Research Progress on Micro (Nano)Plastics Exposure-Induced miRNA-Mediated Biotoxicity
Abstract
:1. Introduction
2. miRNAs Are Involved in Neurotoxicity and Immunotoxicity Caused by Microplastic Exposure
3. Mechanisms of miRNAs in Microplastic-Induced Injury
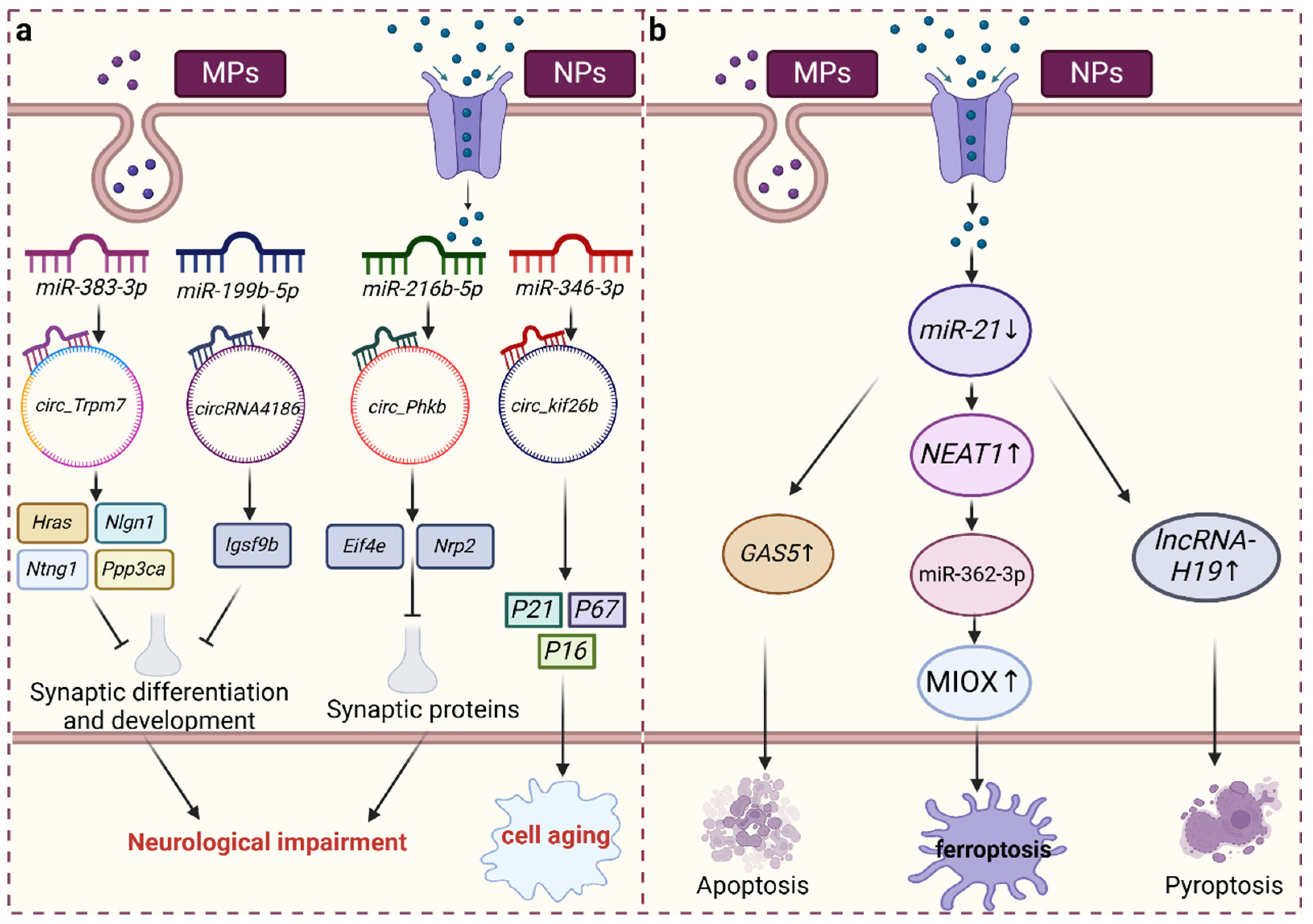
3.1. Role of miRNAs in MNP-Induced Injury
3.2. CircRNA Acts as a “Sponge” of miRNA and Plays a Role in Injury Induced by MNP Exposure
3.3. lncRNAs, as ceRNAs of miRNA, Are Involved in Injury Induced by MNP Exposure
4. miRNA-Mediated Mechanism of MNP Toxicity
5. Changes in miRNAs Induced by MNPs Are Involved in the Occurrence and Development of Related Diseases
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vethaak, A.D.; Legler, J. Microplastics and Human Health. Science 2021, 371, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Nizzetto, L.; Futter, M.; Langaas, S. Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.H.D. Microplastics in Agricultural Soils in China: Sources, Impacts and Solutions. Environ. Pollut. 2023, 322, 121235. [Google Scholar] [CrossRef]
- Prüst, M.; Meijer, J.; Westerink, R.H.S. The Plastic Brain: Neurotoxicity of Micro- and Nanoplastics. Part. Fibre Toxicol. 2020, 17, 24. [Google Scholar] [CrossRef]
- Yang, W.; Jannatun, N.; Zeng, Y.; Liu, T.; Zhang, G.; Chen, C.; Li, Y. Impacts of Microplastics on Immunity. Front. Toxicol. 2022, 4, 956885. [Google Scholar] [CrossRef]
- Li, J.; Yin, K.; Hou, L.; Zhang, Y.; Lu, H.; Ma, C.; Xing, M. Polystyrene Microplastics Mediate Inflammatory Responses in the Chicken Thymus by Nrf2/NF-κB Pathway and Trigger Autophagy and Apoptosis. Environ. Toxicol. Pharmacol. 2023, 100, 104136. [Google Scholar] [CrossRef]
- Xuan, L.; Wang, Y.; Qu, C.; Yi, W.; Yang, J.; Pan, H.; Zhang, J.; Chen, C.; Bai, C.; Zhou, P.-K.; et al. Exposure to Polystyrene Nanoplastics Induces Abnormal Activation of Innate Immunity via the cGAS-STING Pathway. Ecotoxicol. Environ. Saf. 2024, 275, 116255. [Google Scholar] [CrossRef] [PubMed]
- Marcuello, C. Present and Future Opportunities in the Use of Atomic Force Microscopy to Address the Physico-Chemical Properties of Aquatic Ecosystems at the Nanoscale Level. Int. Aquatic Res. 2022, 14, 231–240. [Google Scholar] [CrossRef]
- Zuri, G.; Karanasiou, A.; Lacorte, S. Human Biomonitoring of Microplastics and Health Implications: A Review. Environ. Res. 2023, 237, 116966. [Google Scholar] [CrossRef]
- Li, J.; Weng, H.; Liu, S.; Li, F.; Xu, K.; Wen, S.; Chen, X.; Li, C.; Nie, Y.; Liao, B.; et al. Embryonic Exposure of Polystyrene Nanoplastics Affects Cardiac Development. Sci. Total Environ. 2024, 906, 167406. [Google Scholar] [CrossRef]
- Zhu, Z.; Liao, R.; Shi, Y.; Li, J.; Cao, J.; Liao, B.; Wu, J.; Li, G. Polystyrene Nanoplastics Induce Apoptosis of Human Kidney Proximal Tubular Epithelial Cells via Oxidative Stress and MAPK Signaling Pathways. Environ. Sci. Pollut. Res. Int. 2023, 30, 110579–110589. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, W.; Jiang, H.; Chen, F.; Li, Y.; Gardea-Torresdey, J.L.; Zhou, X.-X.; Yan, B. Surface-Charge-Driven Ferroptosis and Mitochondrial Dysfunction Is Involved in Toxicity Diversity in the Marine Bivalve Exposed to Nanoplastics. ACS Nano 2024, 18, 2370–2383. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Huang, Y.; Zhong, Y.; Li, Z.; Ye, R.; Wang, B.; Zhang, B.; Meng, H.; Lin, X.; Du, J.; et al. Brain Single-Nucleus Transcriptomics Highlights That Polystyrene Nanoplastics Potentially Induce Parkinson’s Disease-like Neurodegeneration by Causing Energy Metabolism Disorders in Mice. J. Hazard. Mater. 2022, 430, 128459. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microRNA Biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Jandura, A.; Krause, H.M. The New RNA World: Growing Evidence for Long Noncoding RNA Functionality. Trends Genet. 2017, 33, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sui, Z.; Zhang, H.; Wang, Y.; Yu, Z. Integrated Analysis of lncRNA-Mediated ceRNA Network in Lung Adenocarcinoma. Front. Oncol. 2020, 10, 554759. [Google Scholar] [CrossRef] [PubMed]
- Kapsimali, M.; Kloosterman, W.P.; de Bruijn, E.; Rosa, F.; Plasterk, R.H.A.; Wilson, S.W. MicroRNAs Show a Wide Diversity of Expression Profiles in the Developing and Mature Central Nervous System. Genome Biol. 2007, 8, R173. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yang, C.; Jiang, C.; Li, L.; Pan, M.; Li, D.; Han, X.; Ding, J. Evaluation of Neurotoxicity in BALB/c Mice Following Chronic Exposure to Polystyrene Microplastics. Environ. Health Perspect. 2022, 130, 107002. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Zhang, Y.; Liu, Q.; Pang, Y.; Niu, Y.; Zhang, R. Identification of ceRNA Network to Explain the Mechanism of Cognitive Dysfunctions Induced by PS NPs in Mice. Ecotoxicol. Environ. Saf. 2022, 241, 113785. [Google Scholar] [CrossRef]
- Rhee, K.-D.; Yu, J.; Zhao, C.Y.; Fan, G.; Yang, X.-J. Dnmt1-Dependent DNA Methylation Is Essential for Photoreceptor Terminal Differentiation and Retinal Neuron Survival. Cell Death Dis. 2012, 3, e427. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, H.; Xiong, Y.; Liu, J. Exosomal miR-199a-5p Derived from Endothelial Cells Attenuates Apoptosis and Inflammation in Neural Cells by Inhibiting Endoplasmic Reticulum Stress. Brain Res. 2020, 1726, 146515. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, J.; Fang, Q.; Shao, H.; Yang, D.; Sun, J.; Gao, L. MiRNA-199a-5p Targets WNT2 to Regulate Depression through the CREB/BDNF Signaling in Hippocampal Neuron. Brain Behav. 2021, 11, e02107. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Yang, X.; Zhao, L.; Calhoun, V.D.; Perrone-Bizzozero, N.; Liu, S.; Jiang, R.; Jiang, T.; Sui, J.; Ma, X. MicroRNA132 Associated Multimodal Neuroimaging Patterns in Unmedicated Major Depressive Disorder. Brain 2018, 141, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; You, X.; Zhou, H.; He, W.; Li, Z.; Li, B.; Xia, J.; Zhu, H.; Zhao, Y.; Yu, G.; et al. MiR-16-5p Regulates Postmenopausal Osteoporosis by Directly Targeting VEGFA. Aging 2020, 12, 9500–9514. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Aceves, M.A.; Cohen, A.; Smith, Y.; Faggio, C. A Potential microRNA Regulation of Immune-Related Genes in Invertebrate Haemocytes. Sci. Total Environ. 2018, 621, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Deng, H.; Zhang, M. Whole Transcriptome Sequencing Analysis Revealed Key RNA Profiles and Toxicity in Mice after Chronic Exposure to Microplastics. Chemosphere 2022, 304, 135321. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Shi, W.; Tang, Y.; Han, Y.; Du, X.; Zhou, W.; Hu, Y.; Zhou, C.; Liu, G. Immunotoxicity of Petroleum Hydrocarbons and Microplastics Alone or in Combination to a Bivalve Species: Synergic Impacts and Potential Toxication Mechanisms. Sci. Total Environ. 2020, 728, 138852. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.; Kim, D.; Kim, H.-Y.; Jeong, S.W.; Lee, S.-G.; Kim, H.-C.; Lee, Y.-J.; Kwon, M.K.; Hwang, J.-S.; Han, J.E.; et al. Microglial Phagocytosis of Polystyrene Microplastics Results in Immune Alteration and Apoptosis in Vitro and in Vivo. Sci. Total Environ. 2022, 807, 150817. [Google Scholar] [CrossRef] [PubMed]
- Diao, L.; Ding, M.; Sun, H.; Xu, Y.; Yin, R.; Chen, H. Micro-Algal Astaxanthin Ameliorates Polystyrene Microplastics-Triggered Necroptosis and Inflammation by Mediating Mitochondrial Ca2+ Homeostasis in Carp’s Head Kidney Lymphocytes (Cyprinus carpio L.). Fish. Shellfish. Immunol. 2023, 143, 109205. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Xiao, T.; Sun, X.; Song, Y.; Shi, W.; Lu, K.; Chen, D.; Sun, C.; Bian, Q. The Regulation of circRNA_kif26b on Alveolar Epithelial Cell Senescence via miR-346-3p Is Involved in Microplastics-Induced Lung Injuries. Sci. Total Environ. 2023, 882, 163512. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An Overview of microRNAs: Biology, Functions, Therapeutics, and Analysis Methods. J. Cell Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Qu, M.; Luo, L.; Yang, Y.; Kong, Y.; Wang, D. Nanopolystyrene-Induced microRNAs Response in Caenorhabditis Elegans after Long-Term and Lose-Dose Exposure. Sci. Total Environ. 2019, 697, 134131. [Google Scholar] [CrossRef]
- Hua, X.; Zhao, Y.; Yuan, Y.; Zhang, L.; Bian, Q.; Wang, D. Nanoplastics Cause Transgenerational Toxicity through Inhibiting Germline microRNA Mir-38 in C. Elegans. J. Hazard. Mater. 2022, 437, 129302. [Google Scholar] [CrossRef]
- Huang, Z.; Weng, Y.; Shen, Q.; Zhao, Y.; Luo, T.; Xiao, Y.; Yang, G.; Jin, Y. Nano- and Micro-Polystyrene Plastics Interfered the Gut Barrier Function Mediated by Exosomal miRNAs in Rats. Environ. Pollut. 2023, 335, 122275. [Google Scholar] [CrossRef]
- Li, Y.; Shi, T.; Li, X.; Sun, H.; Xia, X.; Ji, X.; Zhang, J.; Liu, M.; Lin, Y.; Zhang, R.; et al. Inhaled Tire-Wear Microplastic Particles Induced Pulmonary Fibrotic Injury via Epithelial Cytoskeleton Rearrangement. Environ. Int. 2022, 164, 107257. [Google Scholar] [CrossRef]
- Wu, Q.; Han, X.; Wang, D.; Zhao, F.; Wang, D. Coal Combustion Related Fine Particulate Matter (PM2.5) Induces Toxicity in Caenorhabditis Elegans by Dysregulating microRNA Expression. Toxicol. Res. 2017, 6, 432–441. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Huang, C.C.-Y.; Zheng, C.-M.; Liu, W.-C.; Lee, Y.-H.; Chiu, H.-W. Polystyrene Microplastic-Induced Extracellular Vesicles Cause Kidney-Related Effects in the Crosstalk between Tubular Cells and Fibroblasts. Ecotoxicol. Environ. Saf. 2024, 273, 116098. [Google Scholar] [CrossRef]
- Pucci, M.; Reclusa Asiáin, P.; Duréndez Sáez, E.; Jantus-Lewintre, E.; Malarani, M.; Khan, S.; Fontana, S.; Naing, A.; Passiglia, F.; Raez, L.E.; et al. Extracellular Vesicles As miRNA Nano-Shuttles: Dual Role in Tumor Progression. Target. Oncol. 2018, 13, 175–187. [Google Scholar] [CrossRef]
- Letellier, M.; Lagardère, M.; Tessier, B.; Janovjak, H.; Thoumine, O. Optogenetic Control of Excitatory Post-Synaptic Differentiation through Neuroligin-1 Tyrosine Phosphorylation. Elife 2020, 9, e52027. [Google Scholar] [CrossRef]
- Yao, H.; Yang, S.-R.; Edirisinghe, I.; Rajendrasozhan, S.; Caito, S.; Adenuga, D.; O’Reilly, M.A.; Rahman, I. Disruption of P21 Attenuates Lung Inflammation Induced by Cigarette Smoke, LPS, and fMLP in Mice. Am. J. Respir. Cell Mol. Biol. 2008, 39, 7–18. [Google Scholar] [CrossRef]
- Gong, Q.; Li, W.; Ali, T.; Hu, Y.; Mou, S.; Liu, Z.; Zheng, C.; Gao, R.; Li, A.; Li, T.; et al. eIF4E Phosphorylation Mediated LPS Induced Depressive-like Behaviors via Ameliorated Neuroinflammation and Dendritic Loss. Transl. Psychiatry 2023, 13, 352. [Google Scholar] [CrossRef] [PubMed]
- Larrue, R.; Fellah, S.; Van der Hauwaert, C.; Hennino, M.-F.; Perrais, M.; Lionet, A.; Glowacki, F.; Pottier, N.; Cauffiez, C. The Versatile Role of miR-21 in Renal Homeostasis and Diseases. Cells 2022, 11, 3525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, Z.; Watabe, K.; Zhang, X.; Bai, C.; Xu, M.; Wu, F.; Mo, Y.-Y. Negative Regulation of lncRNA GAS5 by miR-21. Cell Death Differ. 2013, 20, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-J.; Yang, J.; Wu, J.-W.; Sun, X.-R.; Gao, X.-J. Polyethylene Microplastics Induced Inflammation via the miR-21/IRAK4/NF-κB Axis Resulting to Endoplasmic Reticulum Stress and Apoptosis in Muscle of Carp. Fish. Shellfish. Immunol. 2024, 145, 109375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, M.; Cui, X.; O’Connell, D.; Yang, Y. Long Noncoding RNA NEAT1 Promotes Ferroptosis by Modulating the miR-362-3p/MIOX Axis as a ceRNA. Cell Death Differ. 2022, 29, 1850–1863. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xue, S.; Liu, Y.; Peng, M.; Guo, B. The Correlation of Long Non-Coding RNA NEAT1 and Its Targets microRNA (miR)-21, miR-124, and miR-125a with Disease Risk, Severity, and Inflammation of Allergic Rhinitis. Medicine 2021, 100, e22946. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Geng, G.; Zhao, C.; Gao, T.; Wei, B. LncRNA MEG3 Promotes Cisplatin Sensitivity of Cervical Cancer Cells by Regulating the miR-21/PTEN Axis. BMC Cancer 2022, 22, 1145. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Huang, F.-Z.; Liu, H.-Z.; Zhang, T.-Y.; Yang, M.-S.; Sun, C.-Z. LncRNA MEG3 Functions as a ceRNA in Regulating Hepatic Lipogenesis by Competitively Binding to miR-21 with LRP6. Metabolism 2019, 94, 1–8. [Google Scholar] [CrossRef]
- Zhu, M.-R.; Wang, H.-R.; Han, F.-X.; Cai, Z.-L.; Wang, J.-J.; Guo, M.-Y. Polyethylene Microplastics Cause Apoptosis via the MiR-132/CAPN Axis and Inflammation in Carp Ovarian. Aquat. Toxicol. 2023, 265, 106780. [Google Scholar] [CrossRef]
- Marques-Rocha, J.L.; Samblas, M.; Milagro, F.I.; Bressan, J.; Martínez, J.A.; Marti, A. Noncoding RNAs, Cytokines, and Inflammation-Related Diseases. FASEB J. 2015, 29, 3595–3611. [Google Scholar] [CrossRef]
- Chu, Q.; Yan, X.; Liu, L.; Xu, T. The Inducible microRNA-21 Negatively Modulates the Inflammatory Response in Teleost Fish via Targeting IRAK4. Front. Immunol. 2019, 10, 1623. [Google Scholar] [CrossRef]
- Guinea-Viniegra, J.; Jiménez, M.; Schonthaler, H.B.; Navarro, R.; Delgado, Y.; Concha-Garzón, M.J.; Tschachler, E.; Obad, S.; Daudén, E.; Wagner, E.F. Targeting miR-21 to Treat Psoriasis. Sci. Transl. Med. 2014, 6, 225re1. [Google Scholar] [CrossRef]
- Loboda, A.; Sobczak, M.; Jozkowicz, A.; Dulak, J. TGF-Β1/Smads and miR-21 in Renal Fibrosis and Inflammation. Mediators Inflamm. 2016, 2016, 8319283. [Google Scholar] [CrossRef]
- Abdelrahman, S.A.; El-Shal, A.S.; Abdelrahman, A.A.; Saleh, E.Z.H.; Mahmoud, A.A. Neuroprotective Effects of Quercetin on the Cerebellum of Zinc Oxide Nanoparticles (ZnoNps)-Exposed Rats. Tissue Barriers 2023, 11, 2115273. [Google Scholar] [CrossRef]
- Meisgen, F.; Xu, N.; Wei, T.; Janson, P.C.; Obad, S.; Broom, O.; Nagy, N.; Kauppinen, S.; Kemény, L.; Ståhle, M.; et al. MiR-21 Is up-Regulated in Psoriasis and Suppresses T Cell Apoptosis. Exp. Dermatol. 2012, 21, 312–314. [Google Scholar] [CrossRef]
- Kurowska-Stolarska, M.; Alivernini, S.; Ballantine, L.E.; Asquith, D.L.; Millar, N.L.; Gilchrist, D.S.; Reilly, J.; Ierna, M.; Fraser, A.R.; Stolarski, B.; et al. MicroRNA-155 as a Proinflammatory Regulator in Clinical and Experimental Arthritis. Proc. Natl. Acad. Sci. USA 2011, 108, 11193–11198. [Google Scholar] [CrossRef]
- Klöting, N.; Berthold, S.; Kovacs, P.; Schön, M.R.; Fasshauer, M.; Ruschke, K.; Stumvoll, M.; Blüher, M. MicroRNA Expression in Human Omental and Subcutaneous Adipose Tissue. PLoS ONE 2009, 4, e4699. [Google Scholar] [CrossRef]
- Zhao, D.-W.; Hou, Y.-S.; Sun, F.-B.; Han, B.; Li, S.-J. Effects of miR-132 on Proliferation and Apoptosis of Pancreatic Cancer Cells via Hedgehog Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1978–1985. [Google Scholar] [CrossRef]
- Halappanavar, S.; Jackson, P.; Williams, A.; Jensen, K.A.; Hougaard, K.S.; Vogel, U.; Yauk, C.L.; Wallin, H. Pulmonary Response to Surface-Coated Nanotitanium Dioxide Particles Includes Induction of Acute Phase Response Genes, Inflammatory Cascades, and Changes in microRNAs: A Toxicogenomic Study. Environ. Mol. Mutagen. 2011, 52, 425–439. [Google Scholar] [CrossRef]
- Hwang, T.I.-S.; Cuiu, Y.-C.; Chen, Y.-C.; Chen, P.-C.; Tsai, T.-F.; Chou, K.-Y.; Ho, C.-Y.; Chen, H.-E.; Chang, P.-H.; Chang, A.-C. Tumor Suppressive Functions of hsa-miR-34a on Cell Cycle, Migration and Protective Autophagy in Bladder Cancer. Int. J. Oncol. 2023, 62, 66. [Google Scholar] [CrossRef]
- Cao, M.; Peng, B.; Chen, H.; Yang, M.; Chen, P.; Ye, L.; Wang, H.; Ren, L.; Xie, J.; Zhu, J.; et al. miR-34a Induces Neutrophil Apoptosis by Regulating Cdc42-WASP-Arp2/3 Pathway-Mediated F-Actin Remodeling and ROS Production. Redox Rep. 2022, 27, 167–175. [Google Scholar] [CrossRef]
- Zhang, Q.; Xia, W.; Zhou, X.; Yang, C.; Lu, Z.; Wu, S.; Lu, X.; Yang, J.; Jin, C. PS-MPs or Their Co-Exposure with Cadmium Impair Male Reproductive Function through the miR-199a-5p/HIF-1α-Mediated Ferroptosis Pathway. Environ. Pollut. 2023, 339, 122723. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Chai, B.; Wu, Z.; Gu, Z.; Zou, H.; Zhang, H.; Li, Y.; Sun, Q.; Fang, W.; et al. miR-199a-3p/5p Regulate Tumorgenesis via Targeting Rheb in Non-Small Cell Lung Cancer. Int. J. Biol. Sci. 2022, 18, 4187–4202. [Google Scholar] [CrossRef]
- Esteves, M.; Abreu, R.; Fernandes, H.; Serra-Almeida, C.; Martins, P.A.T.; Barão, M.; Cristóvão, A.C.; Saraiva, C.; Ferreira, R.; Ferreira, L.; et al. MicroRNA-124-3p-Enriched Small Extracellular Vesicles as a Therapeutic Approach for Parkinson’s Disease. Mol. Ther. 2022, 30, 3176–3192. [Google Scholar] [CrossRef]
- Bandiera, S.; Pfeffer, S.; Baumert, T.F.; Zeisel, M.B. miR-122--a Key Factor and Therapeutic Target in Liver Disease. J. Hepatol. 2015, 62, 448–457. [Google Scholar] [CrossRef]
- Long, J.-K.; Dai, W.; Zheng, Y.-W.; Zhao, S.-P. miR-122 Promotes Hepatic Lipogenesis via Inhibiting the LKB1/AMPK Pathway by Targeting Sirt1 in Non-Alcoholic Fatty Liver Disease. Mol. Med. 2019, 25, 26. [Google Scholar] [CrossRef]
- Chen, K.; Lin, T.; Yao, W.; Chen, X.; Xiong, X.; Huang, Z. Adipocytes-Derived Exosomal miR-122 Promotes Non-Alcoholic Fat Liver Disease Progression via Targeting Sirt1. Gastroenterol. Hepatol. 2023, 46, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.R. The Balance of Th17 versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 2018, 19, 730. [Google Scholar] [CrossRef]
- Murugaiyan, G.; da Cunha, A.P.; Ajay, A.K.; Joller, N.; Garo, L.P.; Kumaradevan, S.; Yosef, N.; Vaidya, V.S.; Weiner, H.L. MicroRNA-21 Promotes Th17 Differentiation and Mediates Experimental Autoimmune Encephalomyelitis. J. Clin. Investig. 2015, 125, 1069–1080. [Google Scholar] [CrossRef]
- Zhang, M.; Johnson-Stephenson, T.K.; Wang, W.; Wang, Y.; Li, J.; Li, L.; Zen, K.; Chen, X.; Zhu, D. Mesenchymal Stem Cell-Derived Exosome-Educated Macrophages Alleviate Systemic Lupus Erythematosus by Promoting Efferocytosis and Recruitment of IL-17+ Regulatory T Cell. Stem Cell Res. Ther. 2022, 13, 484. [Google Scholar] [CrossRef]
- Khoshmirsafa, M.; Kianmehr, N.; Falak, R.; Mowla, S.J.; Seif, F.; Mirzaei, B.; Valizadeh, M.; Shekarabi, M. Elevated Expression of miR-21 and miR-155 in Peripheral Blood Mononuclear Cells as Potential Biomarkers for Lupus Nephritis. Int. J. Rheum. Dis. 2019, 22, 458–467. [Google Scholar] [CrossRef]
- Young, N.A.; Valiente, G.R.; Hampton, J.M.; Wu, L.-C.; Burd, C.J.; Willis, W.L.; Bruss, M.; Steigelman, H.; Gotsatsenko, M.; Amici, S.A.; et al. Estrogen-Regulated STAT1 Activation Promotes TLR8 Expression to Facilitate Signaling via microRNA-21 in Systemic Lupus Erythematosus. Clin. Immunol. 2017, 176, 12–22. [Google Scholar] [CrossRef]
- Husakova, M. MicroRNAs in the Key Events of Systemic Lupus Erythematosus Pathogenesis. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2016, 160, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chen, K.; Zhang, M.-H.; Wang, L.-C.; Ma, C.-Y.; Lin, Y.-L.; Zhao, Y.-R. MicroRNA-124 Involves in Ankylosing Spondylitis by Targeting ANTXR2. Mod. Rheumatol. 2015, 25, 784–789. [Google Scholar] [CrossRef] [PubMed]

| MNPs Type and Particle Size | Species/Cell | Changes in miRNA | Phenotype | References |
|---|---|---|---|---|
| PS-MPs (0.1/1 μm) | mice | miR-199a-5p ↓ miR-106a-5p ↓ miR-101a-3p ↑ | Injury to reproduction | [62] |
| PS-MPs (100 nm) | MLE12 cells | miR-346-3p ↓ | cell senescence | [30] |
| TWMP (100 nm) | BEAS-2B cells | miR-1a-3p ↓ miR-206-3p ↓ miR-381-3p ↓ miR-204-5p ↓ | Rearrangement of the cytoskeleton | [35] |
| PE-PMs (8 μm) | Carp | miR-132 ↓ | apoptosis | [49] |
| PS-NPs (50 nm/5 μm) | rat | miR-126a-3p ↓ miR-27a-5p ↓ | inflammation | [34] |
| PE-MPs (8 μm) | Carp | miR-21 ↓ miR-203 ↓ miR-181 ↑ | oxidative stress | [44] |
| PM (250 μM) | Carp | miR-25-5p ↓ | mitochondrial dysfunction | [29] |
| PS-NPs (20 nm) | Caenorhabditis elegans | mir-38 ↓ | transgenerational toxicity | [33] |
| PS-NPs (100 nm) | Caenorhabditis elegans | mir-39 ↓ mir-76 ↓ mir-794 ↓ mir-1830 ↓ | oxidative stress | [32] |
| miR-35 ↑ miR-38 ↑ miR-354 ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Lin, Q.; Gong, C.; Zhao, H.; Peng, R. Research Progress on Micro (Nano)Plastics Exposure-Induced miRNA-Mediated Biotoxicity. Toxics 2024, 12, 475. https://doi.org/10.3390/toxics12070475
Chen T, Lin Q, Gong C, Zhao H, Peng R. Research Progress on Micro (Nano)Plastics Exposure-Induced miRNA-Mediated Biotoxicity. Toxics. 2024; 12(7):475. https://doi.org/10.3390/toxics12070475
Chicago/Turabian StyleChen, Ting, Qizhuan Lin, Changyong Gong, Haiyang Zhao, and Renyi Peng. 2024. "Research Progress on Micro (Nano)Plastics Exposure-Induced miRNA-Mediated Biotoxicity" Toxics 12, no. 7: 475. https://doi.org/10.3390/toxics12070475





