Health Risk Assessment of Heavy Metal(loid)s in the Overlying Water of Small Wetlands Based on Monte Carlo Simulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Water Sampling
2.2. Sample Analysis
2.3. Contamination Assessment
2.4. Health Risk Assessment
2.5. Monte Carlo Simulation
3. Results
3.1. The Concentrations of HMs in the Overlying Water of Small Wetlands
3.2. Contamination Levels of HMs in the Overlying Water of Small Wetlands
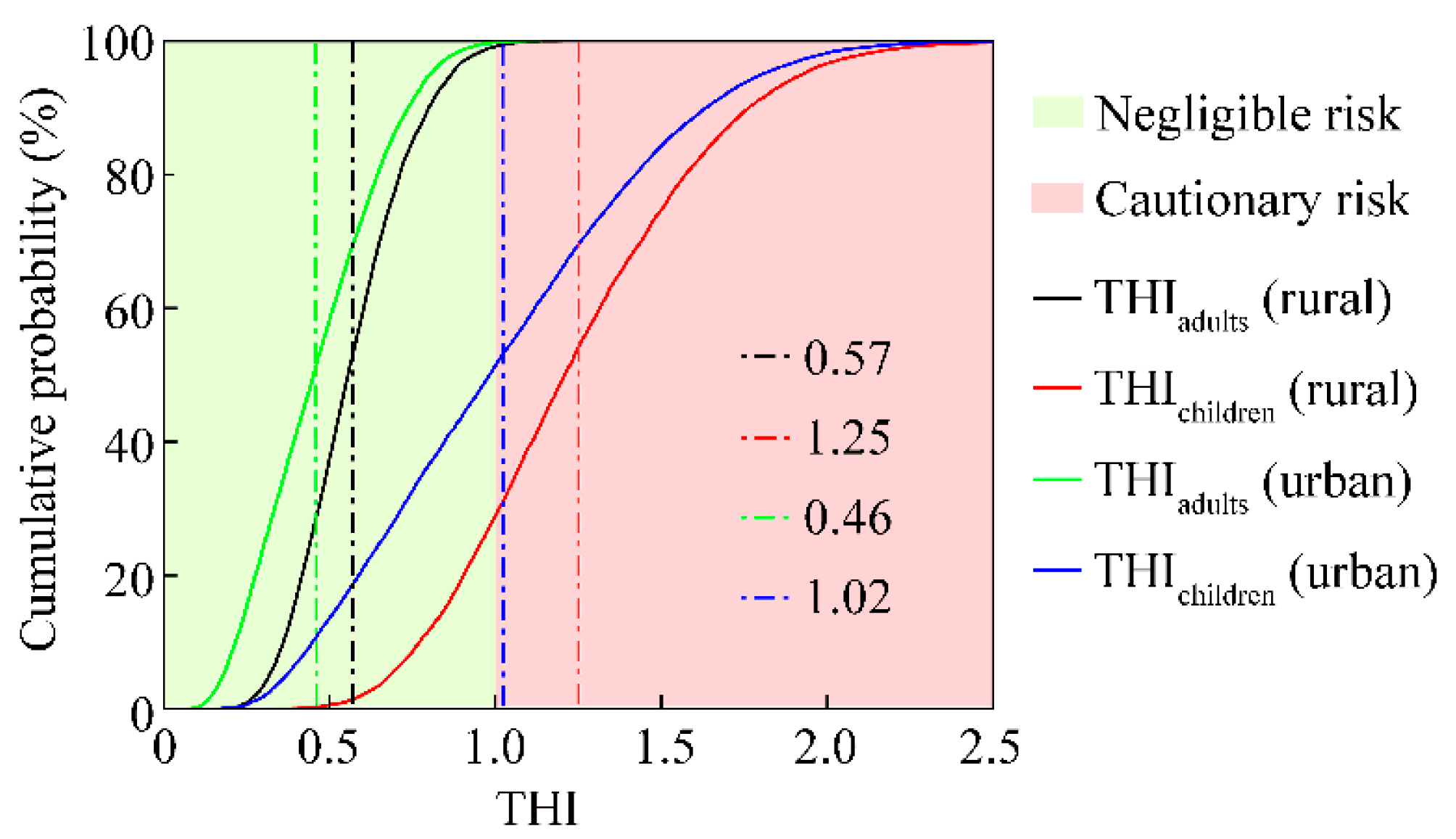
3.3. Non-Carcinogenic Health Risk of HMs in the Overlying Water of Small Wetlands
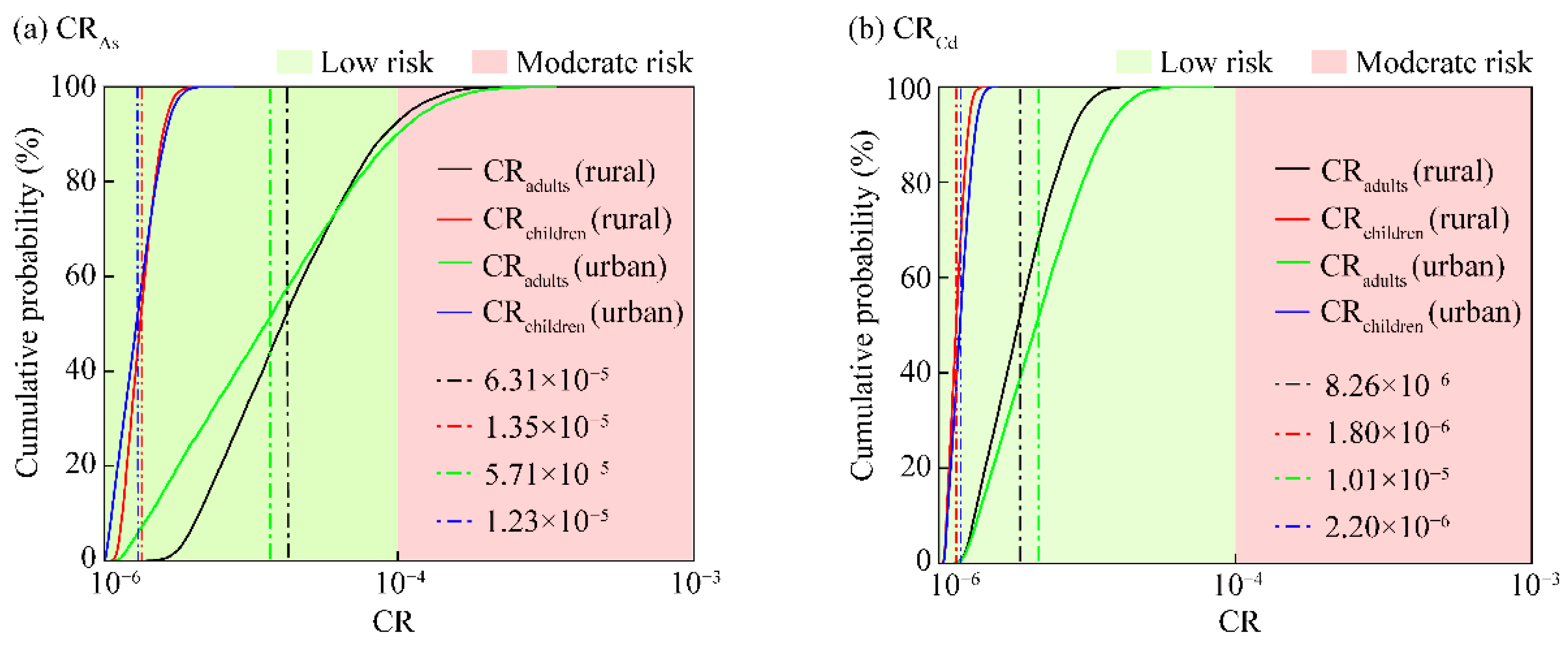
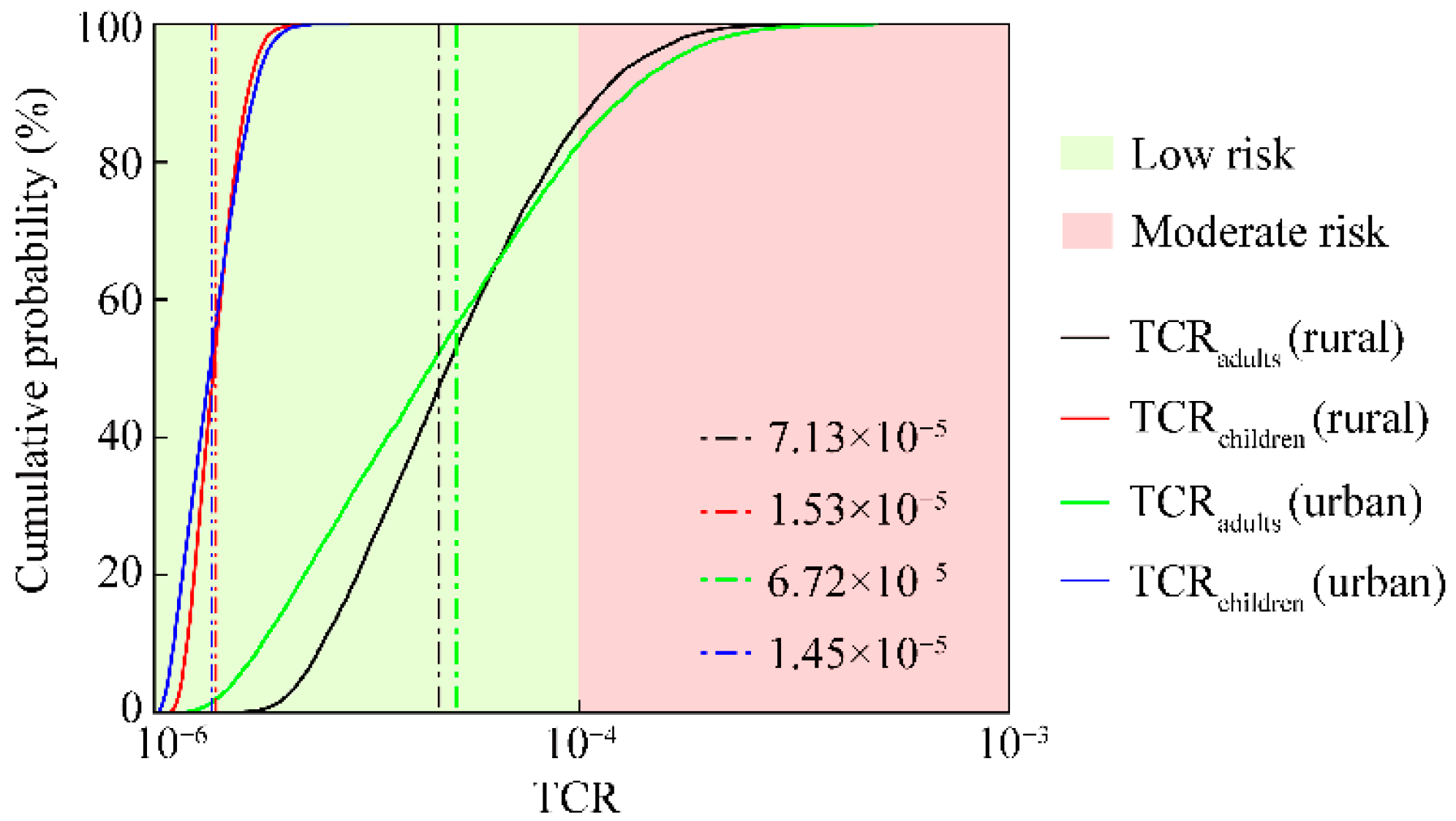
3.4. Carcinogenic Health Risk of HMs in the Overlying Water of Small Wetlands
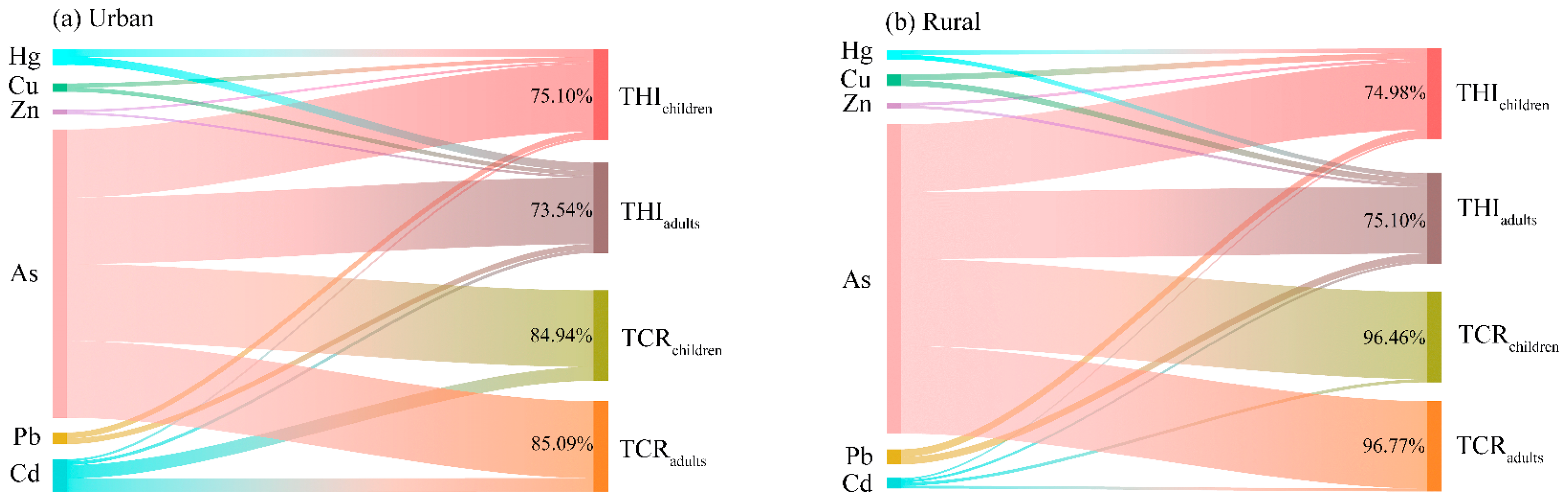
3.5. Sensitivity Analysis
4. Discussion
4.1. Concentration of HMs in the Overlying Water of Small Wetlands
4.2. Comparison of HMs in the Overlying Water of Urban and Rural Small Wetlands
4.3. Identification of the Main Risk Factor in the Overlying Water of Small Wetlands
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, B.W.; Zang, Y.G.; Wu, C.S.; Zhao, Z.H. Spatiotemporal dynamics of wetlands and their future multi-scenario simulation in the Yellow River Delta, China. J. Environ. Manag. 2024, 353, 120193. [Google Scholar] [CrossRef] [PubMed]
- Davidson, N.C.; Fluet-Chouinard, E.; Finlayson, C.M. Global extent and distribution of wetlands: Trends and issues. Mar. Freshw. Res. 2018, 69, 620. [Google Scholar] [CrossRef]
- Junk, W.J.; An, S.Q.; Finlayson, C.M.; Gopal, B.; Květ, J.; Mitchell, S.A.; Mitsch, W.J.; Robarts, R.D. Current state of knowledge regarding the world’s wetlands and their future under global climate change: A synthesis. Aquat. Sci. 2013, 75, 151–167. [Google Scholar] [CrossRef]
- Tanveer, S. Pollutant removal performance of the bioenergy-producing constructed wetlands under unstable loading conditions: Carbon and metallic electrode. J. Water Process Eng. 2024, 58, 104866. [Google Scholar] [CrossRef]
- Rahimi, L.; Malekmohammadi, B.; Yavari, A.R. Assessing and modeling the impacts of wetland land cover changes on water provision and habitat quality ecosystem services. Nat. Resour. Res. 2020, 29, 3701–3718. [Google Scholar] [CrossRef]
- Fatin, S.; Sandra, L.K.; Tala, A.A.; Fadi, Y.; Brendan, W.J.; Sara, M.A.; Baha, K.; Norhan, A. Assessment and management of the water quality and heavy metal pollution of a protected hypersaline wetland in the United Arab Emirates. Water 2023, 15, 1766. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Bernal, B.; Nahlik, A.M.; Mander, U.; Zhang, L.; Anderson, C.J.; Jørgensen, S.E.; Brix, H. Wetlands, carbon, and climate change. Landsc. Ecol. 2013, 28, 583–597. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Christopher, B.C.; Xue, Z.S.; Tong, S.Z.; Lu, X.G. Regulating effects of climate, net primary productivity, and nitrogen on carbon sequestration rates in temperate wetlands, Northeast China. Ecol. Indic. 2016, 70, 114–124. [Google Scholar] [CrossRef]
- Tornwall, B.; Pitt, A.; Brown, B.; Hawley-Howard, J.; Baldwin, R. Diversity patterns associated with varying dispersal capabilities as a function of spatial and local environmental variables in small wetlands in forested ecosystems. Forests 2020, 11, 1146. [Google Scholar] [CrossRef]
- Pandiyan, J.; Arumugam, R.; Al-Ghanim, K.A.; Sachivkina, N.; Nicoletti, M.; Govindarajan, M. Heavy metals in wetland ecosystem: Investigating metal contamination in waterbirds via primary feathers and its effect on population and diversity. Soil Syst. 2023, 7, 104. [Google Scholar] [CrossRef]
- Rehman, K.; Arslan, M.; Müller, J.A.; Saeed, M.; Imran, A.; Amin, I.; Mustafa, T.; Iqbal, S.; Afzal, M. Bioaugmentation—Enhanced remediation of crude oil polluted water in pilot—Scale floating treatment wetlands. Water 2021, 13, 2882. [Google Scholar] [CrossRef]
- Man, Q.L.; Li, H.R.; Ma, X.D.; Gao, P.; Ren, G.B.; Zhou, B.; Liu, H.L. Distribution coefficients of nitrogen pollutants between water and sediment and their environmental risks in Lingang hybrid constructed wetland fed by industrial tailwater, Tianjin, China. Environ. Sci. Pollut. Res. Inter. 2021, 29, 26312–26321. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, G.; Jasrotia, A.; Raj, P.; Kaur, R.; Kumari, A.; Rajput, V.D.; Minkina, T.; Mandzhieva, S.; Kaur, R. Contamination of water and sediments of Harike wetland with phthalate esters and associated risk assessment. Water 2023, 15, 1009. [Google Scholar] [CrossRef]
- Jarf, M.P.; Kamali, A.; Khara, H.; Pourang, N.; Shokrabi, P.H. Microplastic pollution and heavy metal risk assessment in Perca fluviatilis from Anzali wetland: Implications for environmental health and human consumption. Sci. Total. Environ. 2023, 907, 167978. [Google Scholar] [CrossRef] [PubMed]
- Bin, K.; Junga, L.; Jeryang, P. Role of small wetlands on the regime shift of ecological network in a wetlands cape. Environ. Res. 2022, 4, 041006. [Google Scholar] [CrossRef]
- Szpakowska, B.; Świerk, D.; Dudzińska, A.; Pajchrowska, M.; Gołdyn, R. The influence of land use in the catchment area of small waterbodies on the quality of water and plant species composition. Sci. Rep. 2022, 12, 265–7279. [Google Scholar] [CrossRef] [PubMed]
- Seekell, D.A.; Pace, M.L.; Tranvik, L.J.; Verpoorter, C. A fractal-based approach to lake size-distributions. Geophys. Res. Lett. 2013, 40, 517–521. [Google Scholar] [CrossRef]
- Zhang, J.M.; Chu, L.; Zhang, Z.X.; Zhu, B.; Liu, X.Y.; Yang, Q. Evolution of small and micro wetlands and their driving factors in the Yangtze River Delta: A case study of Wuxi Area. Remote Sens. 2023, 15, 1152. [Google Scholar] [CrossRef]
- Scheffer, M.; Van Geest, G.J.; Zimmer, K.D.; Jeppesen, E.; Søndergaard, M.; Butler, M.G.; Hanson, M.A.S.; Declerck, M.L.D. Small habitat size and isolation can promote species richness: Second-order effects on biodiversity in shallow lakes and ponds. Oikos 2006, 112, 227–231. [Google Scholar] [CrossRef]
- Marton, J.M.; Creed, I.F.; Lewis, D.B.; Lane, C.R.; Basu, N.B.; Cohen, M.J.; Craft, C.B. Geographically isolated wetlands are important biogeochemical reactors on the landscape. BioScience 2015, 65, 408–418. [Google Scholar] [CrossRef]
- Schmadel, N.M.; Harvey, J.W.; Schwarz, G.E.; Alexander, R.B.; Gomez-velez, J.D.; Scott, D.; Ator, S.W. Small ponds in headwater catchments are a dominant influence on regional nutrient and sediment budgets. Geophys. Res. Lett. 2019, 46, 9669–9677. [Google Scholar] [CrossRef]
- Holgerson, M.A.; Raymond, P.A. Large contribution to inland water CO2 and CH4 emissions from very small ponds. Nat. Geosci. 2016, 9, 222–226. [Google Scholar] [CrossRef]
- Downing, J.A. Emerging global role of small lakes and ponds: Little things mean a lot. Limnetica 2010, 29, 9–24. [Google Scholar] [CrossRef]
- Cheng, F.Y.; Basu, N.B. Biogeochemical hotspots: Role of small water bodies in landscape nutrient processing. Water Res. 2017, 53, 5038–5056. [Google Scholar] [CrossRef]
- GB/T 42481-2023; Specification on the Conservation and Management of Small Wetlands. State Administration for Market Regulation of China; Standardization Administration of China: Beijing, China, 2023. Available online: https://www.doc88.com/p-23247601745135.html (accessed on 1 October 2023).
- Zhang, J.D.; Li, J.L.; Bao, A.M.; Frankl, A.; Wang, H.Y.; Bai, J.; Shen, Z.F.; Li, L.H.; Maeyer, P.D.; Vande, V.T. Ecological restoration trajectory of the Taitema Lake wetland in arid northwest China: A 36-year wetland health assessment using Landsat time series data. Ecol. Indic. 2024, 161, 111956. [Google Scholar] [CrossRef]
- Céréghino, R.; Boix, D.; Cauchie, H.; Martens, K.; Oertli, B. The ecological role of ponds in a changing world. Hydrobiologia 2013, 723, 1–6. [Google Scholar] [CrossRef]
- Mitsuo, Y.; Tsunoda, H.; Kozawa, G.; Yuma, M. Response of the fish assemblage structure in a small farm pond to management dredging operations. Agric. Ecosyst. Environ. 2014, 188, 93–96. [Google Scholar] [CrossRef]
- Pinhati, F.S.C.; Rodrigues, L.N.; Souza, S.A.D. Modelling the impact of on-farm reservoirs on dry season water availability in an agricultural catchment area of the Brazilian savannah. Agric. Water Manag. 2020, 241, 106296. [Google Scholar] [CrossRef]
- Biggs, J.; von Fumetti, S.; Kelly-Quinn, M. The importance of small waterbodies for biodiversity and ecosystem services: Implications for policy makers. Hydrobiologia 2017, 793, 3–39. [Google Scholar] [CrossRef]
- Huang, J.L.; Wu, Y.Y.; Sun, J.X.; Li, X.; Geng, X.L.; Zhao, M.L.; Sun, T.; Fan, Z.Q. Health risk assessment of heavy metal(loid)s in park soils of the largest megacity in China by using Monte Carlo simulation coupled with Positive matrix factorization model. J. Hazard. Mater. 2021, 415, 125629. [Google Scholar] [CrossRef] [PubMed]
- Muyassar, M.; Mamattursun, E.; Wang, L.L.; Xayida, S.; Wang, N.; Hu, Y.L. Pollution and ecological risk assessment of metal elements in groundwater in the Ibinur Lake Basin of NW China. Water 2023, 15, 4071. [Google Scholar] [CrossRef]
- Sheng, D.R.; Meng, X.H.; Wen, X.H.; Wu, J.; Yu, H.J.; Wu, M. Contamination characteristics, source identification, and source-specific health risks of heavy metal(loid)s in groundwater of an arid oasis region in Northwest China. Sci. Total. Environ. 2022, 841, 156733. [Google Scholar] [CrossRef] [PubMed]
- Mamattursun, E.; Nazupar, S.; Zhong, Q.; Atiyagul, R.; Li, X.G. Distribution, pollution levels, and health risk assessment of heavy metals in groundwater in the main pepper production area of China. Open Geosci. 2023, 15, 20220491. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Maio, M.D.; Singh, P.K.; Mahato, M.K. Evaluation of surface water quality by using GIS and a heavy metal pollution index (HPI) model in a coal mining area, India. Rev. Environ. Contam. Toxic. 2015, 95, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, J.; Gao, X.; Tian, B.; Zhang, J.; Wang, X.; Liu, Z. Human health ambient water quality criteria for 13 heavy metals and health risk assessment in Taihu Lake. Front. Environ. Sci. Eng. 2021, 16, 41. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.L.; Li, J.P.; Wang, Z.H.; Xian, F.Y.; Fan, Y.Y. Spatial distribution characteristics and pollution levels of heavy metals in surface water and sediments of the Heihe cascade reservoir system, China. Water Supply 2022, 22, 8353–8371. [Google Scholar] [CrossRef]
- Qu, L.; Huang, H.; Xia, F.; Liu, Y.; Dahlgren, R.A.; Zhang, M.; Mei, K. Risk analysis of heavy metal concentration in surface waters across the rural-urban interface of the Wen-Rui Tang River, China. Environ. Pollut. 2018, 237, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Nazupar, S.; Mamattursun, E.; Li, X.G.; Wang, Y.H. Spatial distribution, contamination levels, and health risks of trace elements in topsoil along an urbanization gradient in the city of Urumqi, China. Sustainability 2022, 14, 12646–12664. [Google Scholar] [CrossRef]
- GB/T 5750.6-2006; Standard Examination Method for Drinking Water-Metal Parameters. MHPRC (Ministry of Health of the People’s Republic of China): Beijing, China, 2007. (In Chinese)
- Nemerow, N.L. Stream, Lake, Estuary, and Ocean Pollution; Van Nostrand Reinhold Publishing Co.: New York, NY, USA, 1985. [Google Scholar]
- GB 3838-2002; Environmental Quality Standards for Surface Water. MEEPRC (Ministry of Ecology and Environment of the People’s Republic of China): Beijing, China, 2002. (In Chinese)
- US EPA (U.S. Environmental Protection Agency). Regional Screening Levels (RSLs)—User’s Guide. 2016. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-users-guide-may-2016 (accessed on 23 May 2024).
- EPA 540-R-02-002; Risk Assessment Guidance for Superfund: Volume III. Part A: Process for Conducting Probabilistic Risk Assessment. US EPA (U.S. Environmental Protection Agency). United States Environmental Protection Agency: Washington, DC, USA, 2001.
- Rahman, M.M.; Bodrud-Doza, M.; Siddiqua, M.T.; Zahid, A.; Towfiqul Islam, A.R.M. Spatio-temporal distribution of fluoride in drinking water and associated probabilistic human health risk appraisal in the coastal region, Bangladesh. Sci. Total. Environ. 2020, 724, 138316. [Google Scholar] [CrossRef] [PubMed]
- EPA/540/R/99/005; OSWER 9285.7-02EP; PB 99-963312; Risk Assessment Guidance for Superfund., Human Health Evaluation Manual. Part E (Volume1). Supplemental Guidance for Dermal Risk Assessment. US EPA (U.S. Environmental Protection Agency). Office of Superfund Remediation and Technology Innovation: Washington, DC, USA, 2004.
- EPA/540/1-89/002; Risk Assessment Guidance for Superfund. Human Health Evaluation Manual, Part A (Volume 1). US EPA (U.S. Environmental Protection Agency). Office of Emergency and Remedial Response: Washington, DC, USA, 1989.
- Agbasi, J.C.; Chukwu, C.N.; Nweke, N.D.; Uwajingba, H.C.; Khan, M.Y.A.; Egbueri, J.C. Water pollution indexing and health risk assessment due to PTE ingestion and dermal absorption for nine human populations in Southeast Nigeria. Groundw. Sustain. Develop. 2023, 21, 100921. [Google Scholar] [CrossRef]
- Han, Q.; Wang, M.S.; Cao, J.L.; Gui, C.L.; Liu, Y.P.; He, X.D.; He, Y.C.; Liu, Y. Health risk assessment and bioaccessibilities of heavy metals for children in soil and dust from urban parks and schools of Jiaozuo, China. Ecotoxicol. Environ. Saf. 2020, 191, 110157. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.H.; Lu, J.; Wu, J.; Lin, Y.C.; Luo, Y.M. Influence of coastal groundwater salinization on the distribution and risks of heavy metals. Sci. Total Environ. 2019, 652, 267–277. [Google Scholar] [CrossRef]
- Chen, R.H.; Chen, H.Y.; Song, L.T.; Yao, Z.P.; Meng, F.S.; Teng, Y.G. Characterization and source apportionment of heavy metals in the sediments of Lake Tai (China) and its surrounding soils. Sci. Total Environ. 2019, 694, 133819. [Google Scholar] [CrossRef] [PubMed]
- US EPA (U.S. Environmental Protection Agency). Guiding Principles for Monte Carlo Analysis; US EPA: Washington, DC, USA, 1997; pp. 1–35.
- Qin, W.; Han, D.; Song, X.; Liu, S. Sources and migration of heavy metals in a karst water system under the threats of an abandoned Pb–Zn mine, southwest China. Environ. Pollut. 2021, 277, 116774. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Liao, J.; Gai, P.X.; Guo, X.L.; Zheng, W.; Li, X.; Ran, Y.L.; Wang, Z.W.; Chen, J.B. Metabolisms of both inorganic and methyl-mercury in hens reveal eggs as an effective bioindicator for environmental Hg pollution. J. Hazard. Mater. 2024, 465, 133191. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Y.; Zhang, Y.S.; Feng, C.L.; Guo, C.S.; You, J.; Sun, Y.W.; Liu, X.M.; Wu, F.C. Progress, challenge and strategic countermeasures of Chinese pollution risk management of water environment. Res. Environ. Sci. 2021, 34, 1532–1541. (In Chinese) [Google Scholar] [CrossRef]
- Stefan, L.; Jes, J.R.; Angelika, S.; Thomas, K.; Burkhard, G.; Peter, H.; Matthias, S.; Bernd, H.; Ralf, B.S. Specifics and challenges of assessing exposure and effects of pesticides in small water bodies. Hydrobiologia 2016, 793, 213–224. [Google Scholar] [CrossRef]
- Hageman, K.J.; Simonich, S.L.; Campbell, D.H.; Wilson, G.R.; Landers, D.H. Atmospheric deposition of current-use and historic-use pesticides in snow at national parks in the Western United States. Environ. Sci. Technol. 2006, 40, 3174–3180. [Google Scholar] [CrossRef] [PubMed]
- Bereswill, R.; Golla, B.; Streloke, M.; Schulz, R. Entry and toxicity of organic pesticides and copper in vineyard streams: Erosion rills jeopardize the efficiency of riparian buffer strips. Agric. Ecosyst. Environ. 2012, 146, 81–92. [Google Scholar] [CrossRef]
- Stehle, S.; Schulz, R. Agricultural insecticides threaten surface waters at the global scale. Environ. Sci. 2015, 112, 5570–5575. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.Q.; Wu, S.J.; Ma, M.H.; Huang, P.; Wen, Z.F.; Chen, J.L. Small water bodies in China: Spatial distribution and influencing factors. Sci. China Earth. Sci. 2022, 65, 1431–1448. [Google Scholar] [CrossRef]
- Golden, H.E.; Rajib, A.; Lane, C.R.; Christensen, J.R.; Wu, Q.; Mengistu, S. Non-floodplain wetlands affect watershed nutrient dynamics: A critical review. Environ. Sci. Technol. 2019, 53, 7203–7214. [Google Scholar] [CrossRef] [PubMed]
- Picard, C.R.; Fraser, L.H.; Steer, D. The interacting effects of temperature and plant community type on nutrient removal in wetland microcosms. Bioresour. Technol. 2005, 96, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Peris, M.; Micó, C.; Recatalá, L.; Sánchez, R.; Sánchez, J. Heavy metal contents in horticultural crops of a representative area of the European Mediterranean region. Sci. Total Environ. 2007, 378, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.P.; Gao, T.T.; Li, X.Z.; Wang, Y. Didactical approaches and insights into environmental processes and cardiovascular hazards of arsenic contaminants. Chemosphere 2024, 352, 141381. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.M.; He, X.Y.; Chen, M.S.; Qian, B.; Li, M.J.; Yan, Y.L.; Lin, C.; Mao, Z.G. High arsenic pollution of the eutrophic Lake Taihu and its relationship with iron, manganese, and dissolved organic matter: High-resolution synchronous analysis. J. Hazard. Mater. 2024, 467, 133644. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Li, Q.Q.; Cao, Q. A mini-review regarding the control techniques available for arsenic contamination from flue gas and water. J. Environ. Chem. Eng. 2024, 12, 112249. [Google Scholar] [CrossRef]



| Class | Contamination Degree | Pi | NPI |
|---|---|---|---|
| I | No contamination | Pi < 0.7 | NPI < 0.7 |
| II | Slightly contamination | 0.7 < Pi ≤ 1 | 0.7 < NPI ≤ 1 |
| III | Light contamination | 1 < Pi ≤ 2 | 1 < NPI ≤ 2 |
| IV | Moderate contamination | 2 < Pi ≤ 3 | 2 < NPI ≤ 3 |
| V | High contamination | Pi > 3 | NPI > 3 |
| Parameters | Meaning | Units | Children | Adults | References |
|---|---|---|---|---|---|
| IR | Ingestion rate | L/day | Mean = 1.25 SD = 0.3 | Mean = 1.95 SD = 0.3 | [33] |
| EF | Exposure frequency | days/year | Minimum = 180 Most probable = 350 Maximum = 360 | Minimum = 180 Most probable = 350 Maximum = 360 | [43] |
| ED | Exposure duration | year | 2.5 | 26 | [44] |
| BW | Body weight | kg | Mean = 20 SD = 0.15 | Mean = 70 SD = 0.15 | [45] |
| ATnon-cancer | Averaging time of non-cancer risk | days | 912.5 | 9490 | [44] |
| ATcancer | Averaging time of cancer risk | days | 25,550 | 25,550 | [44] |
| SA | Skin surface area | cm2 | 7422 | 18,182 | [33] |
| KP | Absorption coefficient | cm/h | 10−3 for Hg, Cu, As, and Cd, 6 × 10−4 for Zn, and 10−4 for Pb | [46] | |
| Tevent | Absorption coefficient | h/event | 0.58 | 1 | [46] |
| EV | Event frequency | event/day | 1 | 1 | [43] |
| HMs | RfDing (µg/kg/d) | RfDderm (µg/kg/d) | SFing (µg/kg/d)−1 | SFderm (µg/kg/d)−1 |
|---|---|---|---|---|
| Hg | 0.3 | 0.024 | / | / |
| As (for non-cancer) | 0.3 | 0.123 | / | / |
| As (for cancer) | / | / | 1.5 × 10−3 | 3.66 × 10−3 |
| Cu | 40 | 12 | / | / |
| Zn | 300 | 60 | / | / |
| Pb | 1.4 | 0.42 | / | / |
| Cd (for non-cancer) | 0.5 | 0.005 | / | / |
| Cd (for cancer) | / | / | 6.1 × 10−3 | 3.8 × 10−4 |
| Gradients | Statistics | Hg | As | Cu | Zn | Pb | Cd |
|---|---|---|---|---|---|---|---|
| Urban wetlands (n = 20) | Max (μg/L) | 0.88 | 8.30 | 73.0 | 298.0 | 3.53 | 0.34 |
| Min (μg/L) | 0.04 | 0.50 | 1.48 | 7.44 | 0.10 | 0.05 | |
| Average (μg/L) | 0.16 | 2.70 | 37.76 | 93.18 | 1.20 | 0.09 | |
| SD (μg/L) | 0.21 | 1.80 | 20.46 | 54.33 | 0.86 | 0.06 | |
| CV * | 1.29 | 0.67 | 0.54 | 0.58 | 0.72 | 0.73 | |
| Standard-exceeding ratio (%) | 80.0 | 0 | 0 | 0 | 0 | 0 | |
| Rural wetlands (n = 16) | Max (μg/L) | 1.13 | 7.60 | 232.0 | 344.0 | 3.70 | 0.27 |
| Min (μg/L) | 0.04 | 2.10 | 1.22 | 78.60 | 0.97 | 0.05 | |
| Average (μg/L) | 0.25 | 4.15 | 63.29 | 175.65 | 2.18 | 0.14 | |
| SD (μg/L) | 0.31 | 1.79 | 49.42 | 71.00 | 0.89 | 0.06 | |
| CV | 1.25 | 0.43 | 0.78 | 0.40 | 0.41 | 0.43 | |
| Standard-exceeding ratio (%) | 81.25 | 0 | 0 | 0 | 0 | 0 | |
| National standard (μg/L) ** | 0.05 | 50 | 1000 | 1000 | 10 | 5 | |
| Gradients | Statistics | PHg | PAs | PCu | PZn | PPb | PCd | NPI |
|---|---|---|---|---|---|---|---|---|
| Urban wetlands (n = 20) | Max | 17.60 | 0.17 | 0.07 | 0.30 | 0.35 | 0.07 | 12.62 |
| Min | 0.80 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.58 | |
| Average | 3.21 | 0.05 | 0.04 | 0.09 | 0.12 | 0.02 | 2.31 | |
| Rural wetlands (n = 16) | Max | 22.60 | 0.15 | 0.23 | 0.34 | 0.37 | 0.05 | 16.22 |
| Min | 0.80 | 0.04 | 0.00 | 0.08 | 0.10 | 0.01 | 0.58 | |
| Average | 4.98 | 0.08 | 0.06 | 0.18 | 0.22 | 0.03 | 3.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Eziz, M.; Hu, Y.; Subi, X. Health Risk Assessment of Heavy Metal(loid)s in the Overlying Water of Small Wetlands Based on Monte Carlo Simulation. Toxics 2024, 12, 488. https://doi.org/10.3390/toxics12070488
Wang L, Eziz M, Hu Y, Subi X. Health Risk Assessment of Heavy Metal(loid)s in the Overlying Water of Small Wetlands Based on Monte Carlo Simulation. Toxics. 2024; 12(7):488. https://doi.org/10.3390/toxics12070488
Chicago/Turabian StyleWang, Liling, Mamattursun Eziz, Yonglong Hu, and Xayida Subi. 2024. "Health Risk Assessment of Heavy Metal(loid)s in the Overlying Water of Small Wetlands Based on Monte Carlo Simulation" Toxics 12, no. 7: 488. https://doi.org/10.3390/toxics12070488





