Screening of Toxic Effects of Neonicotinoid Insecticides with a Focus on Acetamiprid: A Review
Abstract
:1. Introduction
1.1. Classification of Neonicotinoids
1.2. Mode of Action
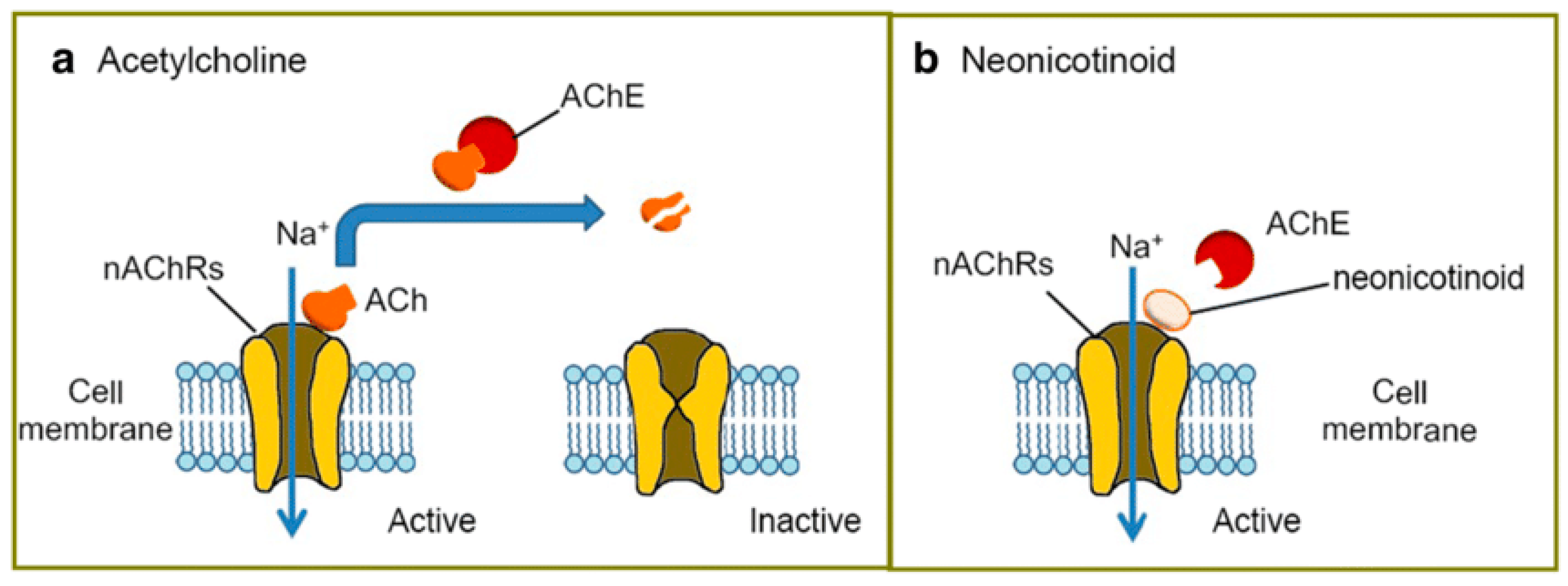
1.3. Toxicity to Non-Target Organisms
1.3.1. Neurotoxicity
1.3.2. Organotoxicity
1.3.3. Other Toxicity Impacts
2. Characterization of Acetamiprid
2.1. Acetamiprid Toxicity
2.1.1. Oxidative Stress
2.1.2. Apoptosis and Genotoxicity
2.1.3. Reproductive Toxicity
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borpatragohain, B.; Sahoo, S.; Rai, A. An Overview on the Impact, Interaction and Fate of Xenobiotic-Soil Organic Matter Complexes. Int. J. Chem. Stud. 2019, 7, 4935–4941. [Google Scholar]
- Aggrawal, A. Agrochemical Poisoning. Forensic. Pathol. Rev. 2006, 4, 261–327. [Google Scholar] [CrossRef]
- Garnett, T.; Wilkes, A. Appetite for Change: Social, Economic and Environmental Transformations in China’s Food System. Food Climate Research Network 2014. Available online: http://www.ccjc-beijing.com/manage/images/2014619213033.pdf (accessed on 5 June 2023).
- Vandenberg, L.N.; Najmi, A.; Mogus, J.P. Agrochemicals with Estrogenic Endocrine Disrupting Properties: Lessons Learned? Mol. Cell. Endocrinol. 2020, 518, 110860. [Google Scholar] [CrossRef] [PubMed]
- FICCI. Safe & Judicious Use of Agrochemicals and Applications of Green Chemistry. In FICCI Knowledge Paper; FICCI: New Delhi, India, 2014; Available online: https://ficci.in/spdocument/20377/agro-knowledge-paper.pdf (accessed on 14 March 2023).
- Gupta, S.; Khanna, R. Agrochemicals as a Potential Cause of Ground Water Pollution: A Review. Int. J. Chem. Stud. 2018, 6, 985–990. [Google Scholar]
- Sharma, A.; Jha, P.; Reddy, G.V.P. Multidimensional Relationships of Herbicides with Insect-Crop Food Webs. Sci. Total Environ. 2018, 643, 1522–1532. [Google Scholar] [CrossRef]
- Wołejko, E.; Jabłońska-Trypuć, A.; Wydro, U.; Butarewicz, A.; Łozowicka, B. Soil Biological Activity as an Indicator of Soil Pollution with Pesticides—A Review. Appl. Soil Ecol. 2020, 147, 103356. [Google Scholar] [CrossRef]
- Salem, H.M.; Abdel-Salam, A.; Abdel-Salam, M.A.; Seleiman, M.F. Soil Xenobiotics and Their Phyto-Chemical Remediation. In Xenobiotics in the Soil Environment: Monitoring, Toxicity and Management; Hashmi, M.Z., Kumar, V., Varma, A., Eds.; Soil Biology; Springer International Publishing: Cham, Switzerland, 2017; pp. 267–280. [Google Scholar] [CrossRef]
- Warra, A.A.; Prasad, M.N.V. Chapter 16—African Perspective of Chemical Usage in Agriculture and Horticulture—Their Impact on Human Health and Environment. In Agrochemicals Detection, Treatment and Remediation; Prasad, M.N.V., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 401–436. [Google Scholar] [CrossRef]
- FAO. Pesticides Use, Pesticides Trade and Pesticides Indicators—Global, Regional and Country Trends, 1990–2020; FAOSTAT Analytical Briefs no. 46; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide Pesticide Usage and Its Impacts on Ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef] [Green Version]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The Global Burden of Pathogens and Pests on Major Food Crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/RP (accessed on 24 March 2023).
- Fenibo, E.O.; Ijoma, G.N.; Matambo, T. Biopesticides in Sustainable Agriculture: A Critical Sustainable Development Driver Governed by Green Chemistry Principles. Front. Sustain. Food Syst. 2021, 5, 619058. [Google Scholar] [CrossRef]
- Thompson, D.A.; Lehmler, H.-J.; Kolpin, D.W.; Hladik, M.L.; Vargo, J.D.; Schilling, K.E.; LeFevre, G.H.; Peeples, T.L.; Poch, M.C.; LaDuca, L.E.; et al. A Critical Review on the Potential Impacts of Neonicotinoid Insecticide Use: Current Knowledge of Environmental Fate, Toxicity, and Implications for Human Health. Environ. Sci. Process. Impacts. 2020, 22, 1315–1346. [Google Scholar] [CrossRef]
- Sgolastra, F.; Medrzycki, P.; Bortolotti, L.; Maini, S.; Porrini, C.; Simon-Delso, N.; Bosch, J. Bees and pesticide regulation: Lessons from the neonicotinoid experience. Biol. Conserv. 2020, 241, 108356. [Google Scholar] [CrossRef]
- Berheim, E.H.; Jenks, J.A.; Lundgren, J.G.; Michel, E.S.; Grove, D.; Jensen, W.F. Effects of neonicotinoid insecticides on physiology and reproductive characteristics of captive female and fawn white-tailed deer. Sci. Rep. 2019, 9, 4534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundoo, A.A.; Dar, S.A.; Mushtaq, M.; Dar, M.S.; Gul, S.; Ali, M.T.; Gulzar, S. Role of Neonicotinoids in Insect Pest Management: A Review. J. Entomol. Zool. 2018, 6, 333–339. [Google Scholar]
- Cimino, A.M.; Boyles, A.L.; Thayer, K.A.; Perry, M.J. Effects of Neonicotinoid Pesticide Exposure on Human Health: A Systematic Review. Environ. Health Perspect. 2017, 125, 155–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bass, C.; Field, L.M. Neonicotinoids. Curr. Biol. 2018, 28, 772–773. [Google Scholar] [CrossRef] [Green Version]
- Costas-Ferreira, C.; Faro, L.R.F. Neurotoxic Effects of Neonicotinoids on Mammals: What Is There beyond the Activation of Nicotinic Acetylcholine Receptors? —A Systematic Review. Int. J. Mol. Sci. 2021, 22, 8413. [Google Scholar] [CrossRef]
- Katić, A.; Kašuba, V.; Kopjar, N.; Lovaković, B.T.; Marjanović Čermak, A.M.; Mendaš, G.; Micek, V.; Milić, M.; Pavičić, I.; Pizent, A.; et al. Effects of low-level imidacloprid oral exposure on cholinesterase activity, oxidative stress responses, and primary DNA damage in the blood and brain of male Wistar rats. Chem. Biol. Interact. 2021, 338, 109287. [Google Scholar] [CrossRef]
- Ellis, C.; Park, K.J.; Whitehorn, P.; David, A.; Goulson, D. The neonicotinoid insecticide thiacloprid impacts upon bumblebee colony development under field conditions. Environ. Sci. Technol. 2017, 51, 1727–1732. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, W.; Pang, S.; Huang, Y.; Mishra, S.; Bhatt, P.; Chen, S. Current approaches to and future perspectives on methomyl degradation in contaminated soil/water environments. Molecules 2020, 25, 738. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Tian, Y.; Shen, X. Human Exposure to Neonicotinoid Insecticides and the Evaluation of Their Potential Toxicity: An Overview. Chemosphere 2018, 192, 59–65. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, S. Human exposure to neonicotinoids and the associated health risks: A review. Environ. Int. 2022, 163, 107201. [Google Scholar] [CrossRef] [PubMed]
- Saka, M.; Tada, N. Acute and Chronic Toxicity Tests of Systemic Insecticides, Four Neonicotinoids and Fipronil, Using the Tadpoles of the Western Clawed Frog Silurana Tropicalis. Chemosphere 2021, 270, 129418. [Google Scholar] [CrossRef] [PubMed]
- Tasman, K.; Rands, S.A.; Hodge, J.J.L. The power of Drosophila melanogaster for modeling neonicotinoid effects on pollinators and identifying novel mechanisms. Front. Physiol. 2021, 12, 659440. [Google Scholar] [CrossRef] [PubMed]
- Bommuraj, V.; Chen, Y.; Birenboim, M.; Barel, S.; Shimshoni, J.A. Concentration- and time-dependent toxicity of commonly encountered pesticides and pesticide mixtures to honeybees (Apis mellifera L.). Chemosphere 2021, 266, 128974. [Google Scholar] [CrossRef]
- Mahmoudi-Dehpahni, B.; Alizadeh, M.; Pourian, H.-R. Exposure Route Affects the Toxicity Class of Thiamethoxam for the Predatory Bug, Orius Albidipennis (Hemiptera: Anthocoridae) by Changing Its Fitness. J. Econ. Entomol. 2021, 114, 684–693. [Google Scholar] [CrossRef]
- Xu, L.; Xu, X.; Guo, L.; Wang, Z.; Wu, X.; Wu, X.; Kuang, H.; Xu, C. Potential environmental health risk analysis of neonicotinoids and a synergist. Environ. Sci. Technol. 2021, 55, 7541–7550. [Google Scholar] [CrossRef]
- Liu, Y.; He, Q.-K.; Xu, Z.-R.; Xu, C.-L.; Zhao, S.-C.; Luo, Y.-S.; Sun, X.; Qi, Z.-Q.; Wang, H.-L. Thiamethoxam exposure induces endoplasmic reticulum stress and affects ovarian function and oocyte development in mice. J. Agric. Food Chem. 2021, 69, 1942–1952. [Google Scholar] [CrossRef]
- Watanabe, E. Review of Sample Preparation Methods for Chromatographic Analysis of Neonicotinoids in Agricultural and Environmental Matrices: From Classical to State-of-the-Art Methods. J. Chromatogr. A 2021, 1643, 462042. [Google Scholar] [CrossRef]
- Hayes, W.J. Pesticides Studied in Man; Williams & Wilkins: Baltimore, MA, USA, 1982; 672p. [Google Scholar]
- Yang, L.; Zhao, Y.-L.; Zhao, C.-Y.; Li, H.-H.; Wang, M.-J.; Morris-Natschke, S.L.; Qian, K.; Lee, K.-H.; Liu, Y.-Q. Design, Synthesis, Crystal Structure, Bioactivity, and Molecular Docking Studies of Novel Sulfonylamidine-Derived Neonicotinoid Analogs. Med. Chem. Res. 2014, 23, 5043–5057. [Google Scholar] [CrossRef]
- Borsuah, J.F.; Messer, T.L.; Snow, D.D.; Comfort, S.D.; Mittelstet, A.R. Literature Review: Global Neonicotinoid Insecticide Occurrence in Aquatic Environments. Water 2020, 12, 3388. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, Y.; Wang, Y.; Lu, Q.; Ruan, H.; Luo, J.; Yang, M. A Comprehensive Review on the Pretreatment and Detection Methods of Neonicotinoid Insecticides in Food and Environmental Samples. Food Chem. X 2022, 15, 100375. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, T.; Motoyama, N.; Ambrose, J.T.; Roe, M.R. Mechanism for the differential toxicity of neonicotinoid insecticides in the honeybee, Apis mellifera. Crop Prot. 2004, 23, 371–378. [Google Scholar] [CrossRef]
- Pisa, L.; Goulson, D.; Yang, E.-C.; Gibbons, D.; Sánchez-Bayo, F.; Mitchell, E.; Aebi, A.; van der Sluijs, J.; MacQuarrie, C.J.K.; Giorio, C.; et al. An Update of the Worldwide Integrated Assessment (WIA) on Systemic Insecticides. Part 2: Impacts on Organisms and Ecosystems. Environ. Sci. Pollut. Res. Int. 2021, 28, 11749–11797. [Google Scholar] [CrossRef] [Green Version]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic Insecticides (Neonicotinoids and Fipronil): Trends, Uses, Mode of Action and Metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef]
- Zapol’skii, V.; Fischer, R.; Namyslo, J.; Kaufmann, D. Chemistry of Polyhalogenated Nitrobutadienes, 8: Nitropolychlorobutadienes-Precursors for Insecticidal Neonicotinoids. Bioorg. Med. Chem. 2009, 17, 4206–4215. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Selective Toxicity of Neonicotinoids Attributable to Specificity of Insect and Mammalian Nicotinic Receptors. Annu. Rev. Entomol. 2003, 48, 339–364. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Neonicotinoid Insecticide Toxicology: Mechanisms of Selective Action. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Jeschke, P.; Nauen, R.; Beck, M.E. Nicotinic Acetylcholine Receptor Agonists: A Milestone for Modern Crop Protection. Angew. Chem. Int. Ed. 2013, 52, 9464–9485. [Google Scholar] [CrossRef]
- Corringer, P.-J.; Novère, N.L.; Changeux, J.-P. Nicotinic Receptors at the Amino Acid Level. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 431–458. [Google Scholar] [CrossRef]
- Ihara, M.; Sattelle, D.B.; Matsuda, K. Probing New Components (Loop G and the α–α Interface) of Neonicotinoid Binding Sites on Nicotinic Acetylcholine Receptors. Pestic. Biochem. Phys. 2015, 121, 47–52. [Google Scholar] [CrossRef]
- Liu, M.Y.; Casida, J.E. High Affinity Binding of [3H] Imidacloprid in the Insect Acetylcholine Receptor. Pestic. Biochem. Physiol. 1993, 46, 40–46. [Google Scholar] [CrossRef]
- Tomizawa, M.; Latli, B.; Casida, J.E. Structure and Function of Insect Nicotinic Acetylcholine Receptors Studied with Nicotinoid Insecticide Affinity Probes. In Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor; Yamamoto, I., Casida, J.E., Eds.; Springer: Tokyo, Japan, 1999; pp. 271–292. [Google Scholar] [CrossRef]
- Yamamoto, I.; Tomizawa, M.; Saito, T.; Miyamoto, T.; Walcott, E.C.; Sumikawa, K. Structural Factors Contributing to Insecticidal and Selective Actions of Neonicotinoids. Arch. Insect Biochem. Physiol. 1998, 37, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, M.; Casida, J.E. Unique Neonicotinoid Binding Conformations Conferring Selective Receptor Interactions. J. Agric. Food Chem. 2011, 59, 2825–2828. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Structure and Diversity of Insect Nicotinic Acetylcholine Receptors. Pest Manag. Sci. 2001, 57, 914–922. [Google Scholar] [CrossRef]
- Thany, S.H. Insect Nicotinic Acetylcholine Receptors; Springer: New York, NY, USA, 2010; 118p. [Google Scholar]
- Taly, A.; Corringer, P.-J.; Guedin, D.; Lestage, P.; Changeux, J.-P. Nicotinic Receptors: Allosteric Transitions and Therapeutic Targets in the Nervous System. Nat. Rev. Drug Discov. 2009, 8, 733–750. [Google Scholar] [CrossRef]
- Yamamoto, I.; Casida, J.E. Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor; Springer: Tokyo, Japan, 1999; 300p. [Google Scholar] [CrossRef]
- Matsuda, K.; Buckingham, S.D.; Kleier, D.; Rauh, J.J.; Grauso, M.; Sattelle, D.B. Neonicotinoids: Insecticides Acting on Insect Nicotinic Acetylcholine Receptors. Trends Pharmacol. Sci. 2001, 22, 573–580. [Google Scholar] [CrossRef]
- Kumar, P.; Meizel, S. Nicotinic Acetylcholine Receptor Subunits and Associated Proteins InHuman Sperm*. J. Biol. Chem. 2005, 280, 25928–25935. [Google Scholar] [CrossRef] [Green Version]
- Kimura-Kuroda, J.; Komuta, Y.; Kuroda, Y.; Hayashi, M.; Kawano, H. Nicotine-Like Effects of the Neonicotinoid Insecticides Acetamiprid and Imidacloprid on Cerebellar Neurons from Neonatal Rats. PLoS ONE 2012, 7, e32432. [Google Scholar] [CrossRef]
- Chen, M.; Tao, L.; McLean, J.; Lu, C. Quantitative Analysis of Neonicotinoid Insecticide Residues in Foods: Implication for Dietary Exposures. J. Agric. Food Chem. 2014, 62, 6082–6090. [Google Scholar] [CrossRef]
- Taillebois, E.; Cartereau, A.; Jones, A.K.; Thany, S.H. Neonicotinoid Insecticides Mode of Action on Insect Nicotinic Acetylcholine Receptors Using Binding Studies. Pestic. Biochem. Phys. 2018, 151, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Houchat, J.-N.; Cartereau, A.; Le Mauff, A.; Taillebois, E.; Thany, S.H. An Overview on the Effect of Neonicotinoid Insecticides on Mammalian Cholinergic Functions through the Activation of Neuronal Nicotinic Acetylcholine Receptors. Int. J. Environ. Res. Public Health 2020, 17, 3222. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Daugherty, L.; Mitchell, A. Bee Afraid, Bee Very Afraid—Neonicotinoids and the nAChRs Family. InterPro Protein Focus. 2013. Available online: https://proteinswebteam.github.io/interpro-blog/potm/2013_9/prot_foc_13_09.pdf (accessed on 3 April 2023).
- Zhang, X.; Huang, Y.; Chen, W.-J.; Wu, S.; Lei, Q.; Zhou, Z.; Zhang, W.; Mishra, S.; Bhatt, P.; Chen, S. Environmental Occurrence, Toxicity Concerns, and Biodegradation of Neonicotinoid Insecticides. Environ. Res. 2023, 218, 114953. [Google Scholar] [CrossRef]
- Liu, T.; Wang, X.; Xu, J.; You, X.; Chen, D.; Wang, F.; Li, Y. Biochemical and Genetic Toxicity of Dinotefuran on Earthworms (Eisenia fetida). Chemosphere 2017, 176, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Lin, Z.; Zhang, W.; Mishra, S.; Bhatt, P.; Chen, S. Insights Into the Microbial Degradation and Biochemical Mechanisms of Neonicotinoids. Front. Microbiol. 2020, 11, 868. [Google Scholar] [CrossRef] [PubMed]
- Mörtl, M.; Kereki, O.; Darvas, B.; Klátyik, S.; Vehovszky, Á.; Győri, J.; Székács, A. Study on Soil Mobility of Two Neonicotinoid Insecticides. J. Chem. 2016, 2016, e4546584. [Google Scholar] [CrossRef] [Green Version]
- Gibbons, D.; Morrissey, C.; Mineau, P. A Review of the Direct and Indirect Effects of Neonicotinoids and Fipronil on Vertebrate Wildlife. Environ. Sci. Pollut. Res. 2015, 22, 103–118. [Google Scholar] [CrossRef] [Green Version]
- Mineau, P.; Palmer, C. The Impact of the Nation’s Most Widely Used Insecticides on Birds; American Bird Conservancy: The Plains, VA, USA, 2013; pp. 1–96. [Google Scholar]
- Sheets, L.P.; Li, A.A.; Minnema, D.J.; Collier, R.H.; Creek, M.R.; Peffer, R.C. A Critical Review of Neonicotinoid Insecticides for Developmental Neurotoxicity. Crit. Rev. Toxicol. 2016, 46, 153–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonare, M.; Kumar, M.; Raut, S.; Badgujar, P.; Doltade, S.; Telang, A. Evaluation of Imidacloprid-Induced Neurotoxicity in Male Rats: A Protective Effect of Curcumin. Neurochem. Int. 2014, 78, 122–129. [Google Scholar] [CrossRef]
- Rodrigues, K.J.A.; Santana, M.B.; Do Nascimento, J.L.M.; Picanço-Diniz, D.L.W.; Maués, L.A.L.; Santos, S.N.; Ferreira, V.M.M.; Alfonso, M.; Durán, R.; Faro, L.R.F. Behavioral and Biochemical Effects of Neonicotinoid Thiamethoxam on the Cholinergic System in Rats. Ecotoxicol. Environ. Saf. 2010, 73, 101–107. [Google Scholar] [CrossRef]
- Özdemir, H.H.; Kara, M.; Yumrutas, O.; Uckardes, F.; Eraslan, E.; Demir, C.F.; Bal, R. Determination of the Effects on Learning and Memory Performance and Related Gene Expressions of Clothianidin in Rat Models. Cogn. Neurodyn. 2014, 8, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, S.; Srivastava, M.K.; Kapoor, U.; Srivastava, L.P. A 90 Days Oral Toxicity of Imidacloprid in Female Rats: Morphological, Biochemical and Histopathological Evaluations. Food Chem. Toxicol. 2010, 48, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- El Okle, O.S.; El Euony, O.I.; Khafaga, A.F.; Lebda, M.A. Thiamethoxam Induced Hepatotoxicity and Pro-Carcinogenicity in Rabbits via Motivation of Oxidative Stress, Inflammation, and Anti-Apoptotic Pathway. Environ. Sci. Pollut. Res. 2018, 25, 4678–4689. [Google Scholar] [CrossRef]
- Badgujar, P.C.; Jain, S.K.; Singh, A.; Punia, J.S.; Gupta, R.P.; Chandratre, G.A. Immunotoxic Effects of Imidacloprid Following 28 Days of Oral Exposure in BALB/c Mice. Environ. Toxicol. Pharm. 2013, 35, 408–418. [Google Scholar] [CrossRef]
- Kapoor, U.; Srivastava, M.K.; Bhardwaj, S.; Srivastava, L.P. Effect of Imidacloprid on Antioxidant Enzymes and Lipid Peroxidation in Female Rats to Derive Its No Observed Effect Level (NOEL). J. Toxicol. Sci. 2010, 35, 577–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, U.; Srivastava, M.K.; Srivastava, L.P. Toxicological Impact of Technical Imidacloprid on Ovarian Morphology, Hormones and Antioxidant Enzymes in Female Rats. Food Chem. Toxicol. 2011, 49, 3086–3089. [Google Scholar] [CrossRef] [PubMed]
- Duzguner, V.; Erdogan, S. Chronic Exposure to Imidacloprid Induces Inflammation and Oxidative Stress in the Liver & Central Nervous System of Rats. Pest. Biochem. Physiol. 2012, 104, 58–64. [Google Scholar] [CrossRef]
- Bal, R.; Naziroğlu, M.; Türk, G.; Yilmaz, Ö.; Kuloğlu, T.; Etem, E.; Baydas, G. Insecticide Imidacloprid Induces Morphological and DNA Damage through Oxidative Toxicity on the Reproductive Organs of Developing Male Rats. Cell Biochem. Funct. 2012, 30, 492–499. [Google Scholar] [CrossRef] [Green Version]
- Ozsahin, A.D.; Bal, R.; Yılmaz, O. Biochemical Alterations in Kidneys of Infant and Adult Male Rats Due to Exposure to the Neonicotinoid Insecticides Imidacloprid and Clothianidin. Toxicol. Res. 2014, 3, 324–330. [Google Scholar] [CrossRef]
- Craddock, H.A.; Huang, D.; Turner, P.C.; Quirós-Alcalá, L.; Payne-Sturges, D.C. Trends in Neonicotinoid Pesticide Residues in Food and Water in the United States, 1999–2015. Environ. Health 2019, 18, 7. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.; Mondal, R.; Mukherjee, S.; Sarkar, M.; Kole, R.K. Persistence of Acetamiprid in Paddy and Soil under West Bengal Agro-Climatic Conditions. Environ. Monit. Assess. 2017, 189, 150. [Google Scholar] [CrossRef]
- Moore, R.; Willoughby, I.H.; Moffat, A.J.; Forster, J. Acetamiprid, Chlorantraniliprole, and in Some Situations the Physical Barriers MultiPro® or Kvaae® Wax, Can Be Alternatives to Traditional Synthetic Pyrethroid Insecticides for the Protection of Young Conifers from Damage by the Large Pine Weevil Hylobius abietis L. Scand. J. For. Res. 2021, 36, 230–248. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the Status and Global Strategy for Neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef] [PubMed]
- Camp, A.A.; Lehmann, D.M. Impacts of Neonicotinoids on the Bumble Bees Bombus Terrestris and Bombus Impatiens Examined through an AOP Framework Lens. Environ Toxicol Chem 2021, 40, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Gaweł, M.; Kiljanek, T.; Niewiadowska, A.; Semeniuk, S.; Goliszek, M.; Burek, O.; Posyniak, A. Determination of Neonicotinoids and 199 Other Pesticide Residues in Honey by Liquid and Gas Chromatography Coupled with Tandem Mass Spectrometry. Food Chem. 2019, 282, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Huang, H.; Li, N.; Li, F.; Wang, D.; Luo, Q. Occurrence and Ecological Risk of Pharmaceuticals and Personal Care Products (PPCPs) and Pesticides in Typical Surface Watersheds, China. Ecotoxicol. Environ. Saf. 2019, 175, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.R. Acetamiprid. In Encyclopedia of Toxicology, 3rd ed.; Academic Press: New York, NY, USA, 2014; pp. 30–32. [Google Scholar] [CrossRef]
- Renaud, M.; Akeju, T.; Natal-da-Luz, T.; Leston, S.; Rosa, J.; Ramos, F.; Sousa, J.P.; Azevedo-Pereira, H.M.V.S. Effects of the Neonicotinoids Acetamiprid and Thiacloprid in Their Commercial Formulations on Soil Fauna. Chemosphere 2018, 194, 85–93. [Google Scholar] [CrossRef]
- EL-Hak, H.N.G.; Al-Eisa, R.A.; Ryad, L.; Halawa, E.; El-Shenawy, N.S. Mechanisms and Histopathological Impacts of Acetamiprid and Azoxystrobin in Male Rats. Environ. Sci. Pollut. Res. 2022, 29, 43114–43125. [Google Scholar] [CrossRef]
- Phogat, A.; Singh, J.; Kumar, V.; Malik, V. Toxicity of the Acetamiprid Insecticide for Mammals: A Review. Environ. Chem. Lett. 2022, 20, 1453–1478. [Google Scholar] [CrossRef]
- Bonmatin, J.-M.; Mitchell, E.A.D.; Glauser, G.; Lumawig-Heitzman, E.; Claveria, F.; Bijleveld van Lexmond, M.; Taira, K.; Sánchez-Bayo, F. Residues of Neonicotinoids in Soil, Water and People’s Hair: A Case Study from Three Agricultural Regions of the Philippines. Sci. Total Environ. 2021, 757, 143822. [Google Scholar] [CrossRef]
- Taira, K.; Fujioka, K.; Aoyama, Y. Qualitative Profiling and Quantification of Neonicotinoid Metabolites in Human Urine by Liquid Chromatography Coupled with Mass Spectrometry. PLoS ONE 2013, 8, e80332. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, G.; Kuribayashi, R.; Ikenaka, Y.; Ichise, T.; Nakayama, S.M.M.; Ishizuka, M.; Taira, K.; Fujioka, K.; Sairenchi, T.; Kobashi, G.; et al. LC-ESI/MS/MS Analysis of Neonicotinoids in Urine of Very Low Birth Weight Infants at Birth. PLoS ONE 2019, 14, e0219208. [Google Scholar] [CrossRef] [Green Version]
- Yeter, O.; Aydın, A. Determination of Acetamiprid and IM-1-2 in PostMortem Human Blood, Liver, Stomach Contents by HPLC-DAD. J. For. Sci. 2014, 59, 287–292. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Xiang, H.; Li, M.; Li, W.; Ma, K.; Wang, X.; Zhang, J. Oxidative Stress: Role in Acetamiprid-Induced Impairment of the Male Mice Reproductive System. Agri. Sci. China 2011, 10, 786–796. [Google Scholar] [CrossRef]
- Ford, K.A.; Casida, J.E. Chloropyridinyl Neonicotinoid Insecticides: Diverse Molecular Substituents Contribute to Facile Metabolism in Mice. Chem. Res. Toxicol. 2006, 19, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Dick, R.A.; Ford, K.A.; Casida, J.E. Enzymes and Inhibitors in Neonicotinoid Insecticide Metabolism. J. Agric. Food Chem. 2009, 57, 4861–4866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noaishi, M.A.; Abd alhafez, H.H. Hepatotoxicity and Nephrotoxicity Evaluation after Repeated Dose of Acetamiprid in Albino Rats. Egypt. J. Chem. Environ. Health 2016, 2, 439–452. [Google Scholar]
- Brunet, J.-L.; Maresca, M.; Fantini, J.; Belzunces, L.P. Intestinal Absorption of the Acetamiprid Neonicotinoid by Caco-2 Cells: Transepithelial Transport, Cellular Uptake and Efflux. J. Environ. Sci. Health B 2008, 43, 261–270. [Google Scholar] [CrossRef]
- Ueyama, J.; Aoi, A.; Ueda, Y.; Oya, N.; Sugiura, Y.; Ito, Y.; Ebara, T.; Kamijima, M. Biomonitoring Method for Neonicotinoid Insecticides in Urine of Non-Toilet-Trained Children Using LC-MS/MS. Food Addit. Contam. Part A 2020, 37, 304–315. [Google Scholar] [CrossRef]
- Gasmi, S.; Kebieche, M.; Rouabhi, R.; Touahria, C.; Lahouel, A.; Lakroun, Z.; Henine, S.; Soulimani, R. Alteration of Membrane Integrity and Respiratory Function of Brain Mitochondria in the Rats Chronically Exposed to a Low Dose of Acetamiprid. Environ. Sci. Pollut. Res. 2017, 24, 22258–22264. [Google Scholar] [CrossRef]
- Kong, D.; Zhang, J.; Hou, X.; Zhang, S.; Tan, J.; Chen, Y.; Yang, W.; Zeng, J.; Han, Y.; Liu, X.; et al. Acetamiprid Inhibits Testosterone Synthesis by Affecting the Mitochondrial Function and Cytoplasmic Adenosine Triphosphate Production in Rat Leydig Cells. Biol. Reprod. 2017, 96, 254–265. [Google Scholar] [CrossRef] [Green Version]
- Shamsi, M.; Soodi, M.; Shahbazi, S.; Omidi, A. Effect of Acetamiprid on Spatial Memory and Hippocampal Glutamatergic System. Environ. Sci. Pollut. Res. 2021, 28, 27933–27941. [Google Scholar] [CrossRef]
- Hataba, A.A.; Keshta, A.T.; Mead, H.I.; El-Shafey, N. Hematological, Biochemical and Histological Alterations Induced by Oral Administration of Thiamethoxam and Acetamiprid in Male Rats. Biochem. Lett. 2014, 10, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Shahin, M. Hepatoprotective Effect of Ginseng, Green Tea, Cinnamon Their Combination against Acetamiprid—Induced Oxidative Stress in Rats. Asian J. Biol. 2018, 5, 1–13. [Google Scholar] [CrossRef]
- Chakroun, S.; Ezzi, L.; Grissa, I.; Kerkeni, E.; Neffati, F.; Bhouri, R.; Sallem, A.; Najjar, M.F.; Hassine, M.; Mehdi, M.; et al. Hematological, Biochemical, and Toxicopathic Effects of Subchronic Acetamiprid Toxicity in Wistar Rats. Environ. Sci. Pollut. Res. 2016, 23, 25191–25199. [Google Scholar] [CrossRef]
- Karaca, B.U.; Arican, Y.E.; Boran, T.; Binay, S.; Okyar, A.; Kaptan, E.; Özhan, G. Toxic Effects of Subchronic Oral Acetamiprid Exposure in Rats. Toxicol. Ind. Health 2019, 35, 679–687. [Google Scholar] [CrossRef]
- Dhouib, I.B.; Annabi, A.; Doghri, R.; Rejeb, I.; Dallagi, Y.; Bdiri, Y.; Lasram, M.M.; Elgaaied, A.; Marrakchi, R.; Fazaa, S.; et al. Neuroprotective Effects of Curcumin against Acetamiprid-Induced Neurotoxicity and Oxidative Stress in the Developing Male Rat Cerebellum: Biochemical, Histological, and Behavioral Changes. Environ. Sci. Pollut. Res. 2017, 24, 27515–27524. [Google Scholar] [CrossRef]
- Wang, X.; Anadón, A.; Wu, Q.; Qiao, F.; Ares, I.; Martínez-Larrañaga, M.-R.; Yuan, Z.; Martínez, M.-A. Mechanism of Neonicotinoid Toxicity: Impact on Oxidative Stress and Metabolism. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 471–507. [Google Scholar] [CrossRef] [PubMed]
- Annabi, E.; Ben Salem, I.; Abid-Essefi, S. Acetamiprid, a Neonicotinoid Insecticide, Induced Cytotoxicity and Genotoxicity in PC12 Cells. Toxicol. Mech. Methods 2019, 29, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Kara, M.; Oztas, E.; Ozhan, G. Acetamiprid Induced Cyto- and Genotoxicity in AR42J Pancreatic Cell Line. Turk. J. Pharm. Sci. 2020, 17, 474. [Google Scholar] [CrossRef]
- Gomez, S.D.; Bustos, P.S.; Sánchez, V.G.; Ortega, M.G.; Guiñazú, N. Trophoblast Toxicity of the Neonicotinoid Insecticide Acetamiprid and an Acetamiprid-Based Formulation. Toxicology 2020, 431, 152363. [Google Scholar] [CrossRef]
- Yan, S.; Meng, Z.; Tian, S.; Teng, M.; Yan, J.; Jia, M.; Li, R.; Zhou, Z.; Zhu, W. Neonicotinoid Insecticides Exposure Cause Amino Acid Metabolism Disorders, Lipid Accumulation and Oxidative Stress in ICR Mice. Chemosphere 2020, 246, 125661. [Google Scholar] [CrossRef] [PubMed]
- Şenyildiz, M.; Kilinc, A.; Ozden, S. Investigation of the Genotoxic and Cytotoxic Effects of Widely Used Neonicotinoid Insecticides in HepG2 and SH-SY5Y Cells. Toxicol. Ind. Health 2018, 34, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Mosbah, R.; Djerrou, Z.; Mantovani, A. Protective Effect of Nigella Sativa Oil against Acetamiprid Induced ReproductiveToxicity in Male Rats. Drug Chem. Toxicol. 2018, 41, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Arıcan, E.Y.; Gökçeoğlu Kayalı, D.; Ulus Karaca, B.; Boran, T.; Öztürk, N.; Okyar, A.; Ercan, F.; Özhan, G. Reproductive Effects of Subchronic Exposure to Acetamiprid in Male Rats. Sci. Rep. 2020, 10, 8985. [Google Scholar] [CrossRef]
- Ibrahim, H.; Banna, S.; Rafea, A. Acetamiprid, insecticide-induced oxidative damage on reproductive parameters male rats. Alex. J. Vet. Sci. 2020, 64, 63. [Google Scholar] [CrossRef]
- Kenfack, A.; Arthénice, J.N.G.; Ngoula, F.; Vemo, B.N.; Osoe, F.P.N.; Pamo, E.T. Reproductive toxicity of acetamiprid in male Guinea pig (Cavia porcellus). J. Anim. Sci. Vet. Med. 2018, 3, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Toghan, R.; Amin, Y.A.; Ali, R.A.; Fouad, S.S.; Ahmed, M.A.-E.B.; Saleh, S.M.M. Protective Effects of Folic Acid against Reproductive, Hematological, Hepatic, and Renal Toxicity Induced by Acetamiprid in Male Albino Rats. Toxicology 2022, 469, 153115. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, J.; Ren, J.; Hou, Y.; Han, Z.; Xiao, J.; Li, Y. Exposure Level of Neonicotinoid Insecticides in the Food Chain and the Evaluation of Their Human Health Impact and Environmental Risk: An Overview. Sustainability 2020, 12, 7523. [Google Scholar] [CrossRef]
- Abou-Zeid, S.M. Developmental Toxicity of Acetamiprid In Rats. World J. Pharm. Pharm. Sci. 2018, 7, 113–126. [Google Scholar]
- Babeľová, J.; Šefčíková, Z.; Čikoš, Š.; Špirková, A.; Kovaříková, V.; Koppel, J.; Makarevich, A.V.; Chrenek, P.; Fabian, D. Exposure to Neonicotinoid Insecticides Induces Embryotoxicity in Mice and Rabbits. Toxicology 2017, 392, 71–80. [Google Scholar] [CrossRef]


| Taxon | Research Object | LC50 or LD50 | References |
|---|---|---|---|
| Aquatic vertebrates | Fish | 1.2–241 mg/L (IMI) >93.6 mg/L (CLO) | Gibbons et al. [67] |
| Birds | Mallards Grey partridges | 283 mg/kg (IMI) 98 mg/kg (ACE) 576 mg/kg (TMX) >752 mg/kg (CLO) 15–41 mg/kg (IMI) 430 mg/kg (CLO) | Mineau and Palmer [68] |
| Mammals | Rats | Oral 450 mg/kg (IMI) 182 mg/kg (ACE) Oral 1563 mg/kg (TMX) >5000 mg/kg (CLO) Oral 640 mg/kg (THC) 2400 mg/kg (DIN) | Sheets et al. [69] |
| Type of Neonicotinoid | Dose | Effect | References |
|---|---|---|---|
| Imidacloprid | 45 and 90 mg/kg b.w. | Pain threshold and locomotor activity decreased in rats. | Lonare et al. [70] |
| 1–100 µM | Cytotoxic to cerebellar neurons: significant excitatory Ca2+ influxes were evoked in neonatal rats. | Kimura-Kuroda et al. [58] | |
| Thiamethoxam | 50 or 100 mg/kg | Increased anxiety behaviour. HACU and acetylcholinesterase significantly decreased in rats. | Rodrigues et al. [71] |
| Clothianidin | 24 mg/kg | Significant deterioration of cognitive function in infant rats. | Özdemir et al. [72] |
| Neonicotinoid | Doses | Effect | References |
|---|---|---|---|
| Imidacloprid | >5 mg/kg | Immunosuppressive effect in mice. | Badgujar et al. [75] |
| 20 mg/kg | Production of radicals and damage to the antioxidant defence system in female rats. | Kapoor et al. [76] | |
| 20 mg/kg/day | Pathomorphological alterations in atretic and antral follicles; decrease in ovarian weight; significant impact to hormone release in female rats. | Kapoor et al. [77] | |
| 1 mg/kg b.w./day | Induced oxidative stress and inflammation in liver and brain of rats. | Duzguner and Erdogan [78] | |
| 8 mg/kg b.w. | Decreased sperm motility and sperm morphology and increased gečrm cell apoptosis in male rats. | Bal et al. [79] | |
| Clothianidin | 4 mg/kg b.w./20 mg/kg b.w. | Changes in kidney biochemical parameters in infant and adult male rats. | Ayse Dilek Ozsahin and Okkes [80] |
| Cell Type | Dose | Results | References | |
|---|---|---|---|---|
| In vitro | Pheochromocytoma adrenal medulla cells (PC12) | 100–700 µM | -↑ Malondialdehyde levels and ROS generation; loss of mitochondrial membrane potential | Annabi et al. [111] |
| Pancreatic cell line (AR42J) | 1–6 mM | -Reduction in glutathione levels | Kara et al. [112] | |
| Isolated trophoblast cells (HTR-8/SVneo) | 10 and 100 µM | -↑ ROS production and superoxides; ↓ Glutathione S-transferase, catalase, and superoxide dismutase | Gomez et al. [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuščíková, L.; Bažány, D.; Greifová, H.; Knížatová, N.; Kováčik, A.; Lukáč, N.; Jambor, T. Screening of Toxic Effects of Neonicotinoid Insecticides with a Focus on Acetamiprid: A Review. Toxics 2023, 11, 598. https://doi.org/10.3390/toxics11070598
Zuščíková L, Bažány D, Greifová H, Knížatová N, Kováčik A, Lukáč N, Jambor T. Screening of Toxic Effects of Neonicotinoid Insecticides with a Focus on Acetamiprid: A Review. Toxics. 2023; 11(7):598. https://doi.org/10.3390/toxics11070598
Chicago/Turabian StyleZuščíková, Lucia, Denis Bažány, Hana Greifová, Nikola Knížatová, Anton Kováčik, Norbert Lukáč, and Tomáš Jambor. 2023. "Screening of Toxic Effects of Neonicotinoid Insecticides with a Focus on Acetamiprid: A Review" Toxics 11, no. 7: 598. https://doi.org/10.3390/toxics11070598






