Interactive Effects of Polyethylene Microplastics and Cadmium on Growth of Microcystis aeruginosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Algae Culture
2.3. Determination of Chlorophyll a
2.4. Determination of MCs
2.5. Enzyme Activity Measurement
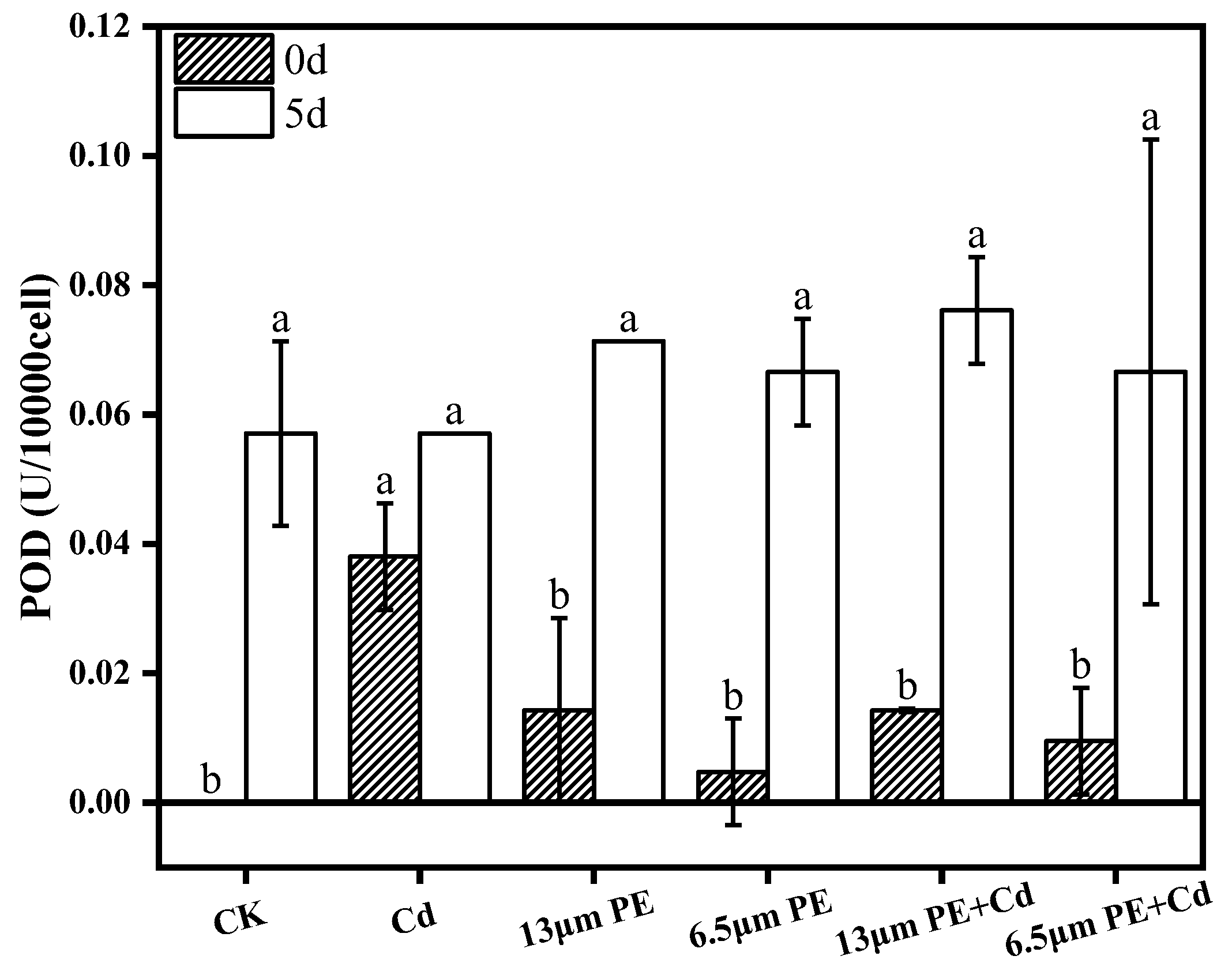
2.5.1. Determination of POD
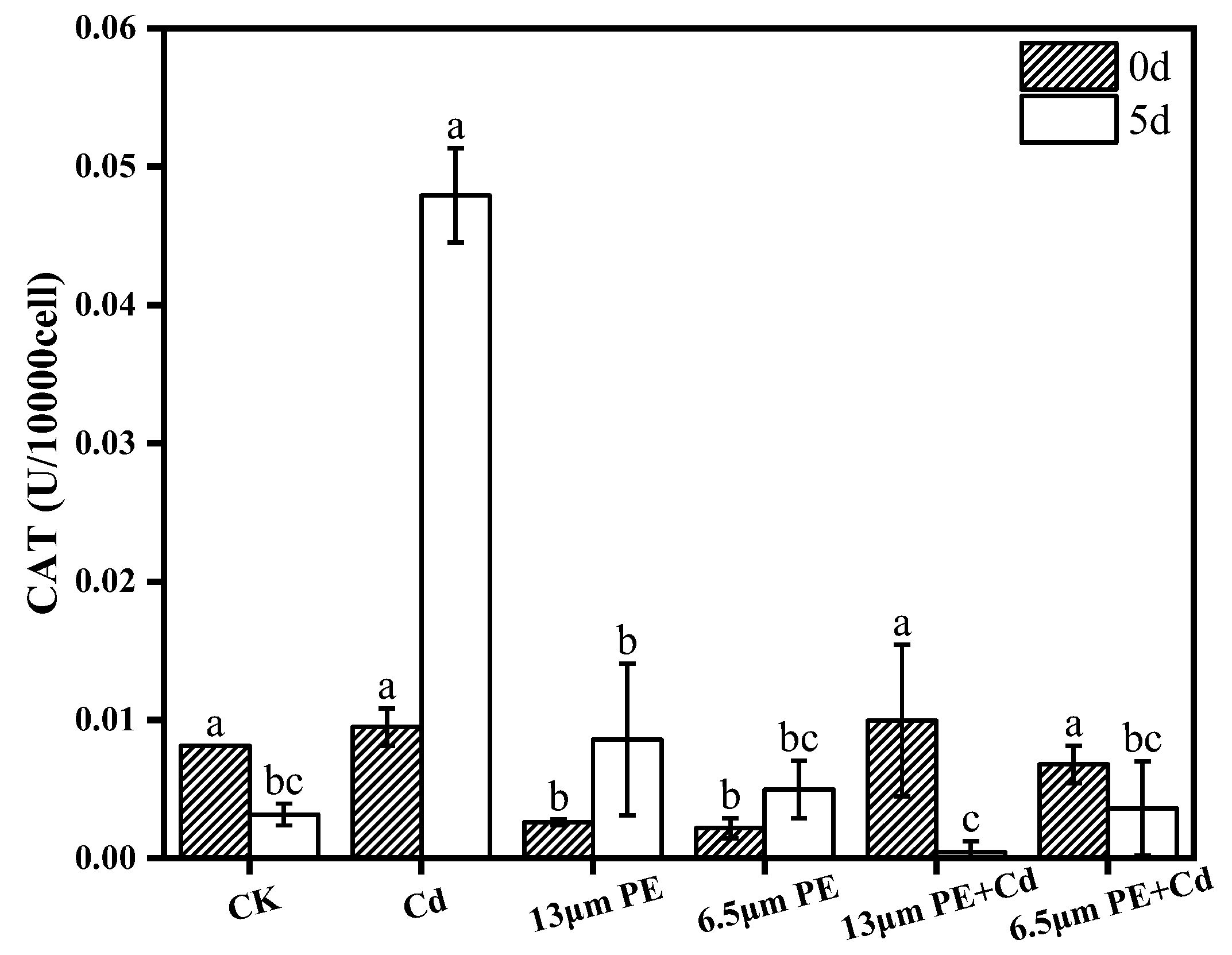
2.5.2. Determination of CAT
2.6. Data Processing
3. Results and Discussion
3.1. Effect of PE and Cd on Chlorophyll a Content
3.2. Effect of PE and Cd on MCs Levels
3.3. Oxidative Damage to Algae by PE and Cd
4. Conclusions
- (1)
- Chlorophyll a content of M. aeruginosa significantly decreased after 5 days of exposure to pollutants, with PE having a greater effect on chlorophyll a content compared to Cd, and the most intensive inhibition observed in the combined PE and Cd treatment group.
- (2)
- The impact of the 6.5 µm PE treatment group on MCs was greater compared to the 13 µm PE treatment group. It is suggested that adsorption of Cd by 6.5 µm PE occurred, resulting in reduced Cd bioavailability. Extracellular MCs content was notably higher than the control group on day 0 and day 5 in all treatment groups except the single Cd treatment group. PE increased algal MCs content, and the involvement of Cd did not significantly affect PE.
- (3)
- POD activity increased in all groups exposure at day 0. There was a synergistic effect of the combined PE and Cd treatment for 5 days on CAT activity stress, leading to decreased CAT activity in the combined treatment group. Oxidative damage caused by Cd was more noticeable. Differential impacts were noted based on PE particle sizes, with the 13 µm PE + Cd treatment group exhibiting the most stress on CAT.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, Z.L.; Wang, J. Current practices and future perspectives of microplastic pollution in freshwater ecosystems in China. Sci. Total Environ. 2019, 691, 697–712. [Google Scholar] [CrossRef]
- Li, J.Y.; Liu, H.H.; Chen, J.P. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef]
- Bowley, J.; Baker-Austin, C.; Porter, A.; Hartnell, R.; Lewis, C. Oceanic Hitchhikers—Assessing Pathogen Risks from Marine Microplastic. Trends Microbiol. 2021, 29, 107–111. [Google Scholar] [CrossRef]
- Carbery, M.; O’Connor, W.; Thavamani, P. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018, 115, 400–440. [Google Scholar] [CrossRef]
- He, L.; Huang, F.; Yin, K. The ecological effect of marine microplastics as a biological vector. J. Trop. Oceanogr. 2018, 37, 1–8. [Google Scholar]
- Sangale, M.K.; Shahnawar, M.; Ade, A.B. Potential of fungi isolated from the dumping sites mangrove rhizosphere soil to degrade polythene. Sci. Rep. 2019, 9, 5390. [Google Scholar] [CrossRef]
- Sendra, M.; Staffieri, E.; Yeste, M.P.; Moreno-Garrido, I.; Gatica, J.M.; Corsi, I.; Blasco, J. Are the primary characteristics of polystyrene nanoplastics responsible for toxicity and ad/absorption in the marine diatom Phaeodactylum tricornutum? Environ. Pollut. 2019, 249, 610–619. [Google Scholar] [CrossRef]
- Mao, Y.; Ai, H.; Chen, Y.; Zhang, Z.; Zeng, P.; Kang, L.; Li, W.; Gu, W.; He, Q.; Li, H. Phytoplankton response to polystyrene microplastics: Perspective from an entire growth period. Chemosphere 2018, 208, 59–68. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Wu, D.; Wang, T.; Wang, J.; Jiang, L.J.; Yin, Y.; Guo, H.Y. Size-dependent toxic effects of polystyrene microplastic exposure on Microcystis aeruginosa growth and microcystin production. Sci. Total Environ. 2021, 761, 143265. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Chen, H.; Zhang, Y. Toxicity effects of microplastics and nanoplastics with cadmium on the alga Microcystis aeruginosa. Environ. Sci. Pollut. Res. 2023, 30, 17360–17373. [Google Scholar] [CrossRef]
- Sjollema, S.B.; Redondo-Hasselerharm, P.; Leslie, H.A.; Kraak, M.H.S.; Vethaak, A.D. Do plastic particles affect microalgal photosynthesis and growth? Aquat. Toxicol. 2016, 170, 259–261. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, D.; Gao, L.; Qi, H.; Su, Y.; Peng, L. Aged microplastics decrease the bioavailability of coexisting heavy metals to microalga Chlorella vulgaris. Ecotoxicol. Environ. Saf. 2021, 217, 112199. [Google Scholar] [CrossRef]
- Lin, W.; Yini, M.; Rong, J. Effects of PS and PVC microplastics on the growth of Chlorella sp. Acta Sci. Circumstantiae 2021, 41, 1538–1544. [Google Scholar]
- Zhao, T.; Tan, L.J.; Huang, W.Q.; Wang, J.T. The interactions between micro polyvinyl chloride (mPVC) and marine dinoflagellate Karenia mikimotoi: The inhibition of growth, chlorophyll and photosynthetic efficiency. Environ. Pollut. 2019, 247, 883–889. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Qin, Z.M.; Fei, J.; Ding, D.J.; Sun, H.M.; Wang, J.; Yin, X.Q. Surfactant-induced adsorption of Pb(II) on the cracked structure of microplastics. J. Colloid Interface Sci. 2022, 621, 91–100. [Google Scholar] [CrossRef]
- Gao, X.; Hassan, I.; Peng, Y.; Huo, S.; Ling, L. Behaviors and influencing factors of the heavy metals adsorption onto microplastics: A review. J. Clean. Prod. 2021, 319, 128777. [Google Scholar] [CrossRef]
- Gao, F.L.; Li, J.X.; Sun, C.J.; Zhang, L.T.; Jiang, F.H.; Cao, W.; Zheng, L. Study on the capability and characteristics of heavy metals enriched on microplastics in marine environment. Mar. Pollut. Bull. 2019, 144, 61–67. [Google Scholar] [CrossRef]
- Tripathi, B.N.; Mehta, S.K.; Amar, A.; Gaur, J.P. Oxidative stress in Scenedesmus sp. during short- and long-term exposure to Cu2+ and Zn2+. Chemosphere 2006, 62, 538–544. [Google Scholar] [CrossRef]
- Jia, P.; Deng, L.J. Progress in the application of photosynthetic bacteria to the treatment ofheavy metal wastewater. Ind. Water Treat. 2011, 31, 13–17. [Google Scholar]
- Guo, J.Y.; Shi, M.K.; Zhao, Y.L.; Ren, G.Y.; Yi, J.P.; Niu, L.L.; Li, J. Response of Nostoc flagelliforme cell to Cu2+, Cr2+ and Pb2+ stress. Acta Microbiol. Sin. 2013, 53, 553–560. [Google Scholar]
- Nie, L.H.; Yang, D.J.; Liu, Y.Q.; Han, B.P.; Ma, X.L.; Cha, G.C. lsolation, identification and effect of heavy metals on the growth of Cylindrospermopsis raciborskii helix from shrimp ponds. Acta Microbiol. Sin. 2019, 59, 1307–1317. [Google Scholar]
- Wang, X.; Wang, P.; Wang, C.; Qian, J.; Feng, T.; Yang, Y. Relationship between Photosynthetic Capacity and Microcystin Production in Toxic Microcystis Aeruginosa under Different Iron Regimes. Int. J. Environ. Res. Public Health 2018, 15, 1954. [Google Scholar] [CrossRef]
- Godoy, V.; Blázquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M.A. The potential of microplastics as carriers of metals. Environ. Pollut. 2019, 255, 113363. [Google Scholar] [CrossRef]
- Yang, G.L.; Zheng, M.M.; Liao, H.M.; Tan, A.J.; Feng, D.; Lv, S.M. Influence of cadmium and microplastics on physiological responses, ultrastructure and rhizosphere microbial community of duckweed. Ecotoxicol. Environ. Saf. 2022, 243, 114011. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, W.T.; Dai, Y.H.; Liu, L.J.; Wang, Z.Y.; Yu, X.Y.; Zhang, J.Z.; Wang, X.K.; Xing, B.S. Uptake, Distribution, and Transformation of CuO NPs in a Floating Plant Eichhornia crassipes and Related Stomatal Responses. Environ. Sci. Technol. 2017, 51, 7686–7695. [Google Scholar] [CrossRef]
- Ho, J.C.; Michalak, A.M.; Pahlevan, N. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef]
- Kaur, M.; Yang, K.; Wang, L.; Xu, M. Interactive effects of polyethylene microplastics and cadmium on growth of Glycine max. Environ. Sci. Pollut. Res. 2023, 30, 101178–101191. [Google Scholar] [CrossRef]
- Kaur, M.; Shen, C.C.; Wang, L.; Xu, M. Exploration of Single and Co-Toxic Effects of Polypropylene Micro-Plastics and Cadmium on Rice (Oryza sativa L.). Nanomaterials 2022, 12, 3967. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Kang, W. Bioremediation of eutrophicated water by Acinetobacter Calcoaceticus. B. Environ. Contam. Tox. 2007, 78, 527–530. [Google Scholar] [CrossRef]
- Han, Z.W.; Osman, R.; Liu, Y.; Wei, Z.D.; Wang, L.; Xu, M. Analyzing the impacts of cadmium alone and in co-existence with polypropylene microplastics on wheat growth. Front. Plant Sci. 2023, 14, 1240472. [Google Scholar] [CrossRef]
- Hu, W.; Ma, J.; Qin, B. Analysis on scientific knowledge graph of global algal bloom studies. Chin. Sci. Bull. Chin. 2023, 68, 3196–3210. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, J.; Dong, W. Influence mechanism of light on common algae and its application. Environ. Eng. 2019, 37, 111–116. [Google Scholar]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, X.H.; Wang, J.T.; Tan, L.J. Toxic effects of microplastic on marine microalgae Skeletonema costatum: Interactions between microplastic and algae. Environ. Pollut. 2017, 220, 1282–1288. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Wang, Y.C.; Liu, Q.H.; Jiao, X.D.; Wang, L. Research Progress on Biological Function of Microcystins. Asian J. Ecotoxicol. 2023, 18, 128–140. [Google Scholar]
- Tanvir, R.U.; Hu, Z.Q.; Zhang, Y.Y.; Lu, J.R. Cyanobacterial community succession and associated cyanotoxin production in hypereutrophic and eutrophic freshwaters. Environ. Pollut. 2021, 290, 118056. [Google Scholar] [CrossRef]
- Feng, L.J.; Sun, X.D.; Zhu, F.P.; Feng, Y.; Duan, J.L.; Xiao, F.; Li, X.Y.; Shi, Y.; Wang, Q.; Sun, J.W.; et al. Nanoplastics Promote Microcystin Synthesis and Release from Cyanobacterial Microcystis aeruginosa. Environ. Sci. Technol. 2020, 54, 3386–3394. [Google Scholar] [CrossRef]
- Feng, A.W.; Sun, Z.T.; Wang, Y.W.; Cao, X.F.; Li, Y.H. Toxic effects of micro/nanoplastics with environmental concentration on Microcystis aeruginosa. Acta Sci. Circumstantiae 2023, 43, 362–370. [Google Scholar]
- Wang, Q.; Wangjin, X.; Zhang, Y.; Wang, N.; Wang, Y.; Meng, G.; Chen, Y. The toxicity of virgin and UV-aged PVC microplastics on the growth of freshwater algae Chlamydomonas reinhardtii. Sci. Total Environ. 2020, 749, 141603. [Google Scholar] [CrossRef]


| Ingredient | Concentration of Each Component in the Medium (mg/L) | Stock Solution (g/L) | Preparation of 1 L Culture Medium Amount of Stock Solution Required (mL) |
|---|---|---|---|
| NaNO3 | 1500 | 150 | Mother liquor 1 10 mL |
| K2HPO4·3H2O | 40 | 4 | |
| Na2CO3 | 20 | 2 | |
| CaCl2·2H2O | 36 | 27.2 | Mother liquor 2 1 mL |
| MgSO4·7H2O | 75 | 7.5 | Mother liquor 3 1 mL |
| C6H8O7 | 6 | 6 | Mother liquor 4 1 mL |
| (NH4)3Fe(C6H5O7)2 | 6 | 6 | |
| Na2EDTA | 1 | 1 | |
| H3BO3 | 2.86 | 2.86 | Mother liquor 5 1 mL |
| MnCl2·4H2O | 1.81 | 1.81 | |
| ZnSO4·7H2O | 0.22 | 0.22 | |
| CuSO4·5H2O | 0.079 | 0.079 | |
| Na2MoO4·2H2O | 0.039 | 0.039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Z.; Xiong, Z.; Wei, Z.; Wang, L.; Xu, M. Interactive Effects of Polyethylene Microplastics and Cadmium on Growth of Microcystis aeruginosa. Toxics 2024, 12, 254. https://doi.org/10.3390/toxics12040254
Xue Z, Xiong Z, Wei Z, Wang L, Xu M. Interactive Effects of Polyethylene Microplastics and Cadmium on Growth of Microcystis aeruginosa. Toxics. 2024; 12(4):254. https://doi.org/10.3390/toxics12040254
Chicago/Turabian StyleXue, Zihan, Zetao Xiong, Zhangdong Wei, Lin Wang, and Ming Xu. 2024. "Interactive Effects of Polyethylene Microplastics and Cadmium on Growth of Microcystis aeruginosa" Toxics 12, no. 4: 254. https://doi.org/10.3390/toxics12040254






