Prenatal PM2.5 Exposure and Its Association with Low Birth Weight: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. PECO
2.2. Search Strategy
2.3. Study Selection
2.4. Outcome Measurement
2.5. Data Extraction and Quality Assessment
- Selection (0–4 stars): Evaluation of exposure, selection of the non-exposed cohort, representativeness of the exposed cohort, and proof that the outcome of interest was absent at the beginning of the trial.
- Comparability (0–2 stars): Evaluation of the confounding variables, such as age, socioeconomic status, and health issues.
- Outcome (0–3 stars): Evaluation of the result, length of the monitoring period, and sufficiency of the monitoring of the groups.
2.6. Ethical Considerations
3. Results
3.1. Study Selection and Characteristics
3.2. PM2.5 Exposure and LBW Diagnostic
3.3. Quality Assessment
3.4. Result and Discussion
3.4.1. Impact of Prenatal PM2.5 Exposure on LBW
3.4.2. Effect of PM2.5 Exposure on LBW across Different Trimesters
3.4.3. Biological Pathways of PM2.5 Exposure in Pregnancy and LBW
3.5. Limitation of this Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amnuaylojaroen, T.; Parasin, N. Perspective on Particulate Matter: From Biomass Burning to the Health Crisis in Mainland Southeast Asia. Toxics 2023, 11, 553. [Google Scholar] [CrossRef] [PubMed]
- Brunekreef, B.; Holgate, S.T. Air pollution and health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., III; Dockery, D.W. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef] [PubMed]
- Perera, F.; Ashrafi, A.; Kinney, P.; Mills, D. Towards a fuller assessment of benefits to children’s health of reducing air pollution and mitigating climate change due to fossil fuel combustion. Environ. Res. 2019, 172, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Son, J.; Cho, Y. Effect of Air Pollution on Low Birth Weight in Seoul of Korea, 1999–2003. Epidemiology 2007, 18, S45. [Google Scholar] [CrossRef]
- Parasin, N.; Amnuaylojaroen, T.; Saokaew, S. Effect of air pollution on weight gain and obesity in children: A systematic review and meta-analysis. Children 2021, 8, 327. [Google Scholar] [CrossRef]
- Amnuaylojaroen, T.; Parasin, N.; Saokaew, S. Exploring the Association Between Air Pollution Exposure and Autism Spectrum Disorders in Children: A Systematic Review and Meta-Analysis of Cohort Studies. Reprod. Toxicol. 2024, 108, 582. [Google Scholar] [CrossRef]
- Liu, S.; Krewski, D.; Shi, Y.; Chen, Y.; Burnett, R.T. Association between maternal exposure to ambient air pollutants during pregnancy and fetal growth restriction. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, Q.; Sui, X.; Ding, L.; Liu, J.; Yang, M.; Cao, J. Associations between air pollution exposure and birth defects: A time series analysis. Environ. Geochem. Health 2021, 43, 4379–4394. [Google Scholar] [CrossRef]
- Parasin, N.; Amnuaylojaroen, T.; Saokaew, S. Exposure to PM10, PM2.5, and NO2 and gross motor function in children: A systematic review and meta-analysis. Eur. J. Pediatr. 2023, 182, 1495–1504. [Google Scholar] [CrossRef]
- Flores-Pajot, M.C.; Ofner, M.; Do, M.T.; Lavigne, E.; Villeneuve, P.J. Childhood autism spectrum disorders and exposure to nitrogen dioxide, PM10, and PM2.5 air pollution: A review and meta-analysis. Environ. Res. 2016, 151, 763–776. [Google Scholar] [CrossRef]
- Hertz-Picciotto, I.; Schmidt, R.J.; Krakowiak, P. Understanding environmental contributions to autism: Causal concepts and the state of science. Autism Res. 2018, 11, 554–586. [Google Scholar] [CrossRef]
- Woodruff, T.J.; Grillo, J.; Schoendorf, K.C. The relationship between selected causes of postneonatal infant mortality and particulate air pollution in the United States. Environ. Health Perspect. 1997, 105, 608–612. [Google Scholar] [CrossRef]
- Block, M.L.; Calderón-Garcidueñas, L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Cole, T.B.; Coburn, J.; Chang, Y.C.; Dao, K.; Roqué, P.J. Neurotoxicity of traffic-related air pollution. Neurotoxicology 2017, 59, 133–139. [Google Scholar] [CrossRef] [PubMed]
- de Melo, J.O.; Soto, S.F.; Katayama, I.A.; Watanabe, I.S.; Fonseca, B.B.; Barbosa, F.; Souza, A.A.; Lopes, R.M.; Heimann, J.C.; Saldiva, P.H.; et al. Inhalation of fine particulate matter during pregnancy increased IL-4 cytokine levels and causes DNA damage in fetuses. Toxicol. Lett. 2015, 232, 618–625. [Google Scholar] [CrossRef]
- Toro-Heredia, J.; Jirau-Colón, H.; Jiménez-Vélez, B.D. Linking PM2.5 organic constituents, relative toxicity and health effects in Puerto Rico. Environ. Chall. 2021, 5, 100350. [Google Scholar] [CrossRef]
- Kalkbrenner, A.E.; Windham, G.C.; Serre, M.L.; Akita, Y.; Wang, X.; Hoffman, K.; Thayer, B.P.; Daniels, J.L. Particulate matter exposure, prenatal and postnatal windows of susceptibility, and autism spectrum disorders. Epidemiology 2015, 26, 30–42. [Google Scholar] [CrossRef]
- Volk, H.E.; Lurmann, F.; Penfold, B.; Hertz-Picciotto, I.; McConnell, R. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry 2013, 70, 71–77. [Google Scholar] [CrossRef]
- Raz, R.; Roberts, A.L.; Lyall, K.; Hart, J.E.; Just, A.C.; Laden, F.; Kloog, I.; Weisskopf, M.G. Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: A nested case-control analysis within the Nurses’ Health Study II cohort. Environ. Health Perspect. 2015, 123, 264–270. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Liu, D.; Ye, F.; Sun, Q.; Huang, Q.; Dong, J.; Pei, T.; He, Y.; Zhang, Q. Prenatal exposure to particulate matter and term low birth weight: Systematic review and meta-analysis. Environ. Sci. Pollut. Res. 2023, 30, 63335–63346. [Google Scholar] [CrossRef] [PubMed]
- Andersen, Z.J.; Andersen, K.K.; Ravnskjær, L.; Brandt, J.; Becker, T.; Ketzel, M.; Hertel, O.; Lynge, E. Long-term Exposure to Fine Particulate Matter and Breast Cancer Incidence: The Danish Nurse Cohort. ISEE Conf. Abstr. 2016, 28, P2–P339. [Google Scholar] [CrossRef]
- Ghosh, R.; Causey, K.; Burkart, K.; Wozniak, S.; Cohen, A.; Brauer, M. Ambient and household PM2.5 pollution and adverse perinatal outcomes: A meta-regression and analysis of attributable global burden for 204 countries and territories. PLoS Med. 2021, 18, e1003718. [Google Scholar] [CrossRef] [PubMed]
- Lyall, K.; Schmidt, R.J.; Hertz-Picciotto, I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int. J. Epidemiol. 2014, 43, 443–464. [Google Scholar] [CrossRef] [PubMed]
- Chaste, P.; Leboyer, M. Autism risk factors: Genes, environment, and gene-environment interactions. Dialogues Clin. Neurosci. 2012, 14, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2013. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 May 2024).
- Rodríguez-Fernández, A.; Ramos-Castillo, N.; Ruiz-De la Fuente, M.; Parra-Flores, J.; Maury-Sintjago, E. Association of prematurity and low birth weight with gestational exposure to PM2.5 and PM10 particulate matter in chileans newborns. Int. J. Environ. Res. Public Health 2022, 19, 6133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fan, C.; Ren, Z.; Feng, H.; Zuo, S.; Hao, J.; Liao, J.; Zou, Y.; Ma, L. Maternal PM2.5 exposure triggers preterm birth: A cross-sectional study in Wuhan, China. Glob. Health Res. Policy 2020, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Golan, R.; Kloog, I.; Almog, R.; Gesser-Edelsburg, A.; Negev, M.; Jolles, M.; Shalev, V.; Eisenberg, V.H.; Koren, G.; Abu Ahmad, W.; et al. Environmental exposures and fetal growth: The Haifa pregnancy cohort study. BMC Public Health 2018, 18, 132. [Google Scholar] [CrossRef] [PubMed]
- Nachman, R.M.; Mao, G.; Zhang, X.; Hong, X.; Chen, Z.; Soria, C.S.; He, H.; Wang, G.; Caruso, D.; Pearson, C.; et al. Intrauterine inflammation and maternal exposure to ambient PM2.5 during preconception and specific periods of pregnancy. Epidemiology 2016, 27, 14–24. [Google Scholar] [CrossRef]
- Gehring, U.; Wijga, A.H.; Brauer, M.; Fischer, P.; de Jongste, J.C.; Kerkhof, M.; Oldenwening, M.; Smit, H.A.; Brunekreef, B. Air pollution exposure and lung function in children: The ESCAPE project. Environ. Health Perspect. 2013, 121, 1357–1364. [Google Scholar] [CrossRef]
- Zhou, S.; Lin, L.; Bao, Z.; Meng, T.; Wang, S.; Chen, G.; Li, Q.; Liu, Z.; Bao, H.; Han, N.; et al. The association of prenatal exposure to particulate matter with infant growth: A birth cohort study in Beijing, China. Environ. Pollut. 2021, 277, 116792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ye, T.; Yu, P.; Xu, R.; Chen, G.; Yu, W.; Song, J.; Guo, Y.; Li, S. Preterm birth and term low birth weight associated with wildfire-specific PM2. 5: A cohort study in New South Wales, Australia during 2016–2019. Environ. Int. 2023, 174, 107879. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Hu, H.; Roussos-Ross, D.; Kim, H.; Roth, J.; Xu, X. The effects of air pollution on adverse birth outcomes. Environ. Res. 2014, 134, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Liang, H.; Yan, L.; Qiu, H.; Yang, Y.; Li, L.; Ma, W. Impact of air pollution exposure during pregnancy on adverse birth outcomes: A cohort study. Sci. Total Environ. 2021, 755, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Ming, X.; Yang, Y.; Hu, Y.; He, Z.; Chen, H.; Zhou, X. Associations between maternal exposure to ambient air pollution and very low birth weight: A birth cohort study in Chongqing, China. Front. Public Health 2023, 11, 1123594. [Google Scholar] [CrossRef] [PubMed]
- Balidemaj, F.; Flanagan, E.; Malmqvist, E.; Rittner, R.; Källén, K.; Åström, D.O.; Oudin, A. Prenatal Exposure to Locally Emitted Air Pollutants Is Associated with Birth Weight: An Administrative Cohort Study from Southern Sweden. Toxics 2022, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Strosnider, H.; Balluz, L.; Qualters, J.R. Geographic variation in the association between ambient fine particulate matter (PM2.5) and term low birth weight in the United States. Environ. Health Perspect. 2016, 124, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Bloemsma, L.D.; Dabelea, D.; Thomas, D.S.; Peel, J.L.; Adgate, J.L.; Allshouse, W.B.; Martenies, S.E.; Magzamen, S.; Starling, A.P. Prenatal exposure to ambient air pollution and traffic and indicators of adiposity in early childhood: The Healthy Start study. Int. J. Obes. 2022, 46, 494–501. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines on Optimal Feeding of Low Birth-Weight Infants in Low-and Middle-Income Countries; World Health Organization: Geneva, Switzerland, 2011; Available online: https://www.who.int/publications/i/item/9789241548366 (accessed on 14 June 2024).
- Spong, C.Y. Defining “term” pregnancy: Recommendations from the Defining “Term” Pregnancy Workgroup. JAMA 2013, 309, 2445–2446. [Google Scholar] [CrossRef]
- Volk, H.E.; Hertz-Picciotto, I.; Delwiche, L.; Lurmann, F.; McConnell, R. Residential proximity to freeways and autism in the CHARGE study. Environ. Health Perspect. 2011, 119, 873–877. [Google Scholar] [CrossRef]
- Šrám, R.J.; Binková, B.; Dejmek, J.; Bobak, M. Ambient air pollution and pregnancy outcomes: A review of the literature. Environ. Health Perspect. 2005, 113, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Luo, X.; Zhao, C.; Zhang, B.; Tao, J.; Yang, Z.; Liu, T. The associations between birth weight and exposure to fine particulate matter (PM2.5) and its chemical constituents during pregnancy: A meta-analysis. Environ. Pollut. 2016, 211, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Janssen, B.G.; Godderis, L.; Pieters, N.; Poels, K.; Kiciński, M.; Cuypers, A.; Fierens, F.; Penders, J.; Plusquin, M.; Gyselaers, W.; et al. Placental DNA hypomethylation in association with particulate air pollution in early life. Part. Fibre Toxicol. 2013, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, C.E.; Kincaid, R.L.; Mirando, M.A. The role of essential trace elements in embryonic and fetal development in livestock. Vet. J. 2003, 166, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P. Fetal and infant origins of adult disease. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A. Living with the past: Evolution, development, and patterns of disease. Science 2004, 305, 1733–1736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gong, X.; Han, B.; Chu, M.; Gong, C.; Yang, J.; Zhang, Y. Ambient PM2.5 exposures and systemic inflammation in women with early pregnancy. Sci. Total Environ. 2022, 829, 154564. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhou, Y.; Zhao, Y.; Zhao, W.; Wang, H.; Li, J.; Zhang, Y. Prenatal fine particulate matter exposure associated with placental small extracellular vesicle derived microRNA and child neurodevelopmental delays. Sci. Total Environ. 2022, 841, 156747. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., 3rd; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Peters, A. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Gurgueira, S.A.; Lawrence, J.; Coull, B.; Murthy, G.G.; González-Flecha, B. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ. Health Perspect. 2002, 110, 749–755. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, X.; Geng, X.; Yue, H.; Li, G.; Sang, N. Maternal PM2.5 exposure and abnormal placental nutrient transport. Ecotoxicol. Environ. Saf. 2021, 207, 111281. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Lesseur, C.; Deyssenroth, M.A.; Kloog, I.; Schwartz, J.D.; Marsit, C.J.; Chen, J. PM2.5 exposure during pregnancy is associated with altered placental expression of lipid metabolic genes in a US birth cohort. Environ. Res. 2022, 211, 113066. [Google Scholar] [CrossRef] [PubMed]
- van den Hooven, E.H.; Jaddoe, V.W.V.; de Kluizenaar, Y.; Hofman, A.; Mackenbach, J.P.; Steegers, E.A.; Brunekreef, B. Residential traffic exposure and pregnancy-related outcomes: A prospective birth cohort study. Environ. Health 2009, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Veras, M.M.; Damaceno-Rodrigues, N.R.; Caldini, E.G.; Ribeiro, A.A.M.; Mayhew, T.M.; Saldiva, P.H.; Dolhnikoff, M. Particulate urban air pollution affects the functional morphology of mouse placenta. Biol. Reprod. 2008, 79, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, J.; Gao, L.; Sang, Y.; Li, X.; Zhou, G.; Ren, L. Maternal exposure to PM2.5 disrupting offspring spermatogenesis through induced sertoli cells apoptosis via inhibin B hypermethylation in mice. Ecotoxicol. Environ. Saf. 2022, 241, 113760. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Song, I.G.; Kim, K.N.; Kim, M.S.; Chung, S.H.; Choi, Y.S.; Bae, C.W. Maternal exposure to particulate matter during pregnancy and adverse birth outcomes in the Republic of Korea. Int. J. Environ. Res. Public Health 2019, 16, 633. [Google Scholar] [CrossRef]
- Clemente, D.B.; Casas, M.; Vilahur, N.; Begiristain, H.; Bustamante, M.; Carsin, A.E.; Nawrot, T.S. Prenatal ambient air pollution, placental mitochondrial DNA content, and birth weight in the INMA (Spain) and ENVIRONAGE (Belgium) birth cohorts. Environ. Health Perspect. 2016, 124, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Postolow, F.; Dakshinamurti, S. Fetal Oxygenation during Maternal Hypoxic Illness. In Hypoxic Respiratory Failure in the Newborn; CRC Press: Boca Raton, FL, USA, 2021; pp. 51–56. [Google Scholar]
- Basu, R.; Harris, M.; Sie, L.; Malig, B.; Broadwin, R.; Green, R. Effects of fine particulate matter and its constituents on low birth weight among full-term infants in California. Environ. Res. 2014, 128, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.; Belanger, K.; Ebisu, K.; Bell, M.L. Fine particulate matter and risk of preterm birth and preeclampsia in Perinatal Collaborative Research Network cohort. Environ. Health Perspect. 2014, 122, 1104–1110. [Google Scholar]
- Khorami-Sarvestani, S.; Vanaki, N.; Shojaeian, S.; Zarnani, K.; Stensballe, A.; Jeddi-Tehrani, M.; Zarnani, A.H. Placenta: An old organ with new functions. Front. Immunol. 2024, 15, 1385762. [Google Scholar] [CrossRef]
- Saenen, N.D.; Plusquin, M.; Bijnens, E.; Janssen, B.G.; Gyselaers, W.; Cox, B.; Nawrot, T.S. In utero fine particle air pollution and placental expression of genes in the brain-derived neurotrophic factor signaling pathway: An ENVIRONAGE birth cohort study. Environ. Health Perspect. 2015, 123, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Huo, X.; Cheng, Z.; Faas, M.M.; Xu, X. Early-life exposure to widespread environmental toxicants and maternal-fetal health risk: A focus on metabolomic biomarkers. Sci. Total Environ. 2020, 739, 139626. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.H.; Nelin, T.; Gorr, M.W.; Wold, L.E. Early life exposure to air pollution: How bad is it? Toxicol. Lett. 2013, 216, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Loftus, C.T.; Ni, Y.; Szpiro, A.A.; Hazlehurst, M.F.; Tylavsky, F.A.; Bush, N.R.; LeWinn, K.Z. Exposure to ambient air pollution and early childhood behavior: A longitudinal cohort study. Environ. Res. 2020, 183, 109075. [Google Scholar] [CrossRef] [PubMed]
- Pardi, G.; Cetin, I. Human fetal growth and organ development: 50 years of discoveries. Am. J. Obstet. Gynecol. 2006, 194, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Stieb, D.M.; Chen, L.; Eshoul, M.; Judek, S. Ambient air pollution, birth weight and preterm birth: A systematic review and meta-analysis. Environ. Res. 2012, 117, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Trasande, L.; Wong, K.; Roy, A.; Savitz, D.A.; Thurston, G. Exploring prenatal outdoor air pollution, birth outcomes and neonatal health care utilization in a nationally representative sample. Environ. Res. 2013, 122, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Amnuaylojaroen, T.; Parasin, N. Pathogenesis of PM2.5-Related Disorders in Different Age Groups: Children, Adults, and the Elderly. Epigenomes 2024, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Vilotić, A.; Nacka-Aleksić, M.; Pirković, A.; Bojić-Trbojević, Ž.; Dekanski, D.; Jovanović Krivokuća, M. IL-6 and IL-8: An overview of their roles in healthy and pathological pregnancies. Int. J. Mol. Sci. 2022, 23, 14574. [Google Scholar] [CrossRef]
- Villarroel, F.; Ponce, N.; Gómez, F.A.; Muñoz, C.; Ramírez, E.; Nualart, F.; Salinas, P. Exposure to Fine Particulate Matter 2.5 from Wood Combustion Smoke Causes Vascular Changes in Placenta and Reduce Fetal Size. Reprod. Toxicol. 2024, 127, 108610. [Google Scholar] [CrossRef]
- Erickson, A.C.; Arbour, L. The shared pathoetiological effects of particulate air pollution and the social environment on fetal-placental development. J. Environ. Public Health 2014, 2014, 901014. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Bunkar, N.; Kumar, R.; Bhargava, A.; Tiwari, R.; Chaudhury, K.; Mishra, P.K. Air pollution associated epigenetic modifications: Transgenerational inheritance and underlying molecular mechanisms. Sci. Total Environ. 2019, 656, 760–777. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Hu, B.; Liu, Y.; Xu, J.; Yang, G.; Xu, D.; Chen, C. Beyond PM2.5: The role of ultrafine particles on adverse health effects of air pollution. Biochim. Biophys. Acta, Gen. Subj. 2016, 1860, 2844–2855. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, D.; Liu, X.; Zhu, J.; Wang, F.; Li, B.; Li, L. Effects of PM2.5 exposure on reproductive system and its mechanisms. Chemosphere 2021, 264, 128436. [Google Scholar] [CrossRef]



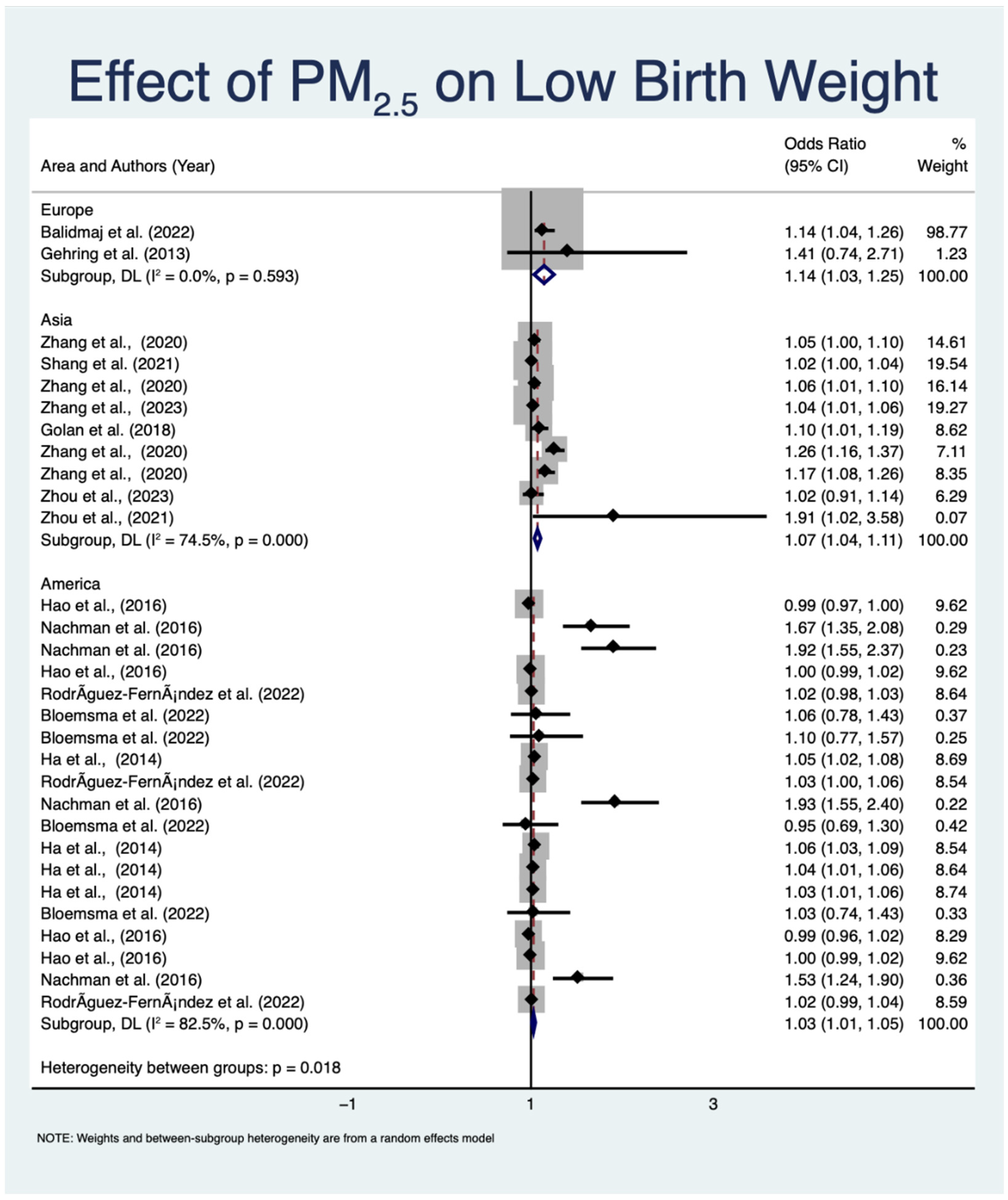
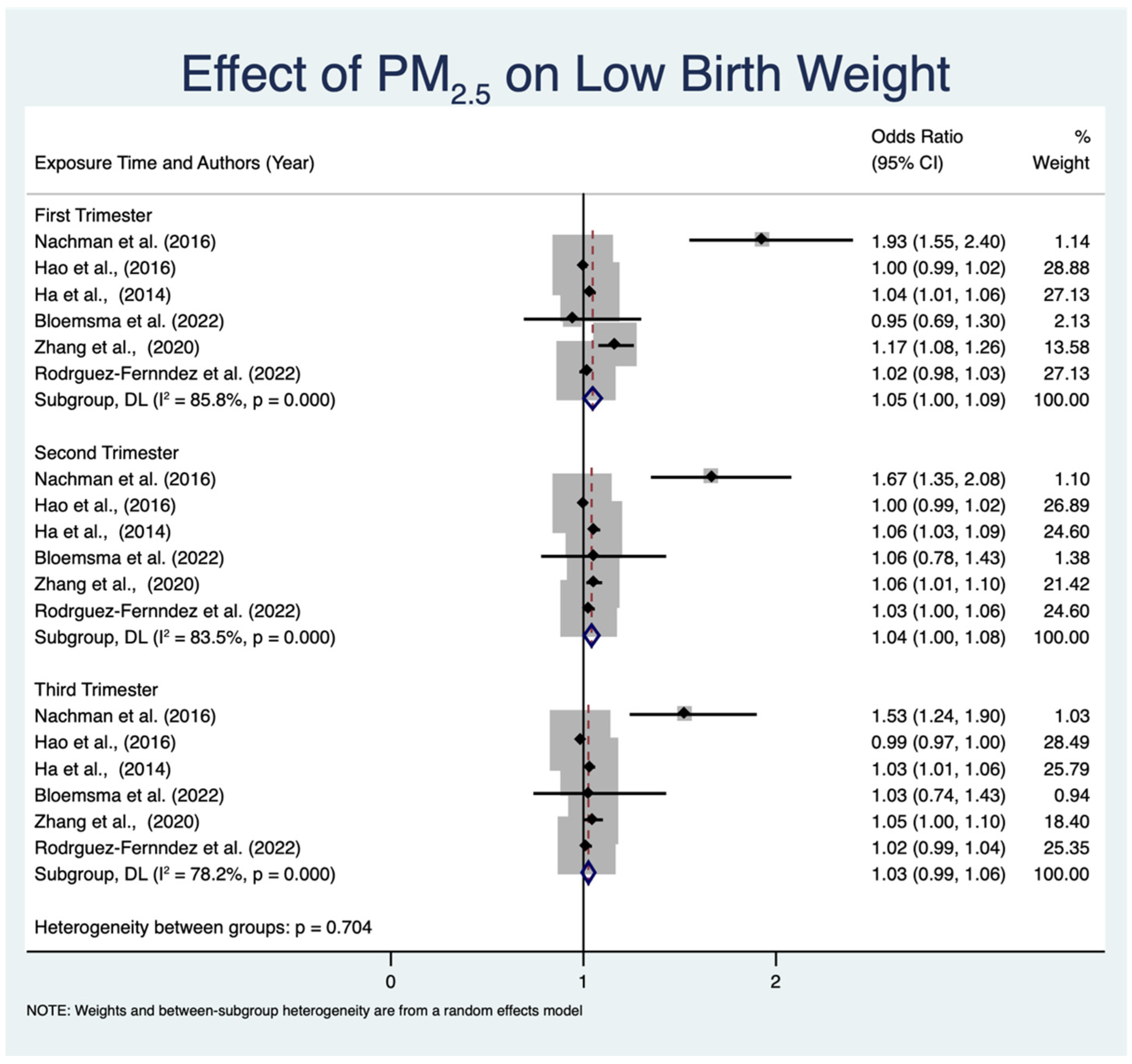
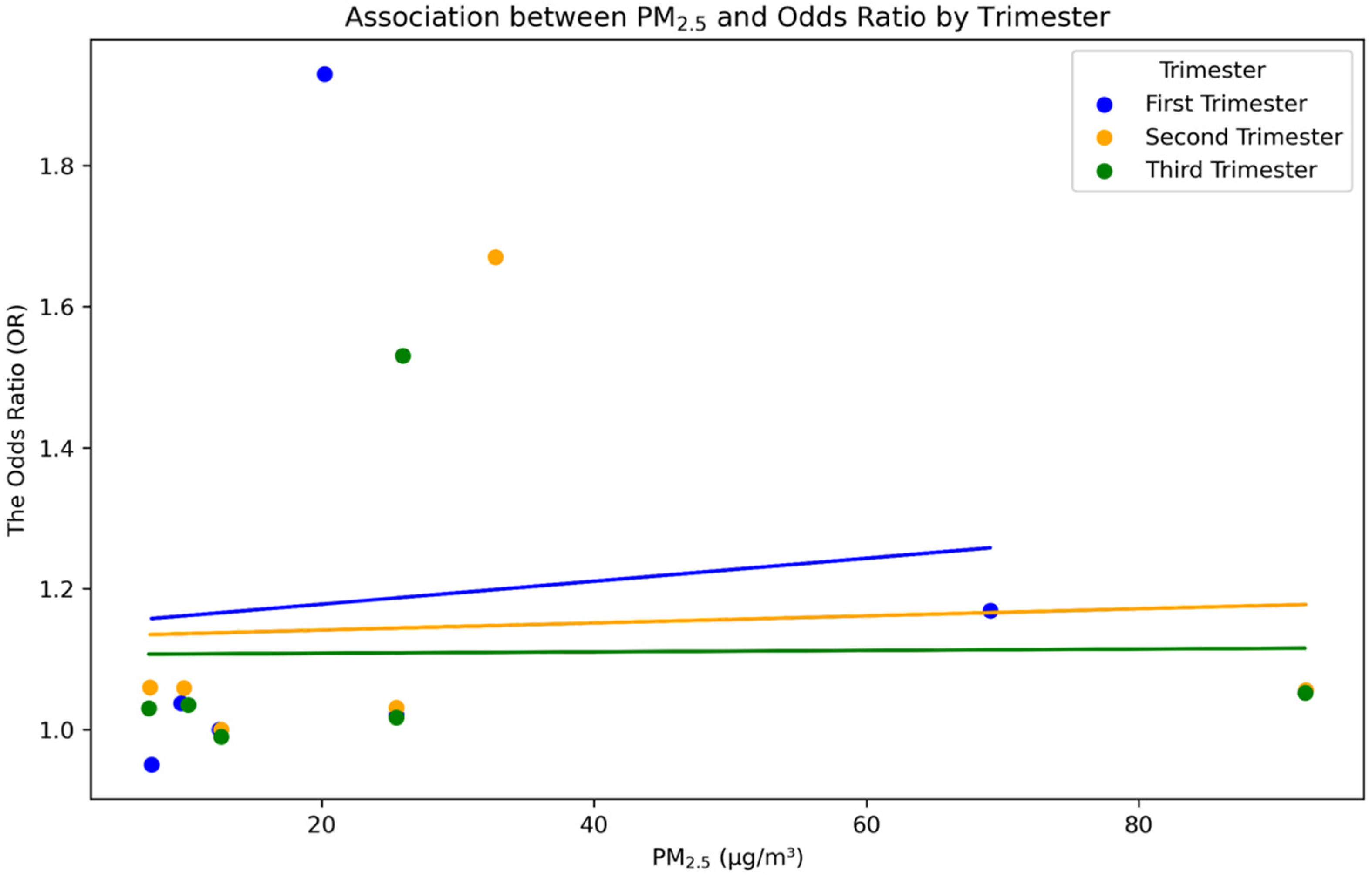
| No. | Author (Year) | Country (Study Design) | Number of Participants | Assessment Tool | Estimated PM2.5 Concentration | Air Pollution Exposure Period | Exposure Assessment | Main Finding |
|---|---|---|---|---|---|---|---|---|
| 1 | Rodríguez-Fernández et al. [27] | Chile (cross-sectional study) | 595,369 | Low birth weight | 25.5 µg/m3 to 55.7 µg/m3 | Specific trimesters | Air Quality Information System data | Maternal intake of PM2.5 within the second trimester of pregnancy increases the probability of giving birth to a baby with LBW. |
| 2 | Zhang et al. [28] | China (cohort study) | 3369 | Low birth weight | 58.53 µg/m3 to 129.53 µg/m3 | Entire pregnancy | Land-use regression models | There is a strong correlation between PM2.5 and LBW. |
| 3 | Golan et al. [29] | Israel (cohort study) | 750,000 | Low birth weight | 12.5 µg/m3 to 25.1 µg/m3 | Entire pregnancy | Air pollution monitoring data | There is a direct correlation between exposure to PM2.5 and LBW. |
| 4 | Nachman et al. [30] | USA (cohort study) | 5059 | Low birth weight | 5.54 µg/m3 to 29 µg/m3 | Entire pregnancy | National Environmental Public Health Tracking Network data | Exposure to PM2.5 is linked to a higher likelihood of LBW. |
| 5 | Gehring et al. [31] | Europe (cohort study) | 5921 | Low birth weight | 15.3 µg/m3 to 21.1 µg/m3 | Entire pregnancy | Land-use regression models | Exposure to PM2.5 is associated with reduced birth weights. |
| 6 | Zhou et al. [32] | China (cohort study) | 13,335 | Low birth weight | 48.6 µg/m3 to 85.5 µg/m3 | Entire pregnancy | Air pollution monitoring data | Elevated susceptibility to reduced birth weight is associated with exposure to PM2.5. |
| 7 | Zhang et al. [33] | Australia (cohort study) | 330,884 | Low birth weight | 0.5 µg/m3 to 4.02 µg/m3 | Entire pregnancy | Satellite-based exposure methods | A strong correlation exists between PM2.5 and LBW. |
| 8 | Ha et al. [34] | USA (cohort study) | 423,719 | Low birth weight | 9.7 µg/m3 to 10.2 µg/m3 | Entire pregnancy | National air pollution monitoring network | Exposure to PM2.5 is linked to reduced birth weight. |
| 9 | Shang et al. [35] | China (cohort study) | 536,993 | Low birth weight | 13.20 µg/m3 to 115.36 µg/m3 | Entire pregnancy | Generalized Additive Model | Exposure to PM2.5 is linked to both LBW and preterm birth. |
| 10 | Zhou et al. [36] | China (cohort study) | 572,106 | Low birth weight | 17.82 µg/m3 to 83.65 µg/m3 | Entire pregnancy and specific trimesters | Generalized Additive Model | There is a strong correlation between exposure to PM2.5 and the occurrence of very LBW. |
| 11 | Balidemaj et al. [37] | Sweden (cohort study) | 43,256 | Low birth weight | 0.3 µg/m3 to 4.2 µg/m3 | Entire pregnancy | Land-use regression models | Exposure to PM2.5 is associated with reduced birth weight. |
| 12 | Hao et al. [38] | USA (cohort study) | 3,389,450 | Low birth weight | 4.7 μg/m3 to 23.8 μg/m3 | Entire pregnancy | Air pollution monitoring data | Exposure to PM2.5 is linked to a higher likelihood of LBW. |
| 13 | Bloemsma et al. [39] | USA (cohort study) | 1410 | Low birth weight | 6.8 µg/m3 to 8.2 µg/m3 | Entire pregnancy | Air pollution monitoring data | There is a direct correlation between exposure to PM2.5 and LBW. |
| No. | Author (Year) | Selection (Max 4) | Comparability (Max 2) | Outcome (Max 3) | Total Score (Max 9) |
|---|---|---|---|---|---|
| 1 | Rodríguez-Fernández et al. [27] | 3 | 1 | 2 * | 6 * |
| 2 | Zhang et al. [28] | 4 | 2 | 3 | 9 |
| 3 | Golan et al. [29] | 3 | 2 | 3 | 8 |
| 4 | Nachman et al. [30] | 4 | 1 | 2 * | 7 * |
| 5 | Gehring et al. [31] | 3 | 2 | 2 * | 7 * |
| 6 | Zhou et al. [32] | 4 | 2 | 3 | 9 |
| 7 | Zhang et al. [33] | 4 | 2 | 3 | 9 |
| 8 | Ha et al. [34] | 4 | 1 | 2 * | 7 * |
| 9 | Shang et al. [35] | 4 | 2 | 3 | 9 |
| 10 | Zhou et al. [36] | 4 | 2 | 3 | 9 |
| 11 | Balidmaj et al. [37] | 3 | 1 | 2 * | 6 * |
| 12 | Hao et al. [38] | 4 | 1 | 2 * | 7 * |
| 13 | Bloemsma et al. [39] | 3 | 2 | 2 * | 7 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parasin, N.; Amnuaylojaroen, T.; Saokaew, S. Prenatal PM2.5 Exposure and Its Association with Low Birth Weight: A Systematic Review and Meta-Analysis. Toxics 2024, 12, 446. https://doi.org/10.3390/toxics12070446
Parasin N, Amnuaylojaroen T, Saokaew S. Prenatal PM2.5 Exposure and Its Association with Low Birth Weight: A Systematic Review and Meta-Analysis. Toxics. 2024; 12(7):446. https://doi.org/10.3390/toxics12070446
Chicago/Turabian StyleParasin, Nichapa, Teerachai Amnuaylojaroen, and Surasak Saokaew. 2024. "Prenatal PM2.5 Exposure and Its Association with Low Birth Weight: A Systematic Review and Meta-Analysis" Toxics 12, no. 7: 446. https://doi.org/10.3390/toxics12070446






