Presence of N, N′-Substituted p-Phenylenediamine-Derived Quinones in Human Urine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Study Population and Sample Collection
2.3. Sample Extraction
2.4. Instrumental Analysis
2.5. Daily Excretion (DE) Calculation
2.6. QA/QC
2.7. Statistical Analysis
3. Results
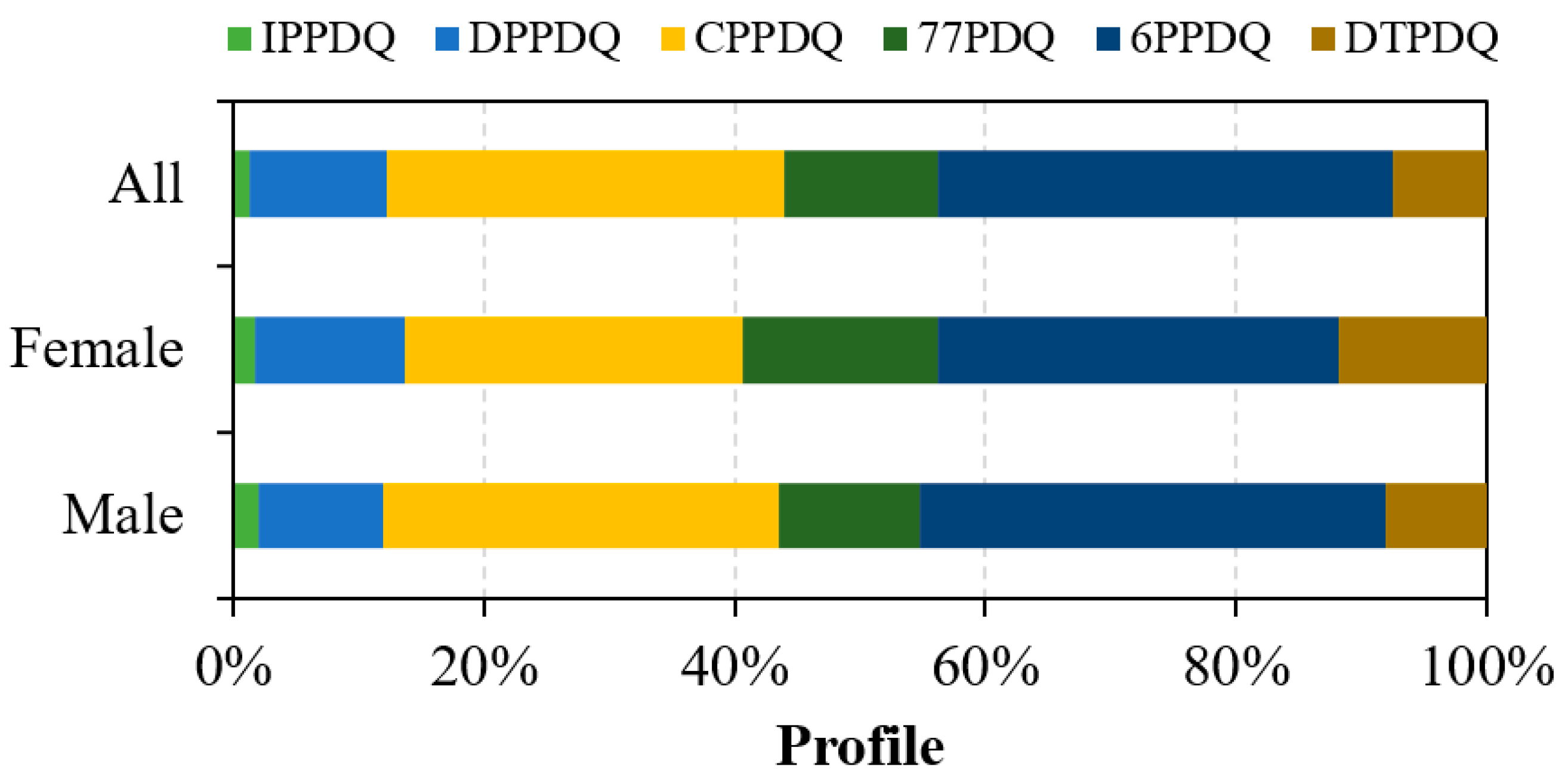
3.1. Concentrations of PPDQs in Human Urine
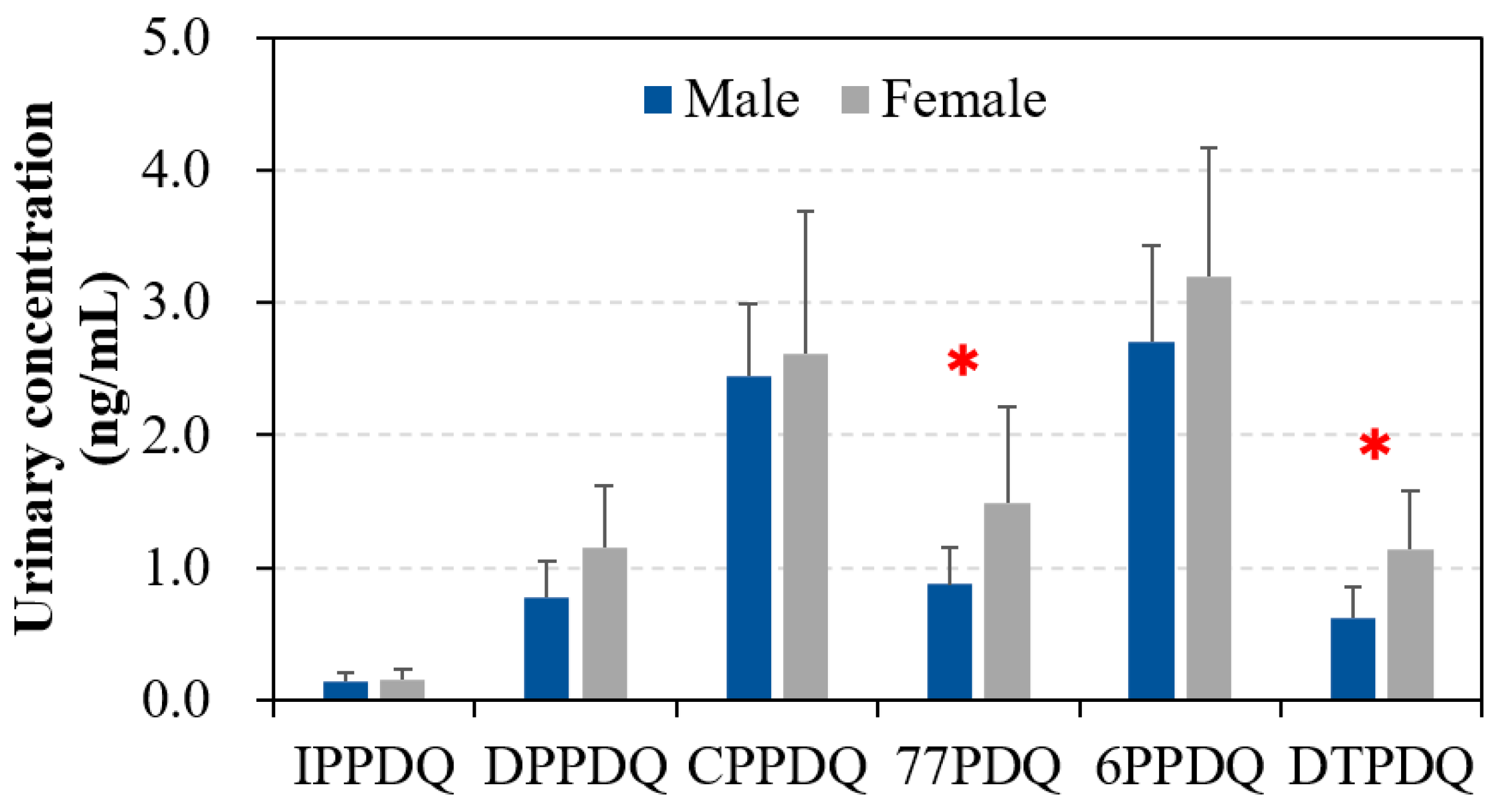
3.2. Gender- and Age-Specific Differences
3.3. Human Daily Excretion (DE) of PPDQs
3.4. Strengths and Limitations of This Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cataldo, F. A study on the reaction between n-substituted p-phenylenediamines and ozone: Experimental results and theoretical aspects in relation to their antiozonant activity. Eur. Polym. J. 2002, 38, 885–893. [Google Scholar]
- Sun, Y.; He, J.; Zhong, B.; Zhu, L.; Liu, F. A synthesized multifunctional rubber additive and its improvements on the curing and antioxidative properties of styrene-butadiene rubber/silica composites. Polym. Degrad. Stab. 2019, 170, 108999. [Google Scholar]
- Zoroufchi Benis, K.; Behnami, A.; Minaei, S.; Brinkmann, M.; McPhedran, K.N.; Soltan, J. Environmental occurrence and toxicity of 6ppd quinone, an emerging tire rubber-derived chemical: A review. Environ. Sci. Technol. Lett. 2023, 10, 815–823. [Google Scholar]
- Chen, X.; He, T.; Yang, X.; Gan, Y.; Qing, X.; Wang, J. Analysis, environmental occurrence, fate and potential toxicity of tire wear compounds 6ppd and 6ppd-quinone. J. Hazard. Mater. 2023, 452, 131245. [Google Scholar]
- Johannessen, C.; Liggio, J.; Zhang, X.; Saini, A.; Harner, T. Composition and transformation chemistry of tire-wear derived organic chemicals and implications for air pollution. Atmos. Pollut. Res. 2022, 13, 101533. [Google Scholar]
- Zhao, H.N.; Hu, X.; Gonzalez, M.; Rideout, C.; Hobby, G.C.; Fisher, M.F.; McCormick, C.J.; Dodd, M.C.; Kim, K.E.; Tian, Z.Y.; et al. Screening p-phenylenediamine antioxidants, their transformation products, and industrial chemical additives in crumb rubber and elastomeric consumer products. Environ. Sci. Technol. 2023, 57, 2779–2791. [Google Scholar] [PubMed]
- Huang, W.; Shi, Y.; Huang, J.; Deng, C.; Tang, S.; Liu, X.; Chen, D. Occurrence of substituted p-phenylenediamine antioxidants in dusts. Environ. Sci. Technol. Lett. 2021, 8, 381–385. [Google Scholar]
- Seiwert, B.; Nihemaiti, M.; Troussier, M.; Weyrauch, S.; Reemtsma, T. Abiotic oxidative transformation of 6-ppd and 6-ppd quinone from tires and occurrence of their products in snow from urban roads and in municipal wastewater. Water Res. 2022, 212, 118122. [Google Scholar]
- Zeng, L.; Li, Y.; Sun, Y.; Liu, L.Y.; Shen, M.; Du, B. Widespread Occurrence and Transport of p-Phenylenediamines and Their Quinones in Sediments across Urban Rivers, Estuaries, Coasts, and Deep-Sea Regions. Environ. Sci. Technol. 2023, 57, 2393–2403. [Google Scholar]
- Zhang, R.; Zhao, S.; Liu, X.; Tian, L.; Mo, Y.; Yi, X.; Liu, S.; Liu, J.; Li, J.; Zhang, G. Aquatic environmental fates and risks of benzotriazoles, benzothiazoles, and p-phenylenediamines in a catchment providing water to a megacity of China. Environ. Res. 2023, 216 Pt 4, 114721. [Google Scholar]
- Tian, Z.; Zhao, H.; Peter, K.T.; Gonzalez, M.; Wetzel, J.; Wu, C.; Hu, X.; Prat, J.; Mudrock, E.; Hettinger, R.; et al. A ubiquitous tire rubber–derived chemical induces acute mortality in coho salmon. Science 2021, 371, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Wang, D. Tire-rubber related pollutant 6-ppd quinone: A review of its transformation, environmental distribution, bioavailability, and toxicity. J. Hazard. Mater. 2023, 459, 132265. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Zhao, Z.; Song, X.; Zhu, J.; Guo, R.; Hangbiao, J. Presence of N, N′-substituted p-phenylenediamine quinones in Tap Water: Implication for human exposure. Environ. Res. 2024, 262, 119817. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, R.; Jiang, S.; Wu, P.; Jin, H. Occurrence of p-phenylenediamine antioxidants (PPDs) and PPDs-derived quinones in indoor dust. Sci. Total Environ. 2024, 912, 169325. [Google Scholar] [CrossRef]
- Cao, G.; Wang, W.; Zhang, J.; Wu, P.; Zhao, X.; Yang, Z.; Hu, D.; Cai, Z. New Evidence of Rubber-Derived Quinones in Water, Air, and Soil. Environ. Sci. Technol. 2022, 56, 4142–4150. [Google Scholar] [CrossRef]
- Wang, W.; Cao, G.; Zhang, J.; Wu, P.; Chen, Y.; Chen, Z.; Qi, Z.; Li, R.; Dong, C.; Cai, Z. Beyond substituted p-Phenylenediamine antioxidants: Prevalence of their quinone derivatives in PM2. 5. Environ. Sci. Technol. 2022, 56, 10629–10637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, T.; Ye, D.; Lin, Z.; Wang, F.; Guo, Y. Widespread n-(1, 3-dimethylbutyl)-n′-phenyl-p-phenylenediamine quinone in size-fractioned atmospheric particles and dust of different indoor environments. Environ. Sci. Technol. Lett. 2022, 9, 420–425. [Google Scholar] [CrossRef]
- Sherman, A.; Masset, T.; Wimmer, L.; Dailey, L.A.; Hüffer, T.; Breider, F. Inhalation of climbing shoe particles is highly relevant for the human exposure to rubber-derived chemicals in indoor facilities. Environ. Sci. Technol. 2023, 22, 123–127. [Google Scholar]
- Zhang, Y.; Xu, C.; Zhang, W.; Qi, Z.; Song, Y.; Zhu, L.; Dong, C.; Chen, J.; Cai, Z. P-phenylenediamine antioxidants in pm(2.5): The underestimated urban air pollutants. Environ. Sci. Technol. 2022, 56, 6914–6921. [Google Scholar] [CrossRef]
- Lyu, Y.; Guo, H.; Cheng, T.; Li, X. Particle size distributions of oxidative potential of lung-deposited particles: Assessing contributions from quinones and water-soluble metals. Environ. Sci. Technol. 2018, 52, 6592–6600. [Google Scholar] [CrossRef]
- Fang, L.; Fang, C.; Di, S.; Yu, Y.; Wang, C.; Wang, X.; Jin, Y. Oral exposure to tire rubber-derived contaminant 6PPD and 6PPD-quinone induce hepatotoxicity in mice. Sci. Total Environ. 2023, 869, 161836. [Google Scholar] [CrossRef] [PubMed]
- Alimonti, A.; Mattei, D. Biomarkers for human biomonitoring. WIT Trans. State—Art Sci. Eng. 2008, 30, 20281. [Google Scholar]
- Gong, J.; Zhu, T.; Kipen, H.; Rich, D.Q.; Huang, W.; Lin, W.-T.; Hu, M.; Zhang, J.F. Urinary polycyclic aromatic hydrocarbon metabolites as biomarkers of exposure to traffic-emitted pollutants. Environ. Int. 2015, 85, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Mao, W.; Liao, K.; Zhang, Y.; Jin, H. Association between urinary bisphenol analogue concentrations and lung cancer in adults: A case-control study. Environ. Pollut. 2022, 315, 120323. [Google Scholar] [CrossRef]
- Du, B.; Liang, B.; Li, Y.; Shen, M.; Liu, L.; Zeng, L. First report on the occurrence of N-(1, 3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (6PPD) and 6PPD-quinone as pervasive pollutants in human urine from south China. Environ. Sci. Technol. Lett. 2022, 9, 1056–1062. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, J.; Ren, L.; Fan, R.; Zhang, J.; Liu, G.; Zhou, L.; Chen, D.; Yu, Y.; Lu, S.Y. Urinary bisphenol analogues and triclosan in children from south china and implications for human exposure. Environ. Pollut. 2018, 238, 299–305. [Google Scholar] [CrossRef]
- Qu, J.; Guo, R.; Liu, L.; Ren, F.; Jin, H. Occurrence of bisphenol analogues and their conjugated metabolites in foodstuff. Sci. Total Environ. 2024, 948, 174922. [Google Scholar] [CrossRef]
- Wang, W.; Kannan, K. Quantitative identification of and exposure to synthetic phenolic antioxidants, including butylated hydroxytoluene, in urine. Environ. Int. 2019, 128, 24–29. [Google Scholar] [CrossRef]
- Frederiksen, H.; Kranich, S.K.; Jørgensen, N.; Taboureau, O.; Petersen, J.H.; Andersson, A.-M. Temporal variability in urinary phthalate metabolite excretion based on spot, morning, and 24-h urine samples: Considerations for epidemiological studies. Environ. Sci. Technol. 2013, 47, 958–967. [Google Scholar] [CrossRef]
- Hyland, C.; Kogut, K.; Gunier, R.B.; Castorina, R.; Curl, C.; Eskenazi, B.; Bradman, A. Organophosphate pesticide dose estimation from spot and 24-hr urine samples collected from children in an agricultural community. Environ. Int. 2021, 146, 106226. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, C.; Miyaura, C.; Ito, A. Dietary bisphenol a suppresses the growth of newborn pups by insufficient supply of maternal milk in mice. J. Health Sci. 2004, 50, 315–318. [Google Scholar] [CrossRef]
- Monaghan, J.; Jaeger, A.; Jai, J.K.; Tomlin, H.; Atkinson, J.; Brown, T.M.; Gill, C.G.; Krogh, E. Automated, High-Throughput Analysis of Tire-Derived p-Phenylenediamine Quinones (PPDQs) in Water by Online Membrane Sampling Coupled to MS/MS. ACS EST Water 2023, 3, 3293–3304. [Google Scholar] [CrossRef] [PubMed]
- Grasse, N.; Seiwert, B.; Massei, R.; Scholz, S.; Fu, Q.; Reemtsma, T. Uptake and Biotransformation of the Tire Rubber-derived Contaminants 6-PPD and 6-PPD Quinone in the Zebrafish Embryo (Danio rerio). Environ. Sci. Technol. 2023, 57, 15598–15607. [Google Scholar] [CrossRef] [PubMed]
- Estlander, T.; Jolanki, R.; Kanerva, L. Dermatitis and urticaria from rubber and plastic gloves. Contact Dermat. 1986, 14, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Bailey, R.E.; Morrall, S.W.; Aardema, M.J.; Stanley, L.A.; Skare, J.A. Dermal penetration and metabolism of p-aminophenol and p-phenylenediamine: Application of the EpiDerm™ human reconstructed epidermis model. Toxicol. Lett. 2009, 188, 119–129. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Fautz, R.; Benech-Kieffer, F.; Toutain, H. Toxicity and human health risk of hair dyes. Food Chem. Toxicol. 2004, 42, 517–543. [Google Scholar] [CrossRef]
- Vogel, T.A.; Coenraads, P.J.; Bijkersma, L.M.; Vermeulen, K.M.; Schuttelaar, M.L.A.; Group, E.F.S. p-Phenylenediamine exposure in real life–a case–control study on sensitization rate, mode and elicitation reactions in the northern N etherlands. Contact Dermat. 2015, 72, 355–361. [Google Scholar] [CrossRef]
- Schwartz, J.B. The influence of sex on pharmacokinetics. Clin. Pharmacokinet. 2003, 42, 107–121. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, F.; Li, J.; Wan, X.; Ge, Y.; Liang, G.; Zhou, Y. P-phenylenediamine antioxidants and their quinone derivatives: A review of their environmental occurrence, accessibility, potential toxicity, and human exposure. Sci. Total Environ. 2024, 948, 174449. [Google Scholar] [CrossRef] [PubMed]
- Soejima, K.; Sato, H.; Hisaka, A. Age-related change in hepatic clearance inferred from multiple population pharmacokinetic studies: Comparison with renal clearance and their associations with organ weight and blood flow. Clin. Pharmacokinet. 2022, 61, 295–305. [Google Scholar] [CrossRef]
- Sohmiya, M.; Kato, Y. Renal clearance, metabolic clearance rate, and half-life of human growth hormone in young and aged subjects. J. Clin. Endocrinol. Metab. 1992, 75, 1487–1490. [Google Scholar]
- Wang, H.; Wang, N.; Qian, J.; Hu, L.; Huang, P.; Su, M.; Yu, X.; Fu, C.; Jiang, F.; Zhao, Q.; et al. Urinary Antibiotics of Pregnant Women in Eastern China and Cumulative Health Risk Assessment. Environ. Sci. Technol. 2017, 51, 3518–3525. [Google Scholar] [CrossRef] [PubMed]

| Detection Frequency | Mean | Median | Concentration Percentile | ||||
|---|---|---|---|---|---|---|---|
| Min | 25th | 75th | Max | ||||
| 6PPDQ | 92% | 2.4 | 2.0 | <LOD | 0.83 | 3.4 | 19 |
| CPPDQ | 84% | 2.1 | 1.6 | <LOD | 0.82 | 2.8 | 24 |
| 77PDQ | 81% | 0.81 | 0.42 | <LOD | 0.15 | 1.1 | 14 |
| DPPDQ | 79% | 0.72 | 0.52 | <LOD | 0.20 | 0.98 | 11 |
| DTPDQ | 63% | 0.49 | 0.37 | <LOD | <LOD | 0.92 | 8.9 |
| IPPDQ | 55% | 0.082 | 0.11 | <LOD | <LOD | 0.28 | 0.65 |
| Mean | Median | Range | |
|---|---|---|---|
| IPPDQ | 4.4 | 3.4 | <0.5–20 |
| DPPDQ | 26 | 13 | <0.5–338 |
| CPPDQ | 68 | 49 | <0.5–516 |
| 77PDQ | 30 | 11 | <0.5–481 |
| 6PPDQ | 81 | 54 | <0.5–475 |
| DTPDQ | 22 | 10 | <0.5–281 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Jin, H.; Zhu, Y.; Guo, R.; Zhou, L.; Wu, X. Presence of N, N′-Substituted p-Phenylenediamine-Derived Quinones in Human Urine. Toxics 2024, 12, 733. https://doi.org/10.3390/toxics12100733
Huang J, Jin H, Zhu Y, Guo R, Zhou L, Wu X. Presence of N, N′-Substituted p-Phenylenediamine-Derived Quinones in Human Urine. Toxics. 2024; 12(10):733. https://doi.org/10.3390/toxics12100733
Chicago/Turabian StyleHuang, Juxiu, Hangbiao Jin, Yingying Zhu, Ruyue Guo, Lisha Zhou, and Xiaoyu Wu. 2024. "Presence of N, N′-Substituted p-Phenylenediamine-Derived Quinones in Human Urine" Toxics 12, no. 10: 733. https://doi.org/10.3390/toxics12100733
APA StyleHuang, J., Jin, H., Zhu, Y., Guo, R., Zhou, L., & Wu, X. (2024). Presence of N, N′-Substituted p-Phenylenediamine-Derived Quinones in Human Urine. Toxics, 12(10), 733. https://doi.org/10.3390/toxics12100733





