposition. Mater. Lett. 2004, 58, 1903–1906. [Google Scholar] [CrossRef]
- Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 2016, 28, 3423–3452. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, A.; Bando, Y.; Golberg, D. Nano boron nitride flatland. Chem. Soc. Rev. 2014, 43, 934–959. [Google Scholar] [CrossRef] [PubMed]
- Bu, F.; Zagho, M.M.; Ibrahim, Y.; Ma, B.; Elzatahry, A.; Zhao, D. Porous MXenes: Synthesis, structures, and applications. Nano Today 2020, 30, 100803. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Ye, G.J.; Ge, Q.; Ou, X.; Wu, H.; Feng, D.; Chen, X.H.; Zhang, Y. Black phosphorus field-effect transistors. Nat. Nanotechnol. 2014, 9, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Baboukani, A.R.; Khakpour, I.; Drozd, V.; Wang, C. Liquid-Based Exfoliation of Black Phosphorus into Phosphorene and Its Application for Energy Storage Devices. Small Struct. 2021, 2, 2000148. [Google Scholar] [CrossRef]
- Lei, W.; Liu, G.; Zhang, J.; Liu, M. Black phosphorus nanostructures: Recent advances in hybridization, doping and functionalization. Chem. Soc. Rev. 2017, 46, 3492–3509. [Google Scholar] [CrossRef]
- Batmunkh, M.; Bat-Erdene, M.; Shapter, J.G. Phosphorene and Phosphorene-Based Materials—Prospects for Future Applications. Adv. Mater. 2016, 28, 8586–8617. [Google Scholar] [CrossRef]
- Wu, S.; Hui, K.S.; Hui, K.N. 2D Black Phosphorus: From Preparation to Applications for Electrochemical Energy Storage. Adv. Sci. 2018, 5, 1700491. [Google Scholar] [CrossRef]
- Eswaraiah, V.; Zeng, Q.; Long, Y.; Liu, Z. Black Phosphorus Nanosheets: Synthesis, Characterization and Applications. Small 2016, 12, 3480–3502. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, M.; Guo, Z.; Miao, L.; Han, S.-T.; Wang, Z.; Zhang, X.; Zhang, H.; Peng, Z. Recent advances in black phosphorus-based photonics, electronics, sensors and energy devices. Mater. Horiz. 2017, 4, 997–1019. [Google Scholar] [CrossRef]
- Xia, F.; Wang, H.; Hwang, J.C.M.; Neto, A.H.C.; Yang, L. Black phosphorus and its isoelectronic materials. Nat. Rev. Phys. 2019, 1, 306–317. [Google Scholar] [CrossRef]
- Feng, W.; Lei, Y.; Wu, X.; Yuan, J.; Chen, J.; Xu, D.; Zhang, X.; Zhang, S.; Liu, P.; Zhang, L.; et al. Tuning the interfacial electronic structure via Au clusters for boosting photocatalytic H2 evolution. J. Mater. Chem. A 2021, 9, 1759–1769. [Google Scholar] [CrossRef]
- Wang, C.; Huang, H.; Weng, B.; Verhaeghe, D.; Keshavarz, M.; Jin, H.; Liu, B.; Xie, H.; Ding, Y.; Gao, Y.; et al. Planar heterojunction boosts solar-driven photocatalytic performance and stability of halide perovskite solar photocatalyst cell. Appl. Catal. B Environ. 2022, 301, 120760. [Google Scholar] [CrossRef]
- Jia, G.; Wang, Z.; Gong, M.; Wang, Y.; Li, L.H.; Dong, Y.; Liu, L.; Zhang, L.; Zhao, J.; Zheng, W.; et al. Ultrathin origami accordion-like structure of vacancy-rich graphitized carbon nitride for enhancing CO2 photoreduction. Carbon Energy 2023, 5, e270. [Google Scholar] [CrossRef]
- Bridgman, P.W. Two New Modifications of Phosphorus. J. Am. Chem. Soc. 1914, 36, 1344–1363. [Google Scholar] [CrossRef]
- Endo, S.; Akahama, Y.; Terada, S.-I.; Narita, S.-I. Growth of Large Single Crystals of Black Phosphorus under High Pressure. Jpn. J. Appl. Phys. 1982, 21, L482. [Google Scholar] [CrossRef]
- Krebs, H.; Weitz, H.P.; Worms, K.H. Über die Struktur und Eigenschaften der Halbmetalle. VIII. Die katalytische Darstellung des schwarzen Phosphors. Z. Anorg. Allg. Chem. 1955, 280, 119–133. [Google Scholar] [CrossRef]
- Maruyama, Y.; Suzuki, S.; Kobayashi, K.; Tanuma, S. Synthesis and some properties of black phosphorus single crystals. Phys. B+C 1981, 105, 99–102. [Google Scholar] [CrossRef]
- Zhang, D.; Yuan, Z.; Zhang, G.; Tian, N.; Liu, D.; Zhang, Y. Preparation and Characterization of Black Phosphorus. Acta Chim. Sin. 2018, 76, 537–542. [Google Scholar] [CrossRef]
- Shin, H.; Zhang, J.; Lu, W. Material structure and chemical bond effect on the electrochemical performance of black phosphorus-graphite composite anodes. Electrochim. Acta 2019, 309, 264–273. [Google Scholar] [CrossRef]
- Zhou, F.; Ouyang, L.; Zeng, M.; Liu, J.; Wang, H.; Shao, H.; Zhu, M. Growth mechanism of black phosphorus synthesized by different ball milling techniques. J. Alloys Compd. 2019, 784, 339–346. [Google Scholar] [CrossRef]
- Lange, S.; Schmidt, P.; Nilges, T. Au3SnP7@Black Phosphorus: An Easy Access to Black Phosphorus. Inorg. Chem. 2007, 46, 4028–4035. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Qian, H.; Niu, X.; Wang, W.; Guan, L.; Sha, J.; Wang, Y. Growth Mechanism and Enhanced Yield of Black Phosphorus Microribbons. Cryst. Growth Des. 2016, 16, 1096–1103. [Google Scholar] [CrossRef]
- Zhao, M.; Niu, X.; Guan, L.; Qian, H.; Wang, W.; Sha, J.; Wang, Y. Understanding the growth of black phosphorus crystals. CrystEngComm 2016, 18, 7737–7744. [Google Scholar] [CrossRef]
- Liu, H.; Neal, A.T.; Zhu, Z.; Luo, Z.; Xu, X.; Tomanek, D.; Ye, P.D. Phosphorene: An Unexplored 2D Semiconductor with a High Hole Mobility. ACS Nano 2014, 8, 4033–4041. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, T.; Sun, Z.; Chen, H.; Guan, J.; Chen, X.; Ji, H.; Du, P.; Yang, S. Black Phosphorus Revisited: A Missing Metal-Free Elemental Photocatalyst for Visible Light Hydrogen Evolution. Adv. Mater. 2017, 29, 1605776. [Google Scholar] [CrossRef]
- Yang, S.; Gong, Y.; Zhang, J.; Zhan, L.; Ma, L.; Fang, Z.; Vajtai, R.; Wang, X.; Ajayan, P.M. Exfoliated Graphitic Carbon Nitride Nanosheets as Efficient Catalysts for Hydrogen Evolution Under Visible Light. Adv. Mater. 2013, 25, 2452–2456. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Georgakilas, V.; Zboril, R.; Steriotis, T.A.; Stubos, A.K. Liquid-Phase Exfoliation of Graphite Towards Solubilized Graphenes. Small 2009, 5, 1841–1845. [Google Scholar] [CrossRef] [PubMed]
- Brent, J.R.; Savjani, N.; Lewis, E.A.; Haigh, S.J.; Lewis, D.J.; O’Brien, P. Production of few-layer phosphorene by liquid exfoliation of black phosphorus. Chem. Commun. 2014, 50, 13338–13341. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, H.; Lu, S.; Wang, Z.; Tang, S.; Shao, J.; Sun, Z.; Xie, H.; Wang, H.; Yu, X.; et al. From Black Phosphorus to Phosphorene: Basic Solvent Exfoliation, Evolution of Raman Scattering, and Applications to Ultrafast Photonics. Adv. Funct. Mater. 2015, 25, 6996–7002. [Google Scholar] [CrossRef]
- Lin, S.; Liu, S.; Yang, Z.; Li, Y.; Ng, T.W.; Xu, Z.; Bao, Q.; Hao, J.; Lee, C.; Surya, C.; et al. Solution-Processable Ultrathin Black Phosphorus as an Effective Electron Transport Layer in Organic Photovoltaics. Adv. Funct. Mater. 2015, 26, 864–871. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Luo, Z.; Tan, H.T.; Li, B.; Sun, S.; Li, Z.; Zong, Y.; Xu, Z.J.; Yang, Y.; et al. An Air-Stable Densely Packed Phosphorene–Graphene Composite Toward Advanced Lithium Storage Properties. Adv. Energy Mater. 2016, 6, 1600453. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Latiff, N.M.; Eng, A.Y.S.; Sofer, Z.; Pumera, M. Black Phosphorus Nanoparticle Labels for Immunoassays via Hydrogen Evolution Reaction Mediation. Anal. Chem. 2016, 88, 10074–10079. [Google Scholar] [CrossRef] [PubMed]
- Baboukani, A.R.; Khakpour, I.; Drozd, V.; Allagui, A.; Wang, C. Single-step exfoliation of black phosphorus and deposition of phosphorene via bipolar electrochemistry for capacitive energy storage application. J. Mater. Chem. A 2019, 7, 25548–25556. [Google Scholar] [CrossRef]
- Xiao, H.; Zhao, M.; Zhang, J.; Ma, X.; Zhang, J.; Hu, T.; Tang, T.; Jia, J.; Wu, H. Electrochemical cathode exfoliation of bulky black phosphorus into few-layer phosphorene nanosheets. Electrochem. Commun. 2018, 89, 10–13. [Google Scholar] [CrossRef]
- Wang, T.; Jin, X.; Yang, J.; Wu, J.; Yu, Q.; Pan, Z.; Shi, X.; Xu, Y.; Wu, H.; Wang, J.; et al. Oxidation-Resistant Black Phosphorus Enable Highly Ambient-Stable Ultrafast Pulse Generation at a 2 μm Tm/Ho-Doped Fiber Laser. ACS Appl. Mater. Interfaces 2019, 11, 36854–36862. [Google Scholar] [CrossRef]
- Yang, Z.; Hao, J.; Yuan, S.; Lin, S.; Yau, H.M.; Dai, J.; Lau, S.P. Field-Effect Transistors Based on Amorphous Black Phosphorus Ultrathin Films by Pulsed Laser Deposition. Adv. Mater. 2015, 27, 3748–3754. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Y.; Lei, Y.; Pan, Y.; Cheng, S.; Ouyang, G.; Yang, X. Redox-Active Moieties in Dissolved Organic Matter Accelerate the Degradation of Nitroimidazoles in SO4•–-Based Oxidation. Environ. Sci. Technol. 2021, 55, 14844–14853. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cheng, F.; He, D.; Zhang, Y.-N.; Qu, J.; Yang, X.; Chen, J.; Peijnenburg, W.J. Effect of UV/chlorine treatment on photophysical and photochemical properties of dissolved organic matter. Water Res. 2021, 192, 116857. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, C.; Liu, S.; Lu, J.; Goh, W.P.; Fang, H.; Qiu, Z.; Tian, B.; Chen, Z.; Yao, C.; et al. Ultrafast Electrochemical Expansion of Black Phosphorus toward High-Yield Synthesis of Few-Layer Phosphorene. Chem. Mater. 2018, 30, 2742–2749. [Google Scholar] [CrossRef]
- Qiu, S.; Zou, B.; Sheng, H.; Guo, W.; Wang, J.; Zhao, Y.; Wang, W.; Yuen, R.K.K.; Kan, Y.; Hu, Y. Electrochemically Exfoliated Functionalized Black Phosphorene and Its Polyurethane Acrylate Nanocomposites: Synthesis and Applications. ACS Appl. Mater. Interfaces 2019, 11, 13652–13664. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Hou, H.; Zhang, Y.; Wang, C.; Qiu, X.; Ji, X. Layer-Tunable Phosphorene Modulated by the Cation Insertion Rate as a Sodium-Storage Anode. Adv. Mater. 2017, 29, 1702372. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-Doped Graphene by Chemical Vapor Deposition and Its Electrical Properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xin, X.; Yan, Q.; Li, Q.; Yang, Y.; Ren, T.-L. Two-step heating synthesis of sub-3 millimeter-sized orthorhombic black phosphorus single crystal by chemical vapor transport reaction method. Sci. China Mater. 2016, 59, 122–134. [Google Scholar] [CrossRef]
- Smith, J.B.; Hagaman, D.; Ji, H.-F. Growth of 2D black phosphorus film from chemical vapor deposition. Nanotechnology 2016, 27, 215602. [Google Scholar] [CrossRef]
- Kumar, S.R.S.; Kurra, N.; Alshareef, H.N. Enhanced high temperature thermoelectric response of sulphuric acid treated conducting polymer thin films. J. Mater. Chem. C 2016, 4, 215–221. [Google Scholar] [CrossRef]
- Li, C.; Wu, Y.; Deng, B.; Xie, Y.; Guo, Q.; Yuan, S.; Chen, X.; Bhuiyan, M.; Wu, Z.; Watanabe, K.; et al. Synthesis of Crystalline Black Phosphorus Thin Film on Sapphire. Adv. Mater. 2018, 30, 1703748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, D.; Lu, J. A review on black-phosphorus-based composite heterojunction photocatalysts for energy and environmental applications. Sep. Purif. Technol. 2023, 307, 122833. [Google Scholar] [CrossRef]
- Li, S.; Wang, P.; Zhao, H.; Wang, R.; Jing, R.; Meng, Z.; Li, W.; Zhang, Z.; Liu, Y.; Zhang, Q.; et al. Fabrication of black phosphorus nanosheets/BiOBr visible light photocatalysts via the co-precipitation method. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125967. [Google Scholar] [CrossRef]
- Zhu, M.; Kim, S.; Mao, L.; Fujitsuka, M.; Zhang, J.; Wang, X.; Majima, T. Metal-Free Photocatalyst for H2 Evolution in Visible to Near-Infrared Region: Black Phosphorus/Graphitic Carbon Nitride. J. Am. Chem. Soc. 2017, 139, 13234–13242. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Jin, D.; Cheng, F.; Zhang, T.; Qu, J.; Zhou, Y.; Yuan, X.; Zhang, Y.-N.; Peijnenburg, W.J. Development of a metal-free black phosphorus/graphitic carbon nitride heterostructure for visible-light-driven degradation of indomethacin. Sci. Total Environ. 2022, 804, 150062. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; He, D.; Lv, Y.; Zhang, K.; Zhang, Z.; Yang, H.; Liu, C.; Qu, J.; Zhang, Y.-N. Preparation of metal-free BP/CN photocatalyst with enhanced ability for photocatalytic tetracycline degradation. Chemosphere 2022, 290, 133317. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, F.; Lu, X.; Zuo, S.; Zhuang, Z.; Yao, C. Black phosphorus quantum dots/attapulgite nanocomposite with enhanced photocatalytic performance. Funct. Mater. Lett. 2017, 10, 1750078. [Google Scholar] [CrossRef]
- Jiang, T.; Shang, C.; Meng, Q.; Jin, M.; Liao, H.; Li, M.; Chen, Z.; Yuan, M.; Wang, X.; Zhou, G. The Ternary Heterostructures of BiOBr/Ultrathin g-C3N4/Black Phosphorous Quantum Dot Composites for Photodegradation of Tetracycline. Polymers 2018, 10, 1118. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Ma, Y.; Li, K.; Mei, Y. In situ formation of red/black phosphorus-modified SiO2@g-C3N4 multi-heterojunction for the enhanced photocatalytic degradation of organic contaminants. RSC Adv. 2023, 13, 13142–13155. [Google Scholar] [CrossRef]
- He, C.; Qian, H.; Li, X.; Yan, X.; Zuo, S.; Qian, J.; Chen, Q.; Yao, C. Visible-light-driven CeO2/black phosphorus heterostructure with enhanced photocatalytic performance. J. Mater. Sci. Mater. Electron. 2019, 30, 593–599. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z. Bismuth-based photocatalytic semiconductors: Introduction, challenges and possible approaches. J. Mol. Catal. A Chem. 2016, 423, 533–549. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Huang, W. Bismuth-based photocatalysts for solar energy conversion. J. Mater. Chem. A 2020, 8, 24307–24352. [Google Scholar] [CrossRef]
- Jing, R.; Yang, J.; Li, S.; Zhao, S.; Wang, P.; Liu, Y.; Liu, A.; Meng, Z.; Huang, H.; Zhang, Z.; et al. Construction of PDDA functionalized black phosphorus nanosheets/BiOI Z-scheme photocatalyst with enhanced visible light photocatalytic activity. J. Colloid Interface Sci. 2020, 576, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Liu, X.; Sui, H.; Sun, H. Enhanced photocatalytic performance of dual Z-scheme BPQDs/g-C3N4/BiFeO3 composites and mechanism insight. Mater. Lett. 2020, 275, 128007. [Google Scholar] [CrossRef]
- Du, F.; Lai, Z.; Tang, H.; Wang, H.; Zhao, C. Construction of dual Z-scheme Bi2WO6/g-C3N4/black phosphorus quantum dots composites for effective bisphenol A degradation. J. Environ. Sci. 2023, 124, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Kuang, P.; Low, J.; Cheng, B.; Yu, J.; Fan, J. MXene-based photocatalysts. J. Mater. Sci. Technol. 2020, 56, 18–44. [Google Scholar] [CrossRef]
- Zhong, Q.; Li, Y.; Zhang, G. Two-dimensional MXene-based and MXene-derived photocatalysts: Recent developments and perspectives. Chem. Eng. J. 2021, 409, 128099. [Google Scholar] [CrossRef]
- Im, J.K.; Sohn, E.J.; Kim, S.; Jang, M.; Son, A.; Zoh, K.-D.; Yoon, Y. Review of MXene-based nanocomposites for photocatalysis. Chemosphere 2021, 270, 129478. [Google Scholar] [CrossRef]
- Tang, R.; Xiong, S.; Gong, D.; Deng, Y.; Wang, Y.; Su, L.; Ding, C.; Yang, L.; Liao, C. Ti3C2 2D MXene: Recent Progress and Perspectives in Photocatalysis. ACS Appl. Mater. Interfaces 2020, 12, 56663–56680. [Google Scholar] [CrossRef]
- You, Z.; Liao, Y.; Li, X.; Fan, J.; Xiang, Q. State-of-the-art recent progress in MXene-based photocatalysts: A comprehensive review. Nanoscale 2021, 13, 9463–9504. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, M.; Liang, H.; Chen, J.; Xu, L.; Niu, J. Novel dual-effective Z-scheme heterojunction with g-C3N4, Ti3C2 MXene and black phosphorus for improving visible light-induced degradation of ciprofloxacin. Appl. Catal. B Environ. 2021, 291, 120105. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Liu, Y.; Alharbi, N.S.; Rabah, S.O.; Wang, S.; Wang, X. Synthesis and fabrication of g-C3N4-based materials and their application in elimination of pollutants. Sci. Total. Environ. 2020, 731, 139054. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Yu, M.; Guo, J.; Zhan, R.; Chen, J.; Li, D.; Zhang, L.; Niu, J. A novel vacancy-strengthened Z-scheme g-C3N4/Bp/MoS2 composite for super-efficient visible-light photocatalytic degradation of ciprofloxacin. Sep. Purif. Technol. 2021, 272, 118891. [Google Scholar] [CrossRef]
- Liu, C.; Sun, S.; Yu, M.; Zhou, Y.; Zhang, X.; Niu, J. Rapid photocatalytic degradation of tetrabromobisphenol A using synergistic p-n/Z-scheme dual heterojunction of black phosphorus nanosheets/FeSe2/g-C3N4. Sep. Purif. Technol. 2023, 311, 123359. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Yu, H.; Yu, J. Emerging S-Scheme Photocatalyst. Adv. Mater. 2022, 34, 2107668. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Gao, X.; Ma, J.; Chen, Z.; Kang, B.; Liu, J.; Li, H.; Feng, Z.; Huang, J. Novel BP/BiOBr S-scheme nano-heterojunction for enhanced visible-light photocatalytic tetracycline removal and oxygen evolution activity. J. Hazard. Mater. 2020, 387, 121690. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Xu, X.; Yu, L.; Yang, T.; Zhang, X.; Zhang, Y.; Zhu, H.; Li, J.; Zhang, J. Zn-P bond induced S-scheme heterojunction for efficient photocatalytic tetracycline degradation synergistic H2 generation. J. Alloys Compd. 2022, 926, 166981. [Google Scholar] [CrossRef]
- Zhang, Z.; He, D.; Liu, H.; Ren, M.; Zhang, Y.; Qu, J.; Lu, N.; Guan, J.; Yuan, X. Synthesis of graphene/black phosphorus hybrid with highly stable P-C bond towards the enhancement of photocatalytic activity. Environ. Pollut. 2019, 245, 950–956. [Google Scholar] [CrossRef]
- Wang, W.; Niu, Q.; Zeng, G.; Zhang, C.; Huang, D.; Shao, B.; Zhou, C.; Yang, Y.; Liu, Y.; Guo, H.; et al. 1D porous tubular g-C3N4 capture black phosphorus quantum dots as 1D/0D metal-free photocatalysts for oxytetracycline hydrochloride degradation and hexavalent chromium reduction. Appl. Catal. B Environ. 2020, 273, 119051. [Google Scholar] [CrossRef]
- Chen, P.; Guo, Z.; Cui, K.; Guo, W.; Li, X.; Chen, Y.; Kuang, K.; Ding, W.; Ding, Y. Photo-induced degradation of norfloxacin by nanosilver modified two-dimensional black phosphorus. Solid State Sci. 2020, 103, 106188. [Google Scholar] [CrossRef]






| Method | Precursor | Experimental Condition | Years | References |
|---|---|---|---|---|
| High-temperature and high-pressure method | WP | 200 °C 1.2 GPa | 1914 | [43] |
| RP | heated to 550 °C, melted at around 900 °C, cooled to 600 °C, 1 GPa | 1982 | [44] | |
| Mercury catalysis and liquid bismuth recrystallization methods | WP | mercury catalyst 360 to 410 °C, 3 d | 1955 | [45] |
| WP | bismuth catalyst 400 °C, 20 h | 1981 | [46] | |
| Ball milling method | RP | 700 r/min, 2 h | 2018 | [47] |
| CVT method | RP | Au, Sn, SnI4 600 °C, 5 to 10 d | 2007 | [50] |
| RP | Sn, SnI4 650 °C, cooled to 480 °C | 2015 | [51] | |
| RP | Sn, I2 600 °C, cooled to 490–120 °C | 2015 | [52] |
| Method | Precursor | Experimental Condition | Years | References |
|---|---|---|---|---|
| Mechanical exfoliation method | BP | SiO2 grid-cutting technology | 2014 | [57] |
| BP | Ball milling technique | 2017 | [58] | |
| Liquid-phase exfoliation method | BP | Ultrasonic treatment and centrifuge with NMP | 2014 | [62] |
| BP | Ultrasonic treatment and centrifuge with NaOH and NMP | 2015 | [63] | |
| BP | Ultrasonic treatment and centrifuge with organic solvent | 2016 | [64,65] | |
| Electrochemical electrode stripping method | BP | Electrochemical electrode stripping With TBA•PF6 | 2018 | [66] |
| BP | Electrochemical electrode stripping With TBA•HSO4 | 2019 | [67] | |
| CVD method | RP | CVD method | 2016 | [77] |
| RP | in situ CVD method | 2016 | [78] | |
| Pulsed laser method | RP | Laser pulse method at 150 °C | 2015 | [70] |
| RP | Laser pulse method at 700 °C and 1.5 GPa | 2018 | [80] |
| Photocatalyst | Photocatalyst Type | Photocatalyst Mass (mg) | ECs | Initial Concentration (mg/L) | Light Source | Removal (%) | Rate Constant (min−1) | References |
|---|---|---|---|---|---|---|---|---|
| BPNS-BiOBr | Type I | 50 | CIP | 10 | 300 W Xenon lamp, >420 nm | 98.2 | 0.0245 | [82] |
| BP-g-C3N4 | Type I | 10 | IDM | 5 | 300 W Xenon lamp, >400 nm | 99.2 | 0.1600 | [84] |
| BP/CN | Type I | 5 | HTC | 5 | 300 W Xenon lamp, >400 nm | 99.2 | - | [85] |
| BPQDs/ATP | Type II | 50 | TPA | 50 | 300 W Xenon lamp, 200–780 nm | 90.0 | - | [86] |
| BiOBr/UCN/BPQDs | Type II | 250 | TC | 30 | 300 W Xenon lamp >420 nm | 92.0 | 0.0410 | [87] |
| BP/RP-g-C3N4/SiO2 | Type II | 5 | OFL | 10 | 350 W Xenon lamp >420 nm | 85.3 | 0.0370 | [88] |
| BP/CeO2 | Z-scheme | 50 | BPA | 50 | 300 W Xenon lamp, 200–780 nm | 82.3 | - | [89] |
| F-BP/BiOI | Z-scheme | 25 | TC | 10 | 300 W Xenon lamp >420 nm | 90.0 | 0.0767 | [92] |
| BPQDs/BiOBr | Z-scheme | - | TC | 20 | 400 W metal halide lamp, >420 nm | 97.5 | 0.4603 | [93] |
| Bi2WO6/g-C3N4/BPQDs | Z-scheme | 40 | BPA | 20 | 300 W Xenon lamp >380 nm | 95.6 | 0.0439 | [94] |
| g-C3N4/Ti3C2 MXene/BP | Z-scheme | 20 | CIP | 20 | 300 W Xenon lamp >420 nm | 99.0 | 0.0480 | [100] |
| g-C3N4/BP/MoS2 | Z-scheme | 20 | CIP | 20 | 300 W Xenon lamp >420 nm | 99.0 | 0.0600 | [102] |
| BPNS/FeSe2/g-C3N4 | Z-scheme | 20 | TBBPA | 10 | 300 W Xenon lamp 380–780 nm | 100.0 | 0.1430 | [103] |
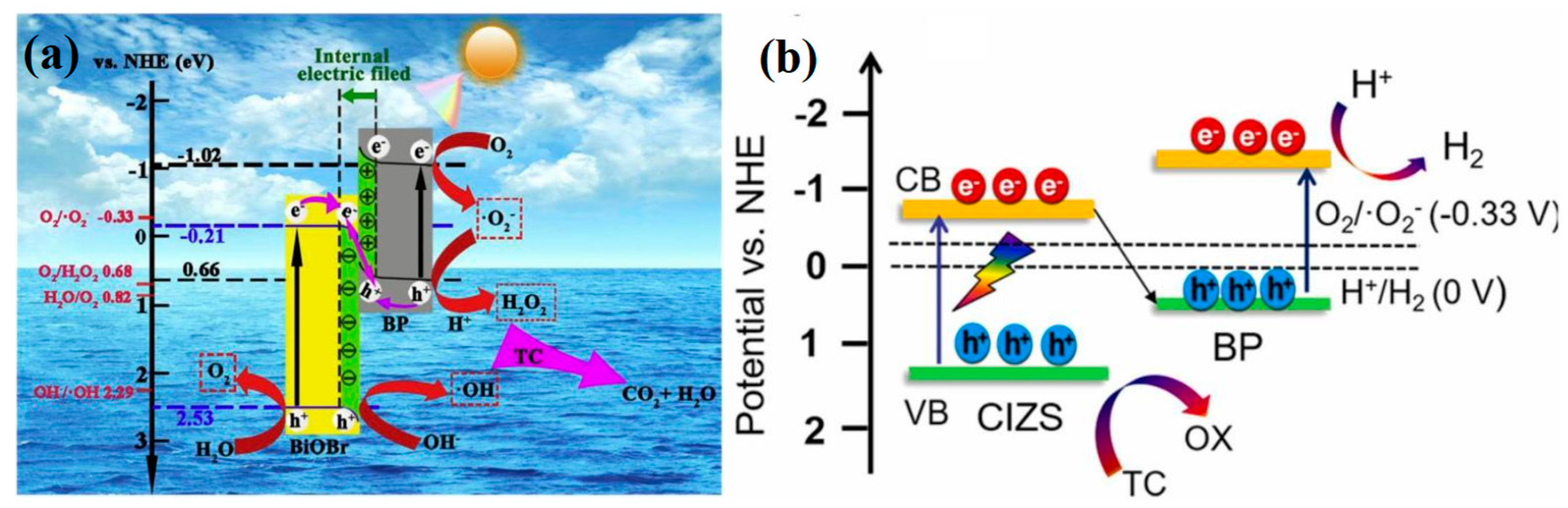
| BP/BiOBr | S-scheme | 100 | TC | 50 | 300 W Xenon lamp 420–780 nm | 85.0 | 0.0210 | [106] |
| BP/CIZS | S-scheme | 35 | TC | 200 | 300 W Xenon lamp 420–780 nm | 82.0 | - | [107] |
| GR-BP | Hybrid | 50 | 2-CP | 10 | 300 W Xenon lamp >420 nm | 87.1 | - | [108] |
| BP-TCN | Hybrid | 30 | OTC-HCl | 10 | 300 W Xenon lamp >420 nm | 81.1 | 0.0276 | [109] |
| AgNPs@BP | Doped | - | NOR | 15 | 300 W Xenon lamp 880 nm | 84.8 | - | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; He, D.; Zhang, K.; Yang, H.; Zhao, S.; Qu, J. Recent Advances in Black Phosphorous-Based Photocatalysts for Degradation of Emerging Contaminants. Toxics 2023, 11, 982. https://doi.org/10.3390/toxics11120982
Zhang Z, He D, Zhang K, Yang H, Zhao S, Qu J. Recent Advances in Black Phosphorous-Based Photocatalysts for Degradation of Emerging Contaminants. Toxics. 2023; 11(12):982. https://doi.org/10.3390/toxics11120982
Chicago/Turabian StyleZhang, Zhaocheng, Dongyang He, Kangning Zhang, Hao Yang, Siyu Zhao, and Jiao Qu. 2023. "Recent Advances in Black Phosphorous-Based Photocatalysts for Degradation of Emerging Contaminants" Toxics 11, no. 12: 982. https://doi.org/10.3390/toxics11120982
APA StyleZhang, Z., He, D., Zhang, K., Yang, H., Zhao, S., & Qu, J. (2023). Recent Advances in Black Phosphorous-Based Photocatalysts for Degradation of Emerging Contaminants. Toxics, 11(12), 982. https://doi.org/10.3390/toxics11120982








